Contents
A total of 73 bitches with ovarian cysts were ovariohysterectomized. Cysts were characterized by gross pathology and endocrine parameters. Therefore, oestradiol-17ß and progesterone concentrations were assessed in cyst-fluid and corresponding blood plasma in each bitch. Our data demonstrated that multiple cysts were often present in a single individual (82%) and that cysts were commonly found on both ovaries (77%). The number of cysts per individual varied from 1 to 35. Most cysts were small in size (range 0.2–4.0 cm in diameter). No cyst was found to produce solely oestradiol-17ß or progesterone. Plasma levels of oestradiol-17ß and progesterone for a given individual were positively correlated with levels of these same hormones in their cyst-fluid (r = 0.334 and p = 0.001 for oestradiol-17ß; r = 0.419 and p < 0.001 for progesterone). Our study is the first to provide a comprehensive evaluation of the gross pathology and endocrinology of ovarian cysts in a larger number of bitches.
Introduction
Hormonally active ovarian cysts are of high clinical relevance in bitches. They are a significant source of hyperoestrogenism in bitches (Johnston et al. 2001), which may result in prolonged oestrus (Arlt et al. 2011; Knauf et al. 2013) and uteropathies (Olson et al. 1989). Per definition, an ovarian cyst is a fluid-filled structure (Olson et al. 1989) of any size present outside physiological prooestrus and oestrus within the ovary (Johnston et al. 2001). It can occur solitaire or multiple on one or on both ovaries (Dow 1960) and is endocrine active or inactive (Olson et al. 1989). There are different types of ovarian cysts: follicular cysts, cysts of subsurface epithelial structures (SES), cystic Rete ovarii, lutein cysts and cystic Corpora lutea (Dow 1960; Johnston et al. 2001; Schlafer and Miller 2007). Follicular cysts are known to be endocrine active (e.g. produce oestradiol-17ß and progesterone; Olson et al. 1989), as well as lutein cysts and cystic Corpora lutea (produce progesterone; McEntee 1990; Johnston et al. 2001). Cysts of subsurface epithelial structures (SES) and cystic Rete ovarii are not associated with the production of hormones (Olson et al. 1989), and clinical signs of disease are uncommon. However, they can replace the surrounding physiological ovarian tissue (Dow 1960; McEntee 1990), which may secondary result in a functional loss.
The physiological oestrous cycle in bitches (both prooestrus and oestrus) is 2–4 weeks long (Feldman and Nelson 2004a) and is followed by metoestrus and anoestrus. At least 4 months can be expected in-between subsequent oestrous cycles (Feldman and Nelson 2004a,b). Per definition, prolonged oestrus occurs when, for a period of longer than 28 days, the bitch is willing to breed (Feldman and Nelson 2004b) or when, for the same time, exfoliate vaginal cytology indicates that vaginal epithelial cells in >90% are superficial cells (Olson et al. 1989). Clinical signs resemble those seen in prooestrus and oestrus for example oedema of the vulva and sero-sanguinous vaginal discharge (Rowley 1980; McEntee 1990; Fayrer-Hosken et al. 1992; Ranganath et al. 1993). However, the initial sero-sanguinous vaginal discharge may already be changed into purulent at the time of presenting to the veterinarian (Fayrer-Hosken et al. 1992; Ranganath et al. 1993; Serin and Ulutas 2007). In severe cases of prolonged oestrus, fatal pancytopenia can occur following prolonged elevations in blood oestrogene (Schwarz et al. 1982; Suttorp et al. 2002). Other clinical signs include skin and coat alterations (Frank 2006; Ghaffari et al. 2009).
