Abstract
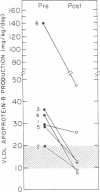
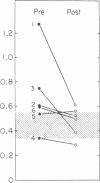
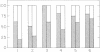
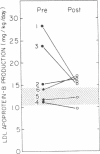
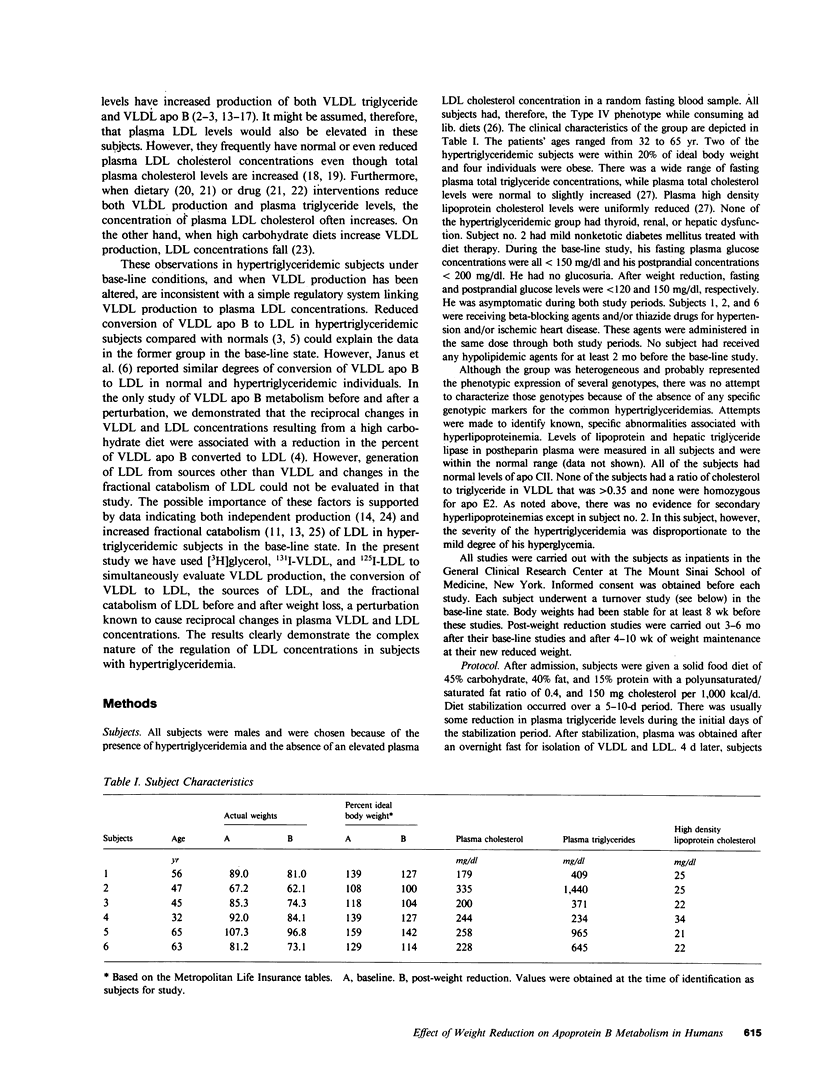
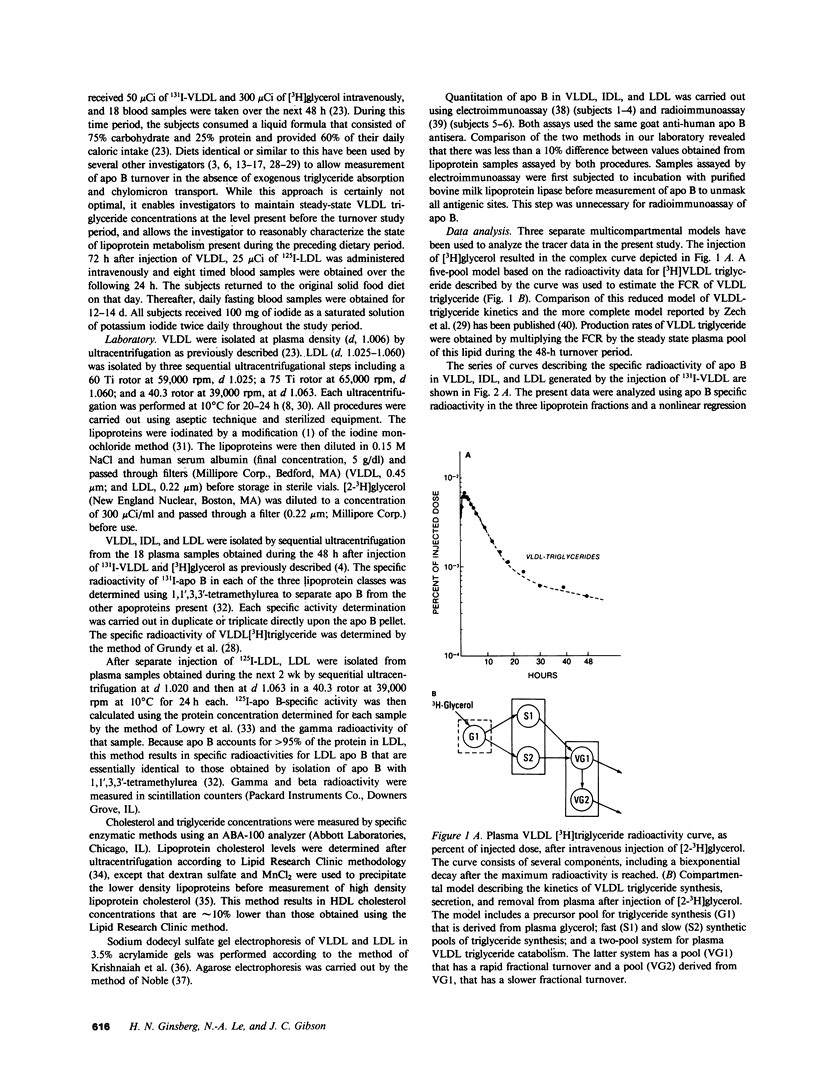
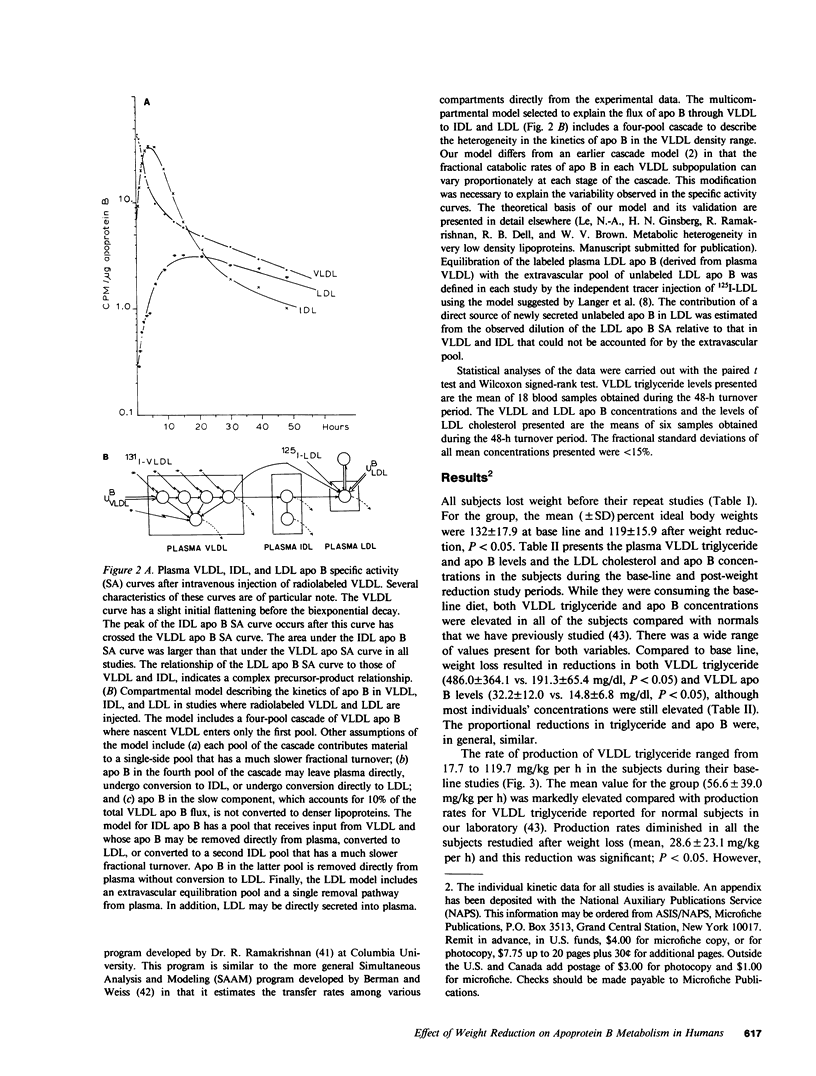
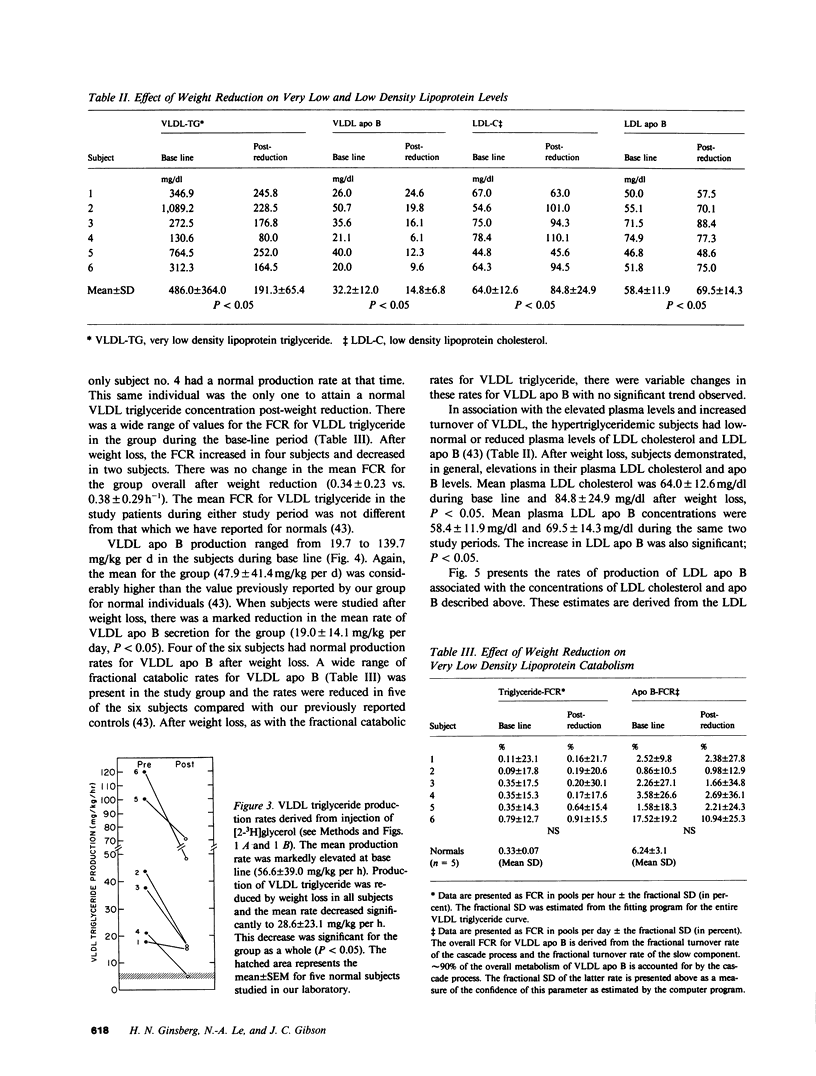
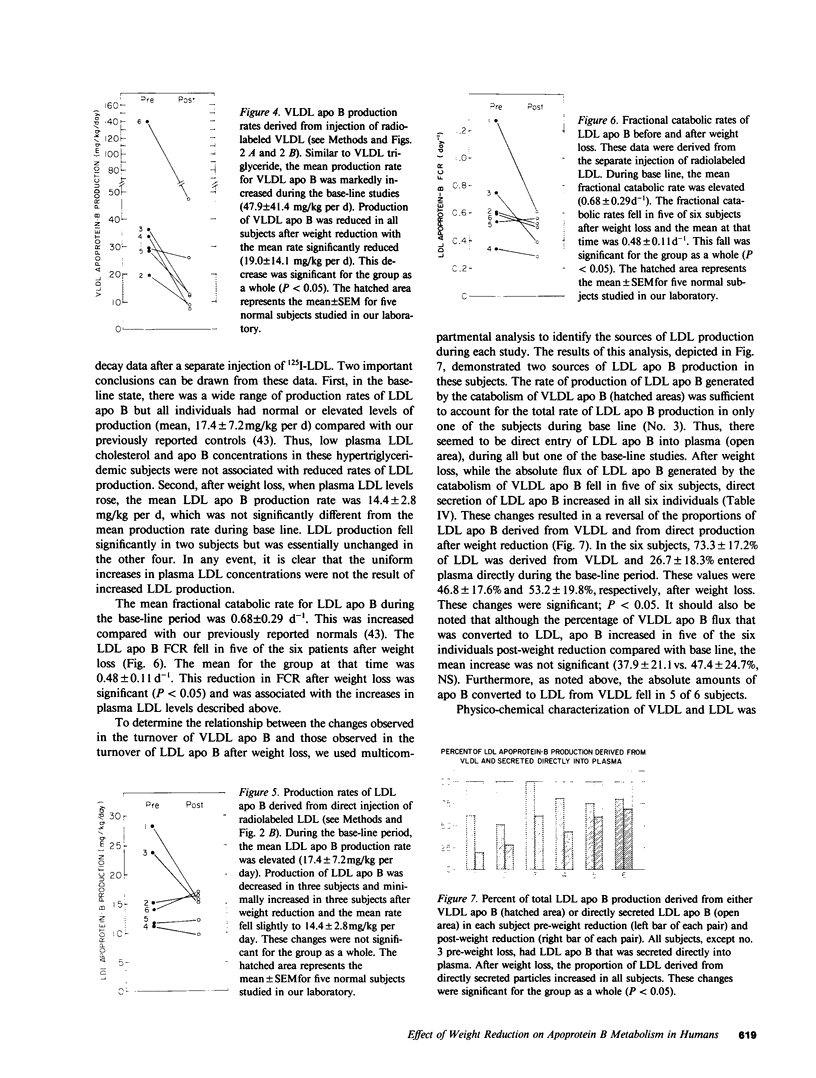
In subjects with hypertriglyceridemia, plasma concentrations of low density lipoprotein (LDL) cholesterol are often normal or reduced. Perturbations that alter plasma very low density lipoprotein (VLDL) concentrations are associated with opposite changes in plasma LDL levels. To determine the mechanisms regulating plasma LDL levels, we used 131I-VLDL and 125I-LDL to measure the fractional catabolic rates (FCR), production rates (PR), and rates of interconversion of apoprotein B (apo B) in VLDL, intermediate density lipoprotein, and LDL in six hypertriglyceridemic subjects pre- and post-weight reduction. [2-3H]glycerol was used to quantitate VLDL triglyceride PR. All data are presented as mean +/- SD. Percent ideal body weight fell from 132 +/- 17.9 to 119 +/- 15.9% in the group, P less than 0.05. After weight loss, plasma VLDL triglyceride (486.0 +/- 364.1 vs. 191.3 +/- 65.4 mg/dl, P less than 0.05) and VLDL apo B (32.2 +/- 12.0 vs. 14.8 +/- 6.8 mg/dl, P less than 0.05) concentrations were reduced. VLDL triglyceride PR also fell after weight reduction (56.6 +/- 39.0 vs. 28.6 +/- 23.1 mg/kg per h, P less than 0.05), as did VLDL apo B PR (47.9 +/- 41.4 vs. 19.0 +/- 14.1 mg/kg per d, P less than 0.05). Pre-weight loss, plasma LDL cholesterol and apo B levels were low-normal or reduced (64.0 +/- 12.6 and 58.4 +/- 11.9 mg/dl, respectively) despite normal or elevated LDL apo B PR (17.4 +/- 7.2 mg/kg per d). The