Abstract
Biological invasions are facilitated by the global transportation of species and climate change. Given that invasions may cause ecological and economic damage and pose a major threat to biodiversity, understanding the mechanisms behind invasion success is essential.
Both the release of non-native populations from natural enemies, such as parasites, and the genetic diversity of these populations may play key roles in their invasion success.
We investigated the roles of parasite communities, through enemy release and parasite acquisition, and genetic diversity in the invasion success of the non-native bumblebee, Bombus hypnorum, in the United Kingdom.
The invasive B. hypnorum had higher parasite prevalence than most, or all native congeners for two high-impact parasites, probably due to higher susceptibility and parasite acquisition. Consequently parasites had a higher impact on B. hypnorum queens’ survival and colony-founding success than on native species. Bombus hypnorum also had lower functional genetic diversity at the sex-determining locus than native species. Higher parasite prevalence and lower genetic diversity have not prevented the rapid invasion of the United Kingdom by B. hypnorum. These data may inform our understanding of similar invasions by commercial bumblebees around the world.
This study suggests that concerns about parasite impacts on the small founding populations common to re-introduction and translocation programs may be less important than currently believed.
Keywords: Apicystis, biological invasion, Bombus hypnorum, enemy release, parasite acquisition, Sphaerularia
Introduction
Biological invasions occur when non-native species successfully establish in a new location and rapidly expand their range (Williamson 1996). Such invasions may affect the diversity and abundance of native species, species interactions (e.g. symbioses) and the provision of ecosystem services (such as pollination), which are important for human well-being (Pimentel, Zuniga & Morrison 2005; Pejchar & Mooney 2009; Vila et al. 2010). The invasion success of a non-native species may be facilitated by a release from natural enemies, such as herbivores, predators and parasites, potentially leading to a rapid increase in distribution and abundance of the invasive species (Elton 1958; Keane & Crawley 2002; Torchin et al. 2003). Evidence supporting the enemy release hypothesis can be found from studies of plant–herbivore interactions (e.g. Agrawal & Kotanen 2003; Colautti et al. 2004; Agrawal et al. 2005; Liu & Stiling 2006) but the evidence from animal–parasite systems is less clear (e.g. Dunn & Dick 1998; MacNeil et al. 2003; Georgiev et al. 2007). Given the historical, current and predicted global impact of invasive species (Elton 1958; Vitousek et al. 1996; Wilcove et al. 1998; Pimentel, Zuniga & Morrison 2005) and the importance of species range expansion due to climate change (Parmesan et al. 1999; Hickling et al. 2006) understanding the mechanisms that facilitate these events is a key challenge (e.g. Phillips et al. 2010; White & Perkins 2012).
Previous studies of the role of parasites in enemy release, in both plant and animal systems, largely examine either parasite prevalence or the impact of individual parasite species (e.g. MacNeil et al. 2003). However, parasites exist in communities (Cloutman 1975; Holmes & Price 1986) and invasive species may host multiple parasite species (e.g. Georgiev et al. 2007). Interactions among parasite species, within a host, include competition for resources (Rigaud, Perrot-Minnot & Brown 2010) and alteration of transmission rates (e.g. castrating parasites reducing the transmission of other parasite species to the offspring of the host, Ben-ami, Rigaud & Ebert 2011). The presence of multiple parasite species can induce a range of host immune responses, which may have divergent impacts on individual parasite species (Schmid-Hempel 1998). In addition, the structure of parasite communities can have significant consequences for assessing the impact of individual parasites (e.g. Rutrecht & Brown 2008). Consequently, understanding the structure of parasite communities and their subsequent impact (Rigaud, Perrot-Minnot & Brown 2010) is essential to establish the role of parasites in invasions.
While invading species may be released from parasites in their new location, the impact of parasites in the invaded communities may, in turn, be modified by invasive species. This may occur through parasite introduction (Dunn 2009) or parasite spillover (Daszak, Cunningham & Hyatt 2000; Kelly et al. 2009) where invading host species introduce non-native parasites and these spillover to infect native hosts. Invasive species may also acquire parasites from congeneric host species in the new location (parasite acquisition: Dunn 2009) which may result in an increase (through invasive species acting as a reservoir for native parasites followed by parasite spillback; reservoir host: Norman et al. 1999; Daszak, Cunningham & Hyatt 2000; Dunn 2009; Kelly et al. 2009; parasite spillback: Daszak, Cunningham & Hyatt 2000; Kelly et al. 2009) or a decrease in parasite abundance in native species (through parasite dilution, where invading hosts provide an additional or alternative host for native parasites ‘diluting’ the parasite prevalence and/or abundance in native hosts: Norman et al. 1999; Ostfeld & Keesing 2000) depending on the competence of the invasive host at transmitting the infective stages of the parasite. These factors may occur individually or in concert, and thus investigating enemy release in the invaded range should take account of these complex interactions.
An additional factor that may play a key role in the host–parasite interactions of invasive species is the genetic diversity and provenance of the invasive host. Invasive species are likely to establish in a new location from only a few propagules or reproductive individuals, and therefore the founding population will have low genetic diversity (Dlugosch & Parker 2008). Low genetic diversity in natural populations is known to be associated with higher rates of parasitism (e.g. Whitehorn et al. 2011) and thus genetically depauperate invasive species may be more likely to acquire parasites from congeners. In addition, invading hosts have not co-evolved with native parasites. Consequently, invading hosts may be maladapted to native parasites and these parasites may therefore have a greater (or lesser) impact on such hosts (Thompson 2005). Relative to native hosts, if the non-native species is less susceptible to parasites and/or these parasites have a smaller impact on fitness, non-native hosts are likely to benefit from enemy release despite the acquisition of generalist parasites from congeners.
While bumblebees (Bombus spp.) are generally considered to be in decline (Goulson, Lye & Darvill 2008; Williams & Osborne 2009), which is concerning as they are important ecological and commercial pollinators, they can also be highly invasive (Dafni 1998; Goulson 2003). In Japan, commercially introduced Bombus terrestris L. have escaped and threaten native congeners and their interactions with native plants (Matsumura, Yokoyama & Washitani 2004; Inoue, Yokoyama & Washitani 2008). Invasive B. terrestris have spread throughout Tasmania in the last 20 years (Allen et al. 2007; Schmid-Hempel et al. 2007) probably from New Zealand, where they were introduced in the 19th century (MacFarlane & Griffin 1990). Most recently, invasive B. terrestris has spread across Argentina and Chile, where it is blamed for rapid declines in the only native bumblebee species, Bombus dahlbomii Guérin-Méneville (Torretta, Medan & Abrahamovich 2006; Plischuk & Lange 2009; Goulson 2010; Arbetman et al. 2013; Morales et al. 2013).
Using the successful establishment of a non-native invasive bumblebee, Bombus hypnorum L., across England and Wales over the last decade (Goulson & Williams 2001; BWARS), we aim to identify the role of parasites and genetic diversity in this invasion. Bombus hypnorum, the tree bumblebee, has expanded across England, Wales and Scotland, to Lennoxtown, Scotland (c. 600 km), to Truro, Cornwall in the South West (c. 300 km) and Pembrokeshire in Wales (c. 320 km) since its first discovery in the New Forest, Wiltshire, England in 2001 (BWARS, Goulson & Williams 2001). The parasite community of bumblebees is composed of generalist parasites and has been well characterized (MacFarlane, Lipa & Liu 1995; Schmid-Hempel 1998; Rutrecht & Brown 2008), making this an excellent opportunity to examine how enemy release and parasite acquisition may impact an invasive species, particularly as recent work has suggested that nest parasites play a role in the dynamics of native bumblebee populations (Antonovics & Edwards 2011).
Enemy release can occur in two ways. First, an invading species, in the invaded range, may escape from the enemies it would have encountered in its native range. A model proposed by Drake (2003) suggests that such enemy release may be important for the establishment of small invading populations. Second, invading species may escape from enemies present in the invaded range, as those enemies are not adapted to exploit it (Dunn 2009). A comparison of enemies of invading species and those of congeneric native species investigates the second mechanism and we take this approach because the origin of our focal species is currently unknown. To investigate the potential release from natural enemies of the non-native B. hypnorum, we determined the parasite community in queens of this invasive bumblebee species and compared it to those of five native bumblebee species with the expectation that B. hypnorum would have lower parasite prevalence and lower parasite species richness than native congeneric species, and thus it should be released from its parasite enemies. In addition to investigating parasite prevalence, parasite species richness and parasite community structure, we also investigated the parasite impact on a proxy for host fitness, and functional genetic diversity at the sex-determining locus, in laboratory-reared colonies of B. hypnorum. We expected that parasites would have a greater impact on fitness in B. hypnorum than in native congeneric species and that the genetic diversity of B. hypnorum would be lower than that of native Bombus species.