Despite their clinical significance, little is known of the inter-relation between gross pathological and endocrine appearance of ovarian cysts in bitches (Knauf and Wehrend 2010). Most previous studies of ovarian cysts in bitches are either case reports, focusing on one or a few individuals (Shille et al. 1984; Ervin and Homans 1986; Arlt et al. 2011; Sontas et al. 2011), or studies focusing on one attribute of the disease or another (e.g. histology or reproductive pathologies at all) (Trasch et al. 2003; Akihara et al. 2007; Ortega-Pacheco et al. 2007). While these previous studies have provided invaluable information about the nature of the disease in dogs, additional details about the connection between gross pathology and endocrinology of ovarian cysts in dogs are needed for a better understanding of their potential for causing disease. We hypothesize that (A) the number of ovarian cysts per individual has no influence on oestradiol-17ß and progesterone concentration in blood plasma and (B) the elevation of oestradiol-17ß and progesterone concentration in ovarian cyst-fluid correlates with the corresponding steroid hormone concentration in blood plasma. The aim of this study was to describe and to evaluate gross pathology and endocrine parameters of ovarian cysts in dogs.
Materials and Methods
Animals and diagnosis
A total of 73 bitches with ovarian cysts were ovariohysterectomized following a standardized protocol described elsewhere (Groeger et al. 2007). Surgery was performed at the Clinic for Obstetrics, Gynaecology and Andrology of Large and Small Animals of the Justus-Liebig-University in Giessen, Germany. Bitches were presented to the clinic due to prolonged oestrus (n = 21) or vaginal discharge outside of physiological oestrous cycle (n = 52). Vulvar discharge was sero-sanguineous or sanguineous-purulent. Diagnosis was based on the dog’s clinical history, as well as clinical and gynaecological examination. The state of oestrous cycle was furthermore determined by exfoliate vaginal cytology. Sampling technique, staining and interpretation of cytological smears followed the description published by Tammer et al. (1994). Systematically, abdominal organs were screened via ultrasound (SonoAce-9900 ultrasound machine with 7.5-MHz convex transducer; Sonoace GmbH, Marl, Germany) using a standardized protocol. Briefly, uterus was examined for content, uterus horn diameter and wall thickness. The sonographic evaluation of the ovaries focused mainly on the imaging of cystic structures.
Gross pathology
Ovarian cysts were defined as fluid-filled cavities within or on the surface of the ovary outside physiological oestrous cycle. The respective location of ovarian cysts in 73 bitches was identified during or directly after ovariohysterectomy in situ. In addition, the number of macroscopic visible cysts was counted, and the diameter of cysts belonging to 52 dogs was measured using a calliper on the isolated organ post-ovariohysterectomy. Furthermore, the presences of Corpora lutea that occurred simultaneously with ovarian cysts were recorded.
Endocrinology
Blood samples were collected from 52 bitches before ovariohysterectomy from the V. cephalica antebrachii and immediately transferred to lithium-heparin-containing tubes (Sarstedt, WDT eG, Garbsen, Germany). Whole-blood samples were centrifuged for 10 min at 3000 × g at room temperature. Plasma was transferred to a sterile tube (4.5 ml; WDT eG, Garbsen, Germany) and stored at −80°C until further processing. Ovarian cysts with diameters >1.0 cm from 32 bitches were sampled for corresponding cyst-fluid which was immediately transferred to a sterile tube (WDT eG) and also stored at −80°C until further processing. We collected fluid from 1 to 15 cysts per dog, which added to a total of 82 cyst-fluid samples. In six of these samples, however, we had just enough fluid material to measure the concentration of one hormone (progesterone) in the sample.
Oestradiol-17ß and progesterone concentrations from plasma and cyst-fluid were measured in duplicates using specific in-house radioimmunoassays (RIA) as described by Hoffmann et al. (1992). Sensitivity and intra-assay coefficients of variation of oestradiol-17ß-RIA were 0.4 pg/ml, 6.0 and 11.4 %, while those of progesterone-RIA were 0.1 ng/ml, 8.8 and 9.6 %. Inter-assay coefficient of variation of oestradiol-17ß was between 13.1 and 13.2 %, and 8.9 and 11.3 % for progesterone.
Hormone concentrations of both, serum and cyst-fluid were classified as endocrine active (>15.0 pg/ml for oestradiol-17ß and >1.5 ng/ml for progesterone; Wehrend 2010) or inactive (<15.0 pg/ml for oestradiol-17ß and <1.5 ng/ml for progesterone; Wehrend 2010).
Statistical analysis
Data were evaluated using the statistic software package BMDP/Dynamic (Release 7.0; Dixon 1993) and prism 6 for Macintosh (Version 6; Graph Pad Software, Inc., La Jolla, CA, USA). Data were tested for normal distribution (D’Agostino & Pearson omnibus normality test). In case of a positive distribution skewed to the right, data were log-transformed. Log Gaussian distributed data are presented as geometric mean (xg) and dispersion factor (DF). In all cases where it was not possible to transform data into normal distribution, results are reported as median and range (minimum–maximum). Correlation between plasma oestradiol-17ß and progesterone and corresponding mean hormone concentrations in cyst-fluid per bitch on the one hand and increasing numbers of cysts per bitch and hormone concentration of plasma on the other hand were analysed using Pearson’s product moment correlation coefficient (r) and linear regression analysis using regression line (y = mx + b). The significance level α = 0.05 was chosen, so p-values were considered statistically significant at p ≤ 0.05.
Results
Breed and age of the bitches, physical findings (e.g. oedema of the vulva and vaginal discharge), predominant vaginal cell population, occurrence of pyometra and coat changes, as well as ultrasonographic imaging of cysts, are summarized in Table S1.
Gross pathology
Macroscopically, 1–35 ovarian cysts (per individual) were detected. Geometric mean of cysts per bitch was 5.2 (2.5) [xg (DF)] (n = 384 cysts in total, Fig.1a). Among the 384 cysts, cyst diameter varied from 0.2 to 4.0 cm (median 0.5 cm, Fig.1b). In 82% of cases (n = 60/73), more than one ovarian cyst was present in a single individual (Table1). Moreover, in 77% of cases (n = 56/73), cysts were detected on both ovaries (Table1). Further details of the individual cyst size and localization in each bitch are provided in Table S1.
Figure 1.

(a) Number of ovarian cysts per bitch (384 cysts counted in 52 bitches), (b) Distribution of diameter measured in 384 ovarian cysts from 52 bitches
Table 1.
Localization and number of ovarian cysts in the bitches (n = 73)
| Localization and number | Number of bitches (%) |
|---|---|
| Multiple cysts on both ovaries | 48 (66) |
| Multiple cysts on a single ovary | 9 (12) |
| Multiple cysts on one ovary and solitary cyst on contralateral ovary | 3 (4) |
| Solitary cysts on both ovaries | 5 (7) |
| Solitary cyst on a single ovary | 8 (11) |
There was no significant correlation between plasma hormone concentrations and the number of ovarian cysts present in a given individual (oestradiol-17ß: correlation coefficient r = 0.26; n = 41, p = 0.265; progesterone: correlation coefficient r = −0.05, n = 40, p = 0.659).
In 36% of the bitches of this study (n = 26/73), Corpora lutea were found in gross examination after removal of the ovaries (post-ovariohysterectomy; see Table S1).
Endocrinology
Blood plasma concentrations of oestradiol-17ß varied widely between bitches (range: 2.0–68.0 pg/ml); geometric mean was 13.0 pg/ml (2.1) [xg (DF)] (n = 51 bitches). Progesterone concentrations were similarly variable (range: 0.1 to 63.0 ng/ml); geometric mean was 4.0 ng/ml (5.4) [xg (DF)] (n = 50 bitches). A quarter of bitches (n = 13/51) exhibited simultaneous elevations in oestradiol-17ß and progesterone plasma levels representing endocrine activity. Detailed information about plasma hormone concentration is provided in the Table S2.
The concentration of both oestradiol-17ß and progesterone exhibited greater variation in cyst-fluid than in plasma (Table S2). Oestradiol-17ß levels in cyst-fluid ranged from 2.0 to 568 000.0 pg/ml (median 545.0 pg/ml; n = 76 cysts from 32 bitches) (see Table S2). In over one-third of cysts (n = 27), oestradiol-17ß levels were in hormonal inactive status, while in over two-thirds of cysts (n = 49), oestradiol-17ß levels represented hormonal activity. Progesterone concentrations in cyst-fluid ranged between 0.1 and 20,138.0 ng/ml (median 31.0 ng/ml; n = 82 cysts from 32 bitches). In 20% of cysts (n = 17), progesterone concentrations showed hormonal inactivity and in nearly 80% of cysts (n = 65) progesterone levels revealed endocrine activity. Based on concentrations of both hormones, 55% of all cysts (n = 45) could be diagnosed as endocrine active. The geometric mean of oestradiol-17ß was 31.0 pg/ml (6.4) [xg (DF)], while progesterone concentrations did not reach Gaussian distribution and had a median of 6.0 ng/ml (range between 1.9 and 935.0 ng/ml). No bitch had ovarian cysts that produced solely oestradiol-17ß or progesterone.
Although weak positive correlated (oestradiol-17ß: r = 0.334, Fig.2a; progesterone: r = 0.419, Fig.2b), p-values for the comparison between individual blood plasma levels of log oestradiol-17ß and log progesterone and the mean log concentrations of these same hormones in cyst-fluid were highly significant (oestradiol-17ß: n = 29, p = 0.001; progesterone: n = 30, p < 0.001). A detailed comparison of hormone concentrations in both, blood and cyst-fluid, is shown in the Table S2.
Figure 2.
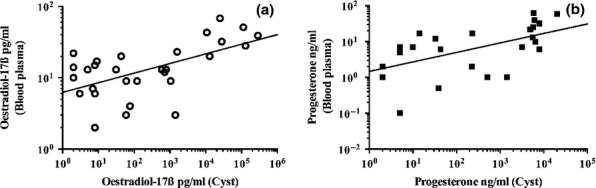
(a) Correlation between blood plasma levels of oestradiol-17ß and corresponding mean hormone concentrations in cyst-fluid from bitches with ovarian cysts (n = 29) is significant (p = 0.001). To improve the graphical presentation, x- and y-axis were presented in logarithmic scale. Regression line results from function log (y) = 0.1344 log (x) + 0.7977. (b) Correlation between blood plasma levels of progesterone and corresponding mean hormone concentrations in cyst-fluid from bitches with ovarian cysts (n = 30) is significant (p < 0.001). To improve the graphical presentation, x- and y-axis were presented in logarithmic scale. Regression line results from function log (y) = 0.2640 log (x) + 0.1709. Five data points are outside the axis limits
Discussion
Our study is the first to provide a comprehensive evaluation of the gross pathology in context with endocrinology of ovarian cysts in a larger number of dogs that originate from one clinical setup. Most previous reports of ovarian cysts in bitches in the literature are case reports that include only a small number of individual dogs (e.g. Shille et al. 1984; Ervin and Homans 1986). Small sample sizes may give mistaken impressions about the nature or presentation of disease, if only extreme (or rare) cases are frequently reported. For example, several previous reports of ovarian cysts in bitches have documented evidence of large cystic structures (e.g. Ervin and Homans 1986: 32.5 × 23.2 cm; Hoffmann et al. 2000: 7.0 cm). However, cystic structures can vary widely in size (Dow 1960; Marino et al. 2010), but in our study, most cysts were ≤0.5 cm. This finding is particularly important for clinicians that use imaging techniques to detect ovarian cysts. In humans, computed tomography (CT) and magnetic resonance imaging (MRI) may provide optimal resolution to identify even smaller cystic structures (Occhipinti et al. 1993), but in veterinary medicine, this however is more of academic practice and not realistic for routine diagnosis. Nöthling et al. (2006) found out that MRI is not suitable for counting follicles or Corpora lutea in the ovaries of bitches. Therefore, ultrasonography needs to be considered as the most feasible imaging technique in bitches (Fontbonne 2011) with the disadvantage of overseeing very small cystic structures.
To identify the endocrine potential of each cyst, the in-house RIA required a minimum of 0.5 ml cyst-fluid to analyse oestradiol-17ß and progesterone. The limiting factor in the collection of cysts fluid was the diameter of cystic structures, and correspondingly the volume of fluid. Yet, sample volume was already diluted to allow each cyst to be measured twice for both hormones. As concentrations in cyst-fluid can be extremely heterogeneous (Table S2), it was not possible to use cyst-fluid in very high dilutions because this may have caused false-negative results for cysts with only low hormonal concentrations, simply because the readout would by far undergo the RIA’s detection limit.
