Abstract
The aim of this study is to determine if weekly application of dehydrated human amnion/chorion membrane allograft reduce time to heal more effectively than biweekly application for treatment of diabetic foot ulcers.
This was an institutional review board‐approved, registered, prospective, randomised, comparative, non‐blinded, single‐centre clinical trial. Patients with non‐infected ulcers of ≥ 4 weeks duration were included for the study. They were randomised to receive weekly or biweekly application of allograft in addition to a non‐adherent, moist dressing with compressive wrapping. All wounds were offloaded. The primary study outcome was mean time to healing.
Overall, during the 12‐week study period, 92·5% (37/40) ulcers completely healed. Mean time to complete healing was 4·1 ± 2·9 versus 2·4 ± 1·8 weeks (P = 0·039) in the biweekly versus weekly groups, respectively. Complete healing occurred in 50% versus 90% by 4 weeks in the biweekly and weekly groups, respectively (P = 0·014). Number of grafts applied to healed wounds was similar at 2·4 ± 1·5 and 2·3 ± 1·8 for biweekly versus weekly groups, respectively (P = 0·841).
These results validate previous studies showing that the allograft is an effective treatment for diabetic ulcers and show that wounds treated with weekly application heal more rapidly than with biweekly application. More rapid healing may decrease clinical operational costs and prevent long‐term medical complications.
Keywords: Amniotic membrane allograft, Diabetic ulcer, Dehydrated amnion/chorion
Introduction
Chronic wounds have a significant impact on public health through increased disability, morbidity and risk of mortality, which result in greater usage of health care resources and higher costs 1, 2. In the USA, 26 million people representing approximately 8·3% of the population have diabetes 3. It is estimated that by 2025, 300 million people worldwide will have diabetes 4. Patients with diabetes are at risk for the development of foot ulcers due to neuropathy, which reduces their sensation of pressure or trauma that leads to a break in the skin. Diabetic ulcers are often slow to resolve, especially in those patients with significant vascular disease 5. Indeed, a weighted healing rate of only 24·2% after 12 weeks of treatment was reported in one large meta‐analysis 6. Approximately one quarter of diabetic patients will develop a foot ulcer over their lifetime 7, 8. Chronic wounds are often defined as those that have not achieved a 50% reduction in wound size after 4 weeks of standard wound care 9. Treatment guidelines frequently use this metric as an indicator for the addition of advanced therapies such as bioengineered skin substitutes, topical growth factors and stromal matrices, among others.
In the USA, lower‐extremity ulcers significantly increase patient resource use and costs, especially among diabetic Medicare beneficiaries. Because diabetic ulcers heal slowly, they are often complicated by infection. The longer the ulcer stays open, the higher incidence of more serious complications such as cellulitis or osteomyelitis with subsequent physician visits, hospitalisation and/or amputation 2. Annually, Medicare patients with a diabetic foot ulcer average 14 visits to their outpatient health care provider and are hospitalised about 1·5 times per year. The cost of care for a patient with a diabetic ulcer is substantial, at about $33 000 for total reimbursement of all Medicare services per year 10. Given the clinical risks and high costs associated with treating lower‐extremity ulcers, the development of treatment strategies to improve healing rates and reduce time to healing is warranted 11, 12.
The results of clinical trials show that human skin equivalents, such as Dermagraft® (human fibroblast‐derived dermal substitute; Shire; Dublin, Ireland) and Apligraf® (living bilayered, cell‐based product; Organogenesis; Canton, MA) promote wound closure, resulting in more frequent and rapid healing of chronic diabetic foot ulcers, when compared with standard therapy 1. Recently, an allograft consisting of dehydrated human amnion/chorion membrane (dHACM) has become commercially available (EpiFix®, MiMedx Group Inc.; Marietta, GA) 13. To ensure safety, the placental tissue is received from screened and tested donors and although processing is uniform, natural variability of the tissue can result in slight colour differences among dHACM allografts. The dHACM material has a stable shelf life of 5 years at ambient temperature. It is available in multiple sizes, which allows the clinician to use a wound size‐appropriate graft and therefore minimise waste. The dHACM has been shown to contain many growth factors that help in wound healing, including platelet‐derived growth factors (PDGF‐AA and PDGF‐BB), basic fibroblast growth factor (bFGF), transforming growth factor beta 1 (TGF‐β1), epidermal growth factor (EGF), vascular endothelial growth factor (VEGF) and placental growth factor (PlGF) 14. In addition to growth factors, cytokines including anti‐inflammatory interleukins (IL‐1Ra, IL‐4 and IL‐10) and the TIMPs (TIMP‐1, TIMP‐2 and TIMP‐4), which help regulate the matrix metalloproteinase (MMP) activity are also present in dHACM 14. Results from both in vitro and in vivo experiments clearly established that dHACM contains one or more soluble factors capable of stimulating mesenchymal stem cell migration and recruitment 14. Prior studies have shown that biweekly application of dHACM promotes rapid and sustained healing of DFU 15, 16, 17. Several case studies and clinical reports on the use of dHACM in various types of wounds in addition to DFU are also found in the literature 18, 19, 20.
The purpose of this study is to examine if applying dHACM to chronic diabetic ulcers weekly versus biweekly can reduce time to healing and to validate results of earlier investigations regarding the efficacy of dHACM.
Materials and methods
A prospective, randomised, comparative, parallel group, non‐blinded clinical trial comparing time to healing with weekly versus biweekly application of dHACM allograft (EpiFix®, MiMedx Group Inc.; Marietta, GA) in addition to a standard protocol of wound care in diabetic patients with a foot ulcer was conducted. The single‐centre trial was performed between September 2012 and October 2013 in Southwest Virginia under the direction of a senior clinician (CMZ) with expertise in diabetic foot care with continuous enrollment of all eligible patients who wished to participate. The study was reviewed and approved by Western IRB (WIRB) and preregistered in ClinicalTrials.gov (NCT01657474). Confidentiality was maintained with all study records and patient information was kept in a locked and secure room with access only to the research coordinator and principle investigator.
Patient screening and eligibility
The study population comprised of patients with a history of type 1 or type 2 diabetes presenting for care of a diabetic ulcer located anywhere on the foot. Patients read and signed an IRB‐approved informed consent form prior to any study involvement. Eligibility requirements are listed in Table 1. Screening evaluations consisted of a medical history and physical examination, an infection assessment, wound‐site measurement, serum creatinine, glycosylated haemoglobin (HbA1c) and a vascular assessment including circulation to the affected extremity [dorsum transcutaneous oxygen test (TcPO2), ABI's or Doppler arterial waveforms] within the last 60 days. After meeting initial eligibility criteria, patients were placed in a 2‐week run‐in period. During the run‐in period, they were instructed to change the collagen–alginate wound dressing (Fibracol®, Systagenix; Gargave, UK) daily followed with a three‐layer compressive dressing including 4 × 4 roll gauze, cast padding and elastic cover from the foot to pretibial area. Patients were given explicit instructions from research staff on how to perform dressing changes. During the 2‐week run‐in period, they were also instructed to use, and provided with, an offloading diabetic cast walker (Active Offloading Walker; Darco of Huntington; Huntington, WV). At the end of the screening period if the wound failed to heal by 20% they were then enrolled into the study. Patients meeting eligibility and screening criteria were randomised to receive the dHACM allograft material on a weekly or biweekly basis in addition to the standard regimen of wound care in a 1:1 ratio. The randomisation schedule was balanced and permuted in blocks of 10.
Table 1.
Major inclusion and exclusion criteria
| Inclusion criteria | Exclusion criteria |
|---|---|
|
|
Study procedures
DHACM was applied weekly or biweekly following surgical debridement of all necrotic tissue according to group assignment. A non‐adherent dressing (Adaptic Touch®, Systagenix, Gargrave, UK) was used to cover the dHACM, followed by a moisture‐retentive dressing (Nugel®, Systagenix, Gargrave, UK) and a compressive padded dressing (Dynaflex®, Systagenix, Gargrave, UK). All wounds were offloaded using a removable cast walker (Active Offloading Walker; Darco of Huntington; Huntington, WV). Follow‐up visits were conducted weekly for dressing change and wound assessment. During each weekly visit, ulcer cleansing with a sterile normal saline solution (rinsing, swabbing or irrigating), ulcer measurement with a graded centimetre ruler (length, width and depth) and a dressing change was conducted. When applicable, measurements and photographic evaluation were carried out after debridement. The wound area was calculated by multiplying the width and length measurements. Debridement and additional wound size‐appropriate dHACM allograft was applied weekly or biweekly according to group assignment if complete epithelialisation had not occurred. The wound dressing techniques described were consistently used for all patients and across all time points. For both treatment groups, the size of each allograft applied was documented.
Study completion, outcomes and data analysis
All randomised patients were seen by the investigator at day 0 (day of first dHACM application) and at least once every 7 days (± 3 days) for up to 12 weeks or 1 week after complete healing, whichever occurred first. Additionally, patients were exited from the study and allowed to seek alternative treatment if the index ulcer did not achieve 50% area reduction after 6 weeks of treatment with dHACM.
The purpose of this investigation was to compare time to complete wound closure and rates of healing with weekly versus biweekly application of dHACM. Primary study outcome was mean time to healing. Secondary outcomes examined were percentage of diabetic ulcers completely healed by 4, 6 and 12 weeks in each group and number of dHACM allografts used. For the purposes of this study, healing was defined as complete reepithelialisation of the wound without drainage or need for dressing. Prior studies 15, 16 evaluated wound healing outcomes of biweekly dHACM application versus standard of care, thus in this study the group receiving biweekly application are considered to be controls.
Parametric and non‐parametric statistics were used as appropriate to compare clinical characteristics between those receiving weekly applications of dHACM to biweekly controls. Percent of ulcers healed at each time point was evaluated with a Fisher's exact test. Wound surface area reduction was evaluated using a Mann–Whitney U‐test. GraphPad InStat v3 was used to perform statistical testing. For the primary outcome of time to healing, a two‐tailed P‐value < 0·05 was considered as significant. For secondary outcomes, adjusted P‐values of < 0·017 were considered significant as the risk of making erroneous false‐positive conclusions is increased when testing multiple hypotheses on a single set of data and the Bonferonni correction was applied.
Results
Patient characteristics
The study comprised individuals who were representative of the types of patients typically seen by clinicians in the community setting (real world). Eligible for inclusion were patients with a history of type 1 or type 2 diabetes receiving treatment for a chronic diabetic foot ulcer that failed to heal for at least 4 weeks. All eligible patients were offered enrollment as long as they met the IRB‐approved study inclusion and exclusion criteria described above. A total of 42 patients entered the screening phase of the study and 40 subjects were ultimately enrolled and randomly assigned to receive dHACM application biweekly (biweekly group, n = 20) or weekly (weekly group, n = 20). Patient characteristics of the biweekly and weekly groups are described in Table 2. No significant differences were observed in gender, age, race, body mass index and tobacco or alcohol use. Mean HbA1c was higher for those patients enrolled in the weekly group 8·7 ± 2·2 versus 7·3 ± 1·5 in the biweekly group, P = 0·036. Groups were similar in regards to wound location, size and duration.
Table 2.
Clinical characteristics at study enrollment
| Biweekly (n = 20) | Weekly (n = 20) | P value | |
|---|---|---|---|
| Male gender (#/%) | 10 (50) | 9 (45) | 1·000 |
| Age (y) | 59·6 ± 13·8 | 60·8 ± 10·9 | 0·758 |
| Caucasian race (#/%) | 19 (95) | 16 (80) | 0·342 |
| African American race (#/%) | 1 (5) | 4 (20) | |
| Tobacco use (#/%) | 1(5) | 1(5) | 1·000 |
| Alcohol use | 3 (15) | 0 | 0·231 |
| Type 1 diabetes (#/%) | 3 (15) | 1 (5) | 0·605 |
| Body mass index | 33·0 ± 5·8 | 36·8 ± 6·7 | 0·065 |
| Glycosylated haemoglobin (HbA1c) | 7·3 ± 1·5 | 8·7 ± 2·2 | 0·036 |
| Ulcer location: | |||
| Forefoot (#/%) | 8 (40) | 10 (50) | 0·751 |
| Hindfoot (#/%) | 3 (15) | 1 (5) | 0·605 |
| Midfoot (#/%) | 2 (10) | 4 (20) | 0·661 |
| Toe (#/%) | 7(35) | 5 (25) | 0·731 |
| Ulcer duration (weeks) |
16·9 ± 21·7 |
17·5 ± 14·5 |
0·480 |
|
9 (4, 99) |
11 (4, 50) |
||
| Baseline wound area (cm2) |
2·4 ± 1·8 |
2·0 ± 1·3 |
0·303 |
|
1·6 (1·1, 8·7) |
1·4 (1·1, 6·4) |
Data presented as mean ± SD, median (minimum, maximum) or #/% as indicated.
Study outcomes
Overall, for the 40 patients treated with dHACM either on a weekly or biweekly basis, complete wound healing was achieved in 37 cases (92·5%) within the 12‐week study period. Mean time to healing (n = 37) was 3·2 ± 2·5 weeks [median 2, range (1, 11)]. At week 1, after one dHACM application, complete healing occurred in 22·5% (9/40) of wounds. By week 2, 42·5% (17/40) were healed. Complete healing had occurred in 67·5% (27/40) by week 3 and 70% (28/40) of wounds were completely healed by week 4. At the 1‐week follow‐up visit, after all patients had received just one dHACM application wounds were found to have reduced in size by a mean of 76·4 ± 21·1%. All but one patient (39/40, 97·5%) had >50% reduction in wound size in the first 28 days of treatment.
Healing characteristics and study outcomes by treatment group are presented in Table 3. Although overall healing rates were similar between the groups, time to healing was shorter for those receiving weekly application of dHACM versus biweekly application (2·4 ± 1·8 weeks versus 4·1 ± 2·9 weeks, respectively), P = 0·039. Illustrating the effect of weekly application, no differences were observed in median wound size between the groups at week 0 (first dHACM application; P = 0·303) and at week 1 follow‐up (P = 0·128), but wound size was significantly smaller by week 2 (P = 0·0007) for those patients receiving a second dHACM application at week 1 (see Table 3). Further evidence for the effectiveness of weekly application is illustrated by the fact that while a similar number of grafts were used on each healed wound (biweekly group = 2·4 ± 1·5 versus 2·3 ± 1·8 – weekly group, P = 0·841), those wounds receiving weekly dHACM healed 41·5% faster than those treated with dHACM biweekly. Rates of healing are compared by week in Figure 1. A significantly greater number of patients receiving weekly dHACM were healed at weeks 2, 3 and 4 compared with those receiving biweekly dHACM application. Examples of healing response with dHACM are presented in Figures 2 and 3.
Table 3.
Healing characteristics and study outcomes
| Biweekly (n = 20) | Weekly (n = 20) | P value | |
|---|---|---|---|
| Completely healed in study period (#/%) | 17 (85) | 20 (100) | 0·231 |
| Completely healed by week 2 (#/%) | 4 (20) | 13 (65) | 0·009 |
| Completely healed by week 4 (#/%) | 10 (50) | 18 (90) | 0·014 |
| Completely healed by week 6 (#/%) | 14 (70) | 19 (95) | 0·091 |
| Completely healed by week 8 (#/%) | 15 (75) | 20 (100) | 0·047 |
| Weeks to primary wound closure |
n = 17 |
n = 20 |
0·039 |
|
4·1 ± 2·9 3 (1, 11) |
2·4 ± 1·8 2 (1, 8) |
||
| Grafts applied to healed wounds |
n = 17 |
n = 20 |
0·841 |
|
2·4 ± 1·5 2 (1, 6) |
2·3 ± 1·8 2 (1, 8) |
||
| Wound size week 0 (cm2) |
2·4 ± 1·8 |
2·0 ± 1·3 |
0·303 |
|
1·6 (1·1, 8·7) |
1·4 (1·1, 6·4) |
||
| Wound size week 1 (cm2) |
0·93 ± 1·4 |
0·37 ± 0·39 |
0·128 |
|
0·36 (0, 6) |
0·25 (0, 1·35) |
||
| Wound size week 2 (cm2) |
0·91 ± 1·4* |
0·09 ± 0·18** |
0·0007 |
|
0·45 (0, 6) |
0 (0, 0·75) |
Data presented as mean ± SD, median (minimum, maximum) or #/% as indicated. * = after one dehydrated human amnion/chorion membrane (dHACM). ** = after two dHACM.
Figure 1.
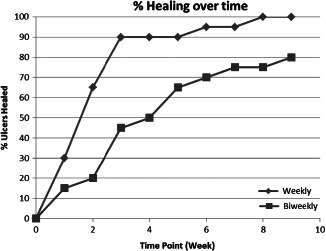
Rates of healing over time for each study group.
Figure 2.
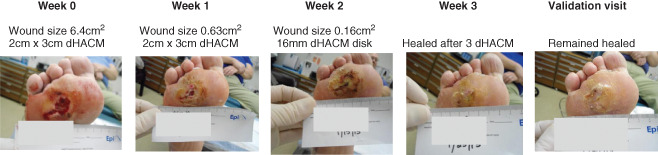
Male, 54 years of age with 6·4 cm2 plantar ulcer of 48 weeks duration. Randomised to receive weekly dehydrated human amnion/chorion membrane (dHACM) application. Complete healing occurred after three applications of dHACM. Wound reduced in size by 90·1% after first dHACM application.
Figure 3.
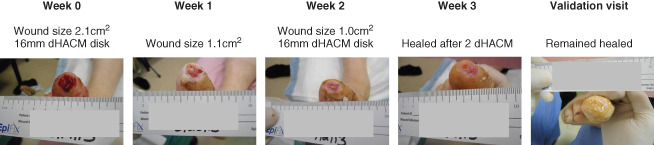
Obese female, 51 years of age with 2·1 cm2 hallux ulcer of 5 weeks duration. Randomised to receive biweekly application of dehydrated human amnion/chorion membrane (dHACM). Completely healed after two biweekly applications. Wound reduced in size by 47·6% after first dHACM application.
Study completion
Three patients, all from the biweekly group, withdrew from the study unhealed at 6, 9 and 12 weeks, respectively. One patient was withdrawn at 6 weeks because of an increase in size of ulcer during the study period. Another withdrew consent at 9 weeks after receiving five biweekly applications of dHACM with their ulcer reducing in size from 8·75 cm2 to 0·32 cm2. The third unhealed patient completed the study through 12 weeks and six applications with a reduction of wound size from 4·0 cm2 to 1·8 cm2.
Adverse events
During the study period, four patients in the biweekly group and two patients in the weekly group experienced a total of eight adverse events and three hospitalisations, although none of these were attributed to the dHACM allograft. Wound related events included three reports of blisters from the offloading boot and one wound infection which was treated with antibiotics and sharp debridement during hospitalisation. One patient experienced two hospitalisations: one for urinary tract infection and one related to Anasarca. One other urinary tract infection was reported. One patient developed anaemia and septicaemia. During hospitalisation, all study procedures and evaluations continued to be conducted by the primary study investigator (CMZ). No patients were withdrawn from the study because of adverse events.
Discussion
Previous studies have established that biweekly application of dHACM is an effective treatment for chronic diabetic foot ulcer 15, 16. This is the first randomised trial to evaluate if weekly versus biweekly dHACM application can reduce time to healing in patients with a chronic DFU. Our results show that diabetic ulcers treated with weekly application of dHACM healed in a significantly more rapid fashion than those treated with biweekly dHACM. The number of dHACM allografts applied to achieve complete healing were similar between the weekly and biweekly groups (median 2 weeks per wound), whereas time to wound closure was significantly longer in the biweekly group (median 3 versus 2 weeks in the weekly group), suggesting that more frequent application can reduce time to wound closure.
Our results are not unprecedented. In a study examining wound healing with Dermagraft, Gentzkow, et al. found a relationship in that weekly frequency of Dermagraft application led to an almost 2·5× greater healing rate when compared with the cohort that received biweekly application 21.
With an overall healing rate of 92·5% for 40 patients treated with weekly or biweekly dHACM, this study also validates the results of earlier studies regarding the efficacy of dHACM. In a randomised clinical trial of chronic DFUs treated with biweekly application of dHACM versus a standard regimen of wound care, primary healing occurred in 92% of ulcers (12/13) treated with dHACM and only 8% of ulcers (1/12) receiving a standard protocol of wound care in the 12‐week study period 15. Those patients that failed to heal in the randomised trial (n = 11) were subsequently treated with dHACM, resulting in a primary healing rate of 91% for diabetic ulcers (10/11) in that study 16. Overall, from these two studies, 91·7% (22/24) of the DFUs treated with biweekly dHACM healed within 12 weeks, similar to the 92·5% rate (37/40) in this study.
A common endpoint in evaluating wound care treatment is velocity of wound healing. A 50% reduction in wound size at 4 weeks is a critical cut‐off point for evaluating diabetic foot ulcer treatment success 9. In this study, 39 of 40 patients (97·5%) had achieved >50% reduction in wound size by 4 weeks. Even more impressive is that by this critical 4‐week cut‐off, 70% of patients receiving dHACM either weekly or biweekly were completely healed. In the previous studies using only biweekly application of dHACM, rates of complete healing by 4 weeks were 77% and 54·5%, whereas in the present study 90% of the patients receiving weekly application of dHACM were healed by 4 weeks 15, 16. These healing rates by 4 weeks after dHACM application are much superior to healing rates reported after 12 weeks with Dermagraft (30% healing rate) or Apligraf (56% healing rate) , and after 20 weeks with becaplermin (50% healing rate) 22, 23, 24.
Advanced wound therapies such as dHACM are provided at a considerably higher cost compared with standard wound care measures, yet the increased cost can be justified. Improved rates of healing reduce morbidity and risk for lower‐extremity amputation with its associated costs 2. A treatment that invokes rapid healing reduces overall medical costs associated with frequent clinic visits and treatments. Use of advanced therapies should be considered if wound size has not reduced by 50% after 4 weeks of standard wound care. Several advanced therapies have been shown to accelerate the healing process but are not a universal remedy; no perfect treatment exists for all patients in all situations 25. Healing rates, time to healing, cost of graft material and ease of use should all be considered when selecting an advanced therapy. DHACM provides several unique advantages over other products in that the allograft is available in many sizes, ranging from 14 mm disks to 9 × 20cm2 sheets, allowing the clinician to choose the graft size most appropriate to the wound, thus reducing cost and eliminating wastage. Other products such as Apligraf and Dermagraft are available in only one size, 44 cm2 and 37·5 cm2, respectively. DHACM is also operationally efficient. It can be transported and stored at ambient temperature for up to 5 years.
In this study, the overall comparison of weekly versus biweekly application of dHACM further illustrates the value of the material as an advanced wound therapy for the treatment of diabetic neurotrophic ulcers. While rates of healing and number of grafts per healed wound were similar between the groups, those patients having the graft applied weekly had their wounds heal more rapidly, despite higher mean HbA1c levels at time of randomisation, which have been shown to reduce healing rates and increase time to healing 26, 27. Reducing the time required for wound healing reduces health care usage – the potential for reduced cumulative as well as episode costs can be assumed.
Limitations of this study are those inherent to small sample size. The lack of a standard care group not receiving dHACM can be perceived as a study weakness, although our intent was solely to examine rates of healing according to frequency of application and not compare with other treatment modalities. Our findings should be confirmed and expanded with subsequent multicentre clinical trials and long‐term follow‐up data to validate the durability of healed wounds. As we did not include other advanced therapies in our study, we do not know if the product is as good as, or better, than other available advanced wound care products. Additional comparative effectiveness studies are required to address those questions.
In conclusion, weekly application of dHACM has demonstrated superior clinical effectiveness when compared with biweekly application in the treatment of chronic neurotropic ulcers of the lower extremity in diabetic patients. These results support those of earlier studies with overall healing rates of over 92%. The low volume of wastage and ease of use is further evidence towards cost effectiveness. Therefore, dHACM is a desirable treatment option from both a clinical and economic perspective that should be considered by clinicians that treat diabetic foot ulcers.
