Abstract
The impact of Parkinson’s disease (PD) dementia is substantial and has major functional and socioeconomic consequences. Early prediction of future cognitive impairment would help target future interventions. The Montreal Cognitive Assessment (MoCA), the Mini-Mental State Examination (MMSE), and fluency tests were administered to 486 patients with PD within 3.5 years of diagnosis, and the results were compared with those from 141 controls correcting for age, sex, and educational years. Eighteen-month longitudinal assessments were performed in 155 patients with PD. The proportion of patients classified with normal cognition, mild cognitive impairment (MCI), and dementia varied considerably, depending on the MoCA and MMSE thresholds used. With the MoCA total score at screening threshold, 47.7%, 40.5%, and 11.7% of patients with PD were classified with normal cognition, MCI, and dementia, respectively; by comparison, 78.7% and 21.3% of controls had normal cognition and MCI, respectively. Cognitive impairment was predicted by lower education, increased age, male sex, and quantitative motor and non-motor (smell, depression, and anxiety) measures. Longitudinal data from 155 patients with PD over 18 months showed significant reductions in MoCA scores, but not in MMSE scores, with 21.3% of patients moving from normal cognition to MCI and 4.5% moving from MCI to dementia, although 13.5% moved from MCI to normal; however, none of the patients with dementia changed their classification. The MoCA may be more sensitive than the MMSE in detecting early baseline and longitudinal cognitive impairment in PD, because it identified 25.8% of those who experienced significant cognitive decline over 18 months. Cognitive decline was associated with worse motor and non-motor features, suggesting that this reflects a faster progressive phenotype.
Keywords: Parkinson’s disease, mild cognitive impairment, dementia, Mini-Mental State Examination, Montreal Cognitive Assessment
Cognitive impairment and dementia are common in Parkinson’s disease (PD), with a long-term cumulative prevalence of 80% for PD dementia (PDD).1 The impact of PDD is substantial and has a major impact on independence, nursing home admission, psychiatric comorbidity, care-giver burden, and mortality.2–4 Consequently, there is interest in a potential transition stage—PD with mild cognitive impairment (PD-MCI) —with which to identify those at increased risk for PDD to facilitate intervention studies.5–11 MCI appears to be common even in newly diagnosed, drug-naive PD patients.12,13 The identification of PD-MCI is important, because patients are at increased risk of developing PDD.7,14,15 Most researchers use psychometric criteria to define PD-MCI by sampling impairments in multiple cognitive domains, because several deficits are implicated as predictors5–11 of progression to PDD.7,16 The difficulty facing clinicians, however, is that markedly heterogeneous criteria for PD-MCI have been applied, leading, in turn, to a lack of consistency in this research field.6,8,10
Movement Disorder Society (MDS) Task Force diagnostic criteria for PD-MCI17 and PDD18,19 suggest that level 1 screening for a diagnosis can be made using a scale of global cognitive abilities, and studies indicate that the Montreal Cognitive Assessment (MoCA)20 is more sensitive than the Mini-Mental State Examination (MMSE) in detecting early PD cognitive impairment.21–23 Level 2 criteria require evidence of cognitive impairment in multiple cognitive domains on lengthier neuropsychological testing.17 Although an MMSE score < 26 was proposed for PDD diagnosis,19 subsequent studies have suggested that this conservative cutoff will be relatively specific, at the cost of sensitivity.24,25 One suggested approach to this problem is longitudinal follow-up and neuropsychological testing for the diagnosis and characterization of PDD and PD-MCI,26 although current guidelines do not inform on how longitudinal testing aids diagnosis.9,18 A recent study of prevalent cases in patients with a wide range of disease duration indicated that MMSE scores, but not MoCA scores, changed significantly over 3 years.26 Our objectives were to evaluate the frequency of cognitive impairment in a prospective, community-acquired sample of patients who had early PD compared with controls; to identify motor and non-motor correlates of impaired baseline cognition; and to assess the performance of screening tools in detecting longitudinal change. This study adds to previous publications, because our sample comes from a cohort of patients that should be more representative of patients with typical PD who have been assessed relatively early in their natural history. In addition, the wide range of other phenotypic variables that have been collected enables correlation with cognitive measures.
Patients and Methods
Patients
Established in September 2010, the Oxford Discovery cohort (http://opdc.medsci.ox.ac.uk) comprises patients with idiopathic PD diagnosed in the previous 3.5 years according to UK PD Society Brain Bank diagnostic criteria27 who were recruited from a 2.9 million population. PD patients were prospectively recruited over 2 years after ethics committee approval and verbal/written consent from participants. Clinical information was prescreened to exclude patients who had atypical parkinsonian disorders. Patients underwent standardized assessment by a movement disorders neurologist/research nurse; then, the patients were diagnosed PD, and an estimated clinical probability for this diagnosis was made by the neurologist. Atypical parkinsonian features were screened using the National Institute of Neurological Disorders and Stroke Parkinson’s tool (NINDS, Bethesda, MD, USA). Patients were excluded from subsequent analysis if they had a < 90% baseline probability of manifesting PD. Control participants were recruited from partners/friends of PD patients. See Appendix for exclusion criteria.
Participants completed a questionnaire that assessed educational/social history, the EQ-5D Health Questionnaire,28 the Rapid Eye Movement (REM) Sleep Behaviour Disorder (RBD) Questionnaire,29 the Leeds Anxiety and Depression Scale,30 and the Epworth Sleepiness Scale.31 Motor assessments were performed by the neurologist while the patients were taking their usual PD medications in a clinically defined on state, including the revised MDS Unified Parkinson’s Disease Rating Scale (UPDRS) parts 1 through 4,32 the Hoehn and Yahr scale,33,34 and the Schwab and England (S&E) scale.35 Levodopa (l-dopa) response was estimated using the Clinical Global Impression (CGI) improvement scale. The l-dopa equivalent daily dose (LEDD) was calculated for each patient with PD who was receiving medication.36 Cognitive assessments included the MMSE (only the serial sevens test, not the WORLD backward test, was used)37 and the MoCA20; and phonemic/semantic verbal fluency tests were performed by a Dementias and Neurodegenerative Diseases Research Network (DeNDRoN) nurse who was trained to administer the tests by a clinical neuropsychologist (C. Murray). An additional point on the total MoCA score was added for patients who had ≤ 12 years of education.20 For phonemic and semantic fluency, the total numbers of words generated beginning with F, A, and S and animal and boys’ names, respectively, were counted over 60 seconds each. The Purdue pegboard test,38 the 16-item Sniffin’ Sticks Odour Identification test,39 the 3-meter Timed Up and Go test40 (3 trials performed), and the Flamingo Balance test41 also were administered. Assessments were repeated after 18 months. Patients were divided into tremor-dominant and postural instability and gait difficulty motor subtypes based on previous work.42
Statistical Analysis
We undertook multivariable linear or logistic regression analyses, controlling for age, education, and sex and for continuous or dichotomous variables for comparisons between patients with PD and controls. Paired t tests were used for within-subject baseline with 18-month follow-up comparisons. Pearson’s correlation (r) was also performed between the S&E scale and motor/non-motor measures. PD and control participants were classified according to their total MoCA scores into groups with normal cognition, MCI, and dementia based on either screening cutoff scores from previous studies in PD and elderly control patients20,21,43 or diagnostic cutoff scores21 (Table1). Similarly, we did the same with MMSE scores using previously suggested screening cutoff scores21,23 or diagnostic MMSE cutoff scores (Table1), although this provided only two groups: a normal cognition group and a combined MCI and dementia group.6,21,23 For cross-sectional and longitudinal analyses, total scores on the MMSE and MoCA were divided into four cognitive-specific domains (Table1).26,44 When comparing cognitive-specific domains between the PD group and the control group, scores on serial subtraction and executive-function domain tests were excluded because of differential weighting within the MMSE and the MoCA. We examined potential motor and non-motor predictors of MoCA scores initially controlling only for age, sex, and educational years. See Appendix for an analysis of skewed variables.
