Abstract
Three Brucella abortus strains were isolated from joint hygromas from cows in northern Togo. Two deletions in the 5′ side of the gene BruAb2_0168 were identified. As this gene is used for species identification, these deletions have consequences for diagnostic procedures. Multiple locus variable number of tandem repeat (VNTR) analysis was therefore performed for species identification. The strains showed unique VNTR profiles, providing some of the first genotypic data from West Africa. More molecular and epidemiological data are needed from the region, in order to better understand transmission patterns and develop suitable diagnostic assays.
Keywords: Brucella, cattle, diagnostics, genotyping, Togo
Although brucellosis is one of the world’s most common zoonoses, there is a lack of data from sub-Saharan Africa 1,2. Brucellosis impacts on human and animal health 3 and has important economic consequences 4. This report describes the isolation and genetic characterization of Brucella abortus strains from Togo, which posed a diagnostic challenge due to deletions in a gene targeted by PCR and the inability to grow on selective medium.
During a brucellosis serosurvey in Togo (2011–2012) 5, joint fluid was aspirated aseptically from nine seropositive cows from five herds with hygromas in the carpi/hocks. Sterile swab tips were soaked in the fluid and sealed in liquid Amies medium. After storage and transportation at 4°C according to international biosafety standards, they were cultivated in Switzerland on tryptic soy agar plates supplemented with 5% sheep blood (BD, Allschwil, Switzerland) and on Brucella-medium base agar supplemented with 5% inactivated horse serum and modified Brucella selective supplement (Oxoid, Basingstoke, UK). The plates were incubated for 10 days at 37°C in 5% CO2. All culture manipulations were performed under Biosafety Level 3 containment. Although there was no growth on Brucella selective medium, mixed cultures grew on blood agar. Brucella-like colonies were purified and isolates were recovered from three cows from three different herds.
Phenotypic testing of the strains was performed. The strains did not require CO2 for growth and grew in the presence of thionin (0.04 mg/mL and 0.02 mg/mL) and basic fuchsine (0.02 mg/mL). They produced H2S, as detected by hydrogen sulphide test strips (Sigma Aldrich, Buchs, Switzerland), and were urease positive (Oxoid). These results indicated B. abortus biovar 3 6.
Molecular identification of the genus Brucella was performed by real-time PCR, as previously described 7. As a species-specific signal for abortus or melitensis could not be obtained but B. abortus was suspected, it was decided to amplify a larger DNA segment of the target locus of the real-time PCR, which was specific for the species abortus, the BruAb2_0168 gene (BAbS19_II01580 in vaccine strain S19). The primer pair used was 0168_Babo_1F and 0168_Babo_1R. The PCR products were sequenced (primers in Table1). Two deletions of 2678 and 263 base pairs were identified, from nucleotide positions 984-3662 and 4295-4558 of the sequence of B. abortus strain S19 (Fig.1) (GenBank accession number: KC847095).
Table 1.
Primers used for the amplification and sequencing of the BruAb2_0168 gene
| Primers | Sequences (5′ to 3′) |
|---|---|
| 0168_Babo_1F | GGCGTGTATGTTGTTGGTAA |
| 0168_Babo_2F | AACATATCGGCGACGCAGTA |
| 0168_Babo_3F | GTGACGGGGACGGGTTGGAC |
| 0168_Babo_4F | TGCGGATAATAATCTGGGTGA |
| 0168_Babo_5F | CGGTTAATGGCACGCTTGAA |
| 0168_Babo_6F | CCTCAATGGTGCGTGGGACAA |
| 0168_Babo_7F | CGGTTCTGGGTGGCACGGTTA |
| 0168_Babo_8F | GCACGGGCAGTCTGACGAAG |
| 0168_Babo_9F | GGCGGCACGACGACGGTTGATG |
| 0168_Babo_11F | GGATACTGACGGCACGCTTGA |
| 0168_Babo_14F | GAACTTCATATCGGTACTGGTG |
| 0168_Babo_15F | CTTGGTGATGACAATTCCAAGA |
| 0168_Babo_16F | GTCTTTTGTGCTCAAGAACAATC |
| 0168_Babo_17F | TGTCGTCAATGGCGGGCGATGGA |
| 0168_Babo_18F | GGGAGTGCAGGCAATCACAG |
| 0168_Babo_19F | CAGGCACGCTGACGCTGA |
| 0168_Babo_20F | ATACACTGACGCTTCAGAACA |
| 0168_Babo_1R | ATCGCCACCAACCATCAGC |
| 0168_Babo_2R | CGCCGTCAGACTGCCCTCCA |
| 0168_Babo_3R | ACCGTCGTTGCGCCCGTA |
| 0168_Babo_4R | AAGTGCCGCCGTTAAACGTCA |
| 0168_Babo_5R | GTGCCTGTGCCGCTCTTCA |
| 0168_Babo_6R | AATTATTCGCCGCATCCTCA |
Figure 1.
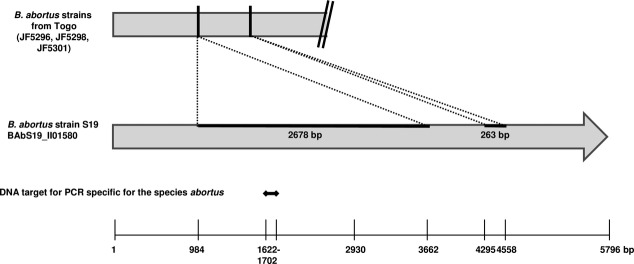
Location of two deletions in the BruAb2_0168 gene of Brucella abortus strains from Togo compared with B. abortus vaccine strain S19. The DNA target for the PCR specific for the abortus species is shown to fall in the range of the first deletion.
The isolated strains displayed features that complicated diagnosis. Firstly, none grew on Brucella selective medium. As some B. melitensis and B. abortus strains are sensitive to the antibiotic concentrations in modified Brucella selective supplement, it is therefore advisable to concurrently inoculate a standard growth medium or modified Thayer-Martin plate 8. Secondly, the deletions in the BruAb2_0168 gene suggest that this may not be a suitable target for an abortus species-specific PCR. Samples for which a species-specific signal cannot be obtained should always be tested for a genus-specific signal to ensure that Brucella sp is not missed. False negatives due to deletions in targets of diagnostic assays can have important public health consequences. In Sweden, a deletion of 377 base pairs in the target of a commercial PCR for the diagnosis of Chlamydia trachomatis was identified following a decrease in the number of human cases detected 9. Genomes can undergo modifications such as deletions, insertions or rearrangements, and this aspect of bacterial evolution must always be considered when performing PCR assays for detection and/or identification. Ideally, more than one target should be tested and unexpected results investigated.
Loci with a variable number of tandem repeats (VNTR) can be used as markers to identify species belonging to the Brucella genus (panel 1 markers) and to discriminate among Brucella strains (panel 2A and 2B markers). Molecular typing by multiple locus VNTR analysis (MLVA) over 16 loci can be used as a molecular epidemiology tool to assess brucellosis transmission patterns 10,11.
MLVA was performed on the three culture lysates and on B. melitensis strain 16MT. For most loci, sequencing confirmed the exact PCR product length. However, five loci could not be sequenced (bruce06, bruce07, bruce09, bruce30, bruce55) and their length was determined by comparing the PCR product bands with those of B. melitensis 16MT on an agarose gel, and by the Agilent 2100 Bioanalyzer using a DNA 1000 LabChip kit (Agilent Technologies, Waldbronn, Germany) followed by comparison with the results of De Santis et al. 12. The MLVA data were analysed using the Brucella aggregated database on MLVAnet (http://mlva.u-psud.fr/) hosted by Université Paris-Sud 13. This database compares queried strains with described strains and performs a clustering analysis using the categorical coefficient and unweighted pair group method with arithmetic mean (UPGMA). The resultant Newick strings were imported into R statistical software Version 2.12.2 (http://www.R-project.org) and a dendogram was drawn using the package ‘ape’ (http://ape-package.ird.fr/). The three strains showed distinct VNTR profiles (Table2) clustering with African strains of B. abortus biovar 3 (Fig.2), confirming the phenotypic results. There is only one other genotyped strain of B. abortus reported from West Africa, isolated from the Gambia 14. Furthermore, the three strains were obtained from a small geographical zone, the sampling sites being only 13–42 km from one another. This demonstrates the diversity of the circulating strains. Given the importance of semi-nomadic herd management and cross-border trade in Togo 15, livestock movements are likely to play a role in this genetic diversity.
Table 2.
MLVA results for three Brucella abortus strains from Togo over 16 loci
| Strain | Number of repeats at each locusa | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Panel 1 | Panel 2A | Panel 2B | ||||||||||||||
| 06 | 08 | 11 | 12 | 42 | 43 | 45 | 55 | 18b | 19 | 21 | 04 | 07 | 09 | 16b | 30 | |
| JF5296 | 3 | 5 | 3 | 11 | 2 | 2 | 3 | 3 | 10 | 41 | 8 | 4 | 2 | 3 | 8 | 4 |
| JF5298 | 3 | 5 | 3 | 11 | 2 | 2 | 3 | 3 | 8 | 41 | 8 | 4 | 2 | 3 | 5 | 4 |
| JF5301 | 3 | 5 | 3 | 11 | 2 | 2 | 3 | 3 | 8 | 41 | 8 | 4 | 2 | 3 | 6 | 4 |
The name of each locus begins with ‘bruce’, followed by the corresponding number.
The three strains differ at loci bruce18 and bruce16.
Figure 2.
Dendogram showing the genetically closest strains to the three Togo strains. The species and biovar are given, followed by the country in which the strain was isolated, the author and the strain reference from the MLVAnet. This analysis was performed using MLVA-8 (panel 1) loci plus bruce18 and bruce21, in order to assess large-scale population structure. *This strain is not listed in MLVAnet 14.
In developing countries, culture is often not feasible due to inadequate laboratory biosafety. At the time of joint fluid collection, several drops were also added to 1 mL of a chaotropic lysis buffer 16. DNA extraction was performed in Switzerland as previously described 17. Real-time PCR 7 confirmed the Brucella genus for six cows, three of which were culture positive. The use of inactivated, buffered samples is therefore both safer and more sensitive than culture, and is recommended for resource-poor, remote areas. Given that DNA extraction and PCR were performed 10 months after collection, rapid sample processing is not required.
More molecular data are needed from sub-Saharan Africa, in order to develop more suitable diagnostic assays for brucellosis and better understand the epidemiology of this important disease. Incoherent diagnostic results should always be further investigated.
Acknowledgments
This study was funded by the National Centre of Competence in Research North-South (NCCR North-South) in Switzerland. The authors acknowledge support from the consortium Afrique One ‘Ecosystem and Population Health: Expanding Frontiers in Health’, which is funded by the Wellcome Trust (WT087535MA). Special thanks are given to Issifa Soulé from the Livestock Production Services of the Savannah Region of Togo for his assistance in sample collection. The Ecole Supérieure d’Agronomie at the University of Lomé is thanked for the administrative support provided.
Transparency Declaration
The authors declare no conflict of interest.