We found that cyst-fluid from different cysts located on the same ovary exhibit differences in hormone concentration, suggesting that the endocrine activity of cysts is independent of one another. These results support the histological findings of Dow (1960) on an additional level. We assumed that these cysts were formed at different stages of follicular development or atresia and therefore have a varied number of granulosa and theca cells, which are producing oestradiol-17ß and progesterone (McNatty et al. 1984; Fortune et al. 2004). At least for cattle, this has been reported (Fortune et al. 2004; Hussein et al. 2013).
Given the great variation in endocrine activity of ovarian cysts in this study, our results suggest that one or a few cysts may have the same endocrine potency as a larger number of cysts. In our sample, increasing numbers of ovarian cysts were not correlated with increasing plasma hormone concentrations for either oestradiol-17ß or progesterone. These results highlight the endocrine potency of even single ovarian cysts for disrupting normal ovarian function in bitches, which can result in hyperoestrogenism and other disorders of hormonal origin (e.g. coat and skin alterations).
Even a single ovarian cyst is part of a dynamic system that interacts directly with the organism’s circulatory system. Steroids can diffuse through the cyst wall and enter the general blood supply. Once there, the concentration of the steroids in the bloodstream may be lower relative to their concentration in cyst-fluid, which may explain the great disparity in cyst-fluid and blood hormone levels in our study.
Results showed a weak positive correlation between oestradiol-17ß- and progesterone-concentration in peripheral blood and cyst-fluid, although p-values were highly significant, which is contrary to the findings published by Bostedt et al. (2013). The larger sample size in the present study, however, could explain the discrepancy.
The presence of Corpora lutea in addition to ovarian cysts has been already described in the work of Dow (1960) and was confirmed in this study. In 36% of the bitches of this work (n = 26/73), Corpora lutea were found in gross examination of the isolated ovaries. This explains also why in the majority of cases (87%, n = 20/23), the plasma progesterone concentration of animals with Corpora lutea, independent of the oestradiol-17ß plasma concentration, corresponded to physiological metoestrus. In the bitch, several follicles ovulate in a certain time period of 1–2 days (Concannon 2011). The ovulation usually occurs at the beginning of oestrus after the LH peak (Concannon 2011). The oestradiol-17ß concentration in the blood decreases after ovulation to basal level (< 15 pg/ml, Wehrend 2010), while the progesterone concentration further increases (Concannon 2011). In case the LH peak was not high enough to stimulate ovulation in all dominant follicles or in case responsiveness of receptors is too low, this may explain genesis of ovarian cysts.
Metoestrus is also partly reflected in the vaginal cytology (see Table S1). While most female dogs (68%) with ovarian cysts showed a typical oestrogen dominated cell profile (n = 40/59 superficial cells), in 20% of female dogs, parabasal and intermediate cells dominated the vaginal smear (n = 12/59), which is typical for metoestrus cycle. In bitches with the open form of pyometra, mainly neutrophil granulocytes (7/59) were found in the vaginal cytology, which corresponds to vaginal discharge of purulent character. The frequent association of ovarian cysts and pyometra (47/73) may be overemphasized and certainly is subject to bias because of a pre-selection of patients that are referred to our clinic. While there is no doubt about the correlation of hormonal imbalance and the risk to develop a pyometra, the overall risk of bitches with ovarian cysts to develop pyometra might be lower in studies that use data of bitches that are exclusively reported to non-referral veterinary practices.
Yet, only the gross pathology and endocrinology of ovarian cysts in bitches were evaluated in the present study. Pursuing research is needed to further characterize cystic structures of the ovaries, which in detail is only possible by histological and immunohistochemical examination (Akihara et al. 2007; Ortega-Pacheco et al. 2007).
In conclusion, our results indicate that most ovarian cysts in bitches are small in size (≤0.5 cm), but that even those smaller cystic structures, in support of our hypothesis, can exhibit substantial endocrine activity with the potential to disrupt normal ovarian function. Our results suggest that in bitches with prolonged oestrus or vaginal discharge outside physiological oestrous cycle, plasma oestradiol-17ß- concentration is not predictive of the number of cystic structures present in a single individual and, if the hormone concentration represents endocrine inactivity, it may not predict the absence of oestradiol-17ß-producing ovarian cysts.
Acknowledgments
The authors thank J. Blad-Stahl, F. Sechser and S. Heerdt from the Clinical Laboratory for their help, as well as W. Damm and S. Feller from the Endocrine Laboratory for conducting the steroid hormone assays. We also thank A. Schaubmar and M. Sparenberg from the Unit for Biomathematics and Data Processing of Justus-Liebig-University for their support in statistical analysis. Sincere thanks are given to Nga Nguyen from California State University Fullerton, Fullerton, CA, USA, for critical revision. We thank the reviewer’s for their comments and suggestions.
Conflict of interest
None of the authors have any conflict of interest to declare.
Author contributions
YK and AW made substantial contributions to the conceptualization and experimental design of this project. SK assisted in the analyses of the results, provided valuable feedback on an earlier draft and helped revise the manuscript substantially throughout. KF analysed the results and helped interpret the data. HB provided funding and support for this study and helped revise the manuscript. YK contributed to the study design, conducted the experiments, analysed the results and wrote the article.
Supporting Information
Additional Supporting Information may be found in the online version of this article:
Table S1 Raw data of 73 bitches with ovarian cysts (online available).
Table S2 Hormone concentration (oestradiol-17ß- and progesterone) in cyst-fluid of individually punctured ovarian cysts (n = 82) of 32 bitches, presented as individual and mean values, and corresponding individual blood plasma hormone concentrations of 52 bitches (online available).
References
- Akihara Y, Shimoyama Y, Kawasako K, Komine M, Hirayama K, Kagawa Y, Omachi T, Matsuda K, Okamoto M, Kadosawa T, Taniyama H. Immunohistochemical evaluation of canine ovarian cysts. J Vet Med Sci. 2007;69:1033–1037. doi: 10.1292/jvms.69.1033. [DOI] [PubMed] [Google Scholar]
- Arlt SP, Spankowsky S, Heuwieser W. Follicular cysts and prolonged oestrus in a female dog after administration of a deslorelin implant. N Z Vet J. 2011;59:87–91. doi: 10.1080/00480169.2011.552858. [DOI] [PubMed] [Google Scholar]
- Bostedt H, Jung C, Wehrend A, Boryzcko Z. Clinical and endocrinological findings of bitches with ovarian cyst syndrome. Schweiz Arch Tierheilkd. 2013;155:543–550. doi: 10.1024/0036-7281/a000510. [DOI] [PubMed] [Google Scholar]
- Concannon PW. Reproductive cycles of the domestic bitch. Anim Reprod Sci. 2011;124:200–210. doi: 10.1016/j.anireprosci.2010.08.028. [DOI] [PubMed] [Google Scholar]
- Dixon WJ. BMDP Statistical Software Manual. 1 and 2. Berkeley, Los Angeles, London: University of California Press; 1993. , Vol. [Google Scholar]
- Dow C. Ovarian abnormalities in the bitch. J Comp Pathol. 1960;70:59–69. doi: 10.1016/s0368-1742(60)80005-7. [DOI] [PubMed] [Google Scholar]
