Abstract
Background
Aortic stenosis in the midgestation fetus with a normal-sized or dilated left ventricle predictably progresses to hypoplastic left heart syndrome when associated with certain physiological findings. Prenatal balloon aortic valvuloplasty may improve left heart growth and function, possibly preventing evolution to hypoplastic left heart syndrome.
Methods and Results
Between March 2000 and October 2008, 70 fetuses underwent attempted aortic valvuloplasty for critical aortic stenosis with evolving hypoplastic left heart syndrome. We analyzed this experience to determine factors associated with procedural and postnatal outcome. The median gestational age at intervention was 23 weeks. The procedure was technically successful in 52 fetuses (74%). Relative to 21 untreated comparison fetuses, subsequent prenatal growth of the aortic and mitral valves, but not the left ventricle, was improved after intervention. Nine pregnancies (13%) did not reach a viable term or preterm birth. Seventeen patients had a biventricular circulation postnatally, 15 from birth. Larger left heart structures and higher left ventricular pressure at the time of intervention were associated with biventricular outcome. A multivariable threshold scoring system was able to discriminate fetuses with a biventricular outcome with 100% sensitivity and modest positive predictive value.
Conclusions
Technically successful aortic valvuloplasty alters left heart valvar growth in fetuses with aortic stenosis and evolving hypoplastic left heart syndrome and, in a subset of cases, appeared to contribute to a biventricular outcome after birth. Fetal aortic valvuloplasty carries a risk of fetal demise. Fetuses undergoing in utero aortic valvuloplasty with an unfavorable multivariable threshold score at the time of intervention are very unlikely to achieve a biventricular circulation postnatally.
Keywords: fetus; heart defects, congenital; hypoplastic left heart syndrome; stenosis; valvuloplasty
Hypoplastic left heart syndrome (HLHS) is a complex anomaly characterized by variable hypoplasia of left-sided cardiac structures, producing a left heart that is insufficient to support the systemic circulation. Outcomes of staged univentricular palliation for HLHS have improved dramatically since Norwood’s initial report,1 with neonatal survival now approaching 90% at many experienced centers.2,3 Nevertheless, there is a steady hazard for death beyond the neonatal period, and all survivors of univentricular palliation are subject to significant ongoing functional limitations.
A subset of patients born with HLHS are diagnosed prenatally in the second trimester with aortic stenosis (AS) and a normal-sized or dilated left ventricle (LV) and progress to HLHS by late gestation.4–6 Among fetuses fitting this profile, those with retrograde flow in the transverse arch, left-to-right flow across the foramen ovale, and monophasic mitral valve (MV) inflow have progressive left heart hypoplasia and dysfunction and are born with HLHS.6 The cause of this progression is not known but may have a “mechanical” component, with poor LV function and output secondary to the obstructed aortic valve leading to further impairment of LV compliance and filling, reducing the load- and/or flow-mediated stimulus for growth of the MV, LV, aortic valve, and ascending aorta. This sequence has not been confirmed in humans, but animal models have demonstrated that altered LV loading in utero can affect left heart growth and functional development without necessarily causing LV hypoplasia.7–12
Fetal aortic valve dilation was first described in 1991, but previously reported successful procedures at other centers were performed in the third trimester.13,14 In 2000, we undertook a program of midgestation fetal aortic valvuloplasty to alter the natural history of evolving HLHS in utero. The basic hypothesis behind this initiative was that relieving LV outflow obstruction in fetuses with AS and evolving HLHS, regardless of the cause(s) of the disease, will mitigate the ongoing process of myocardial damage that takes place in fetuses with severe AS, facilitate growth, and improve function of the LV and supporting left heart structures. This hypothesis is supported by data from various animal models that modifying loading and flow conditions in the developing cardiovascular system leads to altered cardiovascular growth and function.7–11,15–18 In 2004, we reported our preliminary experience with fetal aortic valvuloplasty in 20 fetuses19 and have focused on technical, maternal, and hemodynamic factors in separate reports.20–23 We now have sufficient experience to evaluate the impact of fetal aortic valvuloplasty on left heart growth during the remainder of gestation. In this study, we conducted a retrospective analysis of prospectively collected data with the aim of identifying preintervention predictors of technically successful valvuloplasty and of biventricular outcome postnatally.
Methods
Patients
Since 2000, midgestation fetuses with valvar AS were considered for in utero aortic valvuloplasty if valvar AS appeared to be the primary or dominant anatomic abnormality, progression to HLHS was considered to be highly likely, and the LV was thought to be potentially sufficient to sustain a biventricular circulation. The present study covers our entire experience from March 2000 (the first procedure) through October 2008. Our understanding of the natural history of HLHS changed over this period, and we became more selective in our assessment of candidates with a potentially viable left heart. Over the course of our experience, our criteria for predicting evolution to HLHS did not change, but our guidelines for assessing the ultimate potential for a biventricular circulation were more fluid. Prior and current guidelines for patient selection are summarized in Table 1.
Table 1.
Previous and Current Selection Guidelines for Fetal Aortic Valvuloplasty
| 1. Dominant cardiac anatomic anomaly is valvar AS with all of the following |
| Decreased mobility of valve leaflets |
| Antegrade Doppler color flow jet across aortic valve smaller than the valve annulus diameter |
| No or minimal subvalvar LV outflow obstruction |
| 2. Evolving HLHS |
| LV function qualitatively depressed AND EITHER Retrograde or bidirectional flow in the transverse aortic arch (between the first 2 brachiocephalic vessels) at any time during the cardiac cycle OR two of the following: Monophasic MV inflow (Doppler profile of MV inflow without discrete E and A waves) Left-to-right flow across atrial septum or intact atrial septum (bulging left to right) Bidirectional flow in pulmonary veins |
| 3. Potential for a technically successful procedure and biventricular outcome postnatally |
| Criteria used for most of the patients in the present study (all 3 of the following) |
| LV long-axis Z score ≥−2 |
| LV function qualitatively depressed but generating at least a 10 mm Hg pressure gradient across aortic valve or 15 mm Hg MR jet gradient |
| MV diameter Z score >−3 |
| Modified criteria based on the findings of the present study |
| Unequivocal AS (vs aortic atresia) |
| LV long-axis Z score >−2 |
| Threshold score ≥4 (≥4 of the following) |
| LV long-axis Z score >0 |
| LV short-axis Z score >0 |
| Aortic annulus Z score >−3.5 |
| MV annulus Z score >−2 |
| MR or AS maximum systolic gradient ≥20 mm Hg |
This report excludes aortic valvuloplasty procedures in 10 fetuses with AS, mitral regurgitation (MR), and hydrops (n=9) or established HLHS with an intact atrial septum (n=1)24 that were performed to improve fetal and postnatal survival rather than to promote left heart growth. This study was conducted with approval from the Committee for Clinical Investigation at Children’s Hospital Boston and the Institutional Review Board at Brigham and Women’s Hospital under an innovative therapy protocol or as part of a prospective clinical protocol.
Aortic Valvuloplasty
The technical aspects of fetal aortic valvuloplasty were described in previous reports.19–21 A technically successful aortic valvuloplasty was defined as one in which the aortic valve was crossed and a balloon inflated, with clear evidence of increased flow across the valve and/or new aortic regurgitation (AR) by color Doppler as assessed by 2 echocardiographers.
Follow-Up and Postnatal Management
After the procedure, mothers were hospitalized overnight. The fetus was assessed by ultrasound later in the same day and the following day before planned maternal discharge. Patients received follow-up at either our center or the referring institution. Echocardiography was performed at intervals determined by the primary fetal cardiologist. Anatomic and Doppler variables from the most recent prenatal study were used for analysis. A single echocardiographer independently confirmed all measurements from the primary images. Z scores were calculated relative to estimated gestational age on the basis of unpublished fetal norms that were derived from data collected at Children’s Hospital Boston between 2005 and 2007 on 232 normal fetuses (see the online-only Data Supplement for Z-score equations). Because all of the relevant left heart structures are normally related to gestational age in a linear fashion, growth rates may be estimated as the change in dimension per unit of time (e.g., millimeters per week). “High” LV pressure was defined as a maximum instantaneous MR jet predicting a gradient of ≥20 mm Hg or, if there was no MR, as a maximum instantaneous AS gradient ≥16 mm Hg (in most patients with an MR jet predicting a 20 to 25 mm Hg gradient, the AS gradient was within 4 to 8 mm Hg).
Postnatal management varied with the anatomy at birth and the institution providing care. We characterized outcome among surviving patients as biventricular circulation from birth (no univentricular staging procedures), biventricular circulation after initial univentricular palliation (i.e., neonatal stage 1 procedure, with or without subsequent palliative procedures, later taken down to biventricular circulation), or single-ventricle circulation (ie, a definitive or intermediate univentricular circulation at the time of cross-sectional follow-up). A biventricular circulation was defined as one in which the LV was the sole source of systemic output, with no intracardiac or great arterial shunts except possibly a patent foramen ovale or atrial septal defect.
Data Analysis
Outcomes assessed included technical success, pregnancy outcome, growth of the LV and associated left heart structures, and postnatal survival with a biventricular circulation. The analysis of technical success included all fetuses that underwent attempted intervention, regardless of pregnancy or postnatal outcome. Pregnancy outcomes are presented as descriptive data, which were not analyzed statistically. For analysis of left heart growth, fetuses that underwent successful in utero aortic valvuloplasty were assessed relative to a composite comparison group of fetuses that had similar anatomic and physiological profiles. This group included all 7 fetuses that underwent technically unsuccessful prenatal intervention, had an LV long-axis Z score in the normal range (>−2), and had adequate follow-up fetal echocardiographic data available. It also included 14 midgestation fetuses that met the same anatomic and physiological criteria as intervention fetuses but did not undergo attempted aortic valvuloplasty and were selected from our prior natural history report.6 For analysis of factors associated with biventricular outcome, which included only fetuses that underwent attempted prenatal intervention (ie, not historical comparison group fetuses), newborns who died before neonatal intervention or underwent heart transplant were grouped with patients managed according to a single ventricle strategy, and 2 fetuses that were in utero as of November 2008 were not included.
Continuous and categorical variables were compared within patients or between groups by paired or independent-samples t test or by the McNemar or Fisher exact tests, respectively. Multivariable logistic regression analysis was performed to assess factors associated with biventricular outcome. Receiver-operating characteristic curve analysis determined optimal threshold levels of continuous variables for dichotomization and analysis of sensitivity and specificity for predicting the aforementioned outcomes. In addition, a multivariable model for predicting biventricular outcome was determined from threshold levels of Z scores for left-sided structures and left heart physiological features at the time of intervention. Because the goal was to capture all patients with a chance of biventricular outcome, the model was derived to optimize sensitivity for predicting this outcome; analysis included all fetuses that underwent attempted aortic valvuloplasty, regardless of technical success or failure. Data are presented as mean±SD or median (range).
Results
Patients
Between March 2000 and October 2008, in utero aortic valvuloplasty was attempted in 70 fetuses with severe AS and evolving HLHS. In 5 fetuses, the presence of antegrade aortic flow across the valve was uncertain, and the diagnosis was reported to be AS or aortic atresia (ie, equivocal aortic atresia); all other selection criteria were met, and the decision was made to attempt valvuloplasty. The median gestational age at intervention was 23.2 weeks (range, 20 to 31 weeks); 54 (77%) fetuses were male. Sixty-one patients (87%) were referred from outside the normal geographic catchment of Children’s Hospital Boston, all from the United States.
Preintervention Echocardiographic Data
Left heart anatomic dimensions measured the day before intervention and Z scores are summarized in Table 2.
Table 2.
Anatomic Dimensions on Preintervention/Midgestation Echocardiogram and at Late-Gestation Follow-Up Among Patients and Comparison Fetuses
| Variable | Technically Successful Intervention (n=45)* |
Comparison Fetuses (n=21) |
P | ||||
|---|---|---|---|---|---|---|---|
| Pre-intervention | Follow-Up | P | Mid-gestation | Follow-Up | P | ||
| Gestational age, wk | 23.9±2.1 | 34.0±2.7 | 23.2±3.6 | 32.7±3.1 | 0.31,* 0.11‡ | ||
| Aortic annulus diameter Zscore | −2.5±0.9 | −2.9±1.0 | 0.008 | −2.3±1.2 | −4.3±0.9 | <0.001 | 0.55,† <0.001‡ |
| Ascending aorta Zscore diameter | −0.4±2.0 | −0.6±1.5 | 0.83 | −0.8±1.9 | −2.2±2.1 | <0.001 | 0.44,† 0.001‡ |
| LV long-axis dimension Zscore | 0.9±1.8 | −1.4±1.5 | <0.001 | 0.0±1.3 | −2.7±1.2 | <0.001 | 0.03,† 0.002‡ |
| LV short-axis dimension Zscore | 3.0±2.7 | −0.3±2.2 | <0.001 | 2.4±2.2 | −1.7±1.8 | <0.001 | 0.3,† 0.02‡ |
| MV annulus diameter Zscore | −1.2±1.2 | −2.2±1.3 | <0.001 | −1.3±1.3 | −4.3±1.3 | <0.001 | 0.82,†<0.001‡ |
| RV long-axis dimension Zscore | 1.5±1.4 | 0.5±1.4 | 0.001 | 1.2±1.2 | 0.2±0.9 | 0.16 | 0.30,†0.46‡ |
Follow-up duration was 71.1±23.2 days in fetuses that underwent technically successful intervention and 68.2±24.9 days in comparison group fetuses (P=0.58). Data are presented as mean±SD.