Zelen CM, Serena TE, Snyder RJ. A prospective, randomised comparative study of weekly versus biweekly application of dehydrated human amnion/chorion membrane allograft in the management of diabetic foot ulcers.
References
- 1. Ho C, Tran K, Hux M, Sibbald G, Campbell K. Artificial skin grafts in chronic wound care: a meta‐analysis of clinical efficacy and a review of cost‐effectiveness [Tech. Rep. 52]. Ottawa: Canadian Coordinating Office for Health Technology Assessment; 2005.
- 2. Lavery LA, Armstrong DG, Wunderlich RP, Mohler MJ, Wendel CS, Lipsky BA. Risk factors for foot infections in individuals with diabetes. Diabetes Care 2006;29:1288–93. [DOI] [PubMed] [Google Scholar]
- 3. CDC 2011. National Estimates – 2011 National Diabetes Fact Sheet – Publications – Diabetes DDT. URL http://www.cdc.gov/diabetes/pubs/estimates11.htm [accessed on 16 December 2013].
- 4. Boulton AJ, Vileikyte L, Ragnarson‐Tennvall G, Apelqvist J. The global burden of diabetic foot disease. Lancet 2005;366:1719–24. [DOI] [PubMed] [Google Scholar]
- 5. Snyder RJ, Cardinal M, Dauphinée DM, Stavosky J. A post‐hoc analysis of reduction in diabetic foot ulcer size at 4 weeks as a predictor of healing by 12 weeks. Ostomy Wound Manage 2010;56:44–50. [PubMed] [Google Scholar]
- 6. Margolis D, Kantor J, Berlin J. Healing of neuropathic ulcers: results of a meta‐analysis. Diabetes Care 1999;22:692–5. [DOI] [PubMed] [Google Scholar]
- 7. Boulton AJ, Armstrong DG, Albert SF, Frykberg RG, Hellman R, Kirkman MS, Lavery LA, Lemaster JW, Mills JL Sr, Mueller MJ, Sheehan P, Wukich DK, American Diabetes Association, American Association of Clinical Endocrinologists. Comprehensive foot examination and risk assessment: a report of the task force of the foot care interest group of the ADA, with endorsement by the AACE. Diabetes Care 2008;8:1679–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Singh N, Armstrong DG, Lipsky BA. Preventing foot ulcers in patients with diabetes. JAMA 2005;293:217–28. [DOI] [PubMed] [Google Scholar]
- 9. Warriner RA, Snyder RJ, Cardinal MH. Differentiating diabetic foot ulcers that are unlikely to heal by 12 weeks following achieving 50% percent area reduction at 4 weeks. Int Wound J 2011;8:632–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Margolis DJ, Malay DS, Hoffstad OJ, Leonard CE, MaCurdy T, Tan Y, Molina T, López de NK, Siegel K. Economic burden of diabetic foot ulcers and amputations: Data Points #3. In: 2011 Data Points Publication Series [Internet]. Rockville, MD: Agency for Healthcare Research and Quality (US), 2011. URL http://www.ncbi.nlm.nih.gov/books/NBK65152/. [PubMed] [Google Scholar]
- 11. Holzer SE, Camerota A, Martens L, et al. Costs and duration of care for lower extremity ulcers in patients with diabetes. Clin Ther 1998;20:169–81. [DOI] [PubMed] [Google Scholar]
- 12. Harrington C, Zagari MJ, Corea J, Klitenic J. A cost analysis of diabetic lower‐extremity ulcers. Diabetes Care 2000 Sep;23:1333–8. [DOI] [PubMed] [Google Scholar]
- 13. Fetterolf DE, Snyder RJ. Scientific and clinical support for the use of dehydrated amniotic membrane in wound management. Wounds 2012;24:299–307. [PubMed] [Google Scholar]
