Abstract
Background
The optimal duration of dual antiplatelet therapy (DAPT) after drug-eluting stent (DES) implantation remains controversial. The primary aim of our study was to evaluate the impact of optimal DAPT duration on bleeding events between 6 and 12 months after biodegradable polymer-coated DES implantation. The secondary aim is to determine the predictors and prognostic implications of bleeding.
Methods
This study is a post hoc analysis of the Multi-Center Registry of EXCEL Biodegradable Polymer Drug Eluting Stents (CREATE) study population. A total of 2,040 patients surviving at 6 months were studied, including 1,639 (80.3%) who had received 6-month DAPT and 401 (19.7%) who had received DAPT greater than 6 months. Bleeding events were defined according to the bleeding academic research consortium (BARC) definitions as described previously and were classified as major/minor (BARC 2–5) and minimal (BARC 1). A left censored method with a landmark at 6 months was used to determine the incidence, predictors, and impact of bleeding on clinical prognosis between 6 and 12 months.
Results
At 1-year follow-up, patients who received prolonged DAPT longer than 6 months had a significantly higher incidence of overall (3.0% vs. 5.5%, P = 0.021) and major/minor bleeding (1.1% vs. 2.5%, P = 0.050) compared to the patients who received 6-month DAPT. Multivariate analysis showed that being elderly (OR = 1.882, 95% CI: 1.109–3.193, P = 0.019), having diabetes (OR = 1.735, 95% CI: 1.020–2.952, P = 0.042), having a history of coronary artery disease (OR = 2.163, 95% CI: 1.097–4.266, P = 0.026), and duration of DAPT longer than 6 months (OR = 1.814, 95% CI: 1.064–3.091, P = 0.029) were independent predictors of bleeding. Patients with bleeding events had a significantly higher incidence of cardiac death, myocardial infarction, target lesion revascularization, and stent thrombosis.
Conclusions
Prolonged DAPT (greater than 6 months) after biodegradable polymer-coated DES increases the risk of bleeding, and is associated with adverse cardiac events at 1-year follow-up.
Introduction
Dual antiplatelet therapy (DAPT) with aspirin and a P2Y12 receptor antagonist has played a pivotal role in reducing the risk of recurrent thrombotic events in high-risk settings, such as patients with acute coronary syndromes (ACS) and/or undergoing percutaneous coronary intervention (PCI).1–3 Prolonged (at least 12 months) DAPT is recommended especially for patients treated with drug-eluting stents (DES). This is due to the increased risk of thrombotic occlusion of the DES following early discontinuation of DAPT.4–8 However, in balancing the bleeding versus thrombotic risks, the optimal duration of DAPT following DES implantation remains controversial.9–14 Furthermore, prior literature has underscored the association of postprocedural bleeding events with adverse clinical outcomes in patients with ACS and/or undergoing PCI.15–18 Recent data suggest that the new-generation DES with biodegradable polymer may be effective in reducing the risk of late stent thrombosis, as compared with durable polymer DES. As absorbable polymer may promote vascular healing, these novel devices may permit a shortened duration of DAPT. We had previously reported that 6-month DAPT is feasible and as effective as extended DAPT (>6 months) after biodegradable polymer-coated sirolimus stent (EXCEL™, JW Medical System, Weihai, China) implantation in real-world settings.19,20 However, the effect of such a regimen on bleeding has not been elucidated and reported. The aim of our study was to evaluate the impact of different DAPT durations on bleeding events between 6 and 12 months after EXCEL stent implantation and to identify the predictors and prognostic implications of bleeding.
Method
Study Population
This study is a post hoc analysis of the Multicenter Registry of EXCEL Biodegradable Polymer Drug Eluting Stent (CREATE) Study.19,20 The CREATE Study is a post-marketing surveillance study that enrolled 2,077 real-world patients at 59 medical centers from 4 countries. All lesions were exclusively treated with the EXCEL stent. Patients were excluded if they had device or procedural failure, ≥1 stent (other than the protocol stent), contraindications to DAPT, functional status greater than New York Heart Association Class III, or planned surgery in the near future. A 6-month DAPT regimen (aspirin 100–300 mg per day for 30 days followed by 100 mg per day indefinitely; clopidogrel 75 mg per day for 6 months) was recommended but not mandatory. Prasugrel and ticagrelor were not available at the time of the study, so no new platelet inhibitors were used. The Ethics Committee in all participating centers approved the study protocol, and a signed, informed consent was obtained from every enrolled patient. The study is registered in the National Institutes of Health website as identifier NCT00331578.