- Ervin E, Homans P. Giant ovarian cyst. Compend Contin Educ Pract Vet. 1986;8:698–700. [Google Scholar]
- Fayrer-Hosken RA, Durham DH, Allen S, Miller-Liebl DM, Caudle AB. Follicular cystic ovaries and cystic endometrial hyperplasia in a bitch. J Am Vet Med Assoc. 1992;201:107–108. [PubMed] [Google Scholar]
- Feldman EC, Nelson RW. Ovarian cycle and vaginal cytology. In: Feldman EC, Nelson RW, editors. Canine and Feline Endocrinology and Reproduction. St. Louis, MO: Saunders; 2004a. pp. 752–774. In: (eds), [Google Scholar]
- Feldman EC, Nelson RW. Infertility, associated breeding disorders, and disorders of sexual development. In: Feldman EC, Nelson RW, editors. Canine and Feline Endocrinology and Reproduction. St. Louis, MO: Saunders; 2004b. pp. 868–900. In: (eds), [Google Scholar]
- Fontbonne A. Infertility in bitches and queen: recent advances. Rev Bras Reprod Anim. 2011;35:202–209. [Google Scholar]
- Fortune JE, Rivera GM, Yang MY. Follicular development: the role of the follicular microenvironment in selection of the dominant follicle. Anim Reprod Sci. 2004;82–83:109–126. doi: 10.1016/j.anireprosci.2004.04.031. [DOI] [PubMed] [Google Scholar]
- Frank LA. Comparative dermatology–canine endocrine dermatoses. Clin Dermatol. 2006;24:317–325. doi: 10.1016/j.clindermatol.2006.04.007. [DOI] [PubMed] [Google Scholar]
- Ghaffari MS, Dezfoulian O, Aldavood S, Masoudifard M. Estrogen-related alopecia due to polycystic ovaries in a terrier dog. Comp Clin Pathol. 2009;18:341–343. [Google Scholar]
- Groeger S, Weiss R, Trasch K, Wehrend A. Significance of the bacteriological results from vaginal swabs taken from bitches with overt pyometra. Kleintierprax. 2007;52:426–428. [Google Scholar]
- Hoffmann B, Höveler R, Hasan SH, Failing K. Ovarian and pituitary function in dogs after hysterectomy. J Reprod Fert. 1992;96:837–845. doi: 10.1530/jrf.0.0960837. [DOI] [PubMed] [Google Scholar]
- Hoffmann B, Lemmer W, Bostedt H, Failing K. Application of the antiprogestin Aglepristone for conservative treatment of pyometra in the dog. Tierärztl Prax. 2000;28:323–329. [Google Scholar]
- Hussein HA, Boryczko Z, Bostedt H. Acid-base parameters and steroid concentrations in pre-ovulatory follicles and plasma of lactating dairy cows with spontaneous and synchronized oestrus or follicular cyst. Reprod Domest Anim. 2013;48:833–839. doi: 10.1111/rda.12171. [DOI] [PubMed] [Google Scholar]
- Johnston SD, Kustritz MV, Olson PNS. Disorders of canine ovary. In: Johnston SD, Kustritz MV, Olson PNS, editors. Canine and Feline Theriogenology. Philadelphia: Saunders; 2001. pp. 193–205. In: (eds), [Google Scholar]
- Knauf Y, Wehrend A. Ovarian cysts in the bitch. Tierärztl Prax. 2010;38:333–340. [PubMed] [Google Scholar]
- Knauf Y, Failing K, Knauf S, Wehrend A. Treatment of bitches with ovarian cysts using human chorionic gonadotropin-releasing hormone analogon. A case series of 30 bitches. Tierärztl Prax. 2013;41:93–100. [PubMed] [Google Scholar]
- Marino G, Barna A, Mannarino C, Di Prima ML, Zanghi A. Stromal cysts of the canine ovary: prevalence, diagnosis and clinical implications. Veterinaria. 2010;24:9–15. [Google Scholar]
- McEntee K. Cysts in and around the ovary. In: McEntee K, editor. Reproductive Pathology of Domestic Mammals. San Diego: Academic Press; 1990. pp. 52–67. In: (ed.), [Google Scholar]
- McNatty KP, Heath DA, Henderson S, Lun S, Hurst PR, Ellis LM, Montgomery GW, Morrison L, Thurley DC. Some aspects of thecal and granulose cells function during follicular development in the bovine ovary. J Reprod Fertil. 1984;72:39–53. doi: 10.1530/jrf.0.0720039. [DOI] [PubMed] [Google Scholar]
