Abstract
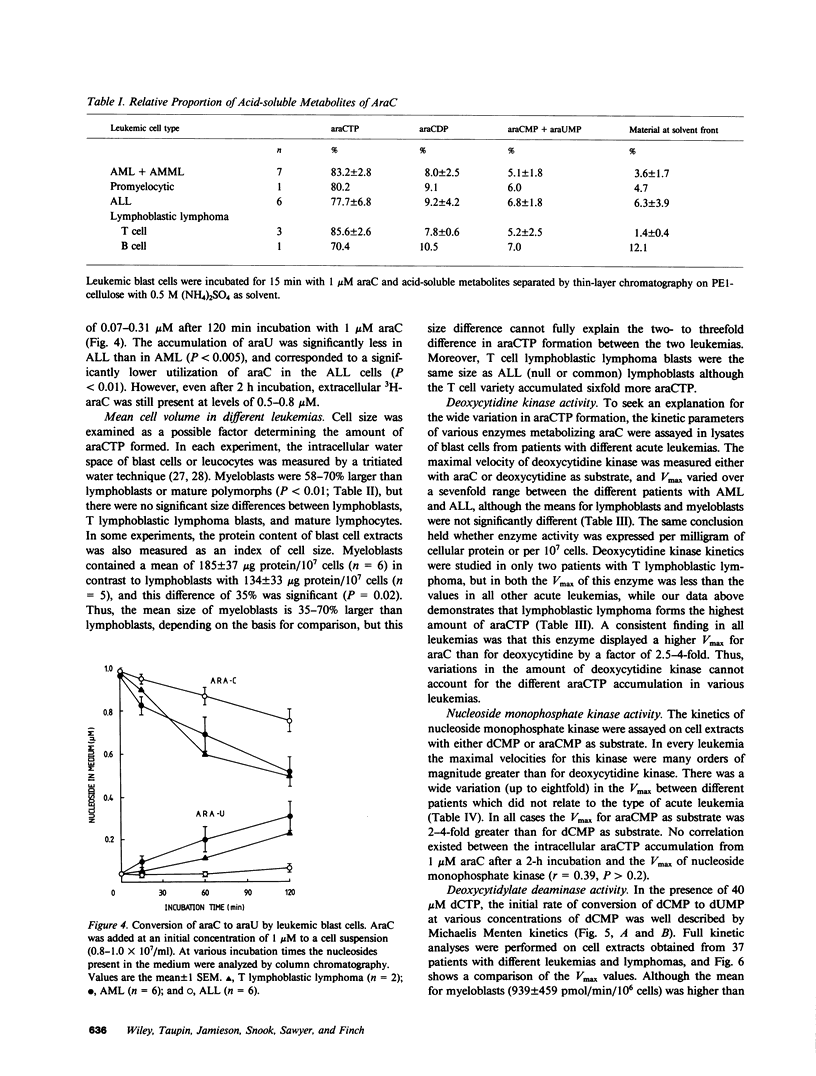
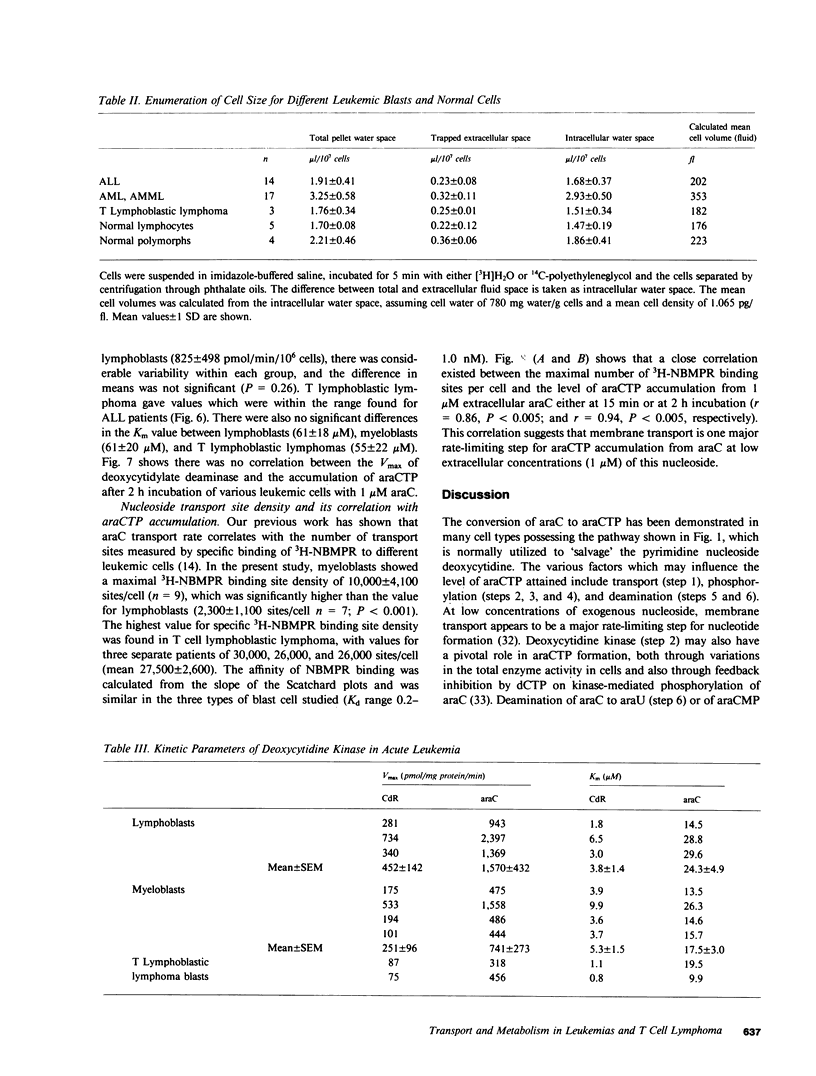
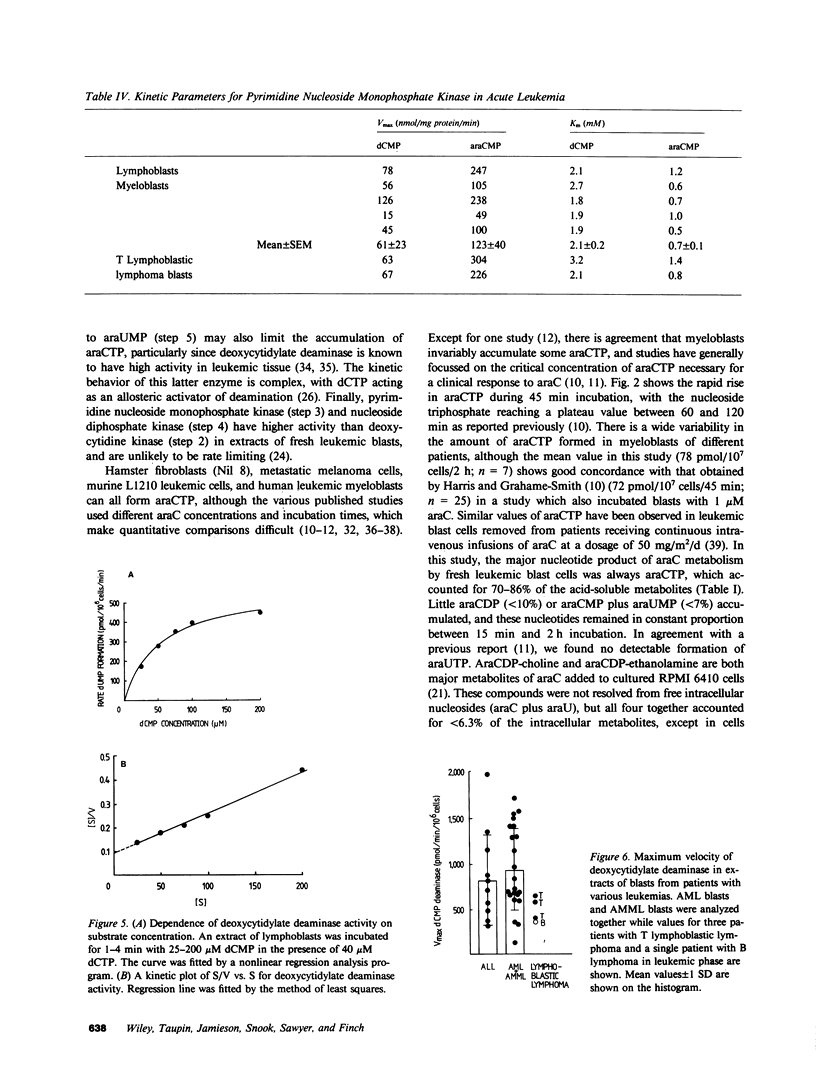
Cytosine arabinoside (araC) has proven efficacy in acute myeloid leukemia (AML), but its place in the treatment of acute lymphoblastic leukemia (ALL) and T lymphoblastic lymphoma is uncertain. The therapeutic potential of araC has been assessed in patients with AML, ALL, and T lymphoblastic lymphoma by measuring the conversion of araC to its active metabolite, the 5'-triphosphate of araC (araCTP), in purified blasts from patients as well as in normal polymorphs and lymphocytes. In all leukemias, araCTP was the major intracellular metabolite of araC. The highest araCTP formation was in blasts from T lymphoblastic lymphoma, which formed threefold more nucleotide than myeloblasts, and in turn myeloblasts formed twofold more araCTP than lymphoblasts from ALL. The mean araCTP formation in myeloblasts was sixfold greater than polymorphs, but in contrast, lymphoblasts and lymphocytes formed low and similar amounts of this nucleotide. Reasons for the sixfold range in araCTP accumulation in the various leukemic blasts were studied. The mean size of myeloblasts was 35-70% larger than lymphoblasts when compared on the basis of protein or intracellular water content, but T lymphoblastic lymphoma blasts and lymphoblasts were the same size. Activities of deoxycytidine kinase, deoxycytidylate deaminase, and pyrimidine nucleoside monophosphate kinase were not different between any of the leukemic cell types. The number of nucleoside transport sites on blasts was estimated by measuring the equilibrium binding of [3H]nitrobenzylthioinosine (NBMPR), which binds with high affinity to the transporter. Scatchard analysis yielded mean values of 27,500 sites/cell for T lymphoblastic lymphoma blasts, 10,000 sites/cell for myeloblasts, and 2,300 sites/cell for lymphoblasts. Our previous work has shown that araC influx correlates with the maximum number of 3H-NBMPR binding sites in leukemic and normal white cells. A strong correlation was observed between the number of nucleoside transport sites per leukemic blast cell and the accumulation of intracellular araCTP from extracellular araC at 1 microM. Membrane transport of araC at the low concentrations (approximately 1 microM), which are achieved therapeutically, is a major rate-limiting step in its conversion to araCTP by leukemic blast cells. Myeloblasts form more araCTP than lymphoblasts because of both higher nucleoside transport capacity and larger cell size. The highest nucleoside transport capacity and largest conversion of araC to araCTP is in T lymphoblastic lymphoma, which suggests that araC may be effective in the treatment of this disease.
Full text
PDF


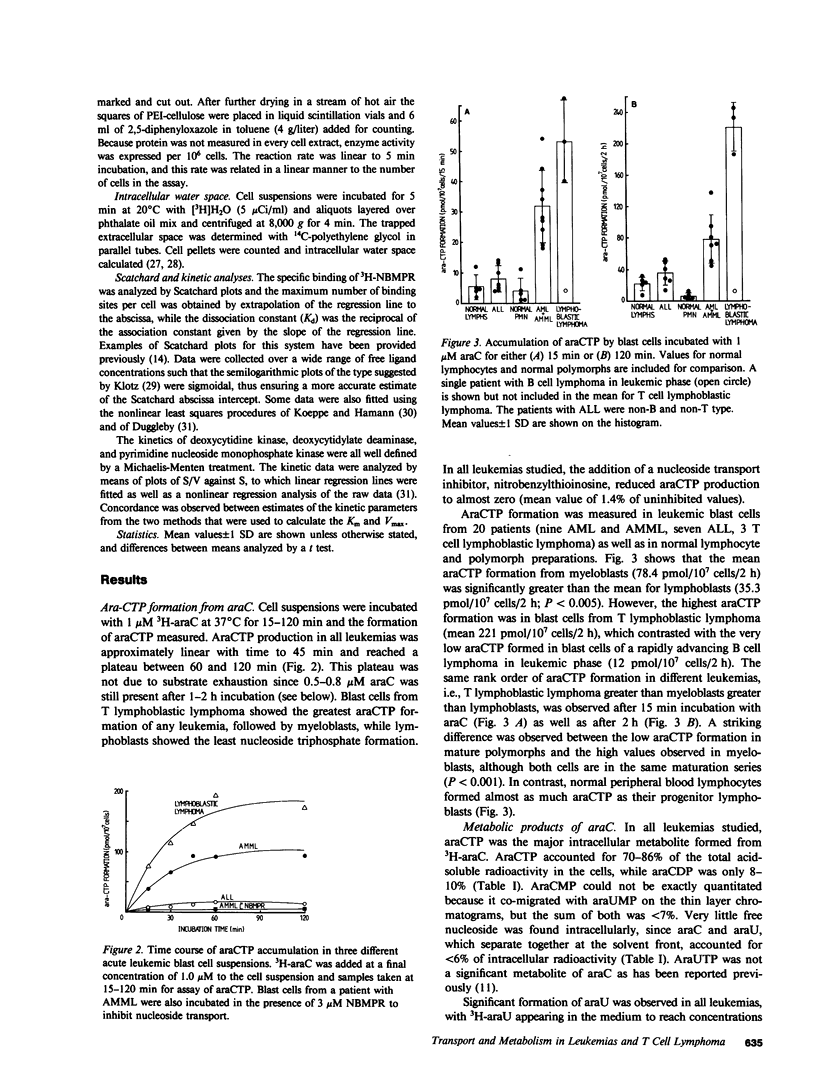



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abe I., Saito S., Hori K., Suzuki M., Sato H. Role of dephosphorylation in accumulation of 1-beta-D-arabinofuranosylcytosine 5'-triphosphate in human lymphoblastic cell lines with reference to their drug sensitivity. Cancer Res. 1982 Jul;42(7):2846–2851. [PubMed] [Google Scholar]
- Bagnara A. S., Finch L. R. Quantitative extraction and estimation of intracellular nucleoside triphosphates of Escherichia coli. Anal Biochem. 1972 Jan;45(1):24–34. doi: 10.1016/0003-2697(72)90004-8. [DOI] [PubMed] [Google Scholar]
- Barlogie B., Plunkett W., Raber M., Latreille J., Keating M., McCredie K. In vivo cellular kinetic and pharmacological studies of 1-beta-D-arabinofuranosylcytosine and 3-deazauridine chemotherapy for relapsing acute leukemia. Cancer Res. 1981 Mar;41(3):1227–1235. [PubMed] [Google Scholar]
- Bennett J. M., Catovsky D., Daniel M. T., Flandrin G., Galton D. A., Gralnick H. R., Sultan C. Proposals for the classification of the acute leukaemias. French-American-British (FAB) co-operative group. Br J Haematol. 1976 Aug;33(4):451–458. doi: 10.1111/j.1365-2141.1976.tb03563.x. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Bryan J. H., Henderson E. S., Leventhal B. G. Cytosine arabinoside and 6-thioguanine in refractory acute lymphocytic leukemia. Cancer. 1974 Feb;33(2):539–544. doi: 10.1002/1097-0142(197402)33:2<539::aid-cncr2820330232>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- Bui A. H., Wiley J. S. Cation fluxes and volume regulation by human lymphocytes. J Cell Physiol. 1981 Jul;108(1):47–54. doi: 10.1002/jcp.1041080107. [DOI] [PubMed] [Google Scholar]
- Cass C. E., Dahlig E., Lau E. Y., Lynch T. P., Paterson A. R. Fluctuations in nucleoside uptake and binding of the inhibitor of nucleoside transport, nitrobenzylthioinosine, during the replication cycle of HeLa cells. Cancer Res. 1979 Apr;39(4):1245–1252. [PubMed] [Google Scholar]
- Cass C. E., Kolassa N., Uehara Y., Dahlig-Harley E., Harley E. R., Paterson A. R. Absence of binding sites for the transport inhibitor nitrobenzylthioinosine on nucleoside transport-deficient mouse lymphoma cells. Biochim Biophys Acta. 1981 Dec 21;649(3):769–777. doi: 10.1016/0005-2736(81)90182-6. [DOI] [PubMed] [Google Scholar]
- Chou T. C., Arlin Z., Clarkson B. D., Phillips F. S. Metabolism of 1-beta-D-arabinofuranosylcytosine in human leukemic cells. Cancer Res. 1977 Oct;37(10):3561–3570. [PubMed] [Google Scholar]
- Coleman C. N., Stoller R. G., Drake J. C., Chabner B. A. Deoxycytidine kinase: properties of the enzyme from human leukemic granulocytes. Blood. 1975 Nov;46(5):791–803. [PubMed] [Google Scholar]
- Dow L. W., Chang L. J., Tsiatis A. A., Melvin S. L., Bowman W. P. Relationship of pretreatment lymphoblast proliferative activity and prognosis in 97 children with acute lymphoblastic leukemia. Blood. 1982 Jun;59(6):1197–1202. [PubMed] [Google Scholar]