Device Description
The Excel stent is a sirolimus-eluting stent coated with a biodegradable polylactic acid (PLA) polymer. The platform is a laser-cut, 316L stainless steel, open-cell design with strut thickness of 0.0047 in. The PLA coating is mixed with sirolimus (C51H79NO13, molecular weight 914.2) (North China Pharmaceutical Group Corporation, Shijiazhuang, China) and coated onto the abluminal surfaces to a thickness of 10–15 µm. The coating has been shown in animal studies to completely degrade into carbon dioxide and water within 6–9 months (based on communications with JW Medical System, October, 2007). There is no adhesive surface between the polymer and the stent struts. Total sirolimus dosage varies from 195 to 376 µg per stent according to the stent length.
Data Collection and Adjudication
Clinical data were prospectively collected on case-report forms and submitted to a data coordination center (located at Shenyang Northern Hospital). Independent study monitors performed audit checks for at least 15% of patients in each center. All adverse clinical events were reviewed and adjudicated by an independent Clinical Events Committee.19
Definitions
This study focused on the incidence, predictors, and prognostic implications of bleeding events between 6 and 12 months after the index procedure. Bleeding complications were defined according to the Bleeding Academic Research Consortium (BARC).21,22 We classified bleeding complications as major/minor (BARC type 2–5) and minimal (BARC type 1). All deaths were considered to be cardiac unless a noncardiac origin could be clearly established by clinical and/or pathological study. Myocardial infarction (MI) was defined as detection of rise and/or fall of cardiac biomarkers (preferably troponin) with at least 1 value above the 99th percentile of the upper reference limit together with evidence of myocardial ischemia with at least 1 of the following: (1) symptoms of ischemia; (2) electrocardiogram (ECG) changes indicative of new ischemia (new ST-T changes or new left bundle branch block); (3) development of pathological Q waves in the ECG; (4) imaging evidence of new loss of viable myocardium or new regional wall motion abnormality. The PCI-related MI was defined by patients with normal baseline troponin values, but showed increases in biomarkers greater than 3 times of 99th percentile upper range limit after the index PCI. We defined target lesion revascularization (TLR) as any repeat intervention inside the stent that had been implanted during the index procedure or within the 5 mm proximal or distal to the stent. Stent thrombosis (ST) was classified as definite, probable, and possible according to definitions proposed by the Academic Research Consortium (ARC).22
Statistical Analysis
Comparisons between continuous variable data, expressed as mean ± SD, were performed with the t-test, while the chi-square or the Fisher’s exact test was used for categorical data, expressed as percentages. The last observation carried forward method of imputation for missing observations was the prospectively defined statistical method. Cumulative incidence of events was calculated according to the Kaplan–Meier method. Survival curves were constructed with Kaplan–Meier estimates and compared by log-rank tests. Multivariate predictor analyses were performed with a binary logistic regression model. All available variables considered potentially relevant were included: age, gender, smoking history, hypertension, diabetes mellitus, hyperlipidemia, heart failure, previous history of MI, family history, and duration of DAPT. A “landmark survival analysis,” with a landmark set at 6 months, was performed to determine the impact of bleeding events between 6 and 12 months on clinical outcomes. Results are presented as odds ratio (OR) with 95% confidence interval (CI). Statistical analyses were performed with the SPSS 20.0 software (SPSS, Inc., Chicago, IL, USA). A 2-side P value of <0.05 was considered statistically significant.
