Abstract
Background
Elevated insulin, C-reactive protein (CRP), tumor necrosis factor (TNF)-α, interleukin (IL)-6, and monocyte chemoattractant protein (MCP)-1 levels and decreased high molecular weight adiponectin (HMW-APN) levels have been reported in Caucasians with gestational diabetes mellitus (GDM). No similar studies have been performed in Chinese women.
Methods
Serum samples were obtained 1 h after a 50-g glucose challenge test (1HGCT) from Chinese–American women at 24–28 gestational weeks and total adiponectin (T-APN), HMW-APN, CRP, TNF-α, IL-6, and MCP-1 concentrations were measured. Correlation coefficients for glucose (1HGCT), HbA1c, insulin, and body mass index (BMI) were calculated against T-APN, HMW-APN, CRP, TNF-α, IL-6, and MCP-1. Significant P-values were determined using Bonferroni adjustments.
Results
Women with GDM had higher insulin and 1HGCT and lower T-APN. In addition, T-APN was lower in non-GDM subjects who had 1HGCT ≥135 mg/dL with no abnormal or one abnormal glucose value on the 3-h oral glucose tolerance test. There were no significant differences in HMW-APN and inflammatory marker levels between non-GDM and GDM groups. There were negative correlations between T-APN and 1HGCT, insulin, BMI, and HbA1c, as well as between HMW-APN and 1HGCT, insulin, and BMI. No significant correlations were observed between 1HGCT, HbA1c, insulin, or BMI and CRP, TNF-α, IL-6, or MCP-1.
Conclusions
T-APN is reduced in Chinese women with GDM and those without GDM but with evidence of glucose intolerance. Unlike results reported for Caucasians, Chinese–American women with GDM do not exhibit elevated levels of CRP, TNF-α, IL-6, or MCP-1, possibly because Chinese women are relatively leaner compared with Caucasians.
Keywords: Chinese pregnant women, gestational diabetes, glucose intolerance, HMW-adiponectin, inflammatory markers, T-adiponectin
Significant findings of the study: Lower levels of total adiponectin (T-APN), which are inversely correlated with an increase in glucose intolerance, may allow for early detection of women at risk of gestational diabetes. Inflammatory markers may not be useful in the assessment of the risk of gestational diabetes in Chinese women.
What this study adds: Lower levels of T-APN, but not markers of inflammation, may be important in the pathogenesis of gestational diabetes in the Chinese population.
Introduction
Gestational diabetes mellitus (GDM) is defined as glucose intolerance first recognized during pregnancy,1 and is associated with both short- and long-term risks, including macrosomia, birth trauma, metabolic complications in the newborn, and subsequent development of diabetes mellitus (T2DM) in the mother.
The prevalence of GDM is significantly higher in Chinese (6.4%) compared with Caucasian (3.8%) women.2 The cause for this difference is unclear. In the present study, we explored adipocytokine and inflammatory markers in pregnant Chinese–American women.
Adiponectin is an adipokine that circulates in the plasma in several forms, including high molecular weight (HMW), medium molecular weight (MMW), and low molecular weight (LMW) forms. The HMW form of adiponectin (HMW-APN) is the active form and is believed to be important in the pathogenesis of GDM in Caucasians.3 Adiponectin has insulin-sensitizing and anti-inflammatory properties. Reduced HMW-APN concentrations have been reported in Caucasian women with GDM.3,4 Retnakaran et al. reported lower HMW-APN levels in pregnant South Asian compared with Caucasian women after adjusting for age, prepregnancy body mass index (BMI), and weight gain during pregnancy.5 Another study suggested that plasma adiponectin levels (the specific assay was not described) were reduced in Asian women with GDM.6 However, an association between decreased plasma total adiponectin (T-APN) levels and GDM has not been clearly established. To our knowledge, there have been no published studies that examined T-APN in Chinese women with GDM.
C-Reactive protein (CRP) is an inflammatory marker that is synthesized by the liver. Concentrations of CRP increase in response to inflammation, activating the complement system.7,8 Interleukin (IL)-6 is an inflammatory cytokine secreted by T cells and macrophages to stimulate the immune response,9 and IL-6 levels are elevated in subjects with insulin resistance and GDM.10 Tumor necrosis factor (TNF)-α is another inflammatory protein produced by macrophages,11 and TNF-α levels have been reported to be elevated in subjects with GDM.12 Monocyte chemoattractant protein (MCP)-1 is a chemokine produced by monocytes and macrophages,13 and it has been reported that MCP-1 levels are elevated in GDM.14 Thus, increased levels of the inflammatory markers CRP, TNF-α, IL-6, and MCP-1 have been associated with GDM in Caucasian women. No similar studies have been performed in Chinese women.
In the present study, we attempted to identify specific biochemical markers associated with GDM in Chinese–American women at 24–28 weeks of pregnancy by exploring the relationships between markers of glucose tolerance (glucose, insulin, HbA1c, and BMI) and T-APN or HMW-APN, CRP, TNF-α, IL-6, and MCP-1.
Methods
The study was approved by Beth Israel Medical Center's Institutional Review Board. Chinese–American women were recruited from obstetrics and gynecology offices located in New York City's Chinatown. The study population was recruited from May 2010 to August 2012. Consent was obtained from all subjects. Consent forms translated into Chinese were given to subjects.
To be eligible for inclusion in the study, the women had to be first-generation immigrants of Chinese ethnicity, between 18 and 40 years of age, and at 24–28 weeks gestation.
Height (in meters) and weight (in kilograms) were obtained at 24–28 weeks gestation when patients came for the two-step screening for GDM. These measurements were obtained by the subjects' examining obstetricians (SW or DT). Electronic records or clinical charts were reviewed by TA, VS, DH, and DS-Y. Body weight divided by height squared was used to calculate BMI. Prepregnancy BMI was not available because the patients came to the clinic for the first time after at least 8–10 weeks of pregnancy.
Women with an established diagnosis of T2DM or GDM, hepatitis B, other infectious diseases (Herpes virus, Streptococcus B carriers, Chlamydia, and Candida), thyroid dysfunction, and a thalassemia carrier state, as well as those with a history of miscarriages or infertility with the use of in vitro fertilization, were excluded from the study.
Blood samples were obtained from subjects 1 h after a 50-g oral glucose load (1-h glucose challenge test; 1HGCT). Serum samples were sent to Quest Diagnostic (New York, NY, USA) for glucose and HbA1c analysis. A separate blood sample was collected and centrifuged at 960 g for 10 min at 4°C. The serum collected was stored at –80°C until analysis (ELISA).
Concentrations of HMW-APN were measured using an American Laboratory Products Company (ALPCO; Salem, NH, USA) ELISA kit. Serum samples were pretreated with protease II, which digests LMW- and MMW-APN. The remaining HMW fractions were treated with a buffer that reduced the HMW multimers into dimers. Therefore, this assay measures the concentration of reduced HMW-APN. The intra- and interassay coefficients of variation (CV) for the HMW-APN assay were 3.3%–5% and 5.7%, respectively.
Concentrations of T-APN were also measured with an ALPCO ELISA kit. Samples were pretreated with citrate buffer and sodium dodecyl sulfate (SDS), which convert multimeric forms, including HMW-, MMW-, and LMW-APN, into dimers. The total adiponectin value obtained by this assay includes all dimeric forms of adiponectin in the sample (in this assay procedure, the monomer is not detected). The intra- and interassay CV for the T-APN assay were 5.3%–5.4% and 5%, respectively.
Other biochemical markers (insulin, CRP, IL-6, TNF-α, MCP-1) were also measured using ELISA kits obtained from ALPCO. The intra- and interassay CV for these assays were as follows: for insulin, 3.2%–10.3% and 6.7%–16.6%, respectively; for CRP, 5.5%–6% and 11.6%–13.8%, respectively; for IL-6, 5.1%–7.7% and 6.5%–9.3%, respectively; for TNF-α, 3.9%–5.2% and 5.9%–8.5%, respectively; and for MCP-1, 4%–7.8% and 6.7%–9.7%, respectively.
Identifying GDM
Patients were instructed to fast for at least 8 h before testing. A two-step test at 24–28 weeks gestation was used to diagnose GDM. Blood samples were obtained 1 h after a 50-g glucose challenge test (1HGCT). A serum glucose concentration of 135 mg/dL was used as a threshold for the 3-h oral glucose tolerance test (3HOGTT).15 Subjects who failed the 1HGCT (glucose ≥135 mg/dL) underwent a 3HOGTT, with glucose levels measured 0, 1, 2, and 3 h after a 100-g glucose load. Finger-stick glucose (FSG) concentrations in capillary blood were measured using a HemoCue (Kuvettgatan, Sweden) HB201-plus glucometer (FSG was used because study subjects refused to have blood drawn four times due to cultural beliefs about phlebotomy being harmful during pregnancy). Two or more glucose values at or above the threshold (i.e. >95 mg/dL at 0 h, >180 mg/dL at 1 h, >155 mg/dL at 2 h, and >140 mg/dL at 3 h) established the diagnosis of GDM.
Non-GDM subjects
Women without GDM were divided into the following three categories.
1HGCT <135 mg/dL glucose. This group consisted of non-GDM subjects who passed the initial glucose challenge test with the 1HGCT glucose value <135 mg/dL.
1HGCT ≥135 mg/dL glucose and no abnormal glucose value on the 3HOGTT. This group consisted of non-GDM subjects with no abnormal glucose values on the 3HOGTT, but 1HGCT glucose ≥135 mg/dL.
1HGCT ≥135 mg/dL and one abnormal glucose value on the 3HOGTT. This group consisted of non-GDM subjects who failed the 1HGCT and had one abnormal glucose value of the four reading on the 3HOGTT.
Statistical analysis
Student's t-test was used to compare mean serum concentrations of various biochemical markers between GDM and non-GDM subjects. Data were further analyzed using SPSS version 16.0 (SPSS Inc., Chicago, IL, USA) to calculate Pearson's correlation coefficients for glucose on 1HGCT, HbA1c, insulin, and BMI against T-APN, HMW-APN, CRP, TNF-α, IL-6, and MCP-1. Significant P-values (P ≤ 0.08), determined by Bonferroni corrections, were used for the multiple comparison of dependent and independent variables. In the present study, P ≤ 0.008 was considered significant at the 95% confidence level/α, where α is the number of variables tested (the two adipokines and four inflammatory variables): 0.05/6 = 0.008.