Table 1.
Screening and diagnostic cutoffs, domain breakdowns, and point values for the Montreal Cognitive Assessment and the Mini-Mental State Examination
| MoCA | Score | MMSE | Score |
|---|---|---|---|
| Cutoff values | Cutoff values | ||
| Screening | Screening | ||
| Normal | 26–30 | Normal | 29–30 |
| MCI | 21–25 | MCI | 27–28 |
| Dementia | ≤20 | Dementia | ≤ 26 |
| Diagnostic | Diagnostic | ||
| Normal | 24–30 | Normal | 24–30 |
| MCI | 22–23 | MCI | ≤ 23 |
| Dementia | ≤21 | Dementia | |
| Domains, no. of pointsa | Domains, no. of pointsa | ||
| Language | Language | ||
| Naming | 3 | Name objects | 2 |
| Sentence | 2 | Three-stage command | 3 |
| Read and obey | 1 | ||
| Write sentence | 1 | ||
| Memory | Memory | ||
| Recall | 5 | Recall | 3 |
| Orientation | 6 | Orientation | 10 |
| Visuospatial and executive | Visuospatial and executive | ||
| Cube | 1 | Copy design | 1 |
| Clock | 3 | ||
| Trails | 1 | ||
| Serial sevens | 3 | Serial sevens | 5 |
| Executive function | Executive function | ||
| Fluency | 1 | Repeat | 1 |
| Trails | 2 | ||
| Digits | 2 | ||
| Letter | 1 | ||
| Abstraction | 2 |
Points for each domain were modified from Lessig et al., 2012.26
MoCA, Montreal Cognitive Assessment; MCI, mild cognitive impairment; MMSE, Mini-Mental State Examination.
Results
Cross-sectional Analysis
Fifty-seven percent of all potentially eligible patients who were approached to take part in our study agreed to participate. Non-participants were significantly older than participants (mean age ± standard deviation [SD], 73.0 ± 10.6 years; P < 0.001), but there were no sex differences. Six hundred consecutive patients with PD were recruited to the study over 2 years, of whom 82% were assessed as having a ≥ 90% probability of manifesting idiopathic PD at their baseline visit, leaving 492 patients with PD. Cognitive assessment was not possible in six patients, which left 486 patients with PD who were available for study analysis. For details regarding the exclusion of control participants, please see Appendix 1.
Clinical characteristics and cognitive scores are summarized in Table2. Controls were younger, they were more likely to be women (because, in the main, they were recruited from patient spouses and partners, and the risk of PD is greater for men), and they had a significantly longer duration of education that the PD group; therefore, subsequent group comparisons between the PD group and the control group were adjusted for age, sex, and educational years. Patients had significantly lower scores on the MoCA and the MMSE (P < 0.001 and P = 0.001 respectively, adjusting for age, sex, and educational years). According to MoCA scores, the PD group had a significantly poorer performance than the control group in all cognitive domains except language; whereas, according to the MMSE scores, performance was poorer in the PD group for memory and visuospatial domains. Semantic and phonemic verbal fluencies also were significantly worse in the PD group compared with the control group.
Table 2.
Clinical characteristics and Montreal Cognitive Assessment and Mini-Mental State Examination total and cognitive subdomain scores for the PD group and the control group at baseline
| Mean ± SD or no. of patients (%) | |||
|---|---|---|---|
| Variable | PD group, n = 486 | Control group, n = 141 | Pa |
| Age, y | 67.8 ± 9.4 | 63.5 ± 8.9 | <0.001 |
| Total education, y | 13.6 ± 3.5 | 15.1 ± 3.5 | <0.001 |
| Education <12 y | 170 (35.4) | 26 (18.5) | <0.001 |
| Men | 298 (61.3) | 50 (35.5) | <0.001 |
| Disease duration, y | 1.5 ± 1.0 | ||
| UPDRS III score | 26.8 ± 11.0 | ||
| H&Y stage | 1.9 ± 0.5 | ||
| LEDD, mgb | 299 ± 212 | ||
| Cognitive tests [maximum score] | |||
| Total MMSE [30] | 27.3 ± 2.2 | 28.4 ± 1.8 | 0.001 |
| Total MoCA [30] | 24.9 ± 3.5 | 27.1 ± 2.2 | <0.001 |
| MMSE subdomains | |||
| Language [7] | 6.7 ± 0.6 | 6.8 ± 0.5 | 0.92 |
| Memory [3] | 2.4 ± 0.8 | 2.8 ± 0.5 | <0.001 |
| Orientation [10] | 9.8 ± 0.5 | 9.9 ± 0.4 | 0.25 |
| Visuospatial [1] | 0.9 ± 0.3 | 1.0 ± 0.2 | 0.05 |
| MoCA subdomains | |||
| Language [5] | 4.4 ± 0.8 | 4.7 ± 0.6 | 0.13 |
| Memory [5] | 2.7 ± 1.6 | 3.9 ± 1.2 | <0.001 |
| Orientation [6] | 5.9 ± 0.4 | 6.0 ± 0.2 | <0.05 |
| Visuospatial [5] | 4.0 ± 1.1 | 4.4 ± 0.7 | <0.03 |
| Total phonemic fluency | 38.8 ± 13.9 | 45.7 ± 13.1 | 0.001 |
| Total semantic fluency | 34.6 ± 9.1 | 41.0 ± 9.1 | <0.001 |
P values were calculated from a regression model adjusting for age, sex, and years of education. Values in bold indicate significant results (P < 0.05) comparing the PD group with the control group.
In total, 11.1% of patients with PD were untreated.
SD, standard deviation; PD, Parkinson’s disease; UPDRS III, Unified Parkinson’s Disease Rating Scale, part III (motor assessment); H&Y, Hoehn and Yahr; LEDD, levodopa equivalent daily dose; MMSE, Mini-Mental Health Examination; MoCA, Montreal Cognitive Assessment.
The bubble plot in Figure 1 compares the ability of the MoCA versus the MMSE to detect cognitive impairment using screening thresholds. The plot indicates that 8.8% of patients with PD who had normal cognition according to MMSE total scores were classified with impaired cognition according to MoCA total scores; conversely, 26.5 % of patients with PD who had normal cognition according to MoCA total scores were classified as cognitively impaired according to MMSE total scores. Using diagnostic thresholds, 3.1% of PD patients who had normal cognition according to the MMSE were impaired according to the MoCA, whereas 0.8% of patients with PD who had normal cognition according to the MoCA were classified as impaired according to the MMSE. Using MMSE total scores at screening threshold, 37.4%, 32.1%, and 30.5% of patients in the PD group and 63.8%, 19.9%, and 16.3% of participants in the control group were classified as having normal cognition, MCI, and dementia, respectively. Using MMSE total scores at diagnostic threshold, 92.8% and 7.2% of PD patients and 98.6% and 1.4% of controls were classified as having normal cognition and PD-MCI/PDD combined, respectively. When MoCA total scores were used at screening threshold, 47.7%, 40.5%, and 11.7% of PD patients were classified as having normal cognition, MCI, and dementia, respectively; whereas 78.7% and 21.3% of controls were classified as having normal cognition and MCI, respectively (no controls were classified with dementia). When the MoCA total score was used at diagnostic threshold, 69.5%, 14.2%, and 16.3% of patients in the PD group were classified as having normal cognition, MCI, and PDD, respectively, compared with 92.9%, 6.4%, and 0.7% of control participants.
Figure 1.
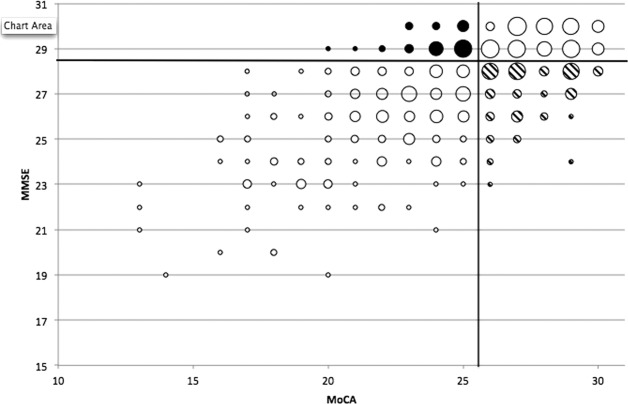
This bubble plot illustrates Montreal Cognitive Assessment (MoCA) versus Mini-Mental State Examination (MMSE) total scores for the Oxford Discovery cohort of 486 patients with early Parkinson’s disease. The size of each bubble is proportional to the number of patients with each corresponding MoCA/MMSE total score. Solid bubbles indicate patients who were classified as cognitively normal on screening MMSE scores but as cognitively impaired using screening MoCA scores; whereas striped bubbles indicate patients who were classified as cognitively normal on screening MoCA scores but as cognitively impaired using screening MMSE scores.
Significant correlations between the S&E scale and motor measures were observed in the PD group (total UPDRS part 2 [activities of daily life], r = −0.55; total UPDRS part 3 [motor evaluation], r = −0.41; Hoehn and Yahr stage, r = −0.36; all P < 0.001). Weaker correlations also were observed with cognitive measures (MMSE total score, r = 0.13; P = 0.004; MoCA total score, r = 0.19; P < 0.001). The difference in S&E scores for participants with dementia/MCI versus normal cognition was much smaller using MMSE diagnostic thresholds (difference, −2.6; P = 0.15) compared with MoCA diagnostic thresholds (difference, −4.2; P = 0.001).
Using a multivariable linear regression model (Table3), we observed that a range of motor and non-motor variables predicted worse MoCA scores in the expected direction: Hoehn and Yahr stage, annualized UPDRS score, Timed Up and Go test, Purdue pegboard, and Flamingo Balance; although total UPDRS score, motor subtypes, LEDD, and the CGI did not. Scores on the Leeds Anxiety and Depression Scale and the Sniffin’ Sticks Odour Identification test also predicted MoCA scores, whereas Epworth Sleepiness Scale/RBD scores did not. These predictors were independent of older age group, male gender, and fewer educational years, all of which were strong predictors of a worse MoCA score. Patients with hyposmia scored worse (−1.12; 95% confidence interval [CI], −1.84 to −0.39) than normosmics. Patients with clinical depression (≥ 7) scored slightly worse (−1.20; 95% CI, −1.96 to −0.44; P = 0.002) than patients with anxiety (≥ 7; −1.10; 95% CI, −1.96 to −0.25; P = 0.01). In the multivariable model, we did not include both the depression and anxiety scores together (correlation coefficient, 0.52) but chose depression, because, depending on the other covariates, this demonstrated a stronger effect (β coefficient for depression: z-score, −0.20; 95% CI, −0.49 to 0.09; P = 0.18). The final model identified effects for both motor and non-motor predictors as well as age and educational years (adjusted R2for final model = 22%). Repeat sensitivity analyses using robust standard errors and a loge-transformed version of the MoCA produced very similar results.
Table 3.
Motor and non-motor predictors of baseline Montreal Cognitive Assessment score
| Simple modela | Final model | |||||
|---|---|---|---|---|---|---|
| Variable | β Coeff | 95% CI | P | β Coeff | 95% CI | P |
| Motor | ||||||
| H&Y stage II vs I | −0.63 | −1.34 to 0.09 | 0.09 | |||
| H&Y stage III vs I | −1.90 | −3.14 to −0.65 | 0.003 | |||
| P value for trend | 0.004 | |||||
| UPDRS III z-score | −0.26 | −0.65 to 0.13 | 0.20 | |||
| Annualized UPDRS, per loge unit | −0.63 | −1.04 to −0.21 | 0.003 | −0.39 | −0.81 to 0.04 | 0.07 |
| Timed up and go test, per quartile | −0.57 | −0.83 to −0.31 | <0.0001 | −0.43 | −0.70 to −0.16 | 0.002 |
| Purdue pegboard test, z-score | 0.99 | 0.62 to 1.36 | <0.0001 | 0.74 | 0.34 to 1.14 | <0.0001 |
| Flamingo balance test, < 30 s | −0.72 | −1.35 to −0.09 | 0.03 | |||
| Postural instability and gait dominant vs tremor dominant | −0.57 | −1.25 to 0.11 | 0.10 | |||
| Indeterminate vs tremor dominant | 0.30 | −0.79 to 1.38 | 0.59 | |||
| CGI change per groupb | −0.04 | −0.46 to 0.38 | 0.85 | |||
| Nonmotor | ||||||
| Leeds anxiety general scale, per quartile | −0.33 | −0.59 to −0.07 | 0.01 | |||
| Leeds depression general scale, per quartile | −0.42 | −0.66 to −0.17 | 0.001 | −0.18 | −0.43 to 0.07 | 0.17 |
| Sniffin odor identification, z-score | 0.49 | 0.13 to 0.84 | 0.008 | 0.49 | 0.14 to 0.84 | 0.007 |
| Epworth sleep score, per quartile | −0.15 | −0.41 to 0.11 | 0.26 | |||
| RBD score, per quartile | −0.17 | −0.42 to 0.08 | 0.18 | |||
| General | ||||||
| Current age, per 10 y | −0.95 | −1.27 to −0.64 | <0.0001 | −0.40 | −0.74 to −0.06 | 0.03 |
| Men | −1.10 | −1.70 to −0.51 | <0.0001 | −0.69 | −1.32 to 0.05 | 0.06 |
| Education years, per y | 0.22 | 0.13 to 0.30 | <0.0001 | 0.16 | 0.08 to 0.25 | <0.0001 |
All variables in the simple model are adjusted for age, sex, and educational years. Variables in the final model are mutually adjusted for each other.
CGI groups were categorized as very much improvement, much improved, minimal, no change, or worsening.
Coeff, coefficient; CI, confidence interval; H&Y, Hoehn and Yahr; UPDRS-III, Unified Parkinson’s Disease Rating Scale part III (motor assessment); CGI; Clinical Global Impression improvement scale; RBD, Rapid Eye Movement Sleep Behavior Disorder Questionnaire.
Longitudinal Analysis
Longitudinal data were available for 155 consecutively recruited patients with PD at 18 months after their baseline assessment (90% overall study retention rate) (Table4). The remaining 314 patients had not yet reached the 18-month follow-up period. Patients who were lost to follow-up showed a non-significant trend toward older age (mean ± SD age, 71.5 ± 9.1 years) but did not differ according to sex compared with patients who were retained in follow-up. Only the MoCA total score showed a significant decline over the 18 months (P < 0.001), and the MMSE total score showed no significant change (P = 0.12) (Table4). An analysis of the MoCA-derived cognitive subdomain scores within the longitudinal PD cohort demonstrated significant longitudinal reductions in memory and orientation domains, whereas only the orientation domain reduced significantly over time using the MMSE-derived cognitive scores. There was a significant reduction in total semantic scores but not in phonemic fluency scores. The mean derived rate of change from baseline to 18 months of follow-up was −0.53 points per year (95% CI, −0.22 to 0.84 points per year) using the MoCA and −0.16 points per year (95% CI, −0.37 to 0.05 points per year) using the MMSE total score.
Table 4.
Montreal Cognitive Assessment and Mini-Mental State Examination total and cognitive subdomain scores for the longitudinal PD cohort (n = 155)
| Mean score ± SD | |||
|---|---|---|---|
| Test | Baseline | 18 Months | Pa |
| Total MMSE score | 27.6 ± 2.1 | 27.4 ± 2.4 | 0.12 |
| Total MoCA score | 25.7 ± 3.0 | 24.9 ± 3.4 | <0.001 |
| MMSE subdomains | |||
| Language | 6.7 ± 0.6 | 6.7 ± 0.6 | 0.83 |
| Memory | 2.4 ± 0.7 | 2.4 ± 0.7 | 0.40 |
| Orientation | 9.9 ± 0.4 | 9.6 ± 0.7 | <0.001 |
| Visuospatial | 0.9 ± 0.3 | 0.9 ± 0.3 | 0.23 |
| MoCA subdomains | |||
| Language | 4.5 ± 0.7 | 4.5 ± 0.7 | 0.24 |
| Memory | 3.0 ± 1.6 | 2.2 ± 1.7 | <0.001 |
| Orientation | 5.9 ± 0.4 | 5.8 ± 0.5 | 0.009 |
| Visuospatial | 4.2 ± 1.0 | 4.1 ± 0.9 | 0.10 |
| Total phonemic fluency | 41.0 ± 13.4 | 41.1 ± 14.0 | 0.89 |
| Total semantic fluency | 36.1 ± 8.4 | 33.4 ± 9.9 | <0.001 |
Values in bold indicate significant results from a paired t test (two-tailed; P < 0.05).
SD, standard deviation; MMSE, Mini-Mental State Examination; MoCA, Montreal Cognitive Assessment.
Of the 155 patients with PD who were studied longitudinally over 18 months using the total MoCA screening cutoff scores, 33 patients (21.3%) moved from normal cognition to MCI; 7 patients (4.5%) moved from MCI to demented; 7 patients (4.5%) and 36 patients (23.2%) stayed in the demented and MCI ranges, respectively; 51 patients (32.9%) remained in the normal cognition range; 21 patients (13.6%) moved from MCI to normal cognition; and none moved from normal cognition to the demented range over the 18-month study period. Using total MMSE screening cutoff scores, 18 patients (11.6%) moved from normal cognition to MCI; 8 patients (5.2%) moved from MCI to demented; 24 patients (15.5%) and 14 patients (9.0%) stayed in the demented and MCI ranges, respectively; 41 patients (26.5%) remained in the normal cognition range; 21 patients (13.6%) moved from MCI to normal cognition; 11 patients (7.1%) moved from normal cognition to the demented range; 15 patients (9.7%) moved from demented to MCI; and 3 patients (1.9%) moved from the demented range to normal cognition.
Discussion
In a well characterized cohort of 486 patients with early PD, we observed that a high proportion already had cognitive impairment in the MCI and dementia range at screening, depending on classification thresholds using the MoCA and the MMSE. Using screening thresholds, the MMSE appeared to be more sensitive than the MoCA in detecting baseline impairment (Fig. 1). However, the improved sensitivity with the MMSE comes at the likely cost of reduced specificity, because the MMSE, but not the MoCA, classified a significant proportion of healthy controls in the dementia range. Consistent with other studies,6,20,21,23 the MoCA appeared to be more sensitive than the MMSE at diagnostic thresholds, because 3.1% of patients with PD who were classified with normal cognition using the MMSE were impaired according to the MoCA, whereas 0.8% of patients with PD who were classified with normal cognition using the MoCA were impaired according to the MMSE. In addition, 21.3% of controls screened in the MCI range (none were in the demented range) using the MoCA, possibly because we did not prescreen for memory symptoms, and the published normative ranges vary considerably.20,45
A key aspect of the current study is longitudinal assessment. One other investigation (n = 98 patients) compared longitudinal changes using global measures in PD26 and reported that MMSE scores, but not MoCA scores, changed significantly, particularly with disease duration > 10 years. Our results, by contrast, suggest that the MoCA may be more sensitive than the MMSE in detecting longitudinal changes and at baseline, in keeping with other studies.6,20,21,23 Furthermore, only one MMSE domain showed deterioration compared with three MoCA domains. Our finding that the semantic domain rather than the phonemic fluency domain may be more sensitive to disease progression is consistent with another investigation that reported similar associations.15 We observed that 25.8% of patients deteriorated across the MoCA classification boundaries, whereas 13.5% improved over 18 months. By comparison, 23.9% deteriorated but 25.2% improved using MMSE thresholds, suggesting a greater degree of misclassification with the MMSE than with the MoCA. In particular, no patients shifted from normal cognition to dementia according to the MoCA, whereas 11 patients had a similar shift according to the MMSE, suggesting either very rapid deterioration or that the MCI range for MMSE may be too narrow to capture the transitional phase. The improvement in cognition seen with both instruments may represent a combination of regression to the mean and/or learning effects observed with repeated cognitive testing or changes in performance due improvement in mood.
The apparent contradictory findings between this study and a previous longitudinal study26 may reflect population differences, because patients in the latter study had longer disease duration than our cohort (mean ± SD, 6.7 ± 5.4 years vs. 1.5 ± 1.0 years), raising the possibility that the MoCA may track cognitive decline better in early PD, whereas the MMSE may be better for tracking patients with longer disease duration. However, this notion is speculative and requires further substantiation. Although the MoCA appeared to be more sensitive to change, it is possible that was is due to a greater proportion of false-positives, and only further follow-up of this cohort, which is planned, will confirm or refute this hypothesis.
Consistent with previous studies,46 patients with who screened in the dementia range scored worse on activities of daily living (ADL) scales. We observed a stronger correlation between the S&E scale and UPDRS/Hoehn and Yahr staging, suggesting that motor function has a bigger impact on ADL than cognition. Only modest (albeit significant) reductions were found in the S&E scale when patients with PD in the demented range were compared with those in the non-demented/MCI range, suggesting that an arbitrary cutoff in this ADL scale may not be helpful in distinguishing demented from non-demented patients with PD.
MoCA total scores correlated with the Purdue pegboard total score, the Timed Up and Go test score, educational years, age, the Sniffin’ Sticks Odour Identification test score, male gender, and anxiety (Table3). The Purdue pegboard assembly has excellent test-retest reliability,47 and patients with MCI and early Alzheimer’s disease perform worse on such fine motor tasks, indicating that higher level hand motor impairment is an important aspect of elderly cognitive decline48,49 and may also be a sensitive marker of early PD cognitive impairment. Consistent with previous studies,50 worsening motoric parkinsonism predicted impaired cognition in our cohort, although only a slower Timed Up and Go test was a significant predictor in our final model, possibly because it is a more objective marker of gait difficulty. Dementia is a known significant predictor of falls in PD,51,52 and PD fallers have reduced thalamic cholinergic innervation compared with non-fallers.53 Hyposmia is common in PD54–56 and predicts cognitive decline in elderly57 patients with PD and Alzheimer’s disease,58–60 because it is linked to cholinergic impairment.61,62 Because they are associated with cortical cholinergic denervation,63–67 depression and anxiety increase the risk of dementia. The correlation between cognitive impairment and motor/non-motor indices in our study may reflect a generalized effect of central cholinergic denervation characterizing prodromal PDD; however, a variety of other neural pathways may also be involved. The findings that reduced educational years, older age, and male gender predict impaired cognition are consistent with previous studies.68–71
A limitation of our study is that we examined patients using cognitive screening instruments and did not have a gold standard clinical diagnosis or a detailed neuropsychological assessment. Therefore, it is possible that the MoCA overestimates MCI and dementia rather than the MMSE under-diagnosing them. We suspect this alternative explanation is unlikely because, if anything, the larger reverse classification observed with the MMSE due to improved scores suggests that it is less specific. However, further follow-up of this cohort is necessary to validate the predictive value of these instruments against a clinical diagnosis. The role of longitudinal testing for level 1 and level 2 diagnostic criteria for PD-MCI and PDD will require future review. Our results are not intended to reflect detailed cognitive subdomains, and further studies comparing the MMSE/MoCA with detailed neuropsychology in the same patients are needed to better delineate the cognitive substrate of PD-MCI. Early detection of cognitive decline, we believe, will be an important strategy for understanding phenotypic heterogeneity and targeting therapies.
Acknowledgments
We are grateful to the patients and their relatives who took part in the study.
Appendix 1— Derived variables and sensitivity analyses
Derived variables: In the PD subjects, an estimate of UPDRS motor change per year was calculated by dividing the baseline MDS-UPDRS part 3 score by the number of years of patient-stated motor symptoms. Cases of depression and anxiety on the Leeds scale were defined by a score ≥ 7 points. Hyposmia was defined as ≤ 9 points on the Sniffin score. The Flamingo Balance Test was dichotomised on whether subjects could or could not balance for 30 seconds.
Sensitivity analyses: As MoCA is negatively skewed, we undertook two sensitivity analyses; repeating the model using robust standard errors to account for heteroskedasticity and by converting the raw score into a positively skewed variable and then using a loge transformation.
Exclusion criteria: Controls were excluded from study analysis if they had any PD family history, past history of stroke, alcohol or drug abuse, a score < 100% on the Schwab and England disability scale, or extrapyramidal features on neurological examination. Neither PD nor control participants were prescreened for memory symptoms. Thirty-seven control subjects were excluded from subsequent analysis (Schwab and England disability score < 100% [n = 20], stroke [n = 3], excessive alcohol history [n = 9], presence of subtle parkinsonian features on examination by the clinician [n = 2], and family history of PD [n = 3]), leaving 141 control subjects for statistical analysis. Educational history was not available in 6 PD patients and 1 control.
Skewed variables: For skewed variables, we either transformed (loge) them or created quartiles, which were entered into the models either as an ordinal variable (1, 2, 3, 4) assuming linearity or as dummy variables (eg 2 vs. 1, 3 vs. 1, 4 vs. 1), which enabled nonlinear associations to emerge. The best predictors, unless very collinear, were then entered into a multivariable analysis to see if their effect remained independent of the other covariates.
References
- Aarsland D, Andersen K, Larsen JP, Lolk A, Kragh-Sorensen P. Prevalence and characteristics of dementia in Parkinson disease: an 8-year prospective study. Arch Neurol. 2003;60:387–392. doi: 10.1001/archneur.60.3.387. [DOI] [PubMed] [Google Scholar]
- Aarsland D, Bronnick K, Ehrt U, et al. Neuropsychiatric symptoms in patients with Parkinson’s disease and dementia: frequency, profile and associated care giver stress. J Neurol Neurosurg Psychiatry. 2007;78:36–42. doi: 10.1136/jnnp.2005.083113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bronnick K, Ehrt U, Emre M, et al. Attentional deficits affect activities of daily living in dementia-associated with Parkinson’s disease. J Neurol Neurosurg Psychiatry. 2006;77:1136–1142. doi: 10.1136/jnnp.2006.093146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy G, Tang MX, Louis ED, et al. The association of incident dementia with mortality in PD. Neurology. 2002;59:1708–1713. doi: 10.1212/01.wnl.0000036610.36834.e0. [DOI] [PubMed] [Google Scholar]
- Aarsland D, Bronnick K, Fladby T. Mild cognitive impairment in Parkinson’s disease. Curr Neurol Neurosci Rep. 2011;11:371–378. doi: 10.1007/s11910-011-0203-1. [DOI] [PubMed] [Google Scholar]
- Dalrymple-Alford JC, Livingston L, MacAskill MR, et al. Characterizing mild cognitive impairment in Parkinson’s disease. Mov Disord. 2011;26:629–636. doi: 10.1002/mds.23592. [DOI] [PubMed] [Google Scholar]
- Janvin CC, Larsen JP, Aarsland D, Hugdahl K. Subtypes of mild cognitive impairment in Parkinson’s disease: progression to dementia. Mov Disord. 2006;21:1343–1349. doi: 10.1002/mds.20974. [DOI] [PubMed] [Google Scholar]
- Liepelt-Scarfone I, Graeber S, Feseker A, et al. Influence of different cut-off values on the diagnosis of mild cognitive impairment in Parkinson’s disease [serial online] Parkinsons Dis. 2011;2011:540843. doi: 10.4061/2011/540843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litvan I, Aarsland D, Adler CH, et al. MDS Task Force on mild cognitive impairment in Parkinson’s disease: critical review of PD-MCI. Mov Disord. 2011;26:1814–1824. doi: 10.1002/mds.23823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marras C, Armstrong MJ, Meaney CA, et al. Measuring mild cognitive impairment in patients with Parkinson’s disease. Mov Disord. 2013;28:626–633. doi: 10.1002/mds.25426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen KF, Larsen JP, Tysnes OB, Alves G. Prognosis of mild cognitive impairment in early Parkinson disease: the Norwegian ParkWest study. JAMA Neurol. 2013;70:580–586. doi: 10.1001/jamaneurol.2013.2110. [DOI] [PubMed] [Google Scholar]
- Poletti M, Frosini D, Ceravolo R, Bonuccelli U. Mild cognitive impairment in de novo Parkinson’s disease according to movement disorder guidelines [letter] Mov Disord. 2012;27:1706; author reply 1707. doi: 10.1002/mds.25120. [DOI] [PubMed] [Google Scholar]
- Poletti M, Frosini D, Pagni C, et al. Mild cognitive impairment and cognitive-motor relationships in newly diagnosed drug-naive patients with Parkinson’s disease. J Neurol Neurosurg Psychiatry. 2012;83:601–606. doi: 10.1136/jnnp-2011-301874. [DOI] [PubMed] [Google Scholar]
- Caviness JN, Driver-Dunckley E, Connor DJ, et al. Defining mild cognitive impairment in Parkinson’s disease. Mov Disord. 2007;22:1272–1277. doi: 10.1002/mds.21453. [DOI] [PubMed] [Google Scholar]
- Williams-Gray CH, Foltynie T, Brayne CE, Robbins TW, Barker RA. Evolution of cognitive dysfunction in an incident Parkinson’s disease cohort. Brain. 2007;130(pt 7):1787–1798. doi: 10.1093/brain/awm111. [DOI] [PubMed] [Google Scholar]
- Janvin CC, Aarsland D, Larsen JP. Cognitive predictors of dementia in Parkinson’s disease: a community-based, 4-year, longitudinal study. J Geriatr Psychiatry Neurol. 2005;18:149–154. doi: 10.1177/0891988705277540. [DOI] [PubMed] [Google Scholar]
- Litvan I, Goldman JG, Troster AI, et al. Diagnostic criteria for mild cognitive impairment in Parkinson’s disease: Movement Disorder Society Task Force guidelines. Mov Disord. 2012;27:349–356. doi: 10.1002/mds.24893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emre M, Aarsland D, Brown RG, et al. Clinical diagnostic criteria for dementia associated with Parkinson’s disease. Mov Disord. 2007;12:1689–1707. doi: 10.1002/mds.21507. [DOI] [PubMed] [Google Scholar]
- Dubois B, Burn D, Goetz C, et al. Diagnostic procedures for Parkinson’s disease dementia: recommendations from the Movement Disorder Society Task Force. Mov Disord. 2007;22:2314–2324. doi: 10.1002/mds.21844. [DOI] [PubMed] [Google Scholar]
- Nasreddine ZS, Phillips NA, Bedirian V, et al. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc. 2005;53:695–699. doi: 10.1111/j.1532-5415.2005.53221.x. [DOI] [PubMed] [Google Scholar]
- Dalrymple-Alford JC, MacAskill MR, Nakas CT, et al. The MoCA: well-suited screen for cognitive impairment in Parkinson disease. Neurology. 2010;75:1717–1725. doi: 10.1212/WNL.0b013e3181fc29c9. [DOI] [PubMed] [Google Scholar]
- Gill DJ, Freshman A, Blender JA, Ravina B. The Montreal Cognitive Assessment as a screening tool for cognitive impairment in Parkinson’s disease. Mov Disord. 2008;23:1043–1046. doi: 10.1002/mds.22017. [DOI] [PubMed] [Google Scholar]