- Duggleby R. G. A nonlinear regression program for small computers. Anal Biochem. 1981 Jan 1;110(1):9–18. doi: 10.1016/0003-2697(81)90104-4. [DOI] [PubMed] [Google Scholar]
- Ellims P. H., Kao A. Y., Chabner B. A. Kinetic behaviour and allosteric regulation of human deoxycytidylate deaminase derived from leukemic cells. Mol Cell Biochem. 1983;57(2):185–190. doi: 10.1007/BF00849195. [DOI] [PubMed] [Google Scholar]
- Ellims P. H., Medley G. Deoxycytidylate deaminase activity in lymphoproliferative disorders. Leuk Res. 1984;8(1):123–128. doi: 10.1016/0145-2126(84)90040-7. [DOI] [PubMed] [Google Scholar]
- Fox R. M., Piddington S. K., Tripp E. H., Tattersall M. H. Ecto-adenosine triphosphatase deficiency in cultured human T and null leukemic lymphocytes. A biochemical basis for thymidine sensitivity. J Clin Invest. 1981 Aug;68(2):544–552. doi: 10.1172/JCI110286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gingrich R. D., Armitage J. O., Burns C. P. Treatment of adult acute lymphoblastic leukemia with cytosine arabinoside, vincristine, and prednisone. Cancer Treat Rep. 1978 Sep;62(9):1389–1391. [PubMed] [Google Scholar]
- Hande K. R., Chabner B. A. Pyrimidine nucleoside monophosphate kinase from human leukemic blast cells. Cancer Res. 1978 Mar;38(3):579–585. [PubMed] [Google Scholar]
- Harris A. L., Grahame-Smith D. G., Potter C. G., Bunch C. Cytosine arabinoside deamination in human leukaemic myeloblasts and resistance to cytosine arabinoside therapy. Clin Sci (Lond) 1981 Feb;60(2):191–198. doi: 10.1042/cs0600191. [DOI] [PubMed] [Google Scholar]
- Harris A. L., Grahame-Smith D. G. The relationship of Ara-C metabolism in vitro to therapeutic response in acute myeloid leukaemia. Cancer Chemother Pharmacol. 1982;9(1):30–35. doi: 10.1007/BF00296758. [DOI] [PubMed] [Google Scholar]
- Harris A. W., Reynolds E. C., Finch L. R. Effect of thymidine on the sensitivity of cultured mouse tumor cells to 1-beta-D-arabinofuranosylcytosine. Cancer Res. 1979 Feb;39(2 Pt 1):538–541. [PubMed] [Google Scholar]
- Hart J. S., Ho D. H., George S. L., Salem P., Gottlieb J. A., Frei E., 3rd Cytokinetic and molecular pharmacology studies of arabinosylcytosine in metastatic melanoma. Cancer Res. 1972 Dec;32(12):2711–2716. [PubMed] [Google Scholar]
- Heichal O., Ish-Shalom D., Koren R., Stein W. D. The kinetic dissection of transport from metabolic trapping during substrate uptake by intact cells. Uridine uptake by quiescent and serum-activated Nil 8 hamster cells and their murine sarcoma virus-transformed counterparts. Biochim Biophys Acta. 1979 Feb 20;551(1):169–186. doi: 10.1016/0005-2736(79)90363-8. [DOI] [PubMed] [Google Scholar]
- Howard J. P., Albo V., Newton W. A., Jr Cytosine arabinoside. Results of a cooperative study in acute childhood leukemia. Cancer. 1968 Mar;21(3):341–345. doi: 10.1002/1097-0142(196803)21:3<341::aid-cncr2820210302>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- Kessel D., Shurin S. B. Transport of two non-metabolized nucleosides, deoxycytidine and cytosine arabinoside, in a sub-line of the L1210 murine leukemia. Biochim Biophys Acta. 1968 Sep 17;163(2):179–187. doi: 10.1016/0005-2736(68)90096-5. [DOI] [PubMed] [Google Scholar]
