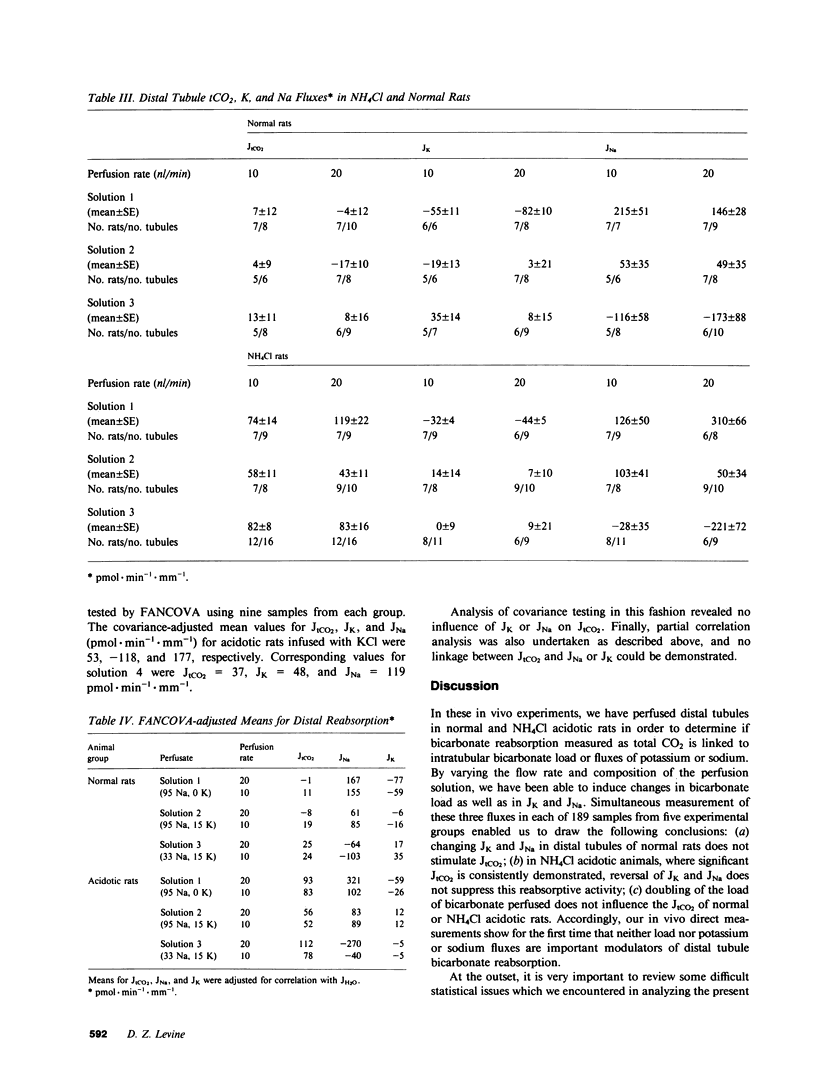
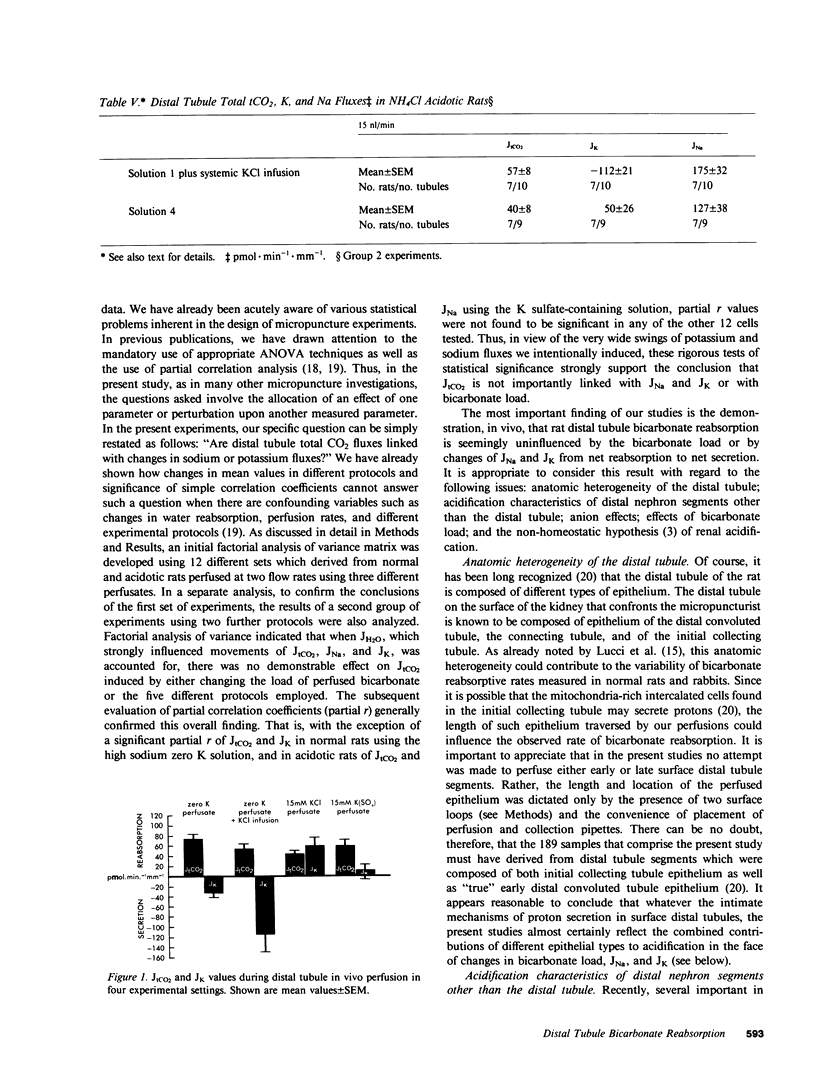
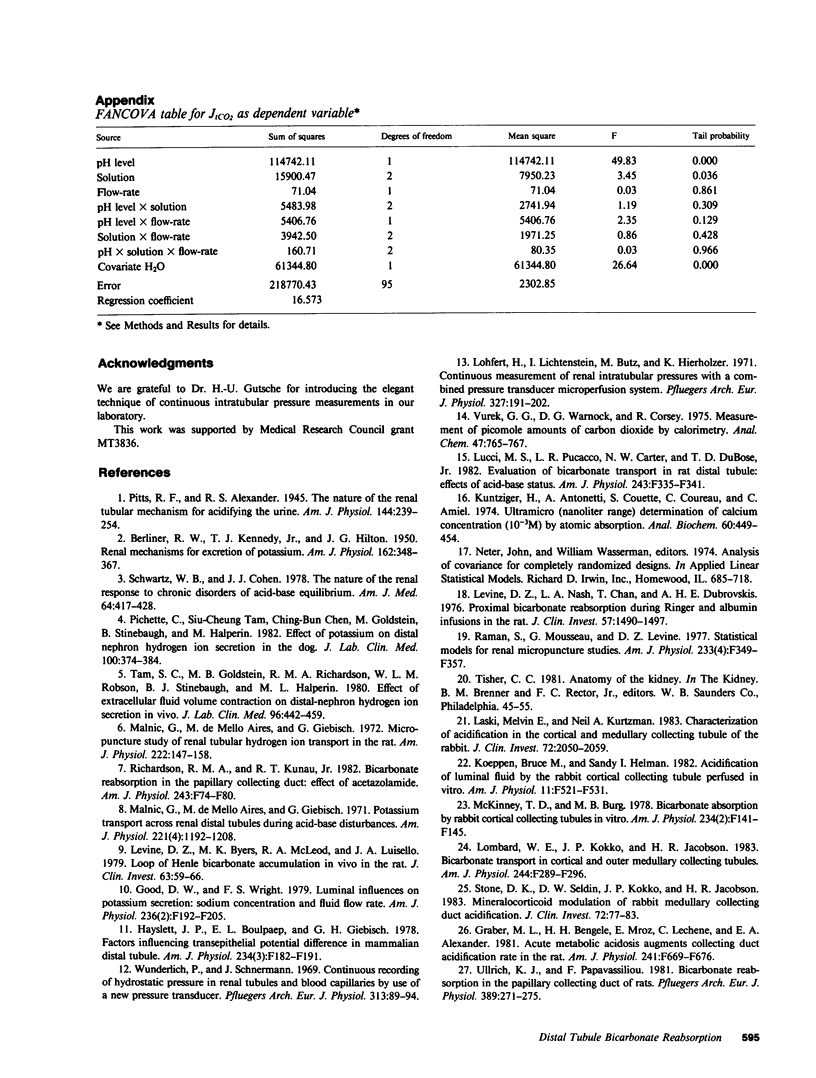
Abstract
For many years it has been thought that distal nephron hydrogen ion secretion can be importantly modulated by factors such as sodium delivery, sodium avidity, and potassium stores. Free flow micropuncture studies have also indicated that the rate of bicarbonate delivery may also alter the rate of bicarbonate reabsorption. The present studies were undertaken to examine possible luminal influences on total CO2 reabsorption in microperfused distal tubules in the rat in vivo. Tubules from normal and acidotic rats were perfused with five solutions in a manner that induced changes in bicarbonate load, sodium and potassium fluxes (JNa, JK), and luminal sulfate concentration. in each collected perfusate, simultaneous analyses were undertaken to determine water reabsorption, Na, and K concentrations using graphite furnace atomic absorption spectroscopy and total CO2 by microcalorimetry. Using factorial analysis of covariance to account for confounding effects on total CO2 flux (JtCO2) such as water reabsorption, distal tubules of acidotic rats reabsorbed CO2 in the range of 50-112 pmol X min-1 X mm-1 X These JtCO2 values were not significantly correlated with HCO3 load, JNa, or JK despite changes in the latter from net reabsorption to net secretion. Distal tubules of rats with normal acid-base status had JtCO2 values which were neither significantly different from zero nor correlated with changes in JK and JNa. Further, doubling the load from 250-500 pmol/min (by doubling the perfusion rate of 25-mM HCO3 solutions) did not stimulate JtCO2 in these normal animals. Accordingly, these acute in vivo microperfusion studies indicate for the first time that neither load nor potassium or sodium fluxes are important modulators of distal tubule bicarbonate reabsorption.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BERLINER R. W., KENNEDY T. J., Jr, HILTON J. G. Renal mechanisms for excretion of potassium. Am J Physiol. 1950 Aug 1;162(2):348–367. doi: 10.1152/ajplegacy.1950.162.2.348. [DOI] [PubMed] [Google Scholar]
- Good D. W., Wright F. S. Luminal influences on potassium secretion: sodium concentration and fluid flow rate. Am J Physiol. 1979 Feb;236(2):F192–F205. doi: 10.1152/ajprenal.1979.236.2.F192. [DOI] [PubMed] [Google Scholar]
- Graber M. L., Bengele H. H., Mroz E., Lechene C., Alexander E. A. Acute metabolic acidosis augments collecting duct acidification rate in the rat. Am J Physiol. 1981 Dec;241(6):F669–F676. doi: 10.1152/ajprenal.1981.241.6.F669. [DOI] [PubMed] [Google Scholar]
- Hayslett J. P., Boulpaep E. L., Giebisch G. H. Factors influencing transepithelial potential difference in mammalian distal tubule. Am J Physiol. 1978 Mar;234(3):F182–F191. doi: 10.1152/ajprenal.1978.234.3.F182. [DOI] [PubMed] [Google Scholar]
- Koeppen B. M., Helman S. I. Acidification of luminal fluid by the rabbit cortical collecting tubule perfused in vitro. Am J Physiol. 1982 May;242(5):F521–F531. doi: 10.1152/ajprenal.1982.242.5.F521. [DOI] [PubMed] [Google Scholar]
- Kuntziger H., Antonetti A., Couette S., Coureau C., Amiel C. Ultramicro (nanoliter range) determination of calcium concentration (10-3 M) by atomic absorption. Anal Biochem. 1974 Aug;60(2):449–454. doi: 10.1016/0003-2697(74)90254-1. [DOI] [PubMed] [Google Scholar]
- Laski M. E., Kurtzman N. A. Characterization of acidification in the cortical and medullary collecting tubule of the rabbit. J Clin Invest. 1983 Dec;72(6):2050–2059. doi: 10.1172/JCI111170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine D. Z., Byers M. K., McLeod R. A., Luisello J. A., Raman S. Loop of Henle bicarbonate accumulation in vivo in the rat. J Clin Invest. 1979 Jan;63(1):59–66. doi: 10.1172/JCI109278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine D. Z., Nash L. A., Chan T., Dubrovskis A. H. Proximal bicarbonate reabsorption during Ringer and albumin infusions in the rat. J Clin Invest. 1976 Jun;57(6):1490–1497. doi: 10.1172/JCI108419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lohfert H., Lichtenstein I., Butz M., Hierholzer K. Continuous measurement of renal intratubular presures with a combined pressure transducer microperfusion system. Pflugers Arch. 1971;327(3):191–202. doi: 10.1007/BF00586858. [DOI] [PubMed] [Google Scholar]
- Lombard W. E., Kokko J. P., Jacobson H. R. Bicarbonate transport in cortical and outer medullary collecting tubules. Am J Physiol. 1983 Mar;244(3):F289–F296. doi: 10.1152/ajprenal.1983.244.3.F289. [DOI] [PubMed] [Google Scholar]
- Lucci M. S., Pucacco L. R., Carter N. W., DuBose T. D., Jr Evaluation of bicarbonate transport in rat distal tubule: effects of acid-base status. Am J Physiol. 1982 Oct;243(4):F335–F341. doi: 10.1152/ajprenal.1982.243.4.F335. [DOI] [PubMed] [Google Scholar]
- Malnic G., De Mello Aires M., Giebisch G. Micropuncture study of renal tubular hydrogen ion transport in the rat. Am J Physiol. 1972 Jan;222(1):147–158. doi: 10.1152/ajplegacy.1972.222.1.147. [DOI] [PubMed] [Google Scholar]
- Malnic G., De Mello Aires M., Giebisch G. Potassium transport across renal distal tubules during acid-base disturbances. Am J Physiol. 1971 Oct;221(4):1192–1208. doi: 10.1152/ajplegacy.1971.221.4.1192. [DOI] [PubMed] [Google Scholar]
- McKinney T. D., Burg M. B. Bicarbonate absorption by rabbit cortical collecting tubules in vitro. Am J Physiol. 1978 Feb;234(2):F141–F145. doi: 10.1152/ajprenal.1978.234.2.F141. [DOI] [PubMed] [Google Scholar]
- Pichette C., Tam S. C., Chen C. B., Goldstein M., Stinebaugh B., Halperin M. Effect of potassium on distal nephron hydrogen ion secretion in the dog. J Lab Clin Med. 1982 Sep;100(3):374–384. [PubMed] [Google Scholar]
- Raman S., Mousseau G., Levine D. Z. Statistical models for renal micropuncture studies. Am J Physiol. 1977 Oct;233(4):F349–F357. doi: 10.1152/ajprenal.1977.233.4.F349. [DOI] [PubMed] [Google Scholar]
- Richardson R. M., Kunau R. T., Jr Bicarbonate reabsorption in the papillary collecting duct: effect of acetazolamide. Am J Physiol. 1982 Jul;243(1):F74–F80. doi: 10.1152/ajprenal.1982.243.1.F74. [DOI] [PubMed] [Google Scholar]
- Schwartz W. B., Cohen J. J. The nature of the renal response to chronic disorders of acid-base equilibrium. Am J Med. 1978 Mar;64(3):417–428. doi: 10.1016/0002-9343(78)90227-9. [DOI] [PubMed] [Google Scholar]
- Stone D. K., Seldin D. W., Kokko J. P., Jacobson H. R. Mineralocorticoid modulation of rabbit medullary collecting duct acidification. A sodium-independent effect. J Clin Invest. 1983 Jul;72(1):77–83. doi: 10.1172/JCI110986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tam S. C., Goldstein M. B., Richardson R. M., Robson W. L., Stinebaugh B. J., Halperin M. L. Effect of extracellular fluid volume contraction on distal-nephron hydrogen ion secretion in vivo. J Lab Clin Med. 1980 Sep;96(3):442–449. [PubMed] [Google Scholar]
- Ullrich K. J., Papavassiliou F. Bicarbonate reabsorption in the papillary collecting duct of rats. Pflugers Arch. 1981 Mar;389(3):271–275. doi: 10.1007/BF00584789. [DOI] [PubMed] [Google Scholar]
- Vurek G. G., Warnock D. G., Corsey R. Measurement of picomole amounts of carbon dioxide by calorimetry. Anal Chem. 1975 Apr;47(4):765–767. doi: 10.1021/ac60354a024. [DOI] [PubMed] [Google Scholar]
- Wunderlich P., Schnermann J. Continuous recording of hydrostatic pressure in renal tubules and blood capillaries by use of a new pressure transducer. Pflugers Arch. 1969;313(1):89–94. doi: 10.1007/BF00586332. [DOI] [PubMed] [Google Scholar]