- Hoops S, Nazem S, Siderowf AD, et al. Validity of the MoCA and MMSE in the detection of MCI and dementia in Parkinson disease. Neurology. 2009;73:1738–1745. doi: 10.1212/WNL.0b013e3181c34b47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barton B, Grabli D, Bernard B, et al. Clinical validation of Movement Disorder Society-recommended diagnostic criteria for Parkinson’s disease with dementia. Mov Disord. 2012;27:248–253. doi: 10.1002/mds.24059. [DOI] [PubMed] [Google Scholar]
- Di Battista ME, Giustini P, Bernardi S, Stirpe P, Vanacore N, Meco G. A simplified algorithm may lead to overestimate dementia in PD. A clinical and epidemiological study using criteria for PD-D proposed by the Movement Disorders Task Force. J Neural Transm. 2011;118:1609–1612. doi: 10.1007/s00702-011-0638-1. [DOI] [PubMed] [Google Scholar]
- Lessig S, Nie D, Xu R, Corey-Bloom J. Changes on brief cognitive instruments over time in Parkinson’s disease. Mov Disord. 2012;27:1125–1128. doi: 10.1002/mds.25070. [DOI] [PubMed] [Google Scholar]
- Hughes AJ, Daniel SE, Kilford L, Lees AJ. Accuracy of the clinical diagnosis of idiopathic Parkinson’s disease: a clinico-pathological study of 100 cases. J Neurol Neurosurg Psychiatry. 1992;55:181–184. doi: 10.1136/jnnp.55.3.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EuroQoL Group. EuroQol—a new facility for the measurement of health-related quality of life. Health Policy. 1990;16:199–208. doi: 10.1016/0168-8510(90)90421-9. [DOI] [PubMed] [Google Scholar]
- Stiasny-Kolster K, Mayer G, Schafer S, Moller JC, Heinzel-Gutenbrunner M, Oertel WH. The REM sleep behavior disorder screening questionnaire—a new diagnostic instrument. Mov Disord. 2007;22:2386–2393. doi: 10.1002/mds.21740. [DOI] [PubMed] [Google Scholar]
- Snaith RP, Bridge GW, Hamilton M. The Leeds scales for the self-assessment of anxiety and depression. Br J Psychiatry. 1976;128:156–165. doi: 10.1192/bjp.128.2.156. [DOI] [PubMed] [Google Scholar]
- Johns MW. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep. 1991;14:540–545. doi: 10.1093/sleep/14.6.540. [DOI] [PubMed] [Google Scholar]
- Goetz CG, Tilley BC, Shaftman SR, et al. Movement Disorder Society-sponsored revision of the Unified Parkinson’s Disease Rating Scale (MDS-UPDRS): scale presentation and clinimetric testing results. Mov Disord. 2008;23:2129–2170. doi: 10.1002/mds.22340. [DOI] [PubMed] [Google Scholar]
- Hoehn MM, Yahr MD. Parkinsonism: onset, progression, and mortality. Neurology. 1967;17:427–442. doi: 10.1212/wnl.17.5.427. [DOI] [PubMed] [Google Scholar]
- Goetz CG, Poewe W, Rascol O, et al. Movement Disorder Society Task Force report on the Hoehn and Yahr staging scale: status and recommendations. Mov Disord. 2004;19:1020–1028. doi: 10.1002/mds.20213. [DOI] [PubMed] [Google Scholar]
- Schwab RS, England RC. Projection technique for evaluating surgery in Parkinson’s disease. In: Gillinghan FH, Donaldson MC, editors. Third Symposium on Parkinson’s Disease. Edinburgh, UK: Livingstone; 1969. pp. 152–157. [Google Scholar]
- Wenzelburger R, Zhang BR, Pohle S, et al. Force overflow and levodopa-induced dyskinesias in Parkinson’s disease. Brain. 2002;125(pt 4):871–879. doi: 10.1093/brain/awf084. [DOI] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Desrosiers J, Hebert R, Bravo G, Dutil E. The Purdue Pegboard Test: normative data for people aged 60 and over. Disabil Rehabil. 1995;17:217–224. doi: 10.3109/09638289509166638. [DOI] [PubMed] [Google Scholar]
- Boesveldt S, Verbaan D, Knol DL, van Hilten JJ, Berendse HW. Odour identification and discrimination in Dutch adults over 45 years. Rhinology. 2008;46:131–136. [PubMed] [Google Scholar]
- Shumway-Cook A, Brauer S, Woollacott M. Predicting the probability for falls in community-dwelling older adults using the Timed Up & Go Test. Phys Ther. 2000;80:896–903. [PubMed] [Google Scholar]
- Tsigilis N, Douda H, Tokmakidis SP. Test-retest reliability of the Eurofit test battery administered to university students. Percept Mot Skills. 2002;95(3 pt 2):1295–1300. doi: 10.2466/pms.2002.95.3f.1295. [DOI] [PubMed] [Google Scholar]
- Jankovic J, McDermott M, Carter J, et al. Variable expression of Parkinson’s disease: a base-line analysis of the DATATOP cohort. The Parkinson Study Group. Neurology. 1990;40:1529–1534. doi: 10.1212/wnl.40.10.1529. [DOI] [PubMed] [Google Scholar]
- Nazem S, Siderowf AD, Duda JE, et al. Montreal Cognitive Assessment performance in patients with Parkinson’s disease with “normal” global cognition according to Mini-Mental State Examination score. J Am Geriatr Soc. 2009;57:304–308. doi: 10.1111/j.1532-5415.2008.02096.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strauss E, Sherman EMS, Spreen O, editors . A Compendium of Neuropsychological Tests: Administration, Norms, and Commentary. 3rd ed. New York: Oxford University Press; 2006. [Google Scholar]
- Rossetti HC, Lacritz LH, Cullum CM, Weiner MF. Normative data for the Montreal Cognitive Assessment (MoCA) in a population-based sample. Neurology. 2011;77:1272–1275. doi: 10.1212/WNL.0b013e318230208a. [DOI] [PubMed] [Google Scholar]
- Leroi I, McDonald K, Pantula H, Harbishettar V. Cognitive impairment in Parkinson disease: impact on quality of life, disability, and caregiver burden. J Geriatr Psychiatry Neurol. 2012;25:208–214. doi: 10.1177/0891988712464823. [DOI] [PubMed] [Google Scholar]
- Reddon JR, Gill DM, Gauk SE, Maerz MD. Purdue Pegboard: test-retest estimates. Percept Mot Skills. 1988;66:503–506. doi: 10.2466/pms.1988.66.2.503. [DOI] [PubMed] [Google Scholar]
- Kluger A, Gianutsos JG, Golomb J, et al. Patterns of motor impairment in normal ageing, mild cognitive decline and early Alzheimer’s disease. J Gerontol. 1997;528:28–39. doi: 10.1093/geronb/52b.1.p28. [DOI] [PubMed] [Google Scholar]
- Scherder E, Dekker W, Eggemont L. Higher-level hand motor function in aging and (preclinical) dementia: its relationship with (instrumental) activities of daily life—a mini-review. Gerontology. 2008;54:333–341. doi: 10.1159/000168203. [DOI] [PubMed] [Google Scholar]
- Ganga G, Alty JE, Clissold BG, et al. Longitudinal study of levodopa in Parkinson’s disease: effects of the advanced disease phase. Mov Disord. 2013;28:476–481. doi: 10.1002/mds.25335. [DOI] [PubMed] [Google Scholar]
- Wielinski CL, Erickson-Davis C, Wichmann R, Walde-Douglas M, Parashos SA. Falls and injuries resulting from falls among patients with Parkinson’s disease and other parkinsonian syndromes. Mov Disord. 2005;20:410–415. doi: 10.1002/mds.20347. [DOI] [PubMed] [Google Scholar]
- Wood BH, Bilclough JA, Bowron A, Walker RW. Incidence and prediction of falls in Parkinson’s disease: a prospective multidisciplinary study. J Neurol Neurosurg Psychiatry. 2002;72:721–725. doi: 10.1136/jnnp.72.6.721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohnen NI, Muller MI, Koeppe RA, et al. History of falls in Parkinson’s disease is associated with reduced cholinergic activity. Neurology. 2009;73:1670–1676. doi: 10.1212/WNL.0b013e3181c1ded6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haehner A, Boesveldt S, Berendse HW, et al. Prevalence of smell loss in Parkinson’s disease—a multicenter study. Parkinsonism Relat Disord. 2009;15:490–494. doi: 10.1016/j.parkreldis.2008.12.005. [DOI] [PubMed] [Google Scholar]
- Kareken DA, Mosnik DM, Doty RL, Dzemidzic M, Hutchins GD. Functional anatomy of human odor sensation, discrimination, and identification in health and ageing. Neuropsychology. 2003;17:482–495. doi: 10.1037/0894-4105.17.3.482. [DOI] [PubMed] [Google Scholar]
- Potagas C, Dellatolas G, Ziegler M, et al. Clinical assessment of olfactory dysfunction in Parkinson’s disease. Mov Disord. 1998;13:394–399. doi: 10.1002/mds.870130304. [DOI] [PubMed] [Google Scholar]
- Swan GE, Carmelli D. Impaired olfaction predicts cognitive decline in nondemented older adults. Neuroepidemiology. 2002;21:58–67. doi: 10.1159/000048618. [DOI] [PubMed] [Google Scholar]
- Wilson RS, Arnold SE, Schneider JA, Bennett DA. The relationship between cerebral Alzheimer’s disease pathology and odour identification in old age. J Neurol Neurosurg Psychiatry. 2007;78:30–35. doi: 10.1136/jnnp.2006.099721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serby M, Larson P, Kalkstein D. The nature and course of olfactory deficits in Alzheimer’s disease. Am J Psychiatry. 1991;148:357–360. doi: 10.1176/ajp.148.3.357. [DOI] [PubMed] [Google Scholar]
- Baba T, Kikuchi A, Hirayama K, et al. Severe olfactory dysfunction is a prodromal symptom of dementia associated with Parkinson’s disease: a 3 year longitudinal study. Brain. 2012;135(pt 1):161–169. doi: 10.1093/brain/awr321. [DOI] [PubMed] [Google Scholar]
- Bohnen NI, Kaufer DI, Ivanco LS, et al. Cortical cholinergic function is more severely affected in parkinsonian dementia than in Alzheimer disease: an in vivo positron emission tomography study. Arch Neurol. 2003;60:1745–1748. doi: 10.1001/archneur.60.12.1745. [DOI] [PubMed] [Google Scholar]
- Hilker R, Thomas AV, Klein JC, et al. Dementia in Parkinson’s disease: functional imaging of cholinergic and dopaminergic pathways. Neurology. 2005;65:1716–1722. doi: 10.1212/01.wnl.0000191154.78131.f6. [DOI] [PubMed] [Google Scholar]
- Marder K, Tang M-X, Cote L, Stern Y, Mayeux R. The frequency and associated risk factors for dementia in patients with Parkinson’s disease. Arch Neurol. 1995;52:695–701. doi: 10.1001/archneur.1995.00540310069018. [DOI] [PubMed] [Google Scholar]
- Lieberman A. Are dementia and depression in Parkinson’s disease related? J Neurol Sci. 2006;248:138–142. doi: 10.1016/j.jns.2006.05.022. [DOI] [PubMed] [Google Scholar]
- Starkstein SE, Bolduc PL, Mayberg HS, Preziosi TJ, Robinson RG. Cognitive impairments and depression in Parkinson’s disease: a follow up study. J Neurol Neurosurg Psychiatry. 1990;53:597–602. doi: 10.1136/jnnp.53.7.597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohnen NI, Kaufer DI, Hendrickson R, Constantine GM, Mathis CA, Moore RY. Cortical cholinergic denervation is associated with depressive symptoms in Parkinson’s disease and parkinsonian dementia. J Neurol Neurosurg Psychiatry. 2007;78:641–643. doi: 10.1136/jnnp.2006.100073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallacher J, Bayer A, Fish M, et al. Does anxiety affect risk of dementia? Findings from the Caerphilly Prospective Study. Psychosom Med. 1999;71:659–666. doi: 10.1097/PSY.0b013e3181a6177c. [DOI] [PubMed] [Google Scholar]
- Hughes TA, Ross HF, Musa S, Bhattacherjee S, Nathan RN, Mindham RHS. A 10-year study of the incidence of and factors predicting dementia in Parkinson’s disease. Neurology. 2000;54:1596–1602. doi: 10.1212/wnl.54.8.1596. [DOI] [PubMed] [Google Scholar]
- Levy G, Schupf N, Tang M-X, Cote LJ, Louis ED. Combined effect of age and severity on the risk of dementia in Parkinson’s disease. Ann Neurol. 2002;51:722–729. doi: 10.1002/ana.10219. [DOI] [PubMed] [Google Scholar]
- Breteler MB, de Groot RM, van Romunde LKJ, Hofman A. Risk of dementia in patients with Parkinson’s disease, epilepsy, and severe head trauma. Am J Epidemiol. 1995;142:1300–1305. doi: 10.1093/oxfordjournals.aje.a117597. [DOI] [PubMed] [Google Scholar]
- Glatt SL, Hubble JP, Lyons KE, et al. Risk factors for dementia in Parkinson’s disease: effect of education. Neuroepidemiology. 1996;15:20–25. doi: 10.1159/000109885. [DOI] [PubMed] [Google Scholar]