Fetal deaths and fetuses still in utero excluded.
Preintervention/midgestation technically successful intervention versus comparison fetuses.
Follow-up technically successful intervention versus comparison fetuses.
Aortic Valvuloplasty
The procedure was performed with percutaneous access in 51 cases (73%); in the other 19, a limited laparotomy without uterine incision or exteriorization was performed, either after failure of percutaneous fetal access (n=10) or without prior attempts at fetal access (n=9). Access was transplacental in 22 cases (31%) with an anterior placenta.
The aortic valvuloplasty was technically successful in 52 fetuses (74%), with 50% success in the first 12 cases but no major variation beyond that initial learning curve (Figure I of the online-only Data Supplement). These 52 fetuses are sometimes referred to here as patients. Factors contributing to the 18 technical failures included unfavorable fetal position or difficulty achieving optimal fetal position, difficulty puncturing the LV (usually with smaller, thicker ventricles), suboptimal cannula trajectory, inability to cross the valve with the wire despite favorable cannula trajectory (5 cases, initially thought to be severe AS with minimal transvalvar flow but most likely were aortic atresia), and fetal hemodynamic instability, as described previously.22 Equivocal aortic atresia and a shorter LV long-axis dimension Z score were associated with technical failure (Table 3). The procedure was technically unsuccessful in 8 of 9 fetuses with an LV long-axis dimension Z score <−2. Gestational age, access via limited laparotomy, transplacental access, and other anatomic factors were not associated with technical outcome.
Table 3.
Comparison of Fetuses That Underwent Technically Successful and Unsuccessful In Utero Aortic Valvuloplasty
| Variable | Technically Successful Intervention (n=52) |
Technically Unsuccessful Intervention (n=18) |
P |
|---|---|---|---|
| Gestational age, wk | 23.8±2.1 | 24.6±3.6 | 0.26 |
| Anatomic features | |||
| Aortic annulus diameter, mm | 2.91±0.45 | 3.01±0.82 | 0.56 |
| Aortic annulus diameter Z score | −2.5±0.9 | −2.6±1.0 | 0.60 |
| Ascending aorta diameter, mm | 4.18±1.08 | 3.76±1.61 | 0.20 |
| Ascending aorta diameter Z score | −0.5±1.9 | −1.7±2.0 | 0.03 |
| LV long-axis dimension, cm | 1.88±0.36 | 1.57±0.63 | 0.01 |
| LV long-axis dimension Z score | 0.9±1.8 | −1.2±1.6 | <0.001 |
| LV short-axis dimension, cm | 1.26±0.33 | 1.20±0.42 | 0.57 |
| LV short-axis dimension Z score | 3.0±2.6 | 1.8±1.7 | 0.09 |
| LV sphericity index | 0.66±0.11 | 0.79±0.13 | <0.001 |
| RV long-axis dimension, cm | 1.84±0.25 | 1.76±0.49 | 0.38 |
| RV long-axis dimension Z score | 1.9±1.6 | 0.2±1.7 | 0.01 |
| Physiological features | |||
| LV ejection fraction, % | 22.8±11.4 | 20.1±10.6 | 0.39 |
| “High” LV pressure (n=29), n (%) | 24 (49) | 5 (36) | 0.38 |
| Moderate or severe MR (n=38), n (%) | 29 (56) | 9 (50) | 0.67 |
| Procedural factors, n (%) | |||
| Limited laparotomy (n=19) | 14 (27) | 5 (28) | 0.94 |
| Transplacental access (n=22) | 16 (32) | 6 (35) | 0.80 |
All fetuses that underwent intervention are included, regardless of pregnancy or postnatal outcome. Data are presented as mean±SD.
The nominal balloon diameter used for aortic valve dilation ranged from 2.5 to 3.0 mm, and in all patients, the balloon was inflated with sufficient pressure that the inflated balloon diameter was greater than the nominal diameter. The ratio of maximally inflated balloon to annulus diameter ranged from 0.73 to 1.42 (median, 1.11).
Several techniques to position the fetus and/or to access the LV were developed during our experience. In 12 cases, a 19-gauge access cannula advanced against the fetal chest wall was used to make minor rotational adjustments of fetal position, and a second cannula was used for LV access; 5 of 12 were technically unsuccessful. In 7 cases in which the LV was difficult to puncture with the 19-gauge cannula, an ultrasharp, thin-walled 22-gauge Chiba needle (Cook Inc, Bloomington, Ind) was introduced through the 19-gauge needle and used to puncture the LV.
Outcomes
Acute Procedural Outcome
Hemodynamic changes (bradycardia and ventricular dysfunction) treated with intramuscular and/or intracardiac medications, and/or hemopericardium for which drainage was attempted occurred in 28 fetuses (40%).22 AR was observed by color Doppler immediately after technically successful aortic valvuloplasty in 31 of 52 fetuses (60%). In 11 of these fetuses, AR was trivial or mild; in 20 (38% of 52), it was moderate or severe (significant). The ratio of balloon to annulus diameters was larger in fetuses that developed significant AR than in those that did not (1.20±0.13 versus 1.08±0.14; P=0.003). Two of 17 fetuses (12%) with a balloon to annulus diameter ratio ≤1.1 developed significant AR compared with 59% of fetuses with a ratio >1.1. Of the 20 patients with significant AR soon after intervention, the AR resolved or improved to mild in all but 1, who continued to have severe AR on late gestation and newborn evaluation.
Pregnancy Outcome
One pregnancy was terminated, and 8 others did not reach a viable term or preterm birth (13% total; 4 after technically unsuccessful procedures): 6 pregnancies ended in fetal death or preterm stillbirth, and 2 fetuses were delivered alive but were nonviable because of extreme prematurity (24 and 25 weeks’ gestation; Figure 1). Perivalvuloplasty treatment was performed for hemodynamic changes or hemopericardium in 5 of these fetuses.22 The median interval from intervention to documentation of death or preterm delivery was 1 day (range, 4 hours to 6 weeks). Of the 8 fetal deaths or nonviable live births (excluding the termination), 1 was deemed the result of a large hemopericardium documented on fetopsy, and in 7, the cause was uncertain. Among the remaining 61 fetuses, 5 were delivered preterm at a viable gestational age (30 to 35 weeks), 54 were delivered at term (36 weeks or later), and 2 were still in utero at the time this manuscript was prepared. Altogether, 67% of fetuses had a technically successful procedure and were born at a viable gestational age.
Figure 1.
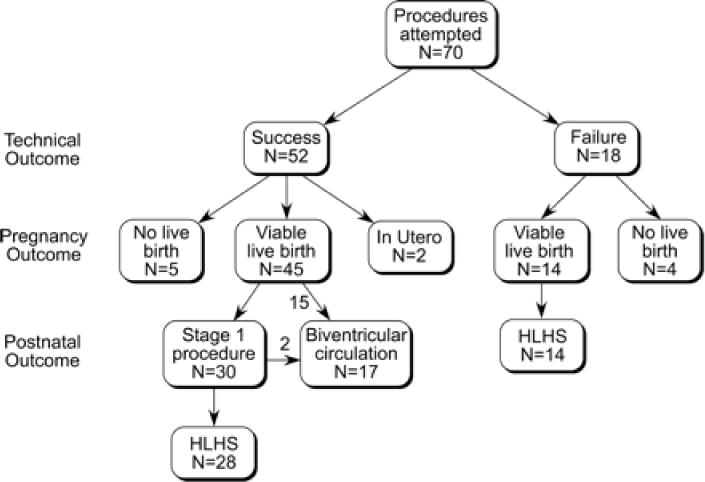
Flow diagram depicting procedural, pregnancy, and postnatal outcomes among 70 fetuses that underwent attempted prenatal aortic valvuloplasty for AS with evolving HLHS.
In Utero Growth of Left Heart Structures
Follow-up anatomic dimensions for the 45 fetuses that underwent technically successful intervention and survived to birth, along with data from 21 comparison fetuses (7 with a technically unsuccessful intervention, LV long-axis Z score >−2, and available follow-up imaging data), are summarized in Table 2 and depicted in Figure 2. After elimination of fetuses with an LV long-axis Z score <−2 (n=8), there were no differences in left heart Z scores, gestational age, or physiological features between control fetuses that underwent unsuccessful intervention and those that did not have an attempted intervention. On the midgestation/preintervention echocardiogram, LV size was normal or dilated overall but modestly longer in patients than in comparison fetuses (Table 2 and Figure 2). From midgestation to late gestation, Z scores decreased for all left heart structures in both patients and comparison fetuses. However, at late-gestation follow-up, there was a clear difference between the sizes of left heart structures in patients and comparison fetuses, with Z scores significantly higher (1 to 2 SD) in patients (Figure 2). The decrease in aortic and MV Z scores was smaller among fetuses that underwent successful intervention than comparison fetuses, but there was no significant difference in the relative decrease in LV long-axis and short-axis Z scores.
Figure 2.

Mean±SD Z scores of the aortic annulus (A), MV annulus (B), LV short axis (C), and LV long axis (D) at the time of prenatal intervention and at the latest follow-up fetal echocardiogram with adequate data in fetuses with a biventricular outcome after technically successful intervention (from birth or after initial univentricular staging), fetuses with a univentricular outcome after technically successful intervention (single ventricle), and comparison fetuses (control).
Generally, there were significant increases in absolute size of the aortic valve, ascending aorta, and MV among both successful interventions and comparison fetuses, but the rate of increase was greater among fetuses that underwent successful intervention (Figure 3). LV long- and short-axis dimensions did not change in most cases, whereas RV length increased to a similar extent in patients and comparison fetuses (Figure 3). The RV length growth rate was significantly higher than the growth rate of the LV long axis among both patients and comparison fetuses (both P<0.001). There was no difference in growth of the LV or other left heart structures between fetuses with and without significant postvalvuloplasty AR.
Figure 3.
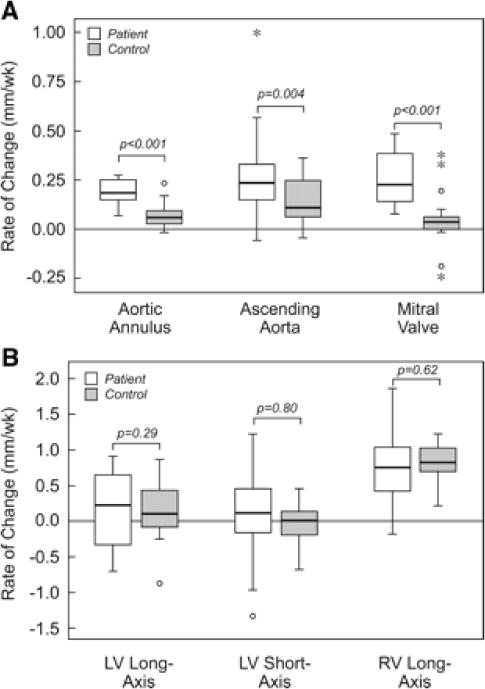
Box plots depicting the rate of change in aortic annulus, ascending aorta, and MV dimensions (A) and LV long- and short-axis and RV long-axis dimensions (B) in fetuses that underwent successful intervention and comparison group fetuses. All measures are expressed in millimeters per week, with positive numbers indicating an increase in size and negative numbers indicating a decrease. For each plot, the central line represents the median rate of change; box, the interquartile range; and whisker bars, 95% confidence intervals. Outliers are shown individually.
Postnatal Outcome
Technically Unsuccessful Fetal Intervention
Of the 14 patients born at a viable gestational age after a technically unsuccessful procedure, all had HLHS postnatally: 1 died in the neonatal period, 2 died after a stage 1 procedure, and 11 are alive after different stages of functionally univentricular palliation. The other 14 comparison fetuses, in which no prenatal intervention was performed, either died in the neonatal period without intervention (n=3) or underwent stage 1 palliation.6
Technically Successful Fetal Intervention
Fifteen fetuses had a functionally biventricular circulation from the time of birth (22% of the 68 patients who underwent attempted intervention, and 30% of the 50 who had a technically successful intervention, not including the 2 fetuses still in utero; Figure 1). Two of these patients underwent no neonatal intervention; the other 13 underwent balloon aortic valvuloplasty (n=12), atrial septal dilation and/or stenting (n=5), coarctation repair (n=4), aortic valve repair (n=1), MV repair (n=1), and/or atrial septal defect closure (n=1). Both of the patients without neonatal intervention subsequently had interventions performed: balloon aortic valvuloplasty at 18 months in 1, and balloon mitral valvuloplasty at 4 months followed by surgical MV repair and resection of LV endocardial fibroelastosis at 5 months in the other. Ten of these 15 patients had >1 intervention.
Of the remaining 30 patients, 27 underwent a stage 1 procedure or modified stage 1 procedure in the newborn period at our center or elsewhere, 2 died without any intervention, and 1 underwent heart transplant. Four patients (12%) died early after a stage 1 operation (n=3) or transplantation (n=1). Two of the remaining 24 patients underwent conversion to a biventricular circulation at 1.9 and 3.3 years of age, each after 3 surgeries, including bidirectional cavopulmonary anastomosis, mitral valvuloplasty, and resection of endocardial fibroelastosis. Thus, a total of 17 patients (25% of 68, 33% of the 51 who underwent technically successful intervention) had a biventricular outcome as of November 2008.
Sixteen of the 17 patients who achieved a biventricular circulation were alive at a median age of 2.1 years (range, 4 months to 7.0 years). The other patient died in a motor vehicle accident at 14 months of age. As depicted in Figure I of the online-only Data Supplement, fetuses that went on to a biventricular outcome were spread across our experience, although there has been a concentration among more recent cases.
Predictors of Biventricular Outcome
Preintervention anatomic and physiological factors in fetuses with univentricular and biventricular outcome postnatally are summarized in Table 4. By multivariable logistic regression, higher LV long-axis Z score (odds ratio, 2.6; 95% confidence interval, 1.4 to 4.9; P=0.003) and “high” LV pressure (odds ratio, 17.0; 95% confidence interval, 2.5 to 117; P=0.004) were independently associated with higher probability of biventricular outcome from the time of birth. Including only the patients who underwent successful prenatal intervention did not change the variables associated with biventricular outcome, nor did including the 2 patients converted to a biventricular circulation after initial stage 1 palliation.
Table 4.
Preintervention Variables Associated With Postnatal Biventricular or Single-Ventricle Outcome After In Utero Aortic Valvuloplasty
| Biventricular Outcome (n=17) |
All Attempted Interventions, Single-Ventricle Outcome (n=51) | Technically Successful Interventions, Single-Ventricle Outcome (n=33) |
P | |
|---|---|---|---|---|
| Gestational age, wk | 23.9±2.6 | 24.0±2.6 | 23.7±2.0 | 0.95, 0.68† |
| Aortic annulus diameter Zscore | −2.2±0.8 | −2.7±0.9 | −2.7±0.9 | 0.03, 0.02† |
| Ascending aorta diameter Z score | 0.6±1.9 | −1.4±1.8 | −1.2±1.6 | <0.001,* 0.002† |
| LV long-axis dimension Zscore | 2.1±1.5 | −0.2±1.8 | 0.3±1.6 | <0.001, <0.001† |
| LV short-axis dimension Zscore | 3.6±2.7 | 2.4±2.3 | 2.7±2.6 | 0.09,* 0.28† |
| LV sphericity | 0.62±0.09 | 0.72±0.13 | 0.69±0.12 | 0.004, 0.04† |
| MV annulus diameter Zscore | −0.6±1.3 | −1.6±1.3 | −1.6±1.1 | 0.009,* 0.01† |
| RV long-axis dimension Zscore | 1.7±1.8 | 1.1±1.2 | 1.4±1.1 | 0.13, 0.46† |
| Female, n (%) | 7 (41) | 9 (18) | 7 (21) | 0.06, 0.14† |
| “High” LV pressure, n (%) | 13 (76) | 15 (29) | 10 (30) | 0.006, 0.004† |
| Moderate or severe MR, n (%) | 10 (59) | 27 (53) | 18 (54) | 0.67, 0.77† |
| MV inflow time (msec) | 124±38 | 115±40 | 118±45 | 0.43, 0.62† |
| MV inflow time Z score | −2.9±1.6 | −3.2±1.7 | −3.1±1.9 | 0.47,* 0.67† |
| Restrictive PFO/intact atrial septum, n (%) | 4 (24) | 4 (8) | 2 (6) | 0.10, 0.16† |
| Acute postdilation AR (moderate or greater), n (%) | 5 (29) | NA | 14 (42) | 0.45† |
PFO indicates patent foramen ovale. Includes patients with a biventricular outcome from birth or after intermediate univentricular palliation. Biventricular outcome vs single-ventricle outcome *including or †excluding technically unsuccessful interventions. Data are presented as mean±SD unless otherwise indicated.
A multivariable threshold score analysis was optimized for positive predictive value after requiring 100% sensitivity for predicting postnatal biventricular outcome. This model included threshold Z scores on the preintervention fetal echocardiogram for LV long-axis dimension, LV short-axis dimension, aortic annulus diameter, and MV annulus diameter, along with a dichotomous measure of LV pressure. A value of 1 was assigned for measurements above the following Z-score thresholds: 0 for the LV diastolic long-axis and short-axis dimensions, −3.5 for the aortic annulus diameter, and −2 for the MV diameter; a value of 1 was also assigned for “high” LV pressure. Scores ranged from 0 to 5. As depicted in Figure 4, 17 of 40 fetuses with a score ≥4 had a biventricular outcome from the time of birth (n=15) or after initial univentricular palliation (n=2) compared with 0 of 28 fetuses with a score <4 (P<0.001). In our observed cohort, a threshold score ≥4 had 100% sensitivity, 53% specificity, 38% positive predictive value, and 100% negative predictive value for identifying fetuses that had a biventricular outcome from the time of birth without an intermediate stage 1 procedure. With all patients who had a biventricular outcome (from birth or after a stage 1 procedure) included, the sensitivity and negative predictive value of this score remained 100%, the specificity was 55%, and the positive predictive value was 43%.
Figure 4.