- 14. Koob TJ, Rennert R, Zabek N, Massee M, Lim JJ, Temenoff JS, Li WW, Gurtner G. Biological properties of dehydrated human amnion/chorion composite graft: implications for chronic wound healing. Int Wound J 2013 Oct;10:493–500. DOI: 10.1111/iwj.12140. Epub 2013 Aug 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Zelen CM, Serena TE, Denoziere G, Fetterolf DE. A prospective randomized comparative parallel study of amniotic membrane wound graft in the management of diabetic foot ulcers. Int Wound J 2013 Oct;10:502–7. DOI: 10.1111/iwj.12097 Epub 2013 Jun 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Zelen CM. An evaluation of dehydrated human amniotic membrane allografts in patients with DFUs. J Wound Care 2013;22 347–8, 350–1. [DOI] [PubMed] [Google Scholar]
- 17. Zelen CM, Serena TE, Fetterolf DE. Dehydrated human amnion/chorion membrane allografts in patients with chronic diabetic foot ulcers: a long‐term follow‐up study. Wound Medicine URL DOI: 10.1016/j.wndm.2013.10.008 [accessed on 19 November 2013] 2013. [Google Scholar]
- 18. Forbes J, Fetterolf DE. Dehydrated amniotic membrane allografts for the treatment of chronic wounds: a case series. J Wound Care 2012;21 290, 292, 294–6. [DOI] [PubMed] [Google Scholar]
- 19. Sheikh ES, Sheikh ES, Fetterolf DE. Use of dehydrated human amniotic membrane allografts to promote healing in patients with refractory non healing wounds. Int Wound J 2013. DOI: 10.1111/iwj.12035[Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Shah A. Using amniotic membrane allografts in the treatment of neuropathic foot ulcers. J Am Podiatr Med Assoc 2014. In press. [DOI] [PubMed] [Google Scholar]
- 21. Gentzkow GD, Iwasaki SD, Hershon KS, Mengel M, Prendergast JJ, Ricotta JJ, Steed DP, Lipkin S. Use of Dermagraft, a cultured human dermis, to treat diabetic foot ulcers. Diabetes Care 1996;19:350–4. [DOI] [PubMed] [Google Scholar]
- 22. Marston WA, Hanft J, Norwood P, Pollak R,; Dermagraft Diabetic Foot Ulcer Study Group . The efficacy and safety of Dermagraft in improving the healing of chronic diabetic foot ulcers: results of a prospective randomized trial. Diabetes Care 2003;26:1701–5. [DOI] [PubMed] [Google Scholar]
- 23. Veves A, Falanga V, Armstrong DG, Sabolinski ML,; Apligraf Diabetic Foot Ulcer Study . Graftskin, a human skin equivalent, is effective in the management of noninfected neuropathic diabetic foot ulcers: a prospective randomized multicenter clinical trial. Diabetes Care 2001;24:290–5. [DOI] [PubMed] [Google Scholar]
- 24. Smiell JM, Wieman TJ, Steed DL, Perry BH, Sampson AR, Schwab BH. Efficacy and safety of becaplermin (recombinant human platelet‐derived growth factor‐BB) in patients with nonhealing, lower extremity diabetic ulcers: a combined analysis of four randomized studies. Wound Repair Regen 1999;7:335–46. [DOI] [PubMed] [Google Scholar]
- 25. Shores JT, Gabriel A, Gupta S. Skin substitutes and alternatives: a review. Adv Skin Wound Care 2007 Sep;20(9 Pt 1):493–508; quiz 509–10. [DOI] [PubMed] [Google Scholar]
- 26. Markuson M, Hanson D, Anderson J, Langemo D, Hunter S, Thompson P, Paulson R, Rustvang D. The relationship between hemoglobin A1c values and healing time for lower extremity ulcers in individuals with diabetes. Adv Skin Wound Care 2009;22:365–72. DOI: 10.1097/01.ASW.0000358639.45784.cd. [DOI] [PubMed] [Google Scholar]
- 27. Marston WA, Dermagraft Diabetic Foot Ulcer Study Group . Risk factors associated with healing chronic diabetic foot ulcers: the importance of hyperglycemia. Ostomy Wound Manage 2006;52 26–8, 30, 32 passim. [PubMed] [Google Scholar]