References
- Dean A, Crump L, Greter H, Schelling E, Zinsstag J. Global burden of human brucellosis: a systematic review of disease frequency. PLoS Negl Trop Dis. 2012;6:e1865. doi: 10.1371/journal.pntd.0001865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pappas G, Papadimitriou P, Akritidis N, Christou L, Tsianos EV. The new global map of human brucellosis. Lancet Infect Dis. 2006;6:91–99. doi: 10.1016/S1473-3099(06)70382-6. [DOI] [PubMed] [Google Scholar]
- Dean A, Crump L, Greter H, et al. Clinical manifestations of human brucellosis: a systematic review and meta-analysis. PLoS Negl Trop Dis. 2012;6:e1929. doi: 10.1371/journal.pntd.0001929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth F, Zinsstag J, Orkhon D, et al. Human health benefits from livestock vaccination for brucellosis: case study. Bull World Health Organ. 2003;81:867–876. [PMC free article] [PubMed] [Google Scholar]
- Dean A, Bonfoh B, Kulo A, et al. Epidemiology of brucellosis and Q Fever in linked human and animal populations in northern Togo. PLoS ONE. 2013;8:e71501. doi: 10.1371/journal.pone.0071501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO, FAO, OIE. Brucellosis in humans and animals. Geneva: World Health Organization; 2006. [Google Scholar]
- Hinic V, Brodard I, Thomann A, et al. Novel identification and differentiation of Brucella melitensis, B. abortus, B. suis, B. ovis, B. canins, and B. neotomae suitable for both conventional and real-time PCR systems. J Microbiol Methods. 2008;75:375–378. doi: 10.1016/j.mimet.2008.07.002. [DOI] [PubMed] [Google Scholar]
- OIE. Manual of Diagnostic Tests and Vaccines. Paris: World Organisation for Animal Health; 2009. Chapter 2.4.3. Bovine Brucellosis. [Google Scholar]
- Ripa T, Nilsson PA. A variant of Chlamydia trachomatis with deletion in cryptic plasmid: implications for use of PCR diagnostic tests. Euro Surveill. 2006;11:3076. doi: 10.2807/esw.11.45.03076-en. [DOI] [PubMed] [Google Scholar]
- Al Dahouk S, Le Flèche P, Nöckler K, et al. Evaluation of Brucella MLVA typing for human brucellosis. J Microbiol Methods. 2007;69:137–145. doi: 10.1016/j.mimet.2006.12.015. [DOI] [PubMed] [Google Scholar]
- Le Flèche P, Jacques I, Grayon M, et al. Evaluation and selection of tandem repeat loci for a Brucella MLVA typing assay. BMC Microbiol. 2006;6:9. doi: 10.1186/1471-2180-6-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Santis R, Ciammaruconi A, Faggioni G, et al. Lab on a chip genotyping for Brucella spp. based on 15-loci multi locus VNTR analysis. BMC Microbiol. 2009;9:66. doi: 10.1186/1471-2180-9-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grissa I, Bouchon P, Pourcel C, Vergnaud G. On-line resources for bacterial micro-evaluatioin studies using MLVA or CRISPR typing. Biochimie. 2008;90:660–668. doi: 10.1016/j.biochi.2007.07.014. [DOI] [PubMed] [Google Scholar]
- Bankole AA, Saegerman C, Berkvens D, et al. Phenotypic and genotypic characterisation of Brucella strains isolated from cattle in the Gambia. Vet Rec. 2010;166:753–756. doi: 10.1136/vr.b4862. [DOI] [PubMed] [Google Scholar]
- Dean AS, Fournié G, Kulo AE, et al. Potential risk of regional disease spread in West Africa through cross-border cattle trade. PLoS ONE. 2013;8:e75570. doi: 10.1371/journal.pone.0075570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bürki S, Vilei EM, Frey J, Wittenbrink MM. Allelic variations of the nox gene of Brachyspira pilosicoli impair its detection by qPCR. Vet Microbiol. 2011;149:291–292. doi: 10.1016/j.vetmic.2010.10.014. [DOI] [PubMed] [Google Scholar]
- Cheng X, Nicolet J, Poumarat F, et al. Insertion element IS1296 in Mycoplasma mycoides subsp. mycoides small colony identifies a European clonal line distinct from African and Australian strains. Microbiology (Reading, Engl) 1995;141(Pt 12):3221–3228. doi: 10.1099/13500872-141-12-3221. [DOI] [PubMed] [Google Scholar]