Data for non-GDM and GDM patients were matched for age, BMI, or age plus BMI. Data on subjects whose age and BMI did not match for these parameters were excluded in this subanalysis. Significant values were calculated as described above.
Data for non-GDM and GDM subjects were also adjusted for age and BMI. A set of regression models (Delta method) testing the difference between the two defined groups (GDM vs non-GDM) on each of the dependent variables controlling for BMI and age was included in the regression model. Subsequently, a contrast on the predicted marginal means was performed. Only T-APN showed a reliable group difference when controlling for BMI and age. None of the other adiponectin values or inflammatory parameters showed any such differences.
Unless indicated otherwise, data are presented as the mean ± SEM.
Results
Comparisons between non-GDM and GDM subjects
In all, 259 pregnant Chinese–American women were enrolled in the study. Their age, BMI, and blood pressure were similar (Table 1). Compared with non-GDM subjects (n = 227), women with GDM (n = 32) had higher levels of glucose on the 1HGCT (113.1 ± 1.5 vs 160 ± 3 mg/dL, respectively; P < 0.001) and insulin (54.6 ± 2.9 vs 120.7 ± 15.5 mU/dL, respectively; P < 0.001), and lower levels of T-APN (8.3 ± 0.4 vs 5.5 ± 0.7 μg/mL, respectively; P < 0.001; Table 1). Blood pressure measurements in non-GDM and GDM women were similar. The average blood pressure (systolic/diastolic) of non-GDM women was 108/66 mmHg, compared with 111/67 mmHg in women with GDM (Table 1).
Table 1.
Comparison of pregnant Chinese–American women with or without gestational diabetes
| Parameters | Non-GDM (n = 227) | GDM (n = 32) |
|---|---|---|
| Age (years) | 30.4 ± 0.3 | 32.1 ± 0.8 |
| BMI (kg/m2) | 24.1 ± 0.9 | 24.9 ± 0.6 |
| SBP/DBP (mmHg) | 108/62 ± 1/1 | 110/66 ± 2/2 |
| HbA1c (%) | 5.20 ± 0.02 | 5.35 ± 0.06 |
| 1HGCT (mg/dL) | 113.1 ± 1.5 | 160 ± 3** |
| Insulin (mU/mL) | 54.6 ± 2.9 | 120.7 ± 15.5** |
| T-APN (μg/mL) | 8.3 ± 0.4 | 5.5 ± 0.7** |
| HMW-APN (μg/mL) | 2.9 ± 0.1 | 2.4 ± 0.2 |
| CRP (μg/mL) | 4.1 ± 0.4 | 3.7 ± 0.6 |
| TNF-α (pg/mL) | 10.9 ± 0.3 | 10.6 ± 0.7 |
| IL-6 (pg/mL) | 2.6 ± 0.2 | 2.9 ± 0.9 |
| MCP-1 (pg/mL) | 62.5 ± 1.9 | 73.7 ± 10.7 |
Data are the mean ± SEM. **P < 0.001 compared with women without gestational diabetes.
GDM, gestational diabetes; BMI, body mass index; SBP/DBP, systolic/diastolic blood pressure; 1HGCT, glucose levels 1 h after a 50-g glucose challenge test; T-APN, total adiponectin; HMW-APN, high molecular weight adiponectin; CRP, C-reactive protein; TNF-α, tumor necrosis factor-α; IL-6, interleukin-6; MCP-1, monocyte chemoattractant protein-1.
Non-GDM women with normal glucose values and with one abnormal glucose value on the 3HOGTT
One non-GDM subgroup (n = 191) of women had glucose values on the 1HGCT ≥135 mg/dL, but had normal 3HOGTT glucose values at all time points. These women had lower T-APN levels than non-GDM women with 1HGCT glucose values <135 mg/dL (6.1 ± 0.5 vs 8.68 ± 0.46 μg/mL, respectively; P < 0.001; Table 2). Similarly, non-GDM women with one abnormal 3HOGTT glucose value had lower T-APN than non-GDM women with 1HGCT glucose values <135 mg/dL (5.5 ± 0.4 vs 8.68 ± 0.46 μg/mL; P < 0.001; Table 2).
Table 2.
Comparison of subgroups of women without gestational diabetes
| Non-gestational diabetes |
|||
|---|---|---|---|
| Parameters | Group 1 (n = 191) | Group 1 (n = 20) | Group 1 (n = 16) |
| Age (years) | 30.0 ± 0.4 | 31.0 ± 0.9 | 33.0 ± 0.4 |
| BMI (kg/m2) | 24.1 ± 0.2 | 24.4 ± 0.8 | 24.0 ± 0.2 |
| HbA1c (%) | 5.20 ± 0.03 | 5.25 ± 0.04 | 5.10 ± 0.02 |
| 1HGCT (mg/dL) | 105.9 ± 1.2 | 149.7 ± 2.3** | 150.3 ± 4.1** |
| Insulin (μU/mL) | 50.4 ± 2.9 | 73.7 ± 9.8 | 81.7 ± 15.7 |
| T-APN (μg/mL) | 8.68 ± 0.46 | 6.1 ± 0.5** | 5.45 ± 0.40** |
| HMW-APN (μg/mL) | 3.0 ± 0.1 | 2.8 ± 0.3 | 2.3 ± 0.3 |
| CRP (μg/mL) | 3.8 ± 0.3 | 6.0 ± 2.6 | 4.7 ± 0.9 |
| TNF-α (pg/mL) | 10.9 ± 0.3 | 11 ± 1 | 11 ± 1 |
| IL-6 (pg/mL) | 2.6 ± 0.2 | 2.4 ± 0.3 | 3.0 ± 0.6 |
| MCP-1 (pg/mL) | 62 ± 2 | 54.2 ± 3.7 | 76.2 ± 9.2 |
Data are the mean ± SEM. **P ≤ 0.001 compared with Group 1 women.
Group 1, women with glucose levels of <135 mg/dL 1 h after a 50-g glucose challenge test (1HGCT); Group 2, women with 1HGCT glucose ≥135 mg/dL and no abnormal readings on the 3-h oral glucose tolerance test (3HOGTT); Group 3, women with 1HGCT glucose ≥135 mg/dL and one abnormal reading on the 3HOGTT.
BMI, body mass index; T-APN, total adiponectin; HMW-APN, high molecular weight adiponectin; CRP, C-reactive protein; TNF-α, tumor necrosis factor-α; IL-6, interleukin-6; MCP-1, monocyte chemoattractant protein-1.
Matching for age, BMI, and age plus BMI
After matching for age, BMI, and age plus BMI, women with GDM had higher levels of glucose on the 1HGCT, higher levels of insulin and lower levels of T-APN (Tables 3).
Table 3.
Comparison of women with and without gestational diabetes after matching for age, body mass index (BMI), and age plus BMI, and adjustment for age and BMI
| Matched for age |
Matched for BMI |
Matched for age plus BMI |
||||
|---|---|---|---|---|---|---|
| Parameters | Non-GDM (n = 197) | GDM (n = 32) | Non-GDM (n = 188) | GDM (n = 31) | Non-GDM (n = 170) | GDM (n = 31) |
| Age (years) | 31.19 ± 0.30 | 32.1 ± 0.8 | 30.25 ± 0.34 | 32.32 ± 0.81 | 31.09 ± 0.33 | 32.32 ± 0.81 |
| BMI (kg/m2) | 24.13 ± 0.20 | 24.9 ± 0.6 | 23.79 ± 0.18 | 24.52 ± 0.52 | 23.87 ± 0.18 | 24.52 ± 0.52 |
| HbA1c (%) | 5.21 ± 0.02 | 5.35 ± 0.06 | 5.20 ± 0.03 | 5.35 ± 0.06 | 5.20 ± 0.03 | 5.35 ± 0.06 |
| 1HGCT (mg/dL) | 113.49 ± 1.66 | 160 ± 3** | 113.38 ± 1.67 | 158.73 ± 3.25** | 114.63 ± 1.80 | 158.73 ± 3.25** |
| Insulin (mU/mL) | 53.12 ± 3.12 | 120.7 ± 15.5** | 54.14 ± 3.21 | 122.64 ± 15.87** | 51.43 ± 3.22 | 122.64 ± 15.87** |
| T-APN (μg/mL) | 8.22 ± 0.42 | 5.5 ± 0.7** | 8.42 ± 0.44 | 5.5 ± 0.5** | 8.42 ± 0.46 | 5.5 ± 0.5** |
| HMW-APN (μg/mL) | 2.90 ± 0.10 | 2.4 ± 0.2 | 2.95 ± 0.11 | 2.46 ± 0.21 | 2.93 ± 0.11 | 2.46 ± 0.21 |
| CRP (μg/mL) | 4.07 ± 0.39 | 3.7 ± 0.6 | 4.13 ± 0.42 | 3.68 ± 0.57 | 4.10 ± 0.44 | 3.68 ± 0.57 |
| TNF-α (pg/mL) | 10.83 ± 0.25 | 10.6 ± 0.7 | 11.08 ± 0.31 | 10.43 ± 0.65 | 11.00 ± 0.28 | 10.43 ± 0.65 |
| IL-6 (pg/mL) | 2.48 ± 0.11 | 2.9 ± 0.6 | 2.7 ± 0.2 | 2.85 ± 0.59 | 2.50 ± 0.12 | 2.85 ± 0.59 |
| MCP-1 (pg/mL) | 61.64 ± 1.98 | 73.7 ± 10.7 | 62.7 ± 2.1 | 73.8 ± 11.9 | 62.79 ± 2.23 | 73.8 ± 11.9 |
Data are the mean ± SEM. **P < 0.001 compared with women without gestational diabetes.
GDM, gestational diabetes; 1HGCT, glucose levels 1 h after a 50-g glucose challenge test; T-APN, total adiponectin; HMW-APN, high molecular weight adiponectin; CRP, C-reactive protein; TNF-α, tumor necrosis factor-α; IL-6, interleukin-6; MCP-1, monocyte chemoattractant protein-1.
Adjustment for age and BMI
As stated in the Methods section, only T-APN showed a reliable group difference (P = 0.047; multiregression models, Delta method) when controlling for BMI and age. All other HMW-APN and inflammatory parameters were not significant in this regard.
Correlation studies
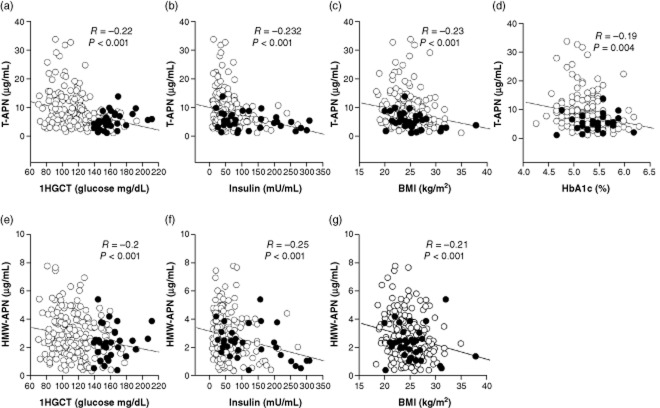
The entire cohort was used for correlation studies. Serum glucose on the 1HGCT, insulin, BMI, or HbA1c was correlated with T-APN, HMW-APN, CRP, IL-6, TNF-α, or MCP-1. As indicated in Fig. 1a–d, there was a negative correlation between T-APN and 1HGCT (R = –0.22; P < 0.001), insulin (R = –0.232; P < 0.001), BMI (R = –0.23; P < 0.001), and HbA1c (R = –019; P = 0.004). Furthermore, there were negative correlations between HMW-APN and 1HGCT (R = –0.2, P < 0.001), insulin (R = –0.25, P < 0.001), and BMI (R = –0.21, P < 0.001; Fig. 1e–g). No significant correlations were found between glucose on the 1HGCT, HbA1c, insulin, or BMI and CPR, TNF-α, IL-6, or MCP-1.
Figure 1.
Correlations between (a–d) total adiponectin (T-APN) or (e–g) high molecular weight adiponectin (HMW-APN) and glucose levels 1 h after a 50-g glucose challenge test (1HGCT), body mass index (BMI), insulin, or HbA1c in pregnant Chinese–American women with (•) or without (○) gestational diabetes.
Discussion
A higher prevalence of GDM is seen in Asian compared with Caucasian populations.1,2 Although GDM typically resolves after delivery, it is associated with complications in both the newborn and mother, including an increased risk of development of T2DM and other metabolic and obstetric complications.
The primary goal of the present study was to identify specific biochemical markers associated with GDM in Chinese–Americans. The GDM subjects were found to have higher 1HGCT, higher insulin levels, and lower T-APN. Among the non-GDM subjects, those with 1HGCT glucose values >135 mg/dL had significantly lower T-APN levels compared with control. No significant difference was observed in HMW-APN levels between non-GDM and GDM subjects. Several dependent (age, BMI, insulin levels, 1HGCT, HbA1c) and independent variable analyses were performed. After Bonferroni correction, HMW-APN did not differ significantly between non-GDM and GDM Chinese women. However, the correlation studies demonstrated that HMW-APN was negatively correlated with 1HGCT, insulin, and BMI.
Existing literature supports a strong association of decreased HMW-APN with insulin resistance and GDM.3–6 Retnakaran et al. compared HMW-APN levels in South Asian to Caucasian women; they reported lower levels of the HMW-APN in pregnant South Asian women after adjusting for age, prepregnancy BMI, and the weight gained during pregnancy.5 Choi et al. reported decreased plasma adiponectin levels in women with GDM;6 however, the specific type of adiponectin and the ethnicities of subjects were not specified in that study.
Of the two forms of adiponectin we measured (T-APN and HMW-APN), T-APN was most consistently reduced in our study, not only in subjects with GDM, but also in subjects with increasing degrees of glucose intolerance. Furthermore, T-APN was negatively correlated with all four markers of glucose intolerance, whereas HMW-APN was negatively correlated only with three of these markers. In this regard, our results agree with the existing literature on Caucasian women.
Our results demonstrated that T-APN, rather than HMW-APN, had a stronger association with increased glucose intolerance in Chinese–Americans. The reasons for this finding are unclear. Possible explanations may include genetic difference between Chinese and Caucasian populations, lower BMI and age among the Chinese women (as discussed below), and/or differences in assay techniques. The assay procedures we used included pretreating the samples with citrate buffer and SDS, which convert multimeric forms, including HMW-, MMW-, and LMW-APN, into dimers. The T-APN value obtained by this assay includes all dimeric forms of adiponectin in the sample (in this assay procedure, the monomer is not detected). In other assays, which measured HMW-, MMW-, and LMW-APN, the samples were not pretreated with SDS; thus the number of dimers differs between the assays performed in previous studies and the present study.