reduced cholesterol and apo B levels were associated with increased FCRs (0.68 +/- 0.29 d-1) and reduced cholesterol/protein ratios (1.01 +/- 0.18) in LDL. The plasma levels of LDL cholesterol and apo B rose after weight reduction (84.8 +/- 24.9, P less than 0.05; and 69.5 +/- 14.3 mg/dl, P less than 0.05, respectively, vs. base line). These increased concentrations resulted from a combination of events. First, the FCR for LDL apo B fell in five of six subjects with a significant reduction for the group as a whole (0.48 +/- 0.11 d-1, P less than 0.05 vs. base line). Second, the cholesterol/protein ratio increased in all six subjects with a significantly greater mean after weight loss (1.25 +/- 0.27, P less than 0.05 vs. base line). In contrast, the LDL apo B PR fell or was essentially unchanged in the six subjects after weight loss (mean, 14.4 +/- 2.8 mg/kg per d; NS vs. pre-weight loss). The changes in LDL catabolism and composition were associated with changes in the source of LDL apo B. Pre-weight loss, 73.3% of LDL was derived from VLDL, while 26.7% was directly secreted into plasma. Post-weight reduction, VLDL-derived LDL fell to 46.8% of total, while direct secretion accounted for 53.2% of LDL production. These changes were significant; P < 0.95. Thus, all subjects had direct secretion of LDL apo B and the magnitude of this source of VLDL triglyceride secretion. These results indicate that the regulation of plasma LDL levels in hypertriglyceridemic subjects is quite complex and that the rise in LDL levels after weight loss results from reduction in the fractional catabolism of this lipoprotein. The fall in the FCR is associated with changes in the source of LDL and in its composition.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bilheimer D. W., Eisenberg S., Levy R. I. The metabolism of very low density lipoprotein proteins. I. Preliminary in vitro and in vivo observations. Biochim Biophys Acta. 1972 Feb 21;260(2):212–221. doi: 10.1016/0005-2760(72)90034-3. [DOI] [PubMed] [Google Scholar]
- Brunzell J. D., Sniderman A. D., Albers J. J., Kwiterovich P. O., Jr Apoproteins B and A-I and coronary artery disease in humans. Arteriosclerosis. 1984 Mar-Apr;4(2):79–83. doi: 10.1161/01.atv.4.2.79. [DOI] [PubMed] [Google Scholar]
- Chait A., Albers J. J., Brunzell J. D. Very low density lipoprotein overproduction in genetic forms of hypertriglyceridaemia. Eur J Clin Invest. 1980 Feb;10(1):17–22. doi: 10.1111/j.1365-2362.1980.tb00004.x. [DOI] [PubMed] [Google Scholar]
- Curry M. D., Alaupovic P., Suenram C. A. Determination of apolipoprotein A and its constitutive A-I and A-II polypeptides by separate electroimmunoassays. Clin Chem. 1976 Mar;22(3):315–322. [PubMed] [Google Scholar]
- Deckelbaum R. J., Eisenberg S., Oschry Y., Butbul E., Sharon I., Olivecrona T. Reversible modification of human plasma low density lipoproteins toward triglyceride-rich precursors. A mechanism for losing excess cholesterol esters. J Biol Chem. 1982 Jun 10;257(11):6509–6517. [PubMed] [Google Scholar]
- Eaton R. P., Allen R. C., Schade D. S. Overproduction of a kinetic subclass of VLDL-apoB, and direct catabolism of VLDL-apoB in human endogenous hypertriglyceridemia: an analytical model solution of tracer data. J Lipid Res. 1983 Oct;24(10):1291–1303. [PubMed] [Google Scholar]
- Finley P. R., Schifman R. B., Williams R. J., Lichti D. A. Cholesterol in high-density lipoprotein: use of Mg2+/dextran sulfate in its enzymic measurement. Clin Chem. 1978 Jun;24(6):931–933. [PubMed] [Google Scholar]
- Fisher W. R. Heterogeneity of plasma low density lipoproteins manifestations of the physiologic phenomenon in man. Metabolism. 1983 Mar;32(3):283–291. doi: 10.1016/0026-0495(83)90194-4. [DOI] [PubMed] [Google Scholar]
- Fisher W. R., Zech L. A., Bardalaye P., Warmke G., Berman M. The metabolism of apolipoprotein B in subjects with hypertriglyceridemia and polydisperse LDL. J Lipid Res. 1980 Aug;21(6):760–774. [PubMed] [Google Scholar]
- Fredrickson D. S., Levy R. I., Lindgren F. T. A comparison of heritable abnormal lipoprotein patterns as defined by two different techniques. J Clin Invest. 1969 Nov;47(11):2446–2457. doi: 10.1172/JCI105927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson J. C., Rubinstein A., Bukberg P. R., Brown W. V. Apolipoprotein E-enriched lipoprotein subclasses in normolipidemic subjects. J Lipid Res. 1983 Jul;24(7):886–898. [PubMed] [Google Scholar]
- Ginsberg H. N., Le N. A., Melish J., Steinberg D., Brown W. V. Effect of a high carbohydrate diet on apoprotein-B catabolism in man. Metabolism. 1981 Apr;30(4):347–353. doi: 10.1016/0026-0495(81)90114-1. [DOI] [PubMed] [Google Scholar]
- Ginsberg H., Davidson N., Le N. A., Gibson J., Ahrens E. H., Jr, Brown W. V. Marked overproduction of low density lipoprotein apolipoprotein B in a subject with heterozygous familial hypercholesterolemia. Effect of portacaval shunting. Biochim Biophys Acta. 1982 Aug 18;712(2):250–257. doi: 10.1016/0005-2760(82)90341-1. [DOI] [PubMed] [Google Scholar]
- Ginsberg H., Le N. A., Mays C., Gibson J., Brown W. V. Lipoprotein metabolism in nonresponders to increased dietary cholesterol. Arteriosclerosis. 1981 Nov-Dec;1(6):463–470. doi: 10.1161/01.atv.1.6.463. [DOI] [PubMed] [Google Scholar]