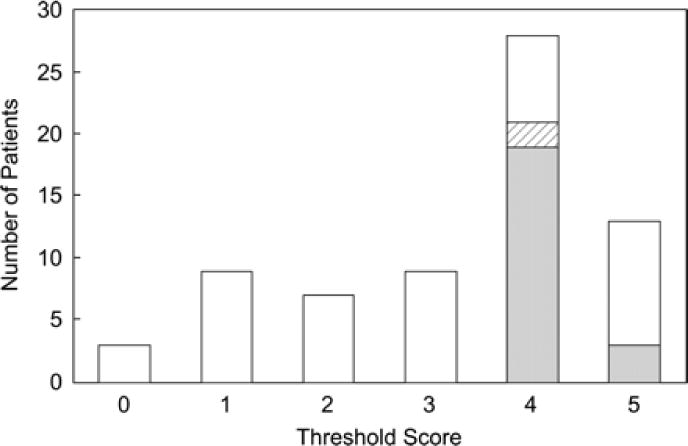
Distribution of threshold scores according to biventricular or univentricular outcome postnatally. White shading indicates fetuses with a univentricular outcome; gray shading, those with a biventricular outcome from birth; and hatched shading, the 2 that initially underwent univentricular staging and were later converted to a biventricular circulation.
Discussion
Hypotheses
When we started our fetal cardiac intervention program 8 years ago, our working hypotheses were simple: (1) In midgestation fetuses with AS and evolving HLHS, the developing left heart is plastic, so alterations in the hemodynamic environment might allow normalization of growth and function; and (2) therefore, in utero aortic valvuloplasty in such fetuses should promote normalization of left heart development and prevent ultimate progression to HLHS. Our faith in these hypotheses was sustained by an apparently dramatic success in the second case we attempted. The procedure was successful; LV function improved; and the patient was born at term with only moderate valvar AS, for which no intervention was performed until 18 months of age. However, it subsequently became clear that such outright success could not be counted on and that our hypotheses were not entirely correct.
Overview of Findings
Here, we report outcomes of our first 70 attempts at prenatal intervention for midgestation fetal AS with evolving HLHS. There are a number of notable findings of this analysis: (1) Aortic valvuloplasty can be performed successfully in approximately three quarters of attempted cases; (2) shorter LV length is associated with higher risk of technical failure; (3) there is an important risk of fetal demise; (4) AR is common and is associated with a larger balloon to annulus diameter ratio but for unknown reasons almost always improves or disappears before birth and does not seem to be associated with outcome; (5) the LV does not grow more after successful prenatal aortic valvuloplasty than in controls, but does contribute to cardiac output, and the supporting left-sided valves and ascending aorta do grow; and (6) a combination of preintervention anatomic and physiological features of the left heart can identify fetuses with and without the potential for a postnatal biventricular outcome.
One of the most important findings of this study was that technically successful fetal aortic valvuloplasty leads to improved growth of the aortic valve, ascending aorta, and MV relative to comparison fetuses but no change in the growth rate of the LV. Whatever the reason—because the condition that we call “fetal AS with evolving HLHS” is intrinsically a disease of the LV myocardium, because the LV is irreversibly damaged early in the disease process, or because the LV is less plastic than the aortic valve and MV—fetal intervention does not effectively promote LV growth in fetuses with this condition. The primary clinical implication of this finding, namely that fetuses with a larger LV at the time of midgestation intervention are more likely to have a left heart sufficient to sustain a biventricular circulation postnatally, was also borne out by our results.
Selection of Fetuses for Aortic Valvuloplasty
Ideally, patient selection should allow identification of fetuses with AS that will progress to HLHS if left untreated, that are too likely to undergo successful intervention, and that have a “salvageable” left heart, in other words, a good chance of thriving with a biventricular circulation postnatally after successful fetal intervention.
Progression to HLHS
Predicting the evolution from AS with a normal-sized or dilated LV in midgestation to HLHS at term is complicated by the fact that HLHS occurs as a spectrum of left heart hypoplasia and dysfunction and that the decision to treat a patient as having HLHS (i.e., with univentricular palliation) may vary between individuals and institutions. Despite models designed to predict left heart adequacy among neonates with AS,25–27 choosing the optimal approach in borderline patients remains difficult. The understanding of fetal AS is even more limited; few studies report serial data, and most include only a small number of fetuses.4–6 Nonetheless, midgestation fetal AS that does not progress to HLHS is rare and can be distinguished from evolving HLHS by a few physiological, rather than anatomic, features: direction of blood flow in the transverse aortic arch, direction of flow across the foramen ovale, and the phasic pattern of MV inflow.6 It is important to reiterate that all of the fetuses in this series demonstrated physiologic findings associated with progression to HLHS. Supporting our preintervention assessment that fetuses selected for fetal intervention truly had evolving HLHS, all of the fetuses that had a technically unsuccessful procedure, as well as the majority that underwent successful intervention, were managed with a univentricular approach postnatally.
Predicting Technical Success
In this series, 74% of attempted aortic valvuloplasty procedures were technically successful. The only anatomic feature associated with inevitable technical failure was equivocal aortic atresia at the time of intervention. Also, only 1 of 9 fetuses with an LV long-axis Z score <−2 underwent technically successful intervention. Overall, 13 of the 18 unsuccessful procedures were in fetuses with an LV long-axis Z score <−2, a threshold score <4, and/or equivocal aortic atresia, and we no longer consider fetuses meeting any of these criteria for routine prenatal aortic valvuloplasty.
Identifying a Salvageable Left Heart
Predicting whether fetal intervention will result in improved left heart growth and ultimately postnatal survival with a biventricular circulation remains a challenge and requires further investigation. Our initial inclusion criteria were deliberately broad. On the basis of the findings of this study, we will now be able to use a combination of anatomic features and LV pressure to identify fetuses that fit our original criteria but have no reasonable chance of a biventricular outcome. Fetuses with a threshold score <4, more than one third of our technically successful interventions and 10 of 18 technically unsuccessful procedures, will no longer be candidates for fetal aortic valvuloplasty to prevent the evolution of critical AS to HLHS. We have modified our selection criteria for identifying a salvageable left heart as indicated in Table 1.
Limitations
This study has several important limitations. We relied on a combination of historical controls and fetuses that underwent technically unsuccessful intervention to serve as a comparison group. This design is less rigorous than a randomized controlled study and may be biased in various ways but was selected on the basis of a combination of scientific and logistical considerations. Because most patients were referred from and managed primarily at other centers, follow-up data were sometimes incomplete, and approaches to postnatal management (i.e., uni- or biventricular management) varied. Although endocardial fibroelastosis is common in this patient population, it can be difficult to quantify or grade reliably in the fetus, and we made no attempt to assess its impact on outcome. MR was not present in some patients, preventing measurement of LV pressure; thus, our assessment of LV pressure was derived from different measures in different patients. Although we expect that the AS gradient will be much higher than the MR gradient in general, in fetuses with both AS and MR gradients and estimated LV pressures in the 20 to 25 mm Hg range, the AS gradient was usually within 5 to 8 mm Hg, so we used a 2 m/s AS gradient as evidence of “high” LV pressure in patients with no MR. Analysis of an evolving experience such as this one is confounded by the fact that the practice is not static, that many small changes were likely incorporated into a collaborative practice at various points, and analytic adjustment for such elements is impractical. The estimates of sensitivity, specificity, and predictive value for our threshold score have not been validated and are almost certainly optimistic.
Conclusions
In fetuses with AS and evolving HLHS, technically successful prenatal aortic valvuloplasty altered the growth and function of some left heart structures, but not the LV. In fetuses with a large LV before intervention, successful aortic valvuloplasty increased the probability of a biventricular outcome after birth. A multivariable threshold scoring system that takes into account the sizes of the aortic valve, MV, and LV, as well as LV pressure, allowed highly sensitive and moderately specific identification of fetuses that survived postnatally with a biventricular circulation. In light of these findings, we have modified our inclusion criteria in fetuses with mid-gestation critical AS and evolving HLHS. The modest positive predictive value of the threshold scoring system remains suboptimal, and additional work is necessary to improve the probability of postnatal biventricular outcome after fetal aortic valvuloplasty.
Fetal intervention for AS with evolving HLHS is generally not a standalone procedure that is either effective or ineffective. Rather, it is 1 element of a broader collaborative strategy for left heart salvage. We are still coming to understand the unique physiologic characteristics of newborns who have undergone successful prenatal intervention and will no doubt need to modify our practice and strategy as we learn more. The potential benefits of fetal intervention must be weighed against the risks of technical failure, fetal demise, AR, and potential long-term adverse events that have yet to be identified.
Supplementary Material
Acknowledgments
This study was supported by contributions from the Kenrose Kitchen Table Foundation and from Brad Lerman and Rita Conroy.
This work was supported by the National Institutes of Health under award number: T32HL007572. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Addendum
Both of the fetuses that were still in utero at the time the manuscript was submitted have been born. In the newborn period, 1 (threshold score, 5) underwent surgical aortic valvuloplasty and is at home with a biventricular circulation and moderate AS; the other underwent a stage 1 operation. In addition, after submission of the manuscript, as of July 2009, 2 more patients (threshold score for both, 4) initially managed with single-ventricle palliation underwent successful conversion to a biventricular circulation. With these 4 patients included, as of July 2009, 20 of the 70 patients (29%) in this series achieved a biventricular circulation, all patients with a threshold score <4 had a single-ventricle outcome, and 50% of patients with a threshold score ≥4 had a biventricular outcome.
The authors had full access to and take full responsibility for the integrity of the data. All authors have read and agree to the manuscript as written.
References
- 1.Mahle WT, Spray TL, Wernovsky G, Gaynor JW, Clark BJ. Survival after reconstructive surgery for hypoplastic left heart syndrome: a 15-year experience from a single institution. Circulation. 2000;102(suppl):III-136–III-141. doi: 10.1161/01.cir.102.suppl_3.iii-136. [DOI] [PubMed] [Google Scholar]
- 2.Tweddell JS, Hoffman GM, Mussatto KA, Fedderly RT, Berger S, Jaquiss RD, Ghanayem NS, Frisbee SJ, Litwin SB. Improved survival of patients undergoing palliation of hypoplastic left heart syndrome: lessons learned from 115 consecutive patients. Circulation. 2002;106(suppl):I-82–II-89. [PubMed] [Google Scholar]
- 3.Tabbutt S, Dominguez TE, Ravishankar C, Marino BS, Gruber PJ, Wernovsky G, Gaynor JW, Nicolson SC, Spray TL. Outcomes after the stage I reconstruction comparing the right ventricular to pulmonary artery conduit with the modified Blalock Taussig shunt. Ann Thorac Surg. 2005;80:1582–1590. doi: 10.1016/j.athoracsur.2005.04.046. [DOI] [PubMed] [Google Scholar]
- 4.Danford DA, Cronican P. Hypoplastic left heart syndrome: progression of left ventricular dilation and dysfunction to left ventricular hypoplasia in utero. Am Heart J. 1992;123:1712–1713. doi: 10.1016/0002-8703(92)90834-i. [DOI] [PubMed] [Google Scholar]
- 5.Simpson JM, Sharland GK. Natural history and outcome of aortic stenosis diagnosed prenatally. Heart. 1997;77:205–210. doi: 10.1136/hrt.77.3.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Makikallio K, McElhinney DB, Levine JC, Marx GR, Colan SD, Marshall AC, Lock JE, Marcus EN, Tworetzky W. Fetal aortic valve stenosis and the evolution of hypoplastic left heart syndrome: patient selection for fetal intervention. Circulation. 2006;113:1401–1405. doi: 10.1161/CIRCULATIONAHA.105.588194. [DOI] [PubMed] [Google Scholar]
- 7.Fishman NH, Hof RB, Rudolph AM, Heymann MA. Models of congenital heart disease in fetal lambs. Circulation. 1978;58:354–364. doi: 10.1161/01.cir.58.2.354. [DOI] [PubMed] [Google Scholar]
- 8.Levin DL, Perkin RM, Parkey M, Mayhew E, Hartwig R. Experimental aortic stenosis in fetal lambs. Circulation. 1980;62:1159–1164. doi: 10.1161/01.cir.62.6.1159. [DOI] [PubMed] [Google Scholar]
- 9.Sedmera D, Hu N, Weiss KM, Keller BB, Denslow S, Thompson RP. Cellular changes in experimental left heart hypoplasia. Anat Rec. 2002;267:137–145. doi: 10.1002/ar.10098. [DOI] [PubMed] [Google Scholar]
- 10.Miller CE, Wong CL, Sedmera D. Pressure overload alters stress-strain properties of the developing chick heart. Am J Physiol Heart Circ Physiol. 2003;285:H1849–H1856. doi: 10.1152/ajpheart.00384.2002. [DOI] [PubMed] [Google Scholar]
- 11.Samson F, Bonnet N, Heimburger M, Rucker-Martin C, Levitsky DO, Mazmanian GM, Mercadier JJ, Serraf A. Left ventricular alterations in a model of fetal left ventricular overload. Pediatr Res. 2000;48:43–49. doi: 10.1203/00006450-200007000-00010. [DOI] [PubMed] [Google Scholar]
- 12.Eghtesady P, Michelfelder E, Altaye M, Ballard E, Hirsh R, Beekman RH. Revisiting animal models of aortic stenosis in the early gestation fetus. Ann Thorac Surg. 2007;83:631–639. doi: 10.1016/j.athoracsur.2006.09.043. [DOI] [PubMed] [Google Scholar]
- 13.Maxwell D, Allan L, Tynan MJ. Balloon dilatation of the aortic valve in the fetus: a report of two cases. Br Heart J. 1991;65:256–258. doi: 10.1136/hrt.65.5.256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kohl T, Sharland G, Allan LD, Gembruch U, Chaoui R, Lopes LM, Zielinsky P, Huhta J, Silverman NH. World experience of percutaneous ultrasound-guided balloon valvuloplasty in human fetuses with severe aortic valve obstruction. Am J Cardiol. 2000;85:1230–1233. doi: 10.1016/s0002-9149(00)00733-5. [DOI] [PubMed] [Google Scholar]
- 15.Harh JY, Paul MH, Gallen WJ, Friedberg DZ, Kaplan S. Experimental production of hypoplastic left heart syndrome in the chick embryo. Am J Cardiol. 1973;31:51–56. doi: 10.1016/0002-9149(73)90810-2. [DOI] [PubMed] [Google Scholar]
- 16.Broekhuizen ML, Hogers B, DeRuiter MC, Poelmann RE, Gittenberger-de Groot AC, Wladimiroff JW. Altered hemodynamics in chick embryos after extraembryonic venous obstruction. Ultrasound Obstet Gynecol. 1999;13:437–445. doi: 10.1046/j.1469-0705.1999.13060437.x. [DOI] [PubMed] [Google Scholar]
- 17.Saiki Y, Konig A, Waddell J, Rebeyka IM. Hemodynamic alteration by fetal surgery accelerates myocyte proliferation in fetal guinea pig hearts. Surgery. 1997;122:412–419. doi: 10.1016/s0039-6060(97)90034-9. [DOI] [PubMed] [Google Scholar]
- 18.Hogers B, DeRuiter MC, Gittenberger-de Groot AC, Poelmann RE. Unilateral vitelline vein ligation alters intracardiac blood flow patterns and morphogenesis in the chick embryo. Circ Res. 1997;80:473–481. doi: 10.1161/01.res.80.4.473. [DOI] [PubMed] [Google Scholar]
- 19.Tworetzky W, Wilkins-Haug L, Jennings RW, van der Velde ME, Marshall AC, Marx GR, Colan SD, Benson CB, Lock JE, Perry SB. Balloon dilation of severe aortic stenosis in the fetus: potential for prevention of hypoplastic left heart syndrome: candidate selection, technique, and results of successful intervention. Circulation. 2004;110:2125–2131. doi: 10.1161/01.CIR.0000144357.29279.54. [DOI] [PubMed] [Google Scholar]
- 20.Marshall AC, Tworetzky W, Bergersen L, McElhinney DB, Benson CB, Jennings RW, Wilkins-Haug LE, Marx GR, Lock JE. Aortic valvuloplasty in the fetus: technical characteristics of successful balloon dilation. J Pediatr. 2005;147:535–539. doi: 10.1016/j.jpeds.2005.04.055. [DOI] [PubMed] [Google Scholar]
- 21.Wilkins-Haug LE, Tworetzky W, Benson CB, Marshall AC, Jennings RW, Lock JE. Factors affecting technical success of fetal aortic valve dilation. Ultrasound Obstet Gynecol. 2006;28:47–52. doi: 10.1002/uog.2732. [DOI] [PubMed] [Google Scholar]
- 22.Mizrahi-Arnaud A, Tworetzky W, Bulich LA, Wilkins-Haug LE, Marshall AC, Benson CB, Lock JE, McElhinney DB. Pathophysiology, management, and outcomes of fetal hemodynamic instability during prenatal cardiac intervention. Pediatr Res. 2007;62:325–330. doi: 10.1203/PDR.0b013e318123fd3a. [DOI] [PubMed] [Google Scholar]
- 23.Selamet Tierney ES, Wald RM, McElhinney DB, Marshall AC, Benson CB, Marcus EN, Marx GR, Levine JC, Wilkins-Haug L, Lock JE, Tworetzky W. Changes in left heart hemodynamics after technically successful in utero aortic valvuloplasty. Ultrasound Obstetr Gynecol. 2007;30:715–720. doi: 10.1002/uog.5132. [DOI] [PubMed] [Google Scholar]
- 24.Marshall AC, van der Velde ME, Tworetzky W, Gomez CA, Wilkins-Haug L, Benson CB, Jennings RW, Lock JE. Creation of an atrial septal defect in utero for fetuses with hypoplastic left heart syndrome and intact or highly restrictive atrial septum. Circulation. 2004;110:253–258. doi: 10.1161/01.CIR.0000135471.17922.17. [DOI] [PubMed] [Google Scholar]
- 25.Rhodes LA, Colan SD, Perry SB, Jonas RA, Sanders SP. Predictors of survival in neonates with critical aortic stenosis. Circulation. 1991;84:2325–2335. doi: 10.1161/01.cir.84.6.2325. [DOI] [PubMed] [Google Scholar]
- 26.Lofland GK, McCrindle BW, Williams WG, Blackstone EH, Tchervenkov CI, Sittiwangkul R, Jonas RA. Critical aortic stenosis in the neonate: a multi-institutional study of management, outcomes, and risk factors: Congenital Heart Surgeons Society. J Thorac Cardiovasc Surg. 2001;121:10–27. doi: 10.1067/mtc.2001.111207. [DOI] [PubMed] [Google Scholar]
- 27.Colan SD, McElhinney DB, Crawford EC, Keane JF, Lock JE. Validation and reevaluation of a discriminant model predicting anatomic suitability for biventricular repair in neonates with aortic stenosis. J Am Coll Cardiol. 2006;47:1858–1865. doi: 10.1016/j.jacc.2006.02.020. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