Methods
Biology of the study system
Most bumblebees are annual eusocial species, passing through a solitary overwintering phase as queens. This makes the queen a key component of the annual life cycle. Interestingly, bumblebee queens are particularly heavily impacted by parasites (Rutrecht & Brown 2008). Consequently, parasites that reduce the survival and colony-founding success of the queen are likely to have a high impact on bumblebee populations and, therefore, in this study we focused on bumblebee queens. The ultimate measure of parasite impact on fitness would be the proportion of sexuals produced by colonies that contribute their genes to the subsequent generation. However, such an analysis is logistically extremely challenging and beyond the scope of this study. Bumblebee gynes (unmated new queens) disperse from their natal nests to mate in late summer, prior to finding a hibernation site. Queens hibernate in individual hibernacula, which can be dispersed or aggregated, depending on the species, and different species favour different hibernation sites (Alford 1969b; Sladen 1912). Variation in hibernation sites may impact the probability of infection by some parasite species (see below) but too little is known about hibernation site choice to make any predictions. Post-hibernation queens disperse again, with estimates of aggregate dispersal of at least 5 km (Lepais et al. 2010), and congregate at florally rich sites to forage for nectar and pollen. Parasites can be acquired from natal nests, interactions with males during mating, during hibernation and through foraging pre- and post-hibernation (Schmid-Hempel 1998).
Sampling scheme
Our sampling methodology was designed around the biology of the system (see above). Bumblebee queens were collected, between February and May 2011, from two primary florally rich sites in Surrey and Berkshire, Windsor Great Park (Lat. 51·41, Long. −0·60) and the Royal Horticultural Society (RHS) Garden, Wisley (Lat. 51·32, Long. −0·58). Additional queens were collected from florally rich sites at the Royal Botanic Gardens, Kew (Lat. 51·47, Long. −0·30); Royal Holloway, University of London (RHUL) (Lat. 51·43, Long. −0·56) and Horsell, Surrey (Lat. 51·32, Long. −0·57). Our sampling area was geographically restricted due to the requirement to catch sufficient queens within a limited time period. However, due to the rapid establishment of this invasive species in the United Kingdom, we believe that the population in South East England is likely to be representative of the UK B. hypnorum population as a whole. The non-native species B. hypnorum and five native species B. jonellus Kirby, B. pratorum L., B. lucorum L., B. pascuorum Scopoli and B. terrestris were collected. The queens were collected using an entomological net and placed in individual plastic vials in a chilled container and transported to RHUL. On each day, sites were collected to exhaustion. The queens were spring queens, foraging after emerging from hibernation, and therefore from the first voltine generation. While abundant species may be the most obvious source of generalist parasites, such parasites are also more likely to infect related host species (Perlman & Jaenike 2003), and our sampling strategy was designed to cover both possibilities, with B. jonellus and B. pratorum being the phylogenetically closest relatives to the invasive B. hypnorum (Cameron, Hines & Williams 2007) and B. lucorum, B. pascuorum and B. terrestris being the most abundant native bumblebee species (Goulson & Darvill 2004; Goulson et al. 2005; Williams 2005).
Parasite – faecal check
Faecal samples were taken and examined using a ×400 phase contrast microscope for the following parasites: Sphaerularia bombi Dufour, a nematode worm; Apicystis bombi, a neogregarine; Crithidia bombi, a trypanosome; and Nosema bombi Fantham & Porter, a microsporidian. All these parasite species can be reliably identified as patent infections using microscopic techniques (e.g. Otterstatter & Thomson 2006; Rutrecht & Brown 2008). While multiple Crithidia spp. have been identified, molecular data show that only Crithidia bombi occurs in this area (M.J.F. Brown unpublished data). These are all generalist parasites with a global distribution (MacFarlane, Lipa & Liu 1995; Schmid-Hempel 1998) and both S. bombi and A. bombi have previously been reported in B. hypnorum (MacFarlane, Lipa & Liu 1995). Hereafter, we refer to these using their generic names. Sphaerularia infects bumblebee queens hibernating in the soil, castrating them and preventing them from founding colonies (Alford 1969a,b; Poinar & van der Laan 1972) and Apicystis kills bumblebee queens before they are able to found colonies (Rutrecht & Brown 2008). Consequently, both of these parasites have a high impact on spring queens. Crithidia reduces overall colony fitness by, on average, 40% (Brown, Schmid-Hempel & Schmid-Hempel 2003a), and Nosema has similar effects (Otti & Schmid-Hempel 2007; Rutrecht & Brown 2009).
Parasite – dissection
The B. jonellus, B. pratorum and B. pascuorum queens were sacrificed by freezing after the faecal check and stored at −80 °C. They were later thawed, dissected and checked again for bumblebee parasites including Sphaerularia,Apicystis,Crithidia, Nosema and Locustacarus buchneri Stammer. L. buchneri is a tracheal mite whose impact on queens is currently unknown, although correlative studies on males and workers show lethargy and the cessation of foraging in workers of B. bimaculatus and reduced life span in B. occidentalis (Husband & Sinha, 1970; Otterstatter & Whidden 2004).
Bee husbandry
Bombus hypnorum queens were reared in the laboratory at a controlled temperature (25–27 °C) and humidity (50–60%), and received sugar-water and pollen ad libitum. The queens were kept in the dark and a red light was used for working. Queens were kept in queen-rearing boxes, with a sugar-water dispenser and a pollen ball to encourage egg-laying. Records were kept of the reproductive output of B. hypnorum queens including eggs laid, number of workers, males and gynes (new queens) produced. Dead queens, either at natural death or at sacrifice, were stored at −80 °C. Queens with no offspring were sacrificed and frozen after 10 weeks in the laboratory. The queens were thawed, dissected and checked for parasites as above.
Sterile procedures were used when handling queens in the laboratory, to prevent cross-contamination. Nevertheless, two B. hypnorum queens that were infected by Crithidia bombi when dissected, but were not infected when the faeces samples were examined, were consequently rejected from the data set due to possible cross-contamination.
Queens of B. terrestris and B. lucorum were reared for other experiments by another researcher but we were still able to assess their parasite status (as described above) and whether they produced normal or diploid male colonies (see below).
Diploid males
Bumblebees are haplodiploid, females being diploid (heterozygous) and males haploid (hemizygous). However, diploid (homozygous) males occur in inbred or genetically depauperate populations, and are indicative of low genetic diversity (Duchateau, Hoshiba & Velthuis 1994). A standard protocol for identifying diploid male production is through the presence of males in the first brood (which is usually just females) at a 50:50 sex ratio (Gerloff & Schmid-Hempel 2005). Consequently, the timing of male production was recorded to assess whether colonies were producing diploid males (Duchateau, Hoshiba & Velthuis 1994).
Analyses
Parasite prevalence was calculated by dividing the number of infected queens by the total number of queens of each species, with 95% confidence intervals using the Clopper–Pearson ‘exact’ method. Here, we report only parasite prevalence, as for the macroparasite Sphaerularia the impact of an individual worm is the same as the impact of multiple worms (Alford 1969a), and nothing is known about whether variation in microparasite infection intensity affects host fitness. The parasite prevalence data and parasite impact on colony-founding data were analysed using Binary Logistic Regressions with the parasite (or parasite impact) as the dependent variable, bumblebee species and site as categorical variables with B. hypnorum set as the indicator species, and the forward log ratio procedure. All analyses were conducted twice, once with the entire data set and once with just the two main sampling sites (Windsor and Wisley), as these were where most of the queens were collected.
Parasite species richness, the number of parasites species in each of the parasite communities (where each bee species is a habitat that hosts a parasite community and each individual bee is a site within that habitat), and the similarity of those parasite communities were analysed using spade (Species Prediction And Diversity Estimation) software (Chao & Shen 2010).
As a measure of genetic diversity at a functionally important locus, the sex-determining locus, we estimated the number of sex alleles in the native and invasive bumblebee populations, and in a continental European population of B. hypnorum (data from Brown, Schmid-Hempel & Schmid-Hempel 2003b), using the formula θ = 2/N where ‘θ’ is the probability of a diploid colony and ‘N’ is the number of sex alleles (Adams et al. 1977; Duchateau, Hoshiba & Velthuis 1994) (and differences tested using Fisher Exact tests). The minimum number of sex alleles was estimated by comparing the number of observed and expected diploid male colonies for a range of values, and determining where they cease to be significantly different.
Statistical analyses of data were performed using IBM spss 19 for Windows and spade (Species Prediction And Diversity Estimation) software (Chao & Shen 2010).
Results
A total of 378 bumblebee queens, collected in 225 h across 45 days, were examined for parasites (59 B. hypnorum, 47 B. jonellus, 104 B. pratorum, 50 B. pascuorum, 61 B. lucorum and 57 B. terrestris) and five parasite species were found (Sphaerularia,Apicystis,Crithidia, Nosema and Locustacarus).
Parasite prevalence
The prevalence of Sphaerularia among bumblebee species differed significantly (Wald = 25·584, d.f. = 5, P < 0·001) and ranged from 29% in B. hypnorum to 0% in B. jonellus (Fig. 1). The prevalence of Sphaerularia in B. hypnorum was significantly higher than its prevalence in B. jonellus (Wald = 7·281, d.f. = 1, P = 0·007, ExpB = 0·058), B. pratorum (Wald = 12·623, d.f. = 1, P < 0·001, ExpB = 0·156), B. pascuorum (Wald = 10·051, d.f. = 1, P = 0·002, ExpB = 0·089) and B. terrestris (Wald = 7·416, d.f. = 1, P = 0·006, ExpB = 0·205) but not significantly higher than in B. lucorum (20%, 12/61; Wald = 0·921, d.f. = 1, P = 0·337, ExpB = 0·654). The prevalence of Sphaerularia across sites differed significantly overall (Wald = 11·887, d.f. = 4, P = 0·018) but in pairwise comparisons, the only significant difference was between Windsor and Horsell (Wald = 8·633, d.f. = 1, P = 0·003). The remaining sites, Wisley (Wald = 3·147, d.f. = 1, P = 0·076), Kew (Wald = 0·697, d.f. = 1, P = 0·404), and RHUL (Wald = 1·457, d.f. = 1, P = 0·227), did not differ significantly to our primary site (Windsor). The prevalence of Sphaerularia was not affected by collection date (this variable was not present in the final model). Qualitatively similar results were found when analyses were restricted to data from the two main sites (Windsor and Wisley; data not shown).
Figure 1.
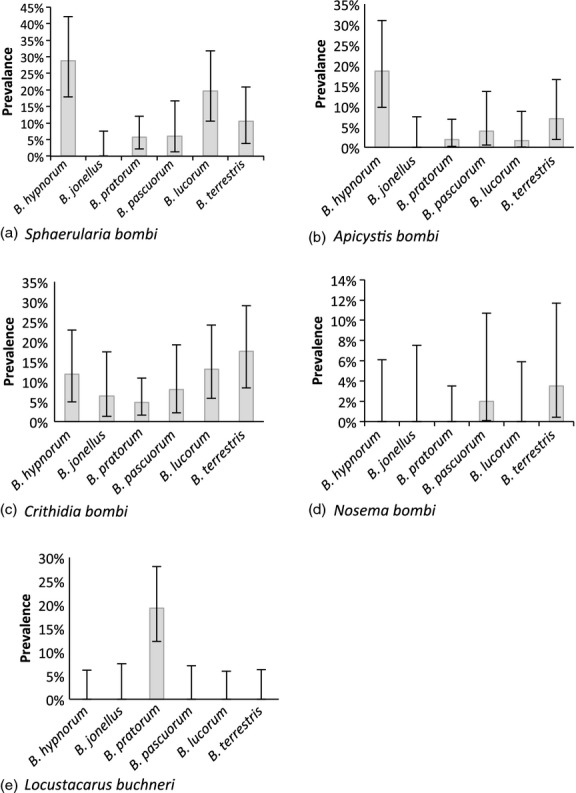
The percentage prevalence of the five parasite species across the native and non-native host species, calculated using the number of infected queens divided by the total number of queens for each Bombus species with 95% confidence intervals: (a) Sphaerularia bombi, (b) Apicystis bombi, (c) Crithidia bombi, (d) Nosema bombi and (e) Locustacarus buchneri.
As with Sphaerularia, the prevalence of Apicystis among bumblebee species differed significantly (Wald = 18·927, d.f. = 5, P = 0·002) and ranged from 18% in B. hypnorum to 0% in B. jonellus (Fig. 1). The prevalence of Apicystis in the non-native B. hypnorum was significantly higher than its prevalence in B. jonellus (Wald = 4·841, d.f. = 1, P = 0·028, ExpB = 0·095), B. pratorum (Wald = 9·216, d.f. = 1, P = 0·002, ExpB = 0·090), B. pascuorum (Wald = 6·120, d.f. = 1, P = 0·013, ExpB = 0·108), B. lucorum (Wald = 6·080, d.f. = 1, P = 0·014, ExpB = 0·072) and B. terrestris (Wald = 4·416, d.f. = 1, P = 0·036, ExpB = 0·244). The prevalence of Apicystis across sites did not differ significantly overall (Wald = 6·454, d.f. = 4, P = 0·168) and was not affected by the collection date. Again, results were qualitatively similar in the site-restricted analysis.
Crithidia was the only parasite found in all six bumblebee species and prevalence ranged from 18% in B. terrestris to 5% in B. pratorum (Fig. 1). The prevalence of Crithidia among bumblebee species did not differ significantly (Wald = 6·846, d.f. = 5, P = 0·232). The prevalence of Crithidia across sites did not differ significantly overall (Wald = 7·722, d.f. = 4, P = 0·102) and, once again, was not affected by the collection date. Again, these results were qualitatively similar in the analysis restricted to the main sampling sites.
Locustacarus was only present in one of the six bumblebee species sampled, B. pratorum, with a prevalence of 16% (N = 104), and Nosema was only present in two B. terrestris, with a prevalence of 4% (N = 57) and one B. pascuorum queen, with a prevalence of 2% (N = 50).
Parasite species richness
Observed parasite species richness differed among the sampled bumblebee species (Kruskal–Wallis H = 24·764, d.f. = 5, P < 0·001, N = 378) and ranged from zero to three parasite species (Fig. 2). Using the spade estimated species richness the number of parasite species in the non-native B. hypnorum (3·0 species, 95% CI = 3·0–3·0) was between the estimate for B. jonellus (1·1 species, 95% CI = 1·0–3·0) and B. pascuorum (4·5 species, 95% CI = 4·0–9·0) (Fig. 3).
Figure 2.
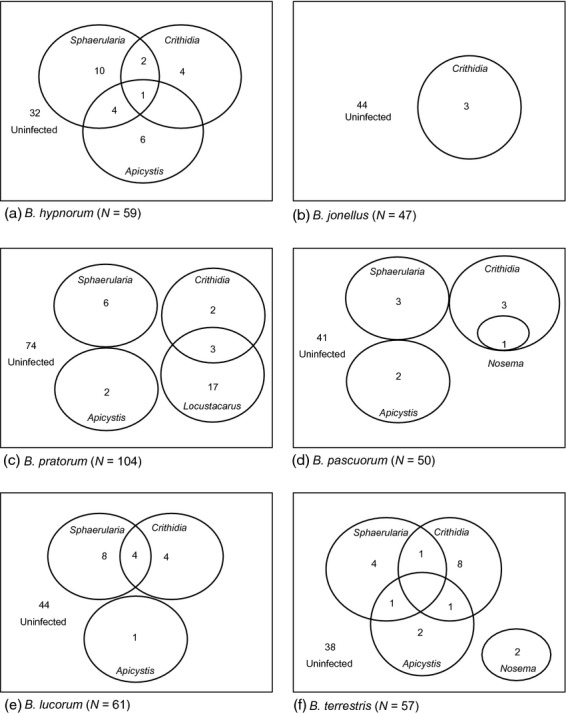
Diagrams of parasite community structure in queens of the six Bombus species showing overlaps where multiple infections occur: (a) B. hypnorum, (b) B. jonellus, (c) B. pratorum, (d) B. pascuorum, (e) B. lucorum and (f) B. terrestris. (Size of ovals is not representative of numbers).
Figure 3.
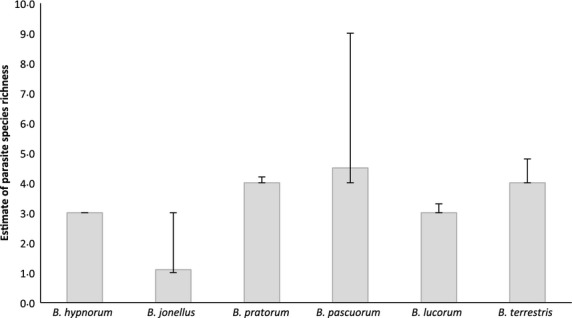
Estimate of parasite species richness (showing 95% confidence intervals) for non-native and native Bombus queens, calculated using spade (Chao & Shen 2010).
Parasite community structure
In contrast to our expectations that the invasive B. hypnorum may have escaped from its parasite enemies, the parasite communities across the non-native and native Bombus species were similar overall (‘Morista similarity’ multiple community measure = 0·597). Interestingly, in pairwise comparisons between the invasive species and the native species, B. hypnorum was more similar to the common species B. pascuorum (0·998), B. lucorum (0·917) and B. terrestris (0·898) than to the closely related species B. jonellus (0·295) or B. pratorum (0·360).
Parasite impact on longevity and colony-founding
As expected, Apicystis in B. hypnorum, B, terrestris and B. lucorum was associated with shorter longevity post-capture (U = 464·000, P < 0·001, N = 177). As found in other studies, queens infected with Apicystis did not found a colony or produce any offspring (Rutrecht & Brown 2008). The mean post-capture life span of Bombus queens infected with Apicystis was 12·31 days (±7·786 SD, N = 16) and for uninfected queens 52·91 days (±35·55 SD, N = 161). Sphaerularia completely inhibited colony foundation in the two native species, as expected. However, the impact of Sphaerularia on B. hypnorum differed: five of the B. hypnorum queens (29%, N = 17) infected with Sphaerularia laid eggs (two produced live offspring) and this differed significantly from the expectation that no queens infected with Sphaerularia would lay eggs (χ2 = 5·8621, d.f. = 1, P = 0·0155). Due to sample sizes, we were not able to assess differences in the impact of the remaining, less abundant parasites. However, previous studies suggest that these have little effect on field caught spring queens (e.g. Crithidia, Shykoff & Schmid-Hempel 1991). Consequently, from hereon we focus on these two high-impact parasites, Apicystis and Sphaerularia.
Parasite community impact
The impact of individual parasites on a host population is modified by the structure of the parasite community (Rigaud, Perrot-Minnot & Brown 2010). Consequently, we determined the overall impact of Apicystis and Sphaerularia on our invasive and native hosts in the context of their parasite community structure. In contrast to an additive scenario, where the impact of parasites might be considered individually, the synergistic scenarios account for co-occurrence of parasite species within hosts. To be conservative, we calculate the community-level impact with and without our knowledge of the differential impact of Sphaerularia across species (see above). Under the additive scenario, where the prevalence of high-impact parasites (Apicystis,c. 19%; Sphaerularia, c. 29%) was simply added, c. 48% of our B. hypnorum queens would be lost from the population of queens potentially able to found a colony (Fig. 4). Under the synergistic ‘community’ scenario, as 8% of B. hypnorum queens were infected by both Sphaerularia and Apicystis (Fig. 3a), c. 40% of queens would be lost (11% with only Apicystis, 21% with only Sphaerularia and 8% with both). As 8% (N = 59) of our B. hypnorum queens infected with Sphaerularia were able to lay eggs they may have been able to produce a colony. Thus, under the synergistic ‘probable’ scenario (Fig. 4) c. 32% of our B. hypnorum queens would be lost from the population of potential colony-founding queens. For the native species, because Apicystis causes early mortality (this study; MacFarlane, Lipa & Liu 1995; Rutrecht & Brown 2008) and Sphaerularia causes complete castration (this study; Alford 1969a; MacFarlane, Lipa & Liu 1995; Rutrecht & Brown 2008; Kelly 2009) the community and probable scenarios are identical.
Figure 4.
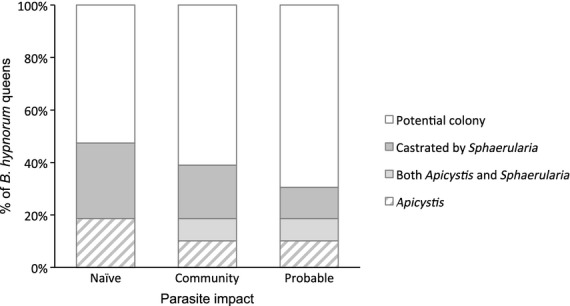
Percentage of Bombus hypnorum queens lost from the potential colony-founding population as a result of ‘high-impact’ parasites. Sphaerularia bombi and Apicystis bombi, are shown additively in ‘naïve’ scenario, with parasite overlap in the ‘community’ scenario and with the actual impact taken into consideration in the ‘probable’ scenario.
If we consider the ‘probable’ impact of Sphaerularia and Apicystis on the non-native B. hypnorum and on the five native bumblebee species (Fig. 5), we find the combined impact of Apicystis and/or Sphaerularia among bumblebee species differed significantly (Wald = 21·668, d.f. = 5, P = 0·001). The combined impact of Apicycstis and/or Sphaerularia on the non-native B. hypnorum was significantly higher than the combined impact of Apicystis and/or Sphaerularia on B. jonellus (Wald = 7·796, d.f. = 1, P = 0·005, ExpB = 0·053), B. pratorum (Wald = 11·759, d.f. = 1, P = 0·001, ExpB = 0·197), B. pascuorum (Wald = 8·138, d.f. = 1, P = 0·004, ExpB = 0·167) and B. terrestris (Wald = 4·636, d.f. = 1, P = 0·031, ExpB = 0·346). The combined impact of Apicycstis and/or Sphaerularia on B. hypnorum was not significantly higher than the impact on B. lucorum (Wald = 0·946, d.f. = 1, P = 0·331, ExpB = 0·658). The combined impact of Apicycstis and/or Sphaerularia on non-native and native Bombus species across sites did not differ significantly (Wald = 9·177, d.f. = 4, P = 0·057). Thus, the number of queens lost from the population of queens potentially able to found a colony is higher for B. hypnorum (c. 32%) than B. lucorum (c. 23%), B. terrestris (c. 18%), B. pascuorum (c. 10%) and B. pratorum (c. 8%). Bombus jonellus were not infected with either Apicystis or Sphaerularia.
Figure 5.
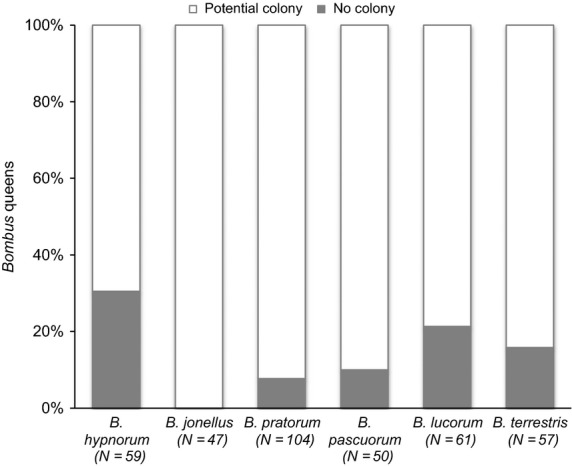
Percentage of Bombus queens lost from the potential colony-founding population as a result of the ‘high-impact’ parasites: Apicystis bombi and Sphaerularia bombi.
Functional genetic diversity at the sex-determining locus
Of the 59 B. hypnorum queens, 13 produced a colony in the laboratory (i.e. produced one or more live offspring) but of these, three produced 50% male offspring from the first brood indicating that they were producing diploid males (Duchateau, Hoshiba & Velthuis 1994; Gerloff & Schmid-Hempel 2005). In total 59 B. terrestris colonies and 57 B. lucorum colonies were reared in the laboratory but none of these produced males from the first brood (M. Fürst, pers. comm.). Consequently, the number of sex alleles in the B. hypnorum population was estimated to be four, compared to at least 32 for B. terrestris and at least 31 for B. lucorum (as shown in Table 1) This compares with an estimate of seven sex alleles for 10 Continental European (Scandinavian) B. hypnorum colonies (also shown in Table 1) using the same method and data from Brown, Schmid-Hempel & Schmid-Hempel (2003b). Although the estimates for B. hypnorum were calculated from a small number of colonies, these estimates indicate that the invading B. hypnorum population in the United Kingdom has lower genetic diversity than the native B. terrestris and B. lucorum populations, and appears to have lower genetic diversity than the Continental European B. hypnorum population.
Table 1.
Estimated number of sex alleles based on the production of diploid males. The transition from significant to non-significant differences gives the minimum number of sex alleles in the population
| Expected | Observed | ||||||
|---|---|---|---|---|---|---|---|
| N | Diploid | Not Diploid | Diploid | Not Diploid | χ2 | Significance | |
| B. terrestris | 31 | 3·8 | 55·2 | 0 | 59 | 3·9264 | * |
| Native | 32 | 3·7 | 55·3 | 0 | 59 | 3·8198 | n.s. |
| UK | |||||||
| B. lucorum | 30 | 3·8 | 53·2 | 0 | 57 | 3·931 | * |
| Native | 31 | 3·7 | 53·3 | 0 | 57 | 3·8241 | n.s. |
| UK | |||||||
| B. hypnorum | 3 | 8·7 | 4·3 | 3 | 10 | 4·9983 | * |
| Invasive | 4 | 6·5 | 6·5 | 3 | 10 | 2·0319 | n.s. |
| UK | |||||||
| B. hypnorum | 6 | 4·0 | 6·0 | 0 | 10 | 3·9521 | * |
| Non-invasive | 7 | 3·3 | 6·7 | 0 | 10 | 3·3918 | n.s. |
| Scandinavian | |||||||
P < 0.05.
‘N’ is the number of sex alleles and each row refers to a given number of sex alleles for each species.
The bold shows the minimum estimate for the number of sex alleles in the population.
Discussion
The successful invasion of the non-native B. hypnorum suggested that this species may have escaped from its natural enemies, benefitting from a lower parasite load than native congeners. However, in sharp contrast, we found that not only was B. hypnorum infected by the same generalist parasite species as native congeners, but that the prevalence of the high-impact species: Apicystis and Sphaerularia was also higher (Apicystis 19% and Sphaerularia 29%) than in native bumblebee species (Apicystis 0–7% and Sphaerularia 0–20%). These results suggest that enemy release is not the main driver for the successful establishment, range expansion and invasion of this non-native species.
Assessing the impact of parasites on invasive species requires the host to be sufficiently established to provide a large enough sample size for analysis. Despite the fact that our sample area was invaded by this species in 2004, only 3 years after the start of the invasion, this was the first year sufficient spring queens could be caught to enable a comparison of parasite communities between it and native species (M.J.F. Brown, unpublished data). While our samples of B. hypnorum were taken from only a portion of its invasive range, given the rapid expansion of this species we believe that our results are likely to be representative of the larger population and provide the first insight into the impact of parasites on invasion in this system.
Before discussing our results further, a number of caveats must be addressed. First, some of our sampled individuals may have been sisters, originating from the same natal colony. This has potential implications for both statistical independence and parasite status. However, given what is known about bumblebee nest density in the United Kingdom (Knight et al. 2005) and queen dispersal (Lepais et al. 2010), and the low rate at which sisters appear in samples of worker populations (Knight et al. 2005), this seems unlikely to be a major concern. Secondly, queens may have emerged from closely aggregated hibernacula, with implications for infection by Sphaerularia. While little quantitative data on hibernacula exist (Alford 1969a,b; Sladen 1912, 1989), by sampling florally rich sites (to which spring queens converge) across multiple locations, and collecting sites to exhaustion on sampling visits, our sampling design minimizes this potential bias. Similarly, any unknown impacts of parasites that make queens more or less likely to be caught should have been avoided. Thirdly, by sampling spring queens we were unable to assess escape from social parasites (the cuckoo bumblebees). While the invasive population of B. hypnorum has definitely escaped the social parasite B. norvegicus, which is absent from the United Kingdom, B. sylvestris, another social parasite of B. hypnorum, is present. It would be interesting to investigate this host/social–parasite interaction further.
The invading non-native population of B. hypnorum supported a similar parasite community at the species level to that of congeneric native host species overall. We note that the potential for non-native parasite strains to be present still exists. Bumblebee parasites can be broadly classified as generalists (MacFarlane, Lipa & Liu 1995; Schmid-Hempel 1998). The most parsimonious explanation, therefore, for this shared community is that B. hypnorum acquired its parasites from native hosts. Firstly, given the likely number of foundress queens in the non-native B. hypnorum population (based on the number of sex alleles in the population) and the prevalence of parasites in spring queens (MacFarlane, Lipa & Liu 1995; Schmid-Hempel 1998; Rutrecht & Brown 2008) it is highly likely that B. hypnorum arrived parasite-free in the United Kingdom, although parasitized queens may have arrived and been unsuccessful in founding a colony. This is supported by the Tasmanian invasion where the low foundress population of B. terrestris had a low parasite load (Allen et al. 2007). Secondly, the most prevalent parasites in B. hypnorum queens were those that either kill queens, or largely prevent colony establishment, thus preventing their potential spread from and within a non-native population (Rutrecht & Brown 2008). The shared parasite community and the hibernation-site transmission route of one of the parasites, Sphaerularia, suggest that the invading B. hypnorum acquired these parasites in the invaded environment. This matches the predictions of Drake’s model (2003), where release from virulent parasites is important for the establishment phase of the invasion. Interestingly, the parasite community in the non-native B. hypnorum was very similar to that of the more abundant congeneric native hosts (B. pascuorum, B. lucorum and B. terrestris) and much less similar to that of B. hypnorum’s closer relatives (B. jonellus and B. pratorum), suggesting that parasite acquisition was not phylogenetically constrained, but was driven by host abundance. Mechanistically, B. hypnorum has probably acquired its parasite community through overlap in the use of floral resources (Durrer & Schmid-Hempel 1994) and hibernation sites (Alford 1969a,b) with the native Bombus species. While the number of parasite species infecting B. hypnorum was similar to that of native congeners and the parasite community in B. hypnorum was similar to the parasite community of the native species overall, prevalence levels, particularly of the high-impact parasites, were higher in the invasive species than in the native species. Higher prevalence could reflect higher susceptibility, which may relate to the low levels of genetic diversity we found in B. hypnorum or to maladaptation to the parasites in its new range. Previous studies have shown that inbreeding in bumblebees correlates with higher parasite prevalence (Whitehorn et al. 2011), but both mechanisms may be at play. Even though infections by one parasite species, Sphaerularia, had a reduced impact in B. hypnorum, this was outweighed by its higher prevalence. Nevertheless, the high prevalence and corresponding impact of acquired parasites does not appear to have constrained the spread of B. hypnorum across the United Kingdom. However, this high prevalence could still affect the native species. Firstly, higher prevalence in the invasive species may actually reflect a parasite dilution effect, where the presence of the new and possibly more susceptible host has lowered parasite prevalence in native species (Norman et al. 1999; Ostfeld & Keesing 2000; Dunn 2009). In the absence of long-term records of parasite prevalence in these, or other bumblebee populations, it is not possible to test this idea. Secondly, the non-native host may also have a detrimental impact on the parasite by preventing transmission. Sphaerularia larvae are usually deposited in the soil at hibernation sites by infected queens, where hibernating queens are infected (Lundberg & Svensson 1975), but, if infected queens found colonies, as we found in this study, such deposition at hibernation sites will not occur, and therefore the parasite’s life cycle would be broken, making B. hypnorum a dead-end host for Sphaerularia. This lack of host competence (Ostfeld & Keesing 2000) is likely to reduce parasite prevalence in native congeners, again through the parasite dilution effect (Ostfeld & Keesing 2000; Dunn 2009). Further studies are needed to determine whether this is in fact happening, and, if so, what quantitative impact it is having on native host–parasite interactions.
In addition to assessing its impact on parasite prevalence, estimating functional genetic diversity at the sex-determining locus enables us to retrospectively assess the number of initial foundress queens in the invasive population (Lundberg & Svensson 1975; Schmid-Hempel et al. 2007). Bombus hypnorum queens can be polyandrous and mate with between one and six males (Pouvreau 1963; Estoup et al. 1995; Schmid-Hempel & Schmid-Hempel 2000; Paxton et al. 2001), thus the B. hypnorum population in the United Kingdom may have been founded by as few as one or two multiply mated queens. Previous studies of both deliberately introduced populations of bumblebees in New Zealand (Lye, Lepais & Goulson 2011), and introduced B. terrestris in Tasmania also found that populations may have been established from as few as one or two mated queens (Schmid-Hempel et al. 2007). Although B. terrestris (and B. lucorum) are usually monandrous, these studies show that bumblebees can establish and become invasive from a small number of founding queens. Finally, diploid-male producing colonies of B. terrestris have been shown to have significantly lower fitness under semi-natural conditions (Whitehorn et al. 2009), and consequently the high proportion of diploid-male producing B. hypnorum colonies found in this study should constrain population expansion.