Both lower molecular weight trimer–dimer (T-APN) and the HMW complex of adiponectin (HMW-APN) have been implicated in the regulation of insulin sensitivity. The role of trimer–dimer complexes in the control of serum glucose is not well understood. Circulating levels of both T-APN and HMW-APN are gender dependent, with women having higher adiponectin levels than men.16 Oligomer formation of adiponectin critically depends on disulfide bond formation mediated by Cys-39.16 Mutation of Cys-39 results in trimers that are subject to proteolytic cleavage. Mutant (C39S) or wild-type Apo30 adiponectin treated with dithiothreitol are significantly more bioactive than the higher-order oligomeric forms of the protein with respect to reduction of serum glucose levels both in vitro and in vivo.16 In Japanese individuals with T2DM, the serum ratio of C1q-adiponectin to T-APN, but not HMW-APN, correlates with polyvascular lesions detected by vascular ultrasonography.17 In a study of Chinese women with polycystic ovarian syndrome (PCOS), serum T-APN levels were found to be negatively correlated with the waist : hip ratio and homeostasis model assessment of insulin resistance (HOMA-IR).18 In summary, these studies, similarly to the present study, suggest that T-APN may have a role in the pathophysiology of glucose intolerance by influencing insulin sensitivity.
In contrast with Caucasian GDM studies,8–10 there were no differences in the present study in the levels of inflammatory markers between GDM and non-GDM subjects. Further, unlike in Caucasians, there were no significant correlations between the markers of glucose tolerance (glucose on 1HGCT, HbA1c, insulin, or BMI) and inflammatory markers (CRP, IL-6, TNF-α, or MCP-1).8–10
The literature on IL-6 in GDM is inconsistent: some studies suggest that high IL-6 levels are associated with GDM independently of other factors,10,14,24 whereas others suggest that increased levels of IL-6 in GDM are possibly due to an increase in adipose tissue in pregnant women.22
The literature on the association of MCP-1 with GDM is very limited. One study showed that MCP-1 levels are decreased in pregnant women regardless of glucose tolerance.25 Other studies of GDM women reported that MCP-1 was increased in the last trimester (28–42 weeks gestation) in GDM.14,24
A possible explanation for the lack of correlation between inflammatory markers and markers of glucose tolerance in the present study may have to do with the lower BMI and young age of our patients. The average age of Chinese women was 30.6 years, with 45% of women younger than 30 years of age. The average BMI in Chinese women was 24.3 kg/m2. The age and BMI were similar in non-GDM and GDM Chinese women. Both BMI and age in our Chinese cohort are lower than those reported in the literature for Caucasians. For example, Ballestero et al. reported that, in Caucasian pregnant women with normal glucose tolerance, average BMI was 29.6 kg/m2 at 27 weeks gestation and that average age was 32 years.26
World Health Organization (WHO) studies of gestational weight gain in South Pacific Sea Asian women suggest that gestational weight gain is lower in Asian than Caucasian women.27 Other than BMI, gestational weight gain is dependent on socioeconomic and educational factors.28 In the present study, the patients came to the clinics at 8–10 weeks of pregnancy and we did not evaluate their educational levels or socioeconomic conditions. In addition, we do not have any simultaneous Caucasian control subjects. Further studies are needed to better understand differences between the gestational weight gain in pregnant Chinese and Caucasian women.
Age and BMI are associated with components of the metabolic syndrome (glucose levels and blood pressure)20 and increased levels of inflammatory markers are observed with increases in both age and BMI.7,21,22,29 For example, TNF-α is associated with persistent impairment of insulin signaling in obese women with previous GDM,21 and increased adiposity and high BMI are associated with elevated TNF-α levels in Caucasian women with GDM.7,21,22,29 One non-randomized study reported that TNF-α was increased mid-trimester in Chinese women with (n = 25) or without (n = 20) GDM.30 Another prospective study reported no difference in TNF-α levels n Mexican women with or without GDM.29 Our data can be interpreted as suggesting that BMI and age may play a role in determining levels of inflammatory markers,31 and may contribute to the development of glucose intolerance in pregnant Chinese–American women.
Another possibility for the lack of increased levels of inflammatory markers in the present study may involve socioeconomic issues. We did not have records for the demographic or social characteristics of our patients, such as education levels, smoking status, physical activity, diet, etc. Our group of patients consists of the first generation of immigrants from China. They are culturally closely related to the population in southern Pacific coastal cities in China and differ from inland and western region Chinese populations.32 The diet of the South Pacific coast of China is relatively leaner compared with the other regions.32
Most of our patients did not speak English (we translated the consent forms into Chinese). In general, the majority of Chinese women do not smoke. The WHO reports that although 61% of men smoke in China, only 4% of women smoke.33 It is likely that our patients had a limited acculturation to the American lifestyle.
A genetic cause for the differences in adiponectin between our study groups cannot be ruled out. Future studies are needed to address this question.
Our study has several additional limitations. First, we included Chinese–American women recruited from only two obstetric and gynecology clinics located in New York City's Chinatown. Therefore, the findings of the study may not be representative of the general Chinese population in the US or China. Second, a HemoCue glucometer was used to measure the glucose values for 3HOGTT because of cultural beliefs, as explained earlier. The HemoCue glucose values may be up to 8% lower than plasma glucose values.34–36 Finally, anther limitation of our study is the lack of a Caucasian control group.
In summary, our study demonstrated two new findings. First, inflammatory markers (CRP, TNF-α, IL-6, and MCP-1) are not significantly elevated in Chinese–American women with GDM, contrary to existing literature on Caucasian women with GDM. Second, T-APN, rather than HMW-APN, is most consistently inversely correlated with increasing degrees of glucose intolerance in pregnant Chinese–American women.
We conclude that although adiponectin appears to be important in the pathogenesis of GDM in Chinese–American women, the role of inflammatory markers is not significant, possibly because both the non-GDM and GDM groups of Chinese patients in our study were relatively young and lean. Further studies are needed to better understand the roles of inflammatory markers in pregnancy in Chinese populations.
Acknowledgments
This study was funded by the Gerald J. and Dorothy R. Friedman New York Foundation for Medical Research, by the Chinese–American Medical Society & Chinese–American Independent Practice Association Research Fund, and by the Yen Family Foundation.
Disclosure
The authors declare no conflict of interests.
References
- 1.Green J, Pawson I, Schumacher L. Glucose tolerance in pregnancy: Ethnic variation and influence of body habits. Am J Obstet Gynecol. 1990;163:86–92. doi: 10.1016/s0002-9378(11)90675-9. [DOI] [PubMed] [Google Scholar]
- 2.Chu SY, Abe K, Hall LR. Gestational diabetes mellitus: All Asians are not alike. Prev Med. 2009;49:265–268. doi: 10.1016/j.ypmed.2009.07.001. et al. [DOI] [PubMed] [Google Scholar]
- 3.Mazaki-Tovi S, Romero R, Valsbuch E. Maternal serum adiponectin multimers in gestational diabetes. J Perinat Med. 2009;37:637–650. doi: 10.1515/JPM.2009.101. et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Low CF, Tohi ERM, Chong PP. Adiponectin SNP45TG is associated with gestational diabetes mellitus. Arch Gynecol Obstet. 2011;283:1255–1260. doi: 10.1007/s00404-010-1548-4. et al. [DOI] [PubMed] [Google Scholar]
- 5.Retnakaran R, Hanley A, Connelly PW. Low serum levels of high molecular weight adiponectin in Indo-Asian women during pregnancy. Diabetes Care. 2006;29:1377–1379. doi: 10.2337/dc06-0413. et al. [DOI] [PubMed] [Google Scholar]
- 6.Choi SH, Kwak SH, Young BS. High plasma retinol binding protein-4 and low plasma adiponectin concentrations are associated with severity of glucose intolerance in women with previous gestational diabetes mellitus. J Clin Endocrinol Metab. 2008;93:3142–3148. doi: 10.1210/jc.2007-1755. et al. [DOI] [PubMed] [Google Scholar]
- 7.Retnakaran R, Hanley A, Raif NW. C-reactive protein and gestational diabetes: The central role of maternal obesity. J Clin Endocrinol Metab. 2003;88:3507–3512. doi: 10.1210/jc.2003-030186. et al. [DOI] [PubMed] [Google Scholar]
- 8.Wolf M, Sandler L, Hsu K. First-trimester C-reactive protein and subsequent gestational diabetes. Diabetes Care. 2003;26:819–824. doi: 10.2337/diacare.26.3.819. et al. [DOI] [PubMed] [Google Scholar]
- 9.Van der Toll T, Keogh CV, Gurao X. Interluekin-6 gen-deficient mice show impaired defense against pneumococcal pneumonia. J Infect Dis. 1997;176:439–444. doi: 10.1086/514062. et al. [DOI] [PubMed] [Google Scholar]
- 10.Morisset AS, Dube MC, Cote JA. Circulating interleukin-6 concentrations during and after gestational diabetes mellitus. Acta Obstet Gynecol Scand. 2011;90:524–530. doi: 10.1111/j.1600-0412.2011.01094.x. et al. [DOI] [PubMed] [Google Scholar]
- 11.Olszewski M, Groot AJ, Dastych J. TNF trafficking to human mast cell granules: Mature chain-dependent endocytosis. J Immunol. 2007;178:5701–5709. doi: 10.4049/jimmunol.178.9.5701. et al. [DOI] [PubMed] [Google Scholar]
- 12.Altinova AE, Toruner F, Bozkurt N. Circulating concentrations of adiponectin and tumor necrosis factor-alpha in gestational diabetes mellitus. Gynecol Endocrinol. 2007;23:161–165. doi: 10.1080/09513590701227960. [DOI] [PubMed] [Google Scholar]
- 13.Tang X, Asano M, O'Reilly A. p53 is an important regulator of CCL2 gene expression. Curr Mol Med. 2012;12:929–943. doi: 10.2174/156652412802480844. et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Klein K, Satler M, Elhenicky M. Circulating levels of MCP-1 are increased in women with gestational diabetes. Prenat Diagn. 2008;28:845–851. doi: 10.1002/pd.2064. et al. [DOI] [PubMed] [Google Scholar]
- 15.Carpenter MW, Coustan DR. Criteria for screening test for gestational diabetes. Am J Obstet Gynecol. 1982;144:768–773. doi: 10.1016/0002-9378(82)90349-0. [DOI] [PubMed] [Google Scholar]
- 16.Pajvani UB, Du X, Combs TP. Structure–function studies of the adipocyte-secreted hormone Acrp30/adiponectin: Implications for metabolic regulation and bioactivity. J Biol Chem. 2003;278:9073–9085. doi: 10.1074/jbc.M207198200. et al. [DOI] [PubMed] [Google Scholar]
- 17.Hirata A, Kishida K, Kobayashi H. Correlation between serum C1q-adiponectin/total adiponectin ratio and polyvascular lesions detected by vascular ultrasonography in Japanese type 2 diabetics. Metabolism. 2013;62:376–385. doi: 10.1016/j.metabol.2012.08.009. et al. [DOI] [PubMed] [Google Scholar]
- 18.Liu X, Zhang J, Li Y. On the relationship between serum total adiponectin and insulin resistance in polycystic ovary syndrome. Sheng Wu Yi Xue Gong Cheng Xue Za Zhi. 2010;27:636–640. et al. (in Chinese) [PubMed] [Google Scholar]
- 19.Bo S, Signorile A, Menato G. C-Reactive protein and tumor necrosis factor-alpha in gestational hyperglycemia. J Endocrinol Invest. 2005;28:779–786. doi: 10.1007/BF03347566. et al. [DOI] [PubMed] [Google Scholar]
- 20.Carpenter MW. Gestational diabetes, pregnancy hypertension and late vascular disease. Diabetes Care. 2007;30(Suppl. 2):S246–250. doi: 10.2337/dc07-s224. [DOI] [PubMed] [Google Scholar]
- 21.Friedman J, Kirwan JP, Jilng M. Increased skeletal muscle tumor necrosis factor-α and impaired insulin signaling persist in obese women with gestational diabetes mellitus 1 year postpartum. Diabetes. 2008;57:606–613. doi: 10.2337/db07-1356. et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lappas M, Permezel M, Rice GE. Release of pro-inflammatory cytokines and 8-isoprostane from placenta, adipose tissue, and skeletal muscle from normal pregnant women and women with gestational diabetes mellitus. J Clin Endocrinol Metab. 2004;89:5627–5633. doi: 10.1210/jc.2003-032097. [DOI] [PubMed] [Google Scholar]
- 23.Kinalski MT, Telejko B, Kuzmicki M. Tumor necrosis factor-alpha system and plasma adiponectin concentration in women with gestational diabetes. Horm Metab Res. 2005;37:450–454. doi: 10.1055/s-2005-870238. et al. [DOI] [PubMed] [Google Scholar]
- 24.Kuzmicki M, Telejko B, Szamatowicz J. High resistin and interleukin-6 are associated with gestational diabetes mellitus. Gynecol Endocrinol. 2009;25:258–263. doi: 10.1080/09513590802653825. et al. [DOI] [PubMed] [Google Scholar]
- 25.Telejko B, Kuzmicki M, Zonenberg A. Circulating monocyte chemoattractant protein-1 in women with gestational diabetes. Folia Histochem Cytobiol. 2007;45(Suppl. 1):S153–156. et al. [PubMed] [Google Scholar]
- 26.Ballestero M, Simon I, Vendrell J. Maternal and cord blood adiponectin multimeric forms in gestational diabetes mellitus. Diabetes Care. 2011;34:2418–2423. doi: 10.2337/dc11-0788. et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ota E, Haruna M, Suzuki M. Gestational weight gain and its association with perinatal outcomes in Viet Nam. Bull World Health Organ. 2011;89:127–136. doi: 10.2471/BLT.10.077982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Holowko N, Mishra G, Koupil I. Social inequality in excessive gestational weight gain. Int J Obes (Lond) 2014;38:91–96. doi: 10.1038/ijo.2013.62. [DOI] [PubMed] [Google Scholar]
- 29.Saucedo R, Zarate A, Basurto L. Relationship between circulating adipokines and insulin resistance during pregnancy and postpartum in women with gestational diabetes. Arch Med Res. 2011;42:318–323. doi: 10.1016/j.arcmed.2011.06.009. et al. [DOI] [PubMed] [Google Scholar]
- 30.Gax XL, Yang HX, Zhao Y. Variations of tumor necrosis factor-α, leptin and adiponectin in mid-trimester of gestational diabetes mellitus. Chin Med J. 2008;121:701–705. [PubMed] [Google Scholar]
- 31.Singh T, Newman AB. Inflammatory markers in population studies of aging. Ageing Res Rev. 2011;10:319–329. doi: 10.1016/j.arr.2010.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wikipedia. National register of historic places in Manhattan, Chinatown, Manhattan. Available from: http://en.wikipedia.org (accessed 8 January 2011)
- 33.Hitchman SC, Fong GT. Gender empowerment and female-to-male smoking prevalence ratios. Bull World Health Organ. 2011;89:195–202. doi: 10.2471/BLT.10.079905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fleming DR. Accuracy of blood glucose monitoring for patients: What it is and how to achieve it. Diabetes Educ. 1994;20:495–500. doi: 10.1177/014572179402000606. [DOI] [PubMed] [Google Scholar]
- 35.Fogh-Anderson N. Evaluation of HemoCue glucose meter (201 plus): Converting B-glucose to P-glucose. Point of Care: The Journal of Near-Patient Testing & Technology. 2004;3:172–175. [Google Scholar]
- 36.Warner JV, Wu JY, Buckingham N, McLeod DSA, Mottram B, Carter AC. Can one point of care glucose meter be used for all pediatric and adult hospital patients? Evaluation of three meters, including recently modified test strips. Diabetes Technol Ther. 2011;13:55–62. doi: 10.1089/dia.2010.0129. [DOI] [PubMed] [Google Scholar]