- Nöthling JO, De Cramer KG, Gerber D, Kammer VR. Luteal and follicular count in bitches: assessment by means of magnetic resonance imaging. Theriogenology. 2006;66:1343–1354. doi: 10.1016/j.theriogenology.2006.05.009. [DOI] [PubMed] [Google Scholar]
- Occhipinti KA, Frankel SD, Hricak H. The ovary. Computed tomography and magnetic resonance imaging. Radiol Clin North Am. 1993;31:1115–1132. [PubMed] [Google Scholar]
- Olson PN, Wrigley RH, Husted PW, Bowen RA, Nett TA. Persistent estrus in the bitch. In: Ettinger SJ, Feldman EC, editors. Textbook of Veterinary Internal Medicine. Philadelphia: WB Saunders; 1989. pp. 1792–1796. In: (eds), [Google Scholar]
- Ortega-Pacheco A, Segura-Correa JC, Jimenez-Coello M, Linde Forsberg C. Reproductive patterns and reproductive pathologies of stray bitches in the tropics. Theriogenology. 2007;67:382–390. doi: 10.1016/j.theriogenology.2006.07.020. [DOI] [PubMed] [Google Scholar]
- Ranganath L, Ranganath BN, Jayagopalareddy NR. Ovarian cyst in a bitch-a report. Indian Vet J. 1993;70:1062–1063. [Google Scholar]
- Rowley J. Cystic ovary in a dog: a case report. Vet Med Small Anim Clin. 1980;75:1888. [PubMed] [Google Scholar]
- Schlafer DH, Miller RB. Female genital system. In: Grant Maxie M, editor. Jubb, Kennedy and Palmer's Pathology of Domestic Animals. Vol. 3. Philadelphia, PA: Saunders Elsevier; 2007. pp. 429–564. In: (ed.), , vol. [Google Scholar]
- Schwarz H, Geyer S, Russe M, Hanichen T. Intoxication caused by estrogen administration in the bitch. Tierärztl Prax. 1982;10:393–402. [PubMed] [Google Scholar]
- Serin G, Ulutas B. Bilateral multiple ovarian cyst and cystic endometrial hyperplasia-Pyometra in a bitch. J Fac Vet Med Istanbul Univ. 2007;33:63–69. [Google Scholar]
- Shille VM, Calderwood-Mays MB, Thatcher MJ. Infertility in a bitch associated with short interestrus intervals and cystic follicles: a case report. J Am Anim Hosp Assoc. 1984;20:171–176. [Google Scholar]
- Sontas BH, Milani C, Romagnoli S, Bertolini G, Caldin M, Caliari D, Zappulli V, Mollo A. A huge ovarian cyst in a hysterectomized bitch. Reprod Domest Anim. 2011;46:1107–1111. doi: 10.1111/j.1439-0531.2011.01797.x. [DOI] [PubMed] [Google Scholar]
- Suttorp M, Hoffmann B, Sippell WG. Prevention of oestradiol-associated toxicosis in a dalmatian by early intervention with granulocyte colony-stimulating factor. Vet Rec. 2002;151:244–245. doi: 10.1136/vr.151.8.244. [DOI] [PubMed] [Google Scholar]
- Tammer I, Blendinger K, Sobiraj A, Bostedt H. The use of exfoliative vaginal cytology for the gynecological evaluation of the bitch. Tierärztl Prax. 1994;22:199–207. [PubMed] [Google Scholar]
- Trasch K, Wehrend A, Bostedt H. Follow-up examination in bitches after conservative treatment of pyometra with antigestagen aglepristone. J Vet Med A Physiol Pathol Clin Med. 2003;50:375–379. doi: 10.1046/j.1439-0442.2003.00557.x. [DOI] [PubMed] [Google Scholar]
- Wehrend A. Der gynäkologische Untersuchungsgang. In: Wehrend A, editor. Leitsymptome Gynäkologie und Geburtshilfe beim Hund. Stuttgart: Enke Verlag; 2010. pp. 32–58. In: (ed.), [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1 Raw data of 73 bitches with ovarian cysts (online available).
Table S2 Hormone concentration (oestradiol-17ß- and progesterone) in cyst-fluid of individually punctured ovarian cysts (n = 82) of 32 bitches, presented as individual and mean values, and corresponding individual blood plasma hormone concentrations of 52 bitches (online available).