- Grundy S. M., Mok H. Y., Zech L., Steinberg D., Berman M. Transport of very low density lipoprotein triglycerides in varying degrees of obesity and hypertriglyceridemia. J Clin Invest. 1979 Jun;63(6):1274–1283. doi: 10.1172/JCI109422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HAVEL R. J., GORDON R. S., Jr Idiopathic hyperlipemia: metabolic studies in an affected family. J Clin Invest. 1960 Dec;39:1777–1790. doi: 10.1172/JCI104202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heiss G., Tamir I., Davis C. E., Tyroler H. A., Rifkand B. M., Schonfeld G., Jacobs D., Frantz I. D., Jr Lipoprotein-cholesterol distributions in selected North American populations: the lipid research clinics program prevalence study. Circulation. 1980 Feb;61(2):302–315. doi: 10.1161/01.cir.61.2.302. [DOI] [PubMed] [Google Scholar]
- Janus E. D., Nicoll A. M., Turner P. R., Magill P., Lewis B. Kinetic bases of the primary hyperlipidaemias: studies of apolipoprotein B turnover in genetically defined subjects. Eur J Clin Invest. 1980 Apr;10(2 Pt 1):161–172. doi: 10.1111/j.1365-2362.1980.tb02076.x. [DOI] [PubMed] [Google Scholar]
- Janus E. D., Nicoll A., Wootton R., Turner P. R., Magill P. J., Lewis B. Quantitative studies of very low density lipoprotein: conversion to low density lipoprotein in normal controls and primary hyperlipidaemic states and the role of direct secretion of low density lipoprotein in heterozygous familial hypercholesterolaemia. Eur J Clin Invest. 1980 Apr;10(2 Pt 1):149–159. doi: 10.1111/j.1365-2362.1980.tb02075.x. [DOI] [PubMed] [Google Scholar]
- Kesaniemi Y. A., Grundy S. M. Significance of low density lipoprotein production in the regulations of plasma cholesterol level in man. J Clin Invest. 1982 Jul;70(1):13–22. doi: 10.1172/JCI110585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kissebah A. H., Alfarsi S., Adams P. W. Integrated regulation of very low density lipoprotein triglyceride and apolipoprotein-B kinetics in man: normolipemic subjects, familial hypertriglyceridemia and familial combined hyperlipidemia. Metabolism. 1981 Sep;30(9):856–868. doi: 10.1016/0026-0495(81)90064-0. [DOI] [PubMed] [Google Scholar]
- Krishnaiah K. V., Walker L. F., Borensztajn J., Schonfeld G., Getz G. S. Apolipoprotein B variant derived from rat intestine. Proc Natl Acad Sci U S A. 1980 Jul;77(7):3806–3810. doi: 10.1073/pnas.77.7.3806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Langer T., Strober W., Levy R. I. The metabolism of low density lipoprotein in familial type II hyperlipoproteinemia. J Clin Invest. 1972 Jun;51(6):1528–1536. doi: 10.1172/JCI106949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le N. A., Melish J. S., Roach B. C., Ginsberg H. N., Brown W. V. Direct measurement of apoprotein B specific activity in 125I-labeled lipoproteins. J Lipid Res. 1978 Jul;19(5):578–584. [PubMed] [Google Scholar]
- Mahley R. W., Weisgraber K. H., Innerarity T. L. Interaction of plasma lipoproteins containing apolipoproteins B and E with heparin and cell surface receptors. Biochim Biophys Acta. 1979 Oct 26;575(1):81–91. doi: 10.1016/0005-2760(79)90133-4. [DOI] [PubMed] [Google Scholar]
- Melish J., Le N. A., Ginsberg H., Steinberg D., Brown W. V. Dissociation of apoprotein B and triglyceride production in very-low-density lipoproteins. Am J Physiol. 1980 Nov;239(5):E354–E362. doi: 10.1152/ajpendo.1980.239.5.E354. [DOI] [PubMed] [Google Scholar]
- Nestel P., Tada N., Billington T., Huff M., Fidge N. Changes in very low density lipoproteins with cholesterol loading in man. Metabolism. 1982 Apr;31(4):398–405. doi: 10.1016/0026-0495(82)90117-2. [DOI] [PubMed] [Google Scholar]
- Noble R. P. Electrophoretic separation of plasma lipoproteins in agarose gel. J Lipid Res. 1968 Nov;9(6):693–700. [PubMed] [Google Scholar]
- Packard C. J., Shepherd J., Joerns S., Gotto A. M., Taunton O. D. Apolipoprotein B metabolism in normal, type IV and type V hyperlipoproteinemic subjects. Metabolism. 1980 Mar;29(3):213–222. doi: 10.1016/0026-0495(80)90062-1. [DOI] [PubMed] [Google Scholar]
- Phillips N. R., Havel R. J., Kane J. P. Levels and interrelationships of serum and lipoprotein cholesterol and triglycerides. Association with adiposity and the consumption of ethanol, tobacco, and beverages containing caffeine. Arteriosclerosis. 1981 Jan-Feb;1(1):13–24. doi: 10.1161/01.atv.1.1.13. [DOI] [PubMed] [Google Scholar]
- Pittman R. C., Carew T. E., Attie A. D., Witztum J. L., Watanabe Y., Steinberg D. Receptor-dependent and receptor-independent degradation of low density lipoprotein in normal rabbits and in receptor-deficient mutant rabbits. J Biol Chem. 1982 Jul 25;257(14):7994–8000. [PubMed] [Google Scholar]
- Ramakrishnan R., Dell R. B., Goodman D. S. On determining the extent of side-pool synthesis in a three-pool model for whole body cholesterol kinetics. J Lipid Res. 1981 Nov;22(8):1174–1180. [PMC free article] [PubMed] [Google Scholar]
- Reardon M. F., Fidge N. H., Nestel P. J. Catabolism of very low density lipoprotein B apoprotein in man. J Clin Invest. 1978 Mar;61(3):850–860. doi: 10.1172/JCI108999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reardon M. F., Poapst M. E., Steiner G. The independent synthesis of intermediate density lipoproteins in type III hyperlipoproteinemia. Metabolism. 1982 May;31(5):421–427. doi: 10.1016/0026-0495(82)90228-1. [DOI] [PubMed] [Google Scholar]
- Shepherd J., Bicker S., Lorimer A. R., Packard C. J. Receptor-mediated low density lipoprotein catabolism in man. J Lipid Res. 1979 Nov;20(8):999–1006. [PubMed] [Google Scholar]