Results
The baseline clinical and procedural characteristics were previously reported in the CREATE Study.19,20 A total of 2,040 patients with 6-month bleeding-free survival after PCI were included in this analysis. Of those patients, 1,639 (80.3%) received 6-month DAPT and 401 (19.7%) received extended DAPT >6 months. The reasons for continuing DAPT beyond 6 months are as follows: (1) some patients underwent a second PCI procedure due to TLR; (2) some patients were not willing to stop DAPT at 6 months; (3) some patients followed local referral physicians’ advice to continue clopidogrel. DAPT >6 months was associated with increased bleeding events (3.0% vs. 5.5%, P = 0.021) and major/minor bleeding (1.1% vs. 2.5%, P = 0.050) between 6 and 12 months (Table1).
Table 1.
Bleeding Events between 6 and 12 Months for Patients with Different DAPT Durations
| Bleeding Events (6–12 Months) | DAPT ≤6 Months (n = 1,639) | DAPT >6 Months (n = 401) | P-Values |
|---|---|---|---|
| All bleeding events | 49 (3.0) | 22 (5.5) | 0.021 |
| Minimal | 31 (1.9) | 12 (3.0) | 0.170 |
| Major/minor | 18 (1.1) | 10 (2.5) | 0.050 |
Predictors of Bleeding Events
Baseline characteristics of patients with and without bleeding between 6 and 12 months are shown in Table2. Patients with bleeding events had longer durations of DAPT and were more likely to be female or elderly. Those who experienced a bleeding event were also more likely to have diabetes, family history of coronary artery disease (CAD), and prior stroke. Multivariate analysis showed that age >65 years (OR = 1.882, 95% CI: 1.109–3.193, P = 0.019), diabetes mellitus (OR = 1.735, 95% CI: 1.020–2.952, P = 0.042), family history of CAD (OR = 2.163, 95% CI: 1.097–4.266, P = 0.026), and DAPT >6 months (OR = 1.814, 95% CI: 1.064–3.091, P = 0.029) were independent predictors of bleeding (Fig. 1). There was no difference in the percentage of patients taking proton pump inhibitors or anticoagulants in the 2 groups. The sites of bleeding and their incidence are listed in Table3.
Table 2.
Univariate Analysis of Predictors of Bleeding between 6 and 12 Months
| Predictors | Any Bleeding between 6 and 12 Months | P-Values | |
|---|---|---|---|
| Yes (n = 71) | No (n = 1,969) | ||
| Female gender | 27 (38.0) | 517 (26.3) | 0.039 |
| Age, years | 63.6 ± 11.7 | 60.6 ± 11.1 | 0.027 |
| Smoking history | 36 (50.7) | 986 (50.1) | 1.000 |
| Diabetes mellitus | 23 (32.4) | 404 (20.5) | 0.025 |
| Hypertension | 39 (54.9) | 1,099 (55.8) | 0.904 |
| Hyperlipidemia | 20 (28.2) | 596 (30.3) | 0.793 |
| Family history of CHD | 11 (16.2) | 151 (8.4) | 0.043 |
| Prior stroke | 19 (26.8) | 328 (16.7) | 0.035 |
| Prior MI | 6 (8.6) | 239 (12.3) | 0.456 |
| Heart failure | 6 (8.5) | 183 (9.3) | 1.000 |
| PCI indications | 0.889 | ||
| Silent ischemia | 4 (5.7) | 77 (3.8) | |
| Stable angina | 4 (5.7) | 127 (6.3) | |
| Unstable angina | 37 (52.9) | 1,068 (53.2) | |
| Recent MI (24 hours to 30 days) | 14 (20.0) | 360 (17.9) | |
| Acute MI within 24 hours | 11 (15.7) | 375 (18.7) | |
| Multivessel stenting | 24 (33.8) | 532 (27.0) | 0.222 |
| Stent no. per patient | 2.00 ± 1.23 | 1.79 ± 1.12 | 0.128 |
| Number of target vessels | 1.59 ± 0.79 | 1.48 ± 0.76 | 0.208 |
| Mean RVD, mm | 3.01 ± 0.47 | 2.99 ± 0.50 | 0.751 |
| Mean lesion length, mm | 26.42 ± 15.25 | 26.96 ± 12.96 | 0.734 |
| Duration of DAPT, days | 217.4 ± 67.0 | 200.0 ± 50.7 | 0.005 |
| Medications | |||
| