Abstract
Type 2 diabetes (T2D) is a major risk factor for late-onset Alzheimer's disease (AD). A variety of metabolic changes related to T2D (e.g. hyperinsulinemia, hyperglycemia, and elevated branched-chain amino acids) have been proposed as mechanistic links, but the basis for this association remains unknown. Retromer-dependent trafficking is implicated in the pathogenesis of AD, and two key retromer proteins, VPS35 and VPS26, are deficient in the hippocampal formation of AD patients. We characterized VPS35 levels in five different mouse models of T2D/obesity to identify specific metabolic factors that could affect retromer in the brain. Mouse models in which hyperleucinemia was present displayed hippocampus-selective retromer deficiency. Wild-type lean mice fed a high leucine diet also developed hippocampal-selective retromer deficiency, and neuronal-like cells grown in high ambient leucine had reduced retromer complex proteins. Our results suggest that hyperleucinemia may account, in part, for the association of insulin resistance/T2D with AD.
Keywords: Alzheimer's disease, Obesity, Diabetes, Retromer
Introduction
Although late-onset Alzheimer's disease (AD) is a pathogenically complex disorder, recent findings have implicated a few unifying mechanisms (Tosto and Reitz, 2013). One of these is endosomal trafficking, in which retromer-dependent transport plays a key role (Gandy and DeKosky, 2013; Siegenthaler and Rajendran, 2012). The retromer, a coat-like complex that traffics cargo out of endosomes, is composed of two functionally distinct subcomplexes. The first is a trimeric complex comprising Vacuolar Protein Sorting 35 (VPS35), VPS26, and VPS29, the core of the retromer that binds cargo at the endosomal membrane. The second is a membrane-associated subcomplex made up of different sorting nexins (SNX 1, 2, 3, 5, 6, or 27) (Bonifacino and Hurley, 2008).
Retromer-dependent trafficking is implicated in late onset AD. Both VPS35 and VPS26 are reduced by approximately 40% in the hippocampal formation of late-onset AD patients (Small et al., 2005). Genetically-engineered animal models with an equivalent deficiency in hippocampal VPS35 and VPS26 show a loss of retromer function and phenocopy features of AD (Muhammad et al., 2008). Allelic variations in genes that cause dysfunction in retromer-dependent trafficking (e.g. SORL1) have also been linked to AD (Rogaeva et al., 2007; Vardarajan et al., 2012).
Type 2 diabetes (T2D) and AD are associated epidemiologically (Luchsinger et al., 2001), specifically via T2D-associated phenotypes such as hyperinsulinemia, hyperglycemia, and obesity (Luchsinger, 2008). Increased circulating concentrations of branched chain amino acids (BCAAs: leucine, isoleucine, valine), which are also associated with insulin resistance and obesity (Adams, 2011; Newgard, 2012; Ridaura et al., 2013; She et al., 2007), are an early biomarker/risk factor for T2D (Wang et al., 2011). However, these complex metabolic phenotypes of T2D render mechanistic analysis of this association difficult. Recent studies have implicated elements of the retromer pathway in T2D, raising the possibility that AD and T2D share a common molecular pathogenic mechanism (Lane et al., 2012). We asked a simple question: Among the many metabolic abnormalities that characterize T2D, do any specifically cause retromer deficiency in the hippocampal formation? To address this question, we screened mouse models of T2D/obesity for alterations in brain retromer levels to identify a shared factor, which was then validated by performing interventional studies in mice and in neuronal cell cultures.
Materials and methods
Mice
Inbred wild type and mutant male C57BL/6 mice were purchased from The Jackson Laboratory (Bar Harbor, ME), with the exception of ΔGly-APN mice (ΔGly-APN +/+ and ΔGly-APN ob/ob) which were generated by breeding male C57BL/6J ΔGly-APN,Lepob/+ with female C57BL/6J Lepob/+ mice to generate +/+ and ob/ob ΔGly-APN littermates as previously described (Kim et al., 2007). Mice were maintained on a 12-h light/dark cycle (lights on at 0700) in a humidity- and temperature-controlled environment with ad libitum access to water and standard laboratory chow. For the high leucine diet (HLD) experiment, both control chow and HLD chow (Supplementary Table 1) were purchased from Research Diets (New Brunswick, NJ). At either 6–8 weeks of age (ΔGly-APN mice) or 14 weeks of age, mice were fasted for 4 h beginning at 9 AM. Tails were pricked to measure blood glucose with a Freestyle Freedom Lite glucometer (Abbott, Alameda, CA), and mice were weighed and ano-nasal length measured, and anesthetized with CO2. Blood for plasma analysis was drawn by cardiac puncture and collected in a tube containing 50 ΔL of aprotinin (Sigma, St. Louis, MO) plus 50 mM EDTA, spun at 5000 g for 5 min at 4 °C, and plasma was isolated and frozen on dry ice. Following cardiac puncture, mice were decapitated and brain regions (hippocampus, cerebellum) and anatomical tissues (liver, inguinal adipose tissue, and epididymal adipose tissue) were harvested simultaneously in separate dissections and all tissues (and plasma) were frozen on dry ice prior to storage at −80 °C. All animal studies were approved by the Institutional Animal Care and Use Committee of Columbia University.
Hormone analysis
Plasma insulin was measured by ELISA (Crystal Chem, Downers Grove, IL). Plasma leptin was measured by AlphaLISA (Perkin Elmer, Waltham, MA). Blood for plasma amino acid analysis was collected at 9 PM (post-prandial) and 9 AM (post-absorptive) 3 days prior to the day of sacrifice for control and HLD-fed mice. Blood for all other mouse models and HLD fasting plasma was obtained after 4 h fasting as described above. Plasma amino acid concentrations were determined using HPLC by the Hormone Analytic Core of the Mouse Metabolic Phenotyping Center at the Vanderbilt University.
Glucose tolerance
Tail blood glucose was measured at 0, 15, 30, 60, and 90 min after a bolus intraperitoneal glucose administration (1 mg/g body weight) to overnight-fasted mice.
Cell culture
Mouse brain neuroblastoma cells (Neuro-2a, CCL-131™) were obtained from the American Type Culture Collection (ATCC, Manassas, VA) and maintained in plating media (low glucose DMEM + 10% FBS; Life Technologies, Grand Island, NY). Cells were seeded at a density of 100/mm2 in plating media overnight. The next day, media was replaced with differentiation media (low glucose DMEM + 2% FBS + 20 µM retinoic acid). Differentiation media was replaced every 24 h for 5 days total. Basal leucine concentration of DMEM media was 0.8 mM; supplemental leucine (100 mM stock; final concentrations of 1.6 mM, 2.8 mM, and 4.8 mM) was added to the appropriate wells for the final 3 days.
Western blots
Mouse brain samples (hippocampi and cerebellums) were dissected according to Paxinos and Watson Mouse Brain Atlas, and then kept at −80 °C or immediately homogenized in ice-cold RIPA buffer containing a Protease Inhibitor Mixture and a Phosphatase Inhibitor cocktail (Roche, USA). ~20–30 g of protein was analyzed by Western blot. Blots were incubated overnight with a primary antibody targeting VPS35 (ab57632, 1:1000 Abcam, Cambridge, MA), followed by an HRP-labeled goat anti-mouse secondary antibody (1:20,000). The secondary antibody was detected with Immobilon Western Chemiluminescent HRP Substrate (Millipore, Billerica, MA) and data were analyzed with ImageJ.
Protein from N2a cell cultures was isolated by removing culture media, rinsing with PBS for 60 s followed by RIPA buffer (Thermo Fisher Scientific, Rockford, IL) supplemented with HALT proteinase and phosphatase inhibitor (Thermo Fisher Scientific, Rockford, IL). Western blots were blocked for 30 min in Odyssey Blocking Buffer (Licor Biotechnology, Lincoln, NE), then incubated overnight with primary antibodies targeting VPS35 (ab57632, 1:1,000; Abcam, Cambridge, MA), VPS26 (ab23892, 1:5,000; Abcam, Cambridge, MA), VPS34 (#3358, 1:500; Cell Signaling Technologies, Danvers, MA) and α-tubulin (#3873, 1:10,000; Cell Signaling Technologies, Danvers, MA), followed by incubation with fluorescent-labeled secondary antibodies (Donkey α-mouse IRDye 680 and Goat α-rabbit IRDye 800; 1:10,000) for 1 h. Blots were scanned and analyzed using the Odyssey Classic imaging system (LI-COR Biotechnology, Lincoln, NE).
Statistical analysis
All statistical analysis was performed using PRISM software (Graphpad, La Jolla, CA). Body weight and IPGTT data were analyzed by two-way analysis of variance (ANOVA) using treatment (HLD vs. control) and time as independent variables; individual time-points were analyzed by Bonferroni post-test. All other data were analyzed by two-tailed Student's t test. P values less than α = 0.05 were considered significant.
Results
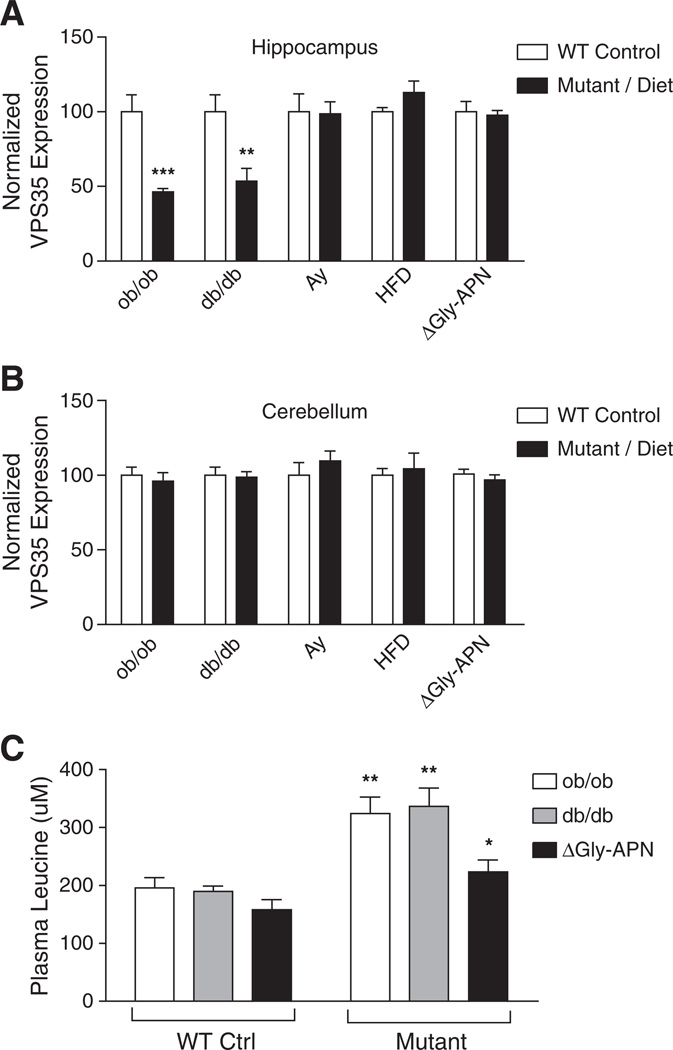
We analyzed levels of VPS35 in the brains of several C57BL/6J mouse models of T2D/obesity: ob/ob (leptin deficient), db/db (leptin receptor deficient), Ay/+ (reduced MC4R signaling), and high fat diet-induced-obese (DIO). We evaluated multiple physiological factors associated with T2D and obesity; all mutant/DIO mice were heavier than their WT/control counterparts and displayed significant increases in body mass, elevations in blood glucose and insulin, and hyperleptinemia (except ob/ob), with the leptin- and leptin receptor-deficient models exhibiting the most severe metabolic phenotypes (Table 1). VPS35 deficiency was only observed in the hippocampus, but not the cerebellum, of ob/ob and db/db mice; no reduction of VPS35 was seen in the hippocampus of Ay/+ and DIO mice (Figs. 1A, B).
Table 1.
Body mass and basal circulating glucose, insulin, and leptin concentrations in obese mouse models.
| Mouse model | Genotype | Body mass (g) | Glucose (mg/dl) | Insulin (ng/mL) | Leptin (ng/mL) |
|---|---|---|---|---|---|
| +/+ | +/+ | 28.9 ± 0.5 | 127.0 ± 8.5 | 0.53 ± 0.20 | 0.6 ± 0.3 |
| ob/ob | ob/ob | 54.9 ± 2.5*** | 251.8 ± 56.6 | 10.69 ± 2.14** | n.d. |
| db/db | db/db | 49.7 ± 0.9*** | 258.4 ± 62.6 | 17.41 ± 2.27*** | 48.5 ± 4.4*** |
| Ay | +/+ | 27.2 ± 0.8 | 95.4 ± 5.5 | 0.74 ± 0.05 | 1.8 ± 0.7 |
| Ay/+ | 33.5 ± 1.4** | 132.8 ± 6.7** | 4.31 ± 0.40*** | 36.3 ± 0.7*** | |
| HFD | Control fed | 26.6 ± 1.2 | 122.8 ± 14.8 | 0.72 ± 0.10 | 2.3 ± 0.2 |
| HFD fed | 35.6 ± 0.3*** | 155.5 ± 22.4 | 1.96 ± 0.37* | 35.8 ± 0.4*** | |
| ΔGly-APN | ΔGly-APN +/+ |
21.3 ± 0.7 | 154.2 ± 7.3 | 1.44 ± 0.80 | 0.4 ± 0.1 |
| ΔGly-APN ob/ob |
43.5 ± 3.0*** | 212.3 ± 18.7* | 6.58 ± 0.88** | n.d. |
Data are presented as mean ± SEM. n.d. indicates value was not detectable.
Indicates P < 0.05 for obese vs. lean control (n = 5–10).
Indicates P < 0.01 for obese vs. lean control (n = 5–10).
Indicates P < 0.001 for obese vs. lean control (n = 5–10).
Fig. 1.
Deficiency of retromer protein levels observed specifically in the hippo-campus of mouse models of T2D/obesity with elevations in plasma leucine. (A, B) VPS35 protein levels in the hippocampus (A) and cerebellum (B) of mouse models of T2D/obesity as determined by Western blot. Data are shown as % of WT/control expression normalized to actin levels (mean ± SEM; n = 5). **P < 0.01, ***P < 0.001. Gly-APN indicates transgenic overexpression of Gly-Adiponectin in both ob/ob and +/+ animals (mean ± SEM; n = 10). (C) Circulating plasma leucine concentrations in leptin signaling-deficient mice compared to controls with normal leptin signaling (mean ± SEM; n = 5). *P < 0.05, **P < 0.01 (vs. paired control).
To determine if leptin deficiency per se, or the metabolic abnormalities associated with leptin deficiency, such as severe insulin resistance, was responsible for the observed retromer deficiency, we measured VPS35 levels in wild type and ob/ob mice overexpressing an adiponectin transgene (ΔGly-APN+/+ vs. ΔGly-APNob/ob) (Combs et al., 2004), which reduces insulin resistance and protects ob/ob mice from diabetes in the presence of unabated morbid obesity (Kimet al., 2007; Yamauchi et al., 2003). Overexpression of the ΔGly-APN transgene improved both the hyperinsulinemia and hyperglycemia in the ob/ob mice (Table 1) and there was no difference in VPS35 expression levels between ΔGly-APN+/+ and ΔGly-APNob/ob mice (Fig. 1A), indicating that the retromer deficiency observed in ob/ob and db/dbmice did not result directly from a lack of leptin signaling, or obesity per se, but rather from metabolic abnormalities associated with leptin deficiency that were rectified by the overexpression of adiponectin.
Analysis of plasma amino acid concentrations in T2D/obese mouse models revealed ~40–110% elevations in leucine, isoleucine, valine, phenylalanine, and tryptophan in ob/ob and db/db, but not the ΔGly-APNob/ob mice or any other T2D/obese models tested (Supplementary Tables 2–6). Since increased circulating concentrations of branched chained amino acids (BCAAs) are associated with insulin resistance/T2D (Melnik, 2012; Rafecas et al., 1991; Wang et al., 2011; Wijekoon et al., 2004), these specific amino acids could represent a mechanistic link between T2D and AD. We focused on the effects of leucine, which was significantly increased only in T2D/obese mouse models that also exhibited hippocampal-specific VPS35 deficiency (Fig. 1). The presence of the ΔGly-APN transgene significantly reduced circulating leucine in ob/ob mice to concentrations that were comparable to those of the non-transgenic wild-type mice (Fig. 1C), supporting leucine as a potential candidate molecule linking T2D and AD-related retromer deficiency.
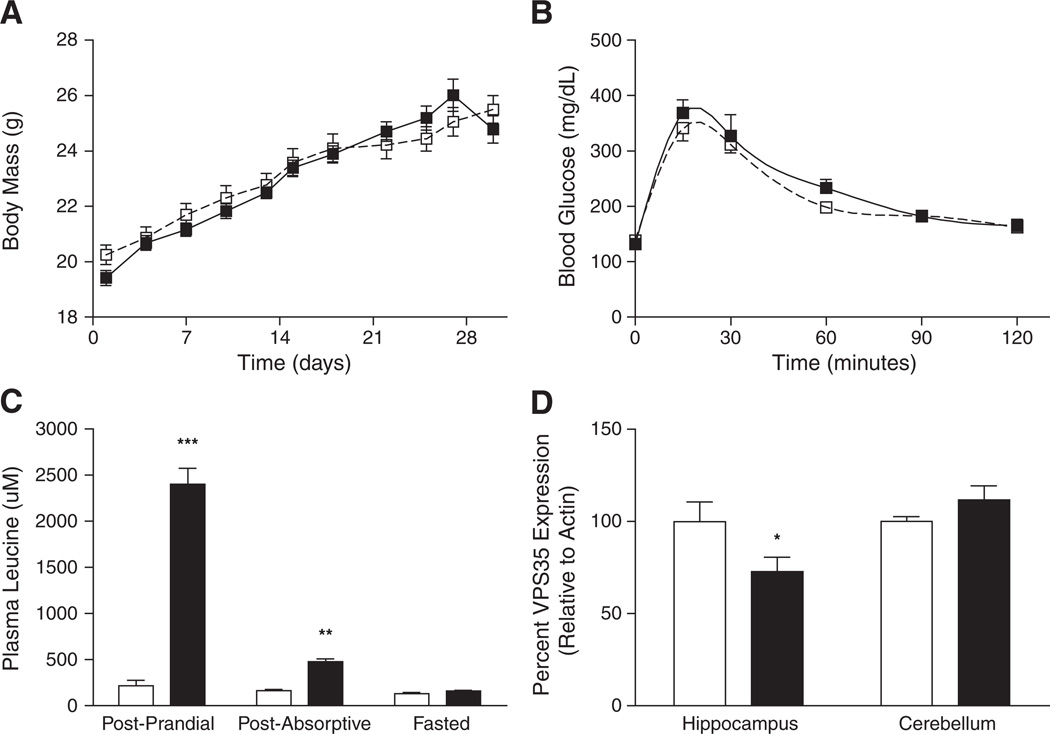
To determine whether elevated circulating leucine is sufficient to cause hippocampal-selective retromer deficiency, we fed non-obese wild-type mice a diet containing a 10-fold excess of leucine (158 mg/g leucine; high leucine diet, HLD) or a control diet with standard leucine content (15.8 mg/g) for 4 weeks. Mice fed HLD did not exhibit any significant differences in body mass or glucose homeostasis compared to controls (Figs. 2A, B), and there were no significant differences in circulating leptin or insulin concentrations in HLD mice, compared to controls (data not shown). HLD mice displayed 1009% and 186% increases in post-prandial and post-absorptive circulating leucine concentrations, respectively (Fig. 2C, Supplementary Table 7). VPS35 protein levels were decreased by 27% specifically in the hippocampus of HLD mice compared to controls (Fig. 2D). Thus, diet-invoked increases in plasma leucine concentrations resulted in decreased retromer protein levels without impairing energy and glucose homeostasis in these mice, suggesting that elevated leucine levels alone are sufficient to disrupt the retromer sorting complex in the brain.
Fig. 2.
Physiological measurements and retromer protein expression in control vs. HLD-fed mice (white vs. black symbols/bars, respectively). (A) Growth curves, (B) glucose tolerance (C) plasma leucine levels, and (D) brain region-specific retromer protein levels are shown for control and HLD-fed mice (mean ± SEM, n = 8 per feeding regimen). *P < 0.05, **P < 0.01, ***P < 0.001.
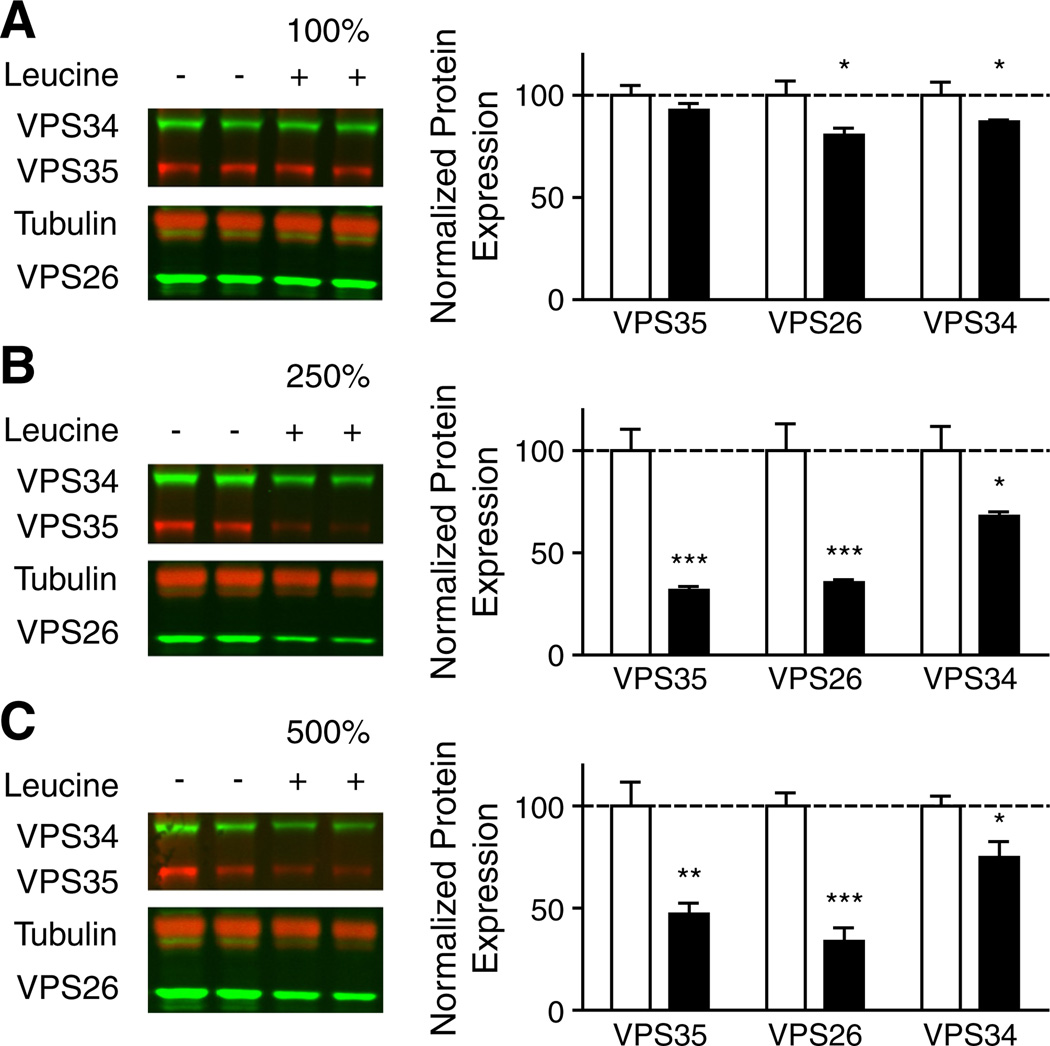
Induced hyperleucinemia could affect the concentration of other metabolites (Araujo et al., 2001). To assess the specificity of the leucine with regard to the reduced retromer protein expression, differentiated neuroblastoma Neuro-2a (N2a) cells were treated with leucine-supplemented media for 72 h. Leucine caused dose-dependent decreases in levels of VPS35 and VPS26 retromer complex proteins (Fig. 3). There were no alterations in total protein levels in cells treated with any dose of leucine, compared to non-treated controls, suggesting that leucine supplementation was not cytotoxic in this context.
Fig. 3.
Dose-dependent retromer protein deficiency in leucine-supplemented differentiated Neuro2A cells (A–C) Representative Western blots of VPS35, VPS26, VPS34, and tubulin are shown for cells treated with 100% (A), 250% (B), and 500% (C) additional leucine (control = 0.8 mM leucine). Average expression of retromer proteins VPS35 and VPS26 and the PI3KIII Kinase VPS34 in cells supplemented with leucine (black bars) compared to non-supplemented controls (white bars) is summarized (mean ± SEM; n = 6). *P < 0.05, **P < 0.01, ***P < 0.001.
The decrease in retromer protein expression observed in leucine-supplemented N2a cell cultures suggests that elevated ambient leucine alone is able to disrupt the retromer sorting pathway. Furthermore, elevated leucine also decreased the expression of VPS34 in a dose-dependent manner (Fig. 3). VPS34 is a class III phosphatidylinositol-3-kinase (PI3K) whose function is essential to normal retromer-dependent trafficking (Burda et al., 2002); VPS34 function has recently been implicated in AD (Morel et al., 2013). Though a decrease in VPS34 protein levels does not prove a decrease in PI3K activity, this result is consistent with the known requirement of VPS34 in retromer-dependent trafficking; VPS34 generates endosomal specific phosphatidylinositol 3-phosphate necessary for the recruitment of the retromer complex to the endosome.
Discussion
In this study, reduced leptin axis activity in mice was accompanied by elevations in circulating BCAAs and a degree of hippocampal VPS35 deficiency similar to that observed in AD patients (Fig. 1). Previous studies have implicated leptin signaling in protection against AD (Greco et al., 2010; Holden et al., 2009; Lieb et al., 2009; Marwarha et al., 2010). However, that functional leptin deficiency or obesity per se is not a necessary or sufficient cause for this hippocampal phenotype is indicated by the lack of hippocampal retromer deficiency in ΔGly-APNob/ob mice (Fig. 1A). Similar reductions in VPS35 expression are observed when leucine is increased in the diet of wild-type animals (Fig. 2) or supplemented in N2a cell culture media in vitro (Fig. 3), indicating that leucine elevation alone is sufficient to cause retromer deficiency independent of reduced leptin signaling or changes in circulating concentrations of glucose or insulin. The finding that HFD and Ay mice show neither hyperleucinemia nor hippocampal retromer deficiency further supports our hypothesis. The milder insulin resistance phenotype observed in these animals relative to the ob/ob and db/db mice may, in part, account for the absence of hyperleucinemia and retromer deficiency in these models (She et al., 2007).
These studies suggest that elevation of circulating leucine, a metabolic phenotype associated with insulin resistance/T2D (Adams, 2011; Newgard, 2012; She et al., 2007), can cause a pattern of retromer deficiency in the hippocampal formation qualitatively and quantitatively similar to changes observed in patients with AD (Small et al., 2005). Previous studies have relied on cognitive and electrophysiological analyses to document that retromer deficiency causes hippocampal dysfunction (Muhammad et al., 2008) and leads to abnormal processing of the amyloid precursor protein (APP) (Bhalla et al., 2012; Muhammad et al., 2008; Rogaeva et al., 2007; Sullivan et al., 2011; Vieira et al., 2010; Wen et al., 2011), recapitulating the behavioral and biochemical phenotypes of AD. Additional studies have described increased misprocessing of APP in mice segregating for the db/db or ob/ob mutations (Li et al., 2012; Son et al., 2012; Takeda et al., 2010), models which also exhibit hippocampal retromer deficiency in the studies reported here.
As dysfunction in retromer trafficking is a pathogenic mechanism in AD, these observations are consistent with the hypothesis that leucine-induced retromer deficiency may account in part for the increased incidence of AD in patients with T2D. At physiological concentrations, brain levels of leucine are ~10% of those in plasma (Kruse et al., 1985). Large neutral amino acids, including the BCAAs, are transported across the blood brain barrier by LAT1 (SLC7A5). The kinetics of this system are such that concentrations of these amino acids can reciprocally influence the brain concentrations of others in this group (Killian and Chikhale, 2001). Hence, the effects of elevated plasma leucine could be conveyed directly and/or by competitive effects on the transport of other amino acids. Increased circulating BCAAs are an early biomarker/risk factor for T2D (Wang et al., 2011). By identifying a novel mechanism linking T2D to AD, these results have epidemiological and clinical implications. This study demonstrates that modifying a single dietary essential amino acid can affect a critical trafficking complex in the brain that has been implicated in AD.
Supplementary Material
Acknowledgments
We thank Kaiying Guo, Charles LeDuc, Rick Rausch, Changyu Zhu, and Joseph Bayne for technical assistance. We also thank Philipp E. Scherer of the University of Texas Southwestern Medical Center for providing ΔGly-APN mice. This work was supported in part by the Russell Berrie Foundation, NIH R01DK52431, R01AG025161, P30DK26687, P30DK63608, and T32DK007647.
Footnotes
Appendix A. Supplementary data
Supplementary data to this article can be found online at http://dx.doi.org/10.1016/j.nbd.2013.12.017.
References
- Adams SH. Emerging perspectives on essential amino acid metabolism in obesity and the insulin-resistant state. Adv. Nutr. 2011;2:445–456. doi: 10.3945/an.111.000737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araujo P, et al. Reduction of large neutral amino acid levels in plasma and brain of hyperleucinemic rats. Neurochem. Int. 2001;38:529–537. doi: 10.1016/s0197-0186(00)00100-5. [DOI] [PubMed] [Google Scholar]
- Bhalla A, et al. The location and trafficking routes of the neuronal retromer and its role in amyloid precursor protein transport. Neurobiol. Dis. 2012;47:126–134. doi: 10.1016/j.nbd.2012.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonifacino JS, Hurley JH. Retromer. Curr. Opin. Cell Biol. 2008;20:427–436. doi: 10.1016/j.ceb.2008.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burda P, et al. Retromer function in endosome-to-Golgi retrograde transport is regulated by the yeast Vps34 PtdIns 3-kinase. J. Cell Sci. 2002;115:3889–3900. doi: 10.1242/jcs.00090. [DOI] [PubMed] [Google Scholar]
- Combs TP, et al. A transgenic mouse with a deletion in the collagenous domain of adiponectin displays elevated circulating adiponectin and improved insulin sensitivity. Endocrinology. 2004;145:367–383. doi: 10.1210/en.2003-1068. [DOI] [PubMed] [Google Scholar]
- Gandy S, DeKosky ST. Toward the treatment and prevention of Alzheimer's disease: rational strategies and recent progress. Annu. Rev. Med. 2013;64:367–383. doi: 10.1146/annurev-med-092611-084441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greco SJ, et al. Leptin reduces pathology and improves memory in a transgenic mouse model of Alzheimer's disease. J. Alzheimers Dis. 2010;19:1155–1167. doi: 10.3233/JAD-2010-1308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holden KF, et al. Serum leptin level and cognition in the elderly: findings from the Health ABC Study. Neurobiol. Aging. 2009;30:1483–1489. doi: 10.1016/j.neurobiolaging.2007.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Killian DM, Chikhale PJ. Predominant functional activity of the large, neutral amino acid transporter (LAT1) isoform at the cerebrovasculature. Neurosci. Lett. 2001;306:1–4. doi: 10.1016/s0304-3940(01)01810-9. [DOI] [PubMed] [Google Scholar]
- Kim JY, et al. Obesity-associated improvements in metabolic profile through expansion of adipose tissue. J. Clin. Invest. 2007;117:2621–2637. doi: 10.1172/JCI31021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruse T, et al. Amino acid transport across the human blood-CSF barrier. An evaluation graph for amino acid concentrations in cerebrospinal fluid. J. Neurol. Sci. 1985;70:129–138. doi: 10.1016/0022-510x(85)90082-6. [DOI] [PubMed] [Google Scholar]
- Lane RF, et al. Vps10 family proteins and the retromer complex in aging-related neurodegeneration and diabetes. J. Neurosci. 2012;32:14080–14086. doi: 10.1523/JNEUROSCI.3359-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, et al. Metformin attenuates Alzheimer's disease-like neuropathology in obese, leptin-resistant mice. Pharmacol. Biochem. Behav. 2012;101:564–574. doi: 10.1016/j.pbb.2012.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieb W, et al. Association of plasma leptin levels with incident Alzheimer disease and MRI measures of brain aging. JAMA. 2009;302:2565–2572. doi: 10.1001/jama.2009.1836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luchsinger JA. Adiposity, hyperinsulinemia, diabetes and Alzheimer's disease: an epidemiological perspective. Eur. J. Pharmacol. 2008;585:119–129. doi: 10.1016/j.ejphar.2008.02.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luchsinger JA, et al. Diabetes mellitus and risk of Alzheimer's disease and dementia with stroke in a multiethnic cohort. Am. J. Epidemiol. 2001;154:635–641. doi: 10.1093/aje/154.7.635. [DOI] [PubMed] [Google Scholar]
- Marwarha G, et al. Leptin reduces the accumulation of Abeta and phosphorylated tau induced by 27-hydroxycholesterol in rabbit organotypic slices. J. Alzheimers Dis. 2010;19:1007–1019. doi: 10.3233/JAD-2010-1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melnik BC. Leucine signaling in the pathogenesis of type 2 diabetes and obesity. World J. Diabetes. 2012;3:38–53. doi: 10.4239/wjd.v3.i3.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morel E, et al. Phosphatidylinositol-3-phosphate regulates sorting and processing of amyloid precursor protein through the endosomal system. Nat. Commun. 2013;4:2250. doi: 10.1038/ncomms3250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muhammad A, et al. Retromer deficiency observed in Alzheimer's disease causes hippocampal dysfunction, neurodegeneration, and Abeta accumulation. Proc. Natl. Acad. Sci. U. S. A. 2008;105:7327–7332. doi: 10.1073/pnas.0802545105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newgard CB. Interplay between lipids and branched-chain amino acids in development of insulin resistance. Cell Metab. 2012;15:606–614. doi: 10.1016/j.cmet.2012.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rafecas I, et al. Plasma amino acids of lean and obese Zucker rats subjected to a cafeteria diet after weaning. Biochem. Int. 1991;25:797–806. [PubMed] [Google Scholar]
- Ridaura VK, et al. Gut microbiota from twins discordant for obesity modulate metabolism in mice. Science. 2013;341:1241214. doi: 10.1126/science.1241214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogaeva E, et al. The neuronal sortilin-related receptor SORL1 is genetically associated with Alzheimer disease. Nat. Genet. 2007;39:168–177. doi: 10.1038/ng1943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- She P, et al. Obesity-related elevations in plasma leucine are associated with alterations in enzymes involved in branched-chain amino acid metabolism. Am. J. Physiol. Endocrinol. Metab. 2007;293:E1552–E1563. doi: 10.1152/ajpendo.00134.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegenthaler BM, Rajendran L. Retromers in Alzheimer's disease. Neurodegener. Dis. 2012;10:116–121. doi: 10.1159/000335910. [DOI] [PubMed] [Google Scholar]
- Small SA, et al. Model-guided microarray implicates the retromer complex in Alzheimer's disease. Ann. Neurol. 2005;58:909–919. doi: 10.1002/ana.20667. [DOI] [PubMed] [Google Scholar]
- Son SM, et al. Altered APP processing in insulin-resistant conditions is mediated by autophagosome accumulation via the inhibition of mammalian target of rapamycin pathway. Diabetes. 2012;61:3126–3138. doi: 10.2337/db11-1735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan CP, et al. Retromer disruption promotes amyloidogenic APP processing. Neurobiol. Dis. 2011;43:338–345. doi: 10.1016/j.nbd.2011.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeda S, et al. Diabetes-accelerated memory dysfunction via cerebrovascular inflammation and Abeta deposition in an Alzheimer mouse model with diabetes. Proc. Natl. Acad. Sci. U. S. A. 2010;107:7036–7041. doi: 10.1073/pnas.1000645107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tosto G, Reitz C. Genome-wide association studies in Alzheimer's disease: a review. Curr. Neurol. Neurosci. Rep. 2013;13:381. doi: 10.1007/s11910-013-0381-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vardarajan BN, et al. Identification of Alzheimer disease-associated variants in Q4genes that regulate retromer function. Neurobiol. Aging. 2012;33:2231.e15–2231.e30. doi: 10.1016/j.neurobiolaging.2012.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vieira SI, et al. Retrieval of the Alzheimer's amyloid precursor protein from the endosome to the TGN is S655 phosphorylation state-dependent and retromer-mediated. Mol. Neurodegener. 2010;5:40. doi: 10.1186/1750-1326-5-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang TJ, et al. Metabolite profiles and the risk of developing diabetes. Nat. Med. 2011;17:448–453. doi: 10.1038/nm.2307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen L, et al. VPS35 haploinsufficiency increases Alzheimer's disease neuropathology. J. Cell Biol. 2011;195:765–779. doi: 10.1083/jcb.201105109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wijekoon EP, et al. Amino acidmetabolismin the Zucker diabetic fatty rat: effects of insulin resistance and of type 2 diabetes. Can. J. Physiol. Pharmacol. 2004;82:506–514. doi: 10.1139/y04-067. [DOI] [PubMed] [Google Scholar]
- Yamauchi T, et al. Globular adiponectin protected ob/ob mice from diabetes and ApoE-deficient mice from atherosclerosis. J. Biol. Chem. 2003;278:2461–2468. doi: 10.1074/jbc.M209033200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.