Nevertheless, despite its high parasite prevalence and low diversity at the sex-determining locus, B. hypnorum has rapidly expanded its range in the United Kingdom. What factors might contribute to this success? One contributing factor may be its association with the ‘urban’ environment (urbanization is increasing in Europe, Eigenbrod et al. 2011), and its use of resources rarely exploited by other bumblebee species such as nesting sites in trees, bird-boxes and buildings (BWARS; C.M. Jones pers. observ.). Bombus hypnorum is also a generalist forager that visits a wide range of flowers (BWARS) and generalists are often associated with biological invasion success (Williamson 1996). Furthermore, B. hypnorum has a bivoltine life cycle (producing two generations per annum) (Edwards & Jenner 2005) and thus their population might increase more rapidly than univoltine species, such as B. lucorum or B. pascuorum. In addition, a second generation B. hypnorum queen could mate and found a colony without hibernating, thus avoiding possible infection by Sphaerularia during hibernation.
A final possible explanation is that the bumblebee species assemblage in Great Britain is depauperate compared with that in Continental Europe, presumably due to the emergence of sea barriers to dispersal at the end of the last Ice Age. In some sense, then, B. hypnorum may simply be invading favourable habitat. Similarly, two related Pyrobombus species, B. pratorum and B. monticola, invaded Ireland, in the 1940s and 1970s, respectively, where the bumblebee species assemblage is even more depauperate than Great Britain (Speight 1974; Fitzpatrick et al. 2007) suggesting that bees from the Pyrobombus sub-genus, such as B. hypnorum, may be successful invaders. Unfortunately, no parasite or genetic data exist from the early stages of these invasions to compare with the current study.
Invasion by B. terrestris of South America (c. 400 km in 8 years, Torretta, Medan & Abrahamovich 2006; Morales et al. 2013), an area with a native bumblebee fauna, has proceeded at a similarly rapid rate as B. hypnorum in the United Kingdom (c. 600 km in 10 years, BWARS). In South America, parasites have been implicated in the invasion success through their impact on the native Bombus species (Torretta, Medan & Abrahamovich 2006; Plischuk & Lange 2009; Arbetman et al. 2013). Our data from the B. hypnorum invasion suggest that it would be extremely valuable to examine the parasite communities and levels of genetic diversity in other invading and native populations to see whether our results are representative of a more general pattern. Unfortunately, while data exist for genetic diversity and parasites in invasive populations in New Zealand and Tasmania (Allen et al. 2007; Schmid-Hempel et al. 2007; Lye, Lepais & Goulson 2011), the absence of a native bumblebee fauna makes it difficult to extrapolate these results to other areas.
To conclude, this study shows that high parasite impact and low functional genetic diversity at the sex-determining locus have not prevented the invasion of a non-native bumblebee. This not only has implications for understanding economically important and ecologically devastating invasions (Inoue, Yokoyama & Washitani 2008; Plischuk & Lange 2009; Arbetman et al. 2013), it also has implications for the successful design of re-introduction programs which begin with low founding populations and low parasite load (IUCN; Frankham, Ballou & Briscoe 2010). While the obvious next steps would be to investigate B. hypnorum in its native range, or the parasite community and genetic diversity of other invasive Bombus species in their invaded ranges, this work provides an important step in understanding the role of parasites and genetic variation in insect invasions. A recent study (Venesky et al. 2012) suggested that captive breeding programs for re-introductions should select for tolerance to natural enemies, to avoid the impact of such enemies in small re-introduced populations with low genetic diversity. Our results, where a genetically depauperate, invasive population has expanded despite high parasite impact, suggest that such complex selection may not be required.
Acknowledgments
We thank M. Fürst and L. Pilling for assistance with fieldwork, M. Fürst and O. Ramos Rodriguez for assistance with bee care and M. Fürst, I. Pedrosa Rovira, G. Baron and D. Stanley for helpful comments on the manuscript. We thank The Crown Estate, The Royal Horticultural Society and The Royal Botanic Gardens for permission to collect. We also thank the anonymous reviewers and the associate editor whose comments significantly enhanced the quality of the manuscript. CMJ and MJFB designed this project, CMJ carried out the research, CMJ and MJFB analysed the data and wrote the paper. CMJ was funded by a BBSRC DTG to the School of Biological Sciences, Royal Holloway, University of London.
References
- Adams J, Rothman ED, Kerr WE. Paulino ZL. Estimation of the number of sex alleles and queen matings from a diploid male frequencies in a population of Apis mellifera. Genetics. 1977;86:583–596. doi: 10.1093/genetics/86.3.583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agrawal AA. Kotanen PM. Herbivores and the success of exotic plants: a phylogenetically controlled experiment. Ecology Letters. 2003;6:712–715. [Google Scholar]
- Agrawal AA, Kotanen PM, Mitchell CE, Power AG, Godsoe W. Klironomos J. Enemy release? An experiment with congeneric plant pairs and diverse above- and belowground enemies. Ecology. 2005;86:2979–2989. [Google Scholar]
- Alford DV. Sphaerularia bombi as a parasite of bumble bees in England. Journal of Apicultural Research. 1969a;8:49–54. [Google Scholar]
- Alford DV. A study of the hibernation of bumblebees (Hymenoptera: Bombidae) in Southern England. Journal of Animal Ecology. 1969b;38:149–170. [Google Scholar]
- Allen GR, Seeman OD, Schmid-Hempel P. Buttermore RE. Low parasite loads accompany the invading population of the bumblebee, Bombus terrestris in Tasmania. Insectes Sociaux. 2007;54:56–63. [Google Scholar]
- Antonovics J. Edwards M. Spatio-temporal dynamics of bumblebee nest parasites (Bombus subgenus Psythirus ssp.) and their hosts (Bombus spp.) Journal of Animal Ecology. 2011;80:999–1011. doi: 10.1111/j.1365-2656.2011.01846.x. [DOI] [PubMed] [Google Scholar]
- Arbetman MP, Meeus I, Morales CL, Aizen MA. Smagghe G. Alien parasite hitchhikes to Patagonia on invasive bumblebee. Biological Invasions. 2013;15:489–494. [Google Scholar]
- Bee Wasps and Ants Recording Society (BWARS) [online] available from: http://www.bwars.com/index.php?q=bee/apidae/bombus-hypnorum (accessed 1 September 2013)
- Ben-ami F, Rigaud T. Ebert D. The expression of virulence during double infections by different parasites with conflicting host exploitation and transmission strategies. Journal of Evolutionary Biology. 2011;24:1307–1316. doi: 10.1111/j.1420-9101.2011.02264.x. [DOI] [PubMed] [Google Scholar]
- Brown MJF, Schmid-Hempel R. Schmid-Hempel P. Strong context-dependent virulence in a host-parasite system: reconciling genetic evidence with theory. Journal of Animal Ecology. 2003a;72:994–1002. [Google Scholar]
- Brown MJF, Schmid-Hempel R. Schmid-Hempel P. Queen-controlled sex ratios and worker reproduction in the bumble bee Bombus hypnorum, as revealed by microsatellites. Molecular Ecology. 2003b;12:1599–1605. doi: 10.1046/j.1365-294x.2003.01840.x. [DOI] [PubMed] [Google Scholar]
- Cameron SA, Hines HM. Williams P. A comprehensive phylogeny of the bumble bee (Bombus. Biological Journal of the Linnean Society. 2007;91:161–188. [Google Scholar]
- Chao A. Shen T-J. 2010. Program SPADE (Species Prediction And Diversity Estimation). Program and User’s Guide published at http://chao.stat.nthu.edu.tw.
- Cloutman DG. Parasite community structure of largemouth bass, warmouth, and blue gill in Lake Fort Smith, Arkansas. Transactions of the American Fisheries Society. 1975;104:277–283. [Google Scholar]
- Colautti RI, Ricciardi A, Grigorovich IA. MacIssac HJ. Is invasion success explained by the enemy release hypothesis? Ecology Letters. 2004;7:721–733. [Google Scholar]
- Dafni A. The threat of Bombus terrestris spread. Bee World. 1998;79:113–114. [Google Scholar]
- Daszak P, Cunningham AA. Hyatt AD. Emerging infectious diseases of wildlife: threats to biodiversity and human health. Science. 2000;287:443. doi: 10.1126/science.287.5452.443. [DOI] [PubMed] [Google Scholar]
- Dlugosch KM. Parker M. Founding events in species invasions: genetic variation, adaptive evolution, and the role of multiple introductions. Molecular Ecology. 2008;17:431–449. doi: 10.1111/j.1365-294X.2007.03538.x. [DOI] [PubMed] [Google Scholar]
- Drake JM. The paradox of the parasites: implications for biological invasion. Proceedings of the Royal Society of London Series B – Biological Sciences. 2003;270:S133–S135. doi: 10.1098/rsbl.2003.0056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duchateau MJ, Hoshiba H. Velthuis HHW. Diploid males in the bumble bee Bombus terrestris: sex determination, sex alleles and variability. Entomologia Experimentalis et Applicata. 1994;71:263–269. [Google Scholar]
- Dunn AM. Parasites and biological invasions. Advances in Parasitology. 2009;68:161–184. doi: 10.1016/S0065-308X(08)00607-6. [DOI] [PubMed] [Google Scholar]
- Dunn AM. Dick JTA. Parasitism and epibiosis in native and non-native gammarids in freshwater in Ireland. Ecography. 1998;21:593–598. [Google Scholar]
- Durrer S. Schmid-Hempel P. Shared use of flowers leads to horizontal pathogen transmission. Proceedings of the Royal Society of London Series B – Biological Sciences. 1994;258:299–302. [Google Scholar]
- Edwards M. Jenner M. Field Guide to the Bumblebees of Great Britain & Ireland. UK: Ocelli; 2005. [Google Scholar]
- Eigenbrod F, Bell VA, Davies HN, Heinemeyer A, Armsworth PR. Gaston KJ. The impact of projected increases in urbanization on ecosystems services. Proceedings of the Royal Society of London Series B – Biological Sciences. 2011;278:3201–3208. doi: 10.1098/rspb.2010.2754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elton CS. The Ecology of Invasions by Plants and Animals. Chicago, Illinois, USA: The University of Chicago Press; 1958. [Google Scholar]
- Estoup A, Scholl A, Pouvreau A. Solignac M. Monoandry and polyandry in bumble bees (Hymenoptera; Bombinae) as evidenced by highly variable microsatellites. Molecular Ecology. 1995;4:89–93. doi: 10.1111/j.1365-294x.1995.tb00195.x. [DOI] [PubMed] [Google Scholar]
- Fitzpatrick U, Murray TE, Paxton RJ, Breen J, Cotton D, Santorum V, et al. Rarity and decline in bumblebees – a test of causes and correlates in the Irish fauna. Biological Conservation. 2007;136:185–194. [Google Scholar]
- Frankham R, Ballou JD. Briscoe DA. Introduction to Conservation Genetics. 2nd edn. Cambridge, UK: Cambridge University Press; 2010. [Google Scholar]
- Georgiev BB, Sánchez MI, Vasileva GP, Nikolov PN. Green AJ. Cestode parasitism in invasive and native brine shrimps (Artemia spp.) as a possible factor promoting the rapid invasion of A. franciscana in the Mediterranean region. Parasitology Research. 2007;101:1647–1655. doi: 10.1007/s00436-007-0708-3. [DOI] [PubMed] [Google Scholar]
- Gerloff CU. Schmid-Hempel P. Inbreeding depression and family variation in a social insect, Bombus terrestris (Hymenoptera: Apidae) Oikos. 2005;111:67–80. [Google Scholar]
- Goulson D. Effects of introduced bees on native ecosystems. Annual Review of Ecology, Evolution and Systematics. 2003;34:1–26. [Google Scholar]
- Goulson D. Impacts of non-native bumblebees in Western Europe and North America. Applied Entomology and Zoology. 2010;45:7–12. [Google Scholar]
- Goulson D. Darvill B. Niche overlap and diet breadth in bumblebees; are rare species more specialized in their choice of flowers? Apidologie. 2004;35:55–63. [Google Scholar]
- Goulson D, Lye GC. Darvill B. Decline and conservation of bumble bees. Annual Review of Entomology. 2008;53:191–208. doi: 10.1146/annurev.ento.53.103106.093454. [DOI] [PubMed] [Google Scholar]
- Goulson D. Williams P. Bombus hypnorum (Hymenoptera: Apidae), a new British bumblebee? British Journal of Entomology and Natural History. 2001;14:129–131. [Google Scholar]
- Goulson D, Hanley ME, Darvill B, Ellis JS. Knight ME. Causes of rarity in bumblebees. Biological Conservation. 2005;122:1–8. [Google Scholar]
- Hickling R, Roy DB, Hill JK, Fox R. Thomas CD. The distribution of a wide range of taxonomic groups are expanding polewards. Global Change Biology. 2006;12:450–455. [Google Scholar]
- Holmes JC, Price PW. Communities of parasites. In: Kittawa J, Anderson DJ, editors. Community Ecology: Patterns and Processes. New York: Blackwell Scientific Publications; 1986. pp. 187–213. [Google Scholar]
- Husband RW. Sinha RN. A Revision of the Genus Locustacarus with a Key to Genera of the Family Podapolipidae (Acarina) Annuals of the Entomological Society of America. 1970;63:1152–1162. [Google Scholar]
- Inoue MN, Yokoyama J. Washitani I. Displacement of Japanese native bumblebees by the recently introduced Bombus terrestris (L.)(Hymenoptera: Apidae) Journal of Insect Conservation. 2008;12:135–146. [Google Scholar]
- IUCN. Guidelines for Re-Introductions. Prepared by the IUCN.SSC Re-introductions Species Group. Gland, Switzerland and Cambridge, UK: IUCN; 1998. [Google Scholar]
- Keane RM. Crawley MJ. Exotic plant invasions and the enemy release hypothesis. Trends in Ecology and Evolution. 2002;17:164–170. [Google Scholar]
- Kelly M. 2009. Investigation into the host-parasite relationship of Sphaerularia bombi and its host(s) Bombus spp. PhD Thesis, Trinity College Dublin.
- Kelly DW, Paterson RA, Townsend CR, Poulin R. Tompkins DM. Parasite spillback: a neglected concept in invasion ecology? Ecology. 2009;90:2047–2056. doi: 10.1890/08-1085.1. [DOI] [PubMed] [Google Scholar]
- Knight ME, Martin AP, Bishop S, Osborne JL, Hale RJ, Sanderson RA. Goulson D. An interspecific comparison of foraging range and nest density of four bumblebee (Bombus) species. Molecular Ecology. 2005;14:1811–1820. doi: 10.1111/j.1365-294X.2005.02540.x. [DOI] [PubMed] [Google Scholar]
- Lepais O, Darvill B, O’Connor S, Osborne JL, Sanderson RA, Cussans J, Goffe L. Goulson D. Estimation of bumblebee queen dispersal distances using sibship reconstruction method. Molecular Ecology. 2010;19:819–831. doi: 10.1111/j.1365-294X.2009.04500.x. [DOI] [PubMed] [Google Scholar]
- Liu H. Stiling P. Testing the enemy release hypothesis: a review and meta-analysis. Biological Invasions. 2006;8:1535–1545. [Google Scholar]
- Lundberg H. Svensson BG. Studies on the behaviour of Bombus Latr. Species (Hym., Apidae) parasitized by Sphaerularia bombi Dufour (Nematoda) in an alpine area. Norwegian Journal of Entomology. 1975;22:129–134. [Google Scholar]
- Lye GC, Lepais O. Goulson D. Reconstructing demographic events from population genetic data: the introduction of bumblebees to New Zealand. Molecular Ecology. 2011;20:2888–2900. doi: 10.1111/j.1365-294X.2011.05139.x. [DOI] [PubMed] [Google Scholar]
- MacFarlane RP. Griffin RP. New Zealand distribution and seasonal incidence of the nematode Sphaerularia bombi Dufour, a parasite of bumble bees. New Zealand Journal of Zoology. 1990;17:191–199. [Google Scholar]
- MacFarlane RP, Lipa JJ. Liu HJ. Bumble bee pathogens and internal enemies. Bee World. 1995;76:130–148. [Google Scholar]
- MacNeil C, Dick JTA, Hatcher MJ, Terry RS, Smith JE. Dunn AM. Parasite-mediated predation between native and invasive amphipods. Proceedings of the Royal Society of London Series B – Biological Sciences. 2003;270:1309–1314. doi: 10.1098/rspb.2003.2358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumura C, Yokoyama J. Washitani I. Invasion status and potential ecological impacts of an invasive alien bumblebee Bombus terrestris L. (Hymenoptera: Apidae) naturalised in Southern Hokkaido, Japan. Global Environmental Research. 2004;8:51–66. [Google Scholar]
- Morales CL, Arbetmann MP, Cameron SA. Aizen MA. Rapid ecological replacement of a native bumble bee by invasive species. Frontiers in Ecology and the Environment. 2013;11:529–534. [Google Scholar]
- Norman R, Bowers RG, Begon M. Hudson PJ. Persistence of tick-borne virus in the presence of multiple host species: tick reservoirs and parasite mediated competition. Journal of Theoretical Biology. 1999;200:111–118. doi: 10.1006/jtbi.1999.0982. [DOI] [PubMed] [Google Scholar]
- Ostfeld RS. Keesing F. Biodiversity and disease risk: the case of Lyme disease. Conservation Biology. 2000;14:722–728. [Google Scholar]
- Otterstatter MC. Thomson JD. Within-host dynamics of an intestinal pathogen of bumble bees. Parasitology. 2006;133:749–761. doi: 10.1017/S003118200600120X. [DOI] [PubMed] [Google Scholar]
- Otterstatter MC. Whidden TL. Patterns of parasitism by tracheal mites (Locustacarus buchneri) in natural bumble bee populations. Apidologie. 2004;35:351–357. [Google Scholar]
- Otti O. Schmid-Hempel P. Nosema bombi: a pollinator parasite with detrimental fitness effects. Journal of Invertebrate Pathology. 2007;96:118–124. doi: 10.1016/j.jip.2007.03.016. [DOI] [PubMed] [Google Scholar]
- Parmesan C, Ryrholm N, Stefanescu C, Hill JK, Thomas CD, Descimon H, et al. Poleward shifts in geographical ranges of butterfly species associated with regional warming. Nature. 1999;388:579–583. [Google Scholar]
- Paxton RJ, Thorn PA, Estoup A. Teng J. Queen-worker conflict over male production and the sex ratio in a facultatively polyandrous bumblebee, Bombus hypnorum: the consequences of nest usurpation. Molecular Ecology. 2001;10:2489–2498. [PubMed] [Google Scholar]
- Pejchar L. Mooney HA. Invasive species, ecosystem services and human well-being. Trends in Ecology and Evolution. 2009;24:497–504. doi: 10.1016/j.tree.2009.03.016. [DOI] [PubMed] [Google Scholar]
- Perlman SJ. Jaenike J. Infection success in novel hosts: an experimental and phylogenetic study of Drosophila-parasitic nematodes. Evolution. 2003;57:544–557. doi: 10.1111/j.0014-3820.2003.tb01546.x. [DOI] [PubMed] [Google Scholar]
- Phillips BL, Keleear C, Pizzatto L, Brown GP, Barton D. Shine R. Parasites and pathogens lag behind their host during periods of host range advance. Ecology. 2010;91:872–881. doi: 10.1890/09-0530.1. [DOI] [PubMed] [Google Scholar]
- Pimentel D, Zuniga R. Morrison D. Update on the environmental and economic costs associated with alien-invasive species in the United States. Ecological Economics. 2005;52:273–288. [Google Scholar]
- Plischuk S. Lange CE. Invasive Bombus terrestris (Hymenoptera: Apidae) parasitized by a flagelleate (Euglenozoa: Kinetoplastea) and a neogregarine (Apicomplexa: Neogregarinorida) Journal of Invertebrate Pathology. 2009;102:263–265. doi: 10.1016/j.jip.2009.08.005. [DOI] [PubMed] [Google Scholar]
- Poinar GOJ. van der Laan PA. Morphology and life history of Sphaerularia bombi. Nematologica. 1972;18:239–252. [Google Scholar]
- Pouvreau A. Observations sur l’accouplement de Bombus hypnorum L. (Hymenoptere, Apidae) en serre. Insectes Sociaux. 1963;10:111–118. [Google Scholar]
- Rigaud T, Perrot-Minnot M-J. Brown MJF. Parasite and host assemblages: embracing the reality will improve our knowledge of parasite transmission and virulence. Proceedings of the Royal Society of London Series B – Biological Sciences. 2010;277:3693–3702. doi: 10.1098/rspb.2010.1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutrecht ST. Brown MJF. The life-history impact and implications of multiple parasites for bumble bee queens. International Journal for Parasitology. 2008;38:799–808. doi: 10.1016/j.ijpara.2007.11.004. [DOI] [PubMed] [Google Scholar]
- Rutrecht ST. Brown MJF. Differential virulence in a multiple-host parasite of bumblebees: resolving the paradox of parasite survival? Oikos. 2009;118:941–949. [Google Scholar]
- Sladen FWL. The Humble-Bee: Its Life-History and How to Domesticate It. London: MacMillan & Co Ltd; 1912. [Google Scholar]
- Schmid-Hempel P. Parasites in Social Insects. Princeton, New Jersey, USA: Princeton University Press; 1998. [Google Scholar]
- Schmid-Hempel R. Schmid-Hempel P. Female mating frequencies in Bombus spp. from Central Europe. Insectes Sociaux. 2000;47:36–41. [Google Scholar]
- Schmid-Hempel P, Schmid-Hempel R, Brunner PC, Seeman OD. Allen GR. Invasion success of the bumblebee, Bombus terrestris, despite a drastic genetic bottleneck. Heredity. 2007;99:414–422. doi: 10.1038/sj.hdy.6801017. [DOI] [PubMed] [Google Scholar]
- Shykoff JA. Schmid-Hempel P. Incidence and effects of four parasites in natural populations of bumble bees in Switzerland. Apidologie. 1991;22:117–125. [Google Scholar]
- Speight MCD. Bombus lapponicus, Parasyrphus lineola, and Phaonia exoleta: insects new to Ireland. Irish Naturalists’ Journal. 1974;18:123–124. [Google Scholar]
- Thompson JN. The Geographic Mosaic of Coevolution. Chicago, Illinois, USA: The University of Chicago Press; 2005. [Google Scholar]
- Torchin ME, Lafferty KD, Dobson AP, McKenzie VJ. Kuris AM. Introduced species and their missing parasites. Nature. 2003;421:628–630. doi: 10.1038/nature01346. [DOI] [PubMed] [Google Scholar]
- Torretta JP, Medan D. Abrahamovich AH. First record of the invasive bumblebee Bombus terrestris (L.)(Hymenoptera, Apidae) in Argentina. Transactions of the American Entomological Society. 2006;132:285–289. [Google Scholar]
- Venesky MD, Mendelson JRIII, Sears BF, Stilling P. Rohr JR. Selecting for tolerance against pathogens and herbivores to enhance success of reintroduction and translocation. Conservation Biology. 2012;26:586–592. doi: 10.1111/j.1523-1739.2012.01854.x. [DOI] [PubMed] [Google Scholar]
- Vila M, Basnou C, Pysek P, Josefsson M, Genovesi P, Gollasch S, et al. DAISIE partners. How well do we understand the impacts of alien invasive species on ecosystems services? A pan-European, cross-taxa assessment. Frontiers in Ecology and the Environment. 2010;8:135–144. [Google Scholar]
- Vitousek PM, D’Antonio CM, Loope LL. Westbooks R. Biological invasions as global environmental change. American Scientist. 1996;84:468–478. [Google Scholar]
- White TA. Perkins SE. The ecoimmunity of invasive species. Functional Ecology. 2012;26:1313–1323. [Google Scholar]
- Whitehorn PR, Tinsley MC, Brown MJF, Darvill B. Goulson D. Impacts of inbreeding on bumblebee colony fitness under field conditions. BMC Evolutionary Biology. 2009;9:152. doi: 10.1186/1471-2148-9-152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitehorn PR, Tinsley MC, Brown MJF, Darvill B. Goulson D. Genetic diversity, parasite prevalence and immunity in wild bumblebees. Proceedings of the Royal Society of London Series B – Biological Sciences. 2011;278:1195–1202. doi: 10.1098/rspb.2010.1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilcove DS, Rothstein D, Dubow J, Phillips A. Losos E. Quantifying threats to imperiled species in the United States. BioScience. 1998;48:607–615. [Google Scholar]
- Williams PH. Osborne JL. Bumblebee vulnerability and conservation world-wide. Apidologie. 2009;40:367–387. [Google Scholar]
- Williams P. Does specialization explain rarity and decline among British bumblebees? A response to Goulson. Biological Conservation. 2005;122:33–43. [Google Scholar]
- Williamson M. Biological Invasions. London: Chapman & Hall; 1996. [Google Scholar]