- Klotz I. M. Numbers of receptor sites from Scatchard graphs: facts and fantasies. Science. 1982 Sep 24;217(4566):1247–1249. doi: 10.1126/science.6287580. [DOI] [PubMed] [Google Scholar]
- Koeppe P., Hamann C. A program for non-linear regression analysis to be used on desk-top computers. Comput Programs Biomed. 1980 Dec;12(2-3):121–128. doi: 10.1016/0010-468x(80)90058-6. [DOI] [PubMed] [Google Scholar]
- Kufe D., Spriggs D., Egan E. M., Munroe D. Relationships among Ara-CTP pools, formation of (Ara-C)DNA, and cytotoxicity of human leukemic cells. Blood. 1984 Jul;64(1):54–58. [PubMed] [Google Scholar]
- Lauzon G. J., Paran J. H., Paterson A. R. Formation of 1-beta-D-arabinofuranosylcytosine diphosphate choline in cultured human leukemic RPMI 6410 cells. Cancer Res. 1978 Jun;38(6):1723–1729. [PubMed] [Google Scholar]
- Lauzon G. J., Paterson A. R. Binding of the nucleoside transport inhibitor nitrobenzylthioinosine to HeLa cells. Mol Pharmacol. 1977 Sep;13(5):883–891. [PubMed] [Google Scholar]
- Madsen N. P. Use of toluene/triton X-100 scintillation mixture for counting C14-protein radioactivity. Anal Biochem. 1969 Jun;29(3):542–544. doi: 10.1016/0003-2697(69)90341-8. [DOI] [PubMed] [Google Scholar]
- Major P. P., Egan E. M., Beardsley G. P., Minden M. D., Kufe D. W. Lethality of human myeloblasts correlates with the incorporation of arabinofuranosylcytosine into DNA. Proc Natl Acad Sci U S A. 1981 May;78(5):3235–3239. doi: 10.1073/pnas.78.5.3235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mancini W. R., Cheng Y. C. Human deoxycytidylate deaminase. Substrate and regulator specificities and their chemotherapeutic implications. Mol Pharmacol. 1983 Jan;23(1):159–164. [PubMed] [Google Scholar]
- Matsumoto S. S., Yu A. L., Bleeker L. C., Bakay B., Kung F. H., Nyhan W. L. Biochemical correlates of the differential sensitivity of subtypes of human leukemia to deoxyadenosine and deoxycoformycin. Blood. 1982 Nov;60(5):1096–1102. [PubMed] [Google Scholar]
- Momparler R. L. Kinetic and template studies with 1- -D-arabinofuranosylcytosine 5'-triphosphate and mammalian deoxyribonucleic acid polymerase. Mol Pharmacol. 1972 May;8(3):362–370. [PubMed] [Google Scholar]
- Nathwani B. N. A critical analysis of the classifications of non-Hodgkin's lymphomas. Cancer. 1979 Aug;44(2):347–384. doi: 10.1002/1097-0142(197908)44:2<347::aid-cncr2820440202>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- Neale G. A., Mitchell A., Finch L. R. Enzymes of pyrimidine deoxyribonucleotide metabolism in Mycoplasma mycoides subsp. mycoides. J Bacteriol. 1983 Dec;156(3):1001–1005. doi: 10.1128/jb.156.3.1001-1005.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohnuma T., Arkin H., Minowada J., Holland J. F. Differential chemotherapeutic susceptibility of human T-lymphocytes and B-lymphocytes in culture. J Natl Cancer Inst. 1978 Apr;60(4):749–752. doi: 10.1093/jnci/60.4.749. [DOI] [PubMed] [Google Scholar]