- Sigurdsson G., Nicoll A., Lewis B. Conversion of very low density lipoprotein to low density lipoprotein. A metabolic study of apolipoprotein B kinetics in human subjects. J Clin Invest. 1975 Dec;56(6):1481–1490. doi: 10.1172/JCI108229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sigurdsson G., Nicoll A., Lewis B. The metabolism of low density lipoprotein in endogenous hypertriglyceridaemia. Eur J Clin Invest. 1976 Mar 31;6(2):151–158. doi: 10.1111/j.1365-2362.1976.tb00506.x. [DOI] [PubMed] [Google Scholar]
- Simons L. A., Reichl D., Myant N. B., Mancini M. The metabolism of the apoprotein of plasma low density lipoprotein in familial hyperbetalipoproteinaemia in the homozygous form. Atherosclerosis. 1975 Mar-Apr;21(2):283–298. doi: 10.1016/0021-9150(75)90087-8. [DOI] [PubMed] [Google Scholar]
- Strisower E. H., Adamson G., Strisower B. Treatment of hyperlipidemias. Am J Med. 1968 Oct;45(4):488–501. doi: 10.1016/0002-9343(68)90165-4. [DOI] [PubMed] [Google Scholar]
- Turner J. D., Le N. A., Brown W. V. Effect of changing dietary fat saturation on low-density lipoprotein metabolism in man. Am J Physiol. 1981 Jul;241(1):E57–E63. doi: 10.1152/ajpendo.1981.241.1.E57. [DOI] [PubMed] [Google Scholar]
- Wilson D. E., Lees R. S. Metabolic relationships among the plasma lipoproteins. Reciprocal changes in the concentrations of very low and low density lipoproteins in man. J Clin Invest. 1972 May;51(5):1051–1057. doi: 10.1172/JCI106896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zech L. A., Grundy S. M., Steinberg D., Berman M. Kinetic model for production and metabolism of very low density lipoprotein triglycerides. Evidence for a slow production pathway and results for normolipidemic subjects. J Clin Invest. 1979 Jun;63(6):1262–1273. doi: 10.1172/JCI109421. [DOI] [PMC free article] [PubMed] [Google Scholar]