- Paterson A. R., Yang S. E., Lau E. Y., Cass C. E. Low specificity of the nucleoside transport mechanism of RPMI 6410 cells. Mol Pharmacol. 1979 Nov;16(3):900–908. [PubMed] [Google Scholar]
- Rivera G., Aur R. J., Dahl G. V., Pratt C. B., Wood A., Avery T. L. Combined VM-26 and cytosine arabinoside in treatment of refractory childhood lymphocytic leukemia. Cancer. 1980 Mar 15;45(6):1284–1288. doi: 10.1002/1097-0142(19800315)45:6<1284::aid-cncr2820450604>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- Rustum Y. M., Preisler H. D. Correlation between leukemic cell retention of 1-beta-D-arabinofuranosylcytosine 5'-triphosphate and response to therapy. Cancer Res. 1979 Jan;39(1):42–49. [PubMed] [Google Scholar]
- Smyth J. F., Robins A. B., Leese C. L. The metabolism of cytosine arabinoside as a predictive test for clinical response to the drug in acute myeloid leukaemia. Eur J Cancer. 1976 Jul;12(7):567–573. doi: 10.1016/0014-2964(76)90164-x. [DOI] [PubMed] [Google Scholar]
- Sylwestrowicz T., Piga A., Murphy P., Ganeshaguru K., Russell N. H., Prentice H. G., Hoffbrand A. V. The effect of deoxycoformycin and deoxyadenosine on deoxyribonucleotide concentrations in leukaemic cells. Br J Haematol. 1982 Aug;51(4):623–630. doi: 10.1111/j.1365-2141.1982.tb02826.x. [DOI] [PubMed] [Google Scholar]
- Tattersall M. H., Ganeshaguru K., Hoffbrand A. V. Mechanisms of resistance of human acute leukaemia cells to cytosine arabinoside. Br J Haematol. 1974 May;27(1):39–46. doi: 10.1111/j.1365-2141.1974.tb06772.x. [DOI] [PubMed] [Google Scholar]
- Traggis D. G., Dohlwitz A., Das L., Jaffe N., Moloney W. C., Hall T. C. Cytosine arabinoside in acute leukemia of childhood. Cancer. 1971 Oct;28(4):815–818. doi: 10.1002/1097-0142(1971)28:4<815::aid-cncr2820280402>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- Vierwinden G., Drenthe-Schonk A. M., Plas A. M., Linssen P. C., Pennings A. H., Holdrinet R. S., van Egmond J., Wessels J. M., Haanen C. A. Variations of the phosphorylation of 1-beta-D-arabinofuranosylcytosine (ARA-C) in human myeloid leukemic cells related to the cell cycle. Leuk Res. 1982;6(2):251–259. doi: 10.1016/0145-2126(82)90031-5. [DOI] [PubMed] [Google Scholar]
- Wiley J. S., Jones S. P., Sawyer W. H. Cytosine arabinoside transport by human leukaemic cells. Eur J Cancer Clin Oncol. 1983 Aug;19(8):1067–1074. doi: 10.1016/0277-5379(83)90029-9. [DOI] [PubMed] [Google Scholar]
- Wiley J. S., Jones S. P., Sawyer W. H., Paterson A. R. Cytosine arabinoside influx and nucleoside transport sites in acute leukemia. J Clin Invest. 1982 Feb;69(2):479–489. doi: 10.1172/JCI110472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiley J. S., Quinn M. A., Connellan J. M. Estimation of platelet size by measurement of intracellular water space using an oil technique. Thromb Res. 1983 Jul 15;31(2):261–268. doi: 10.1016/0049-3848(83)90328-6. [DOI] [PubMed] [Google Scholar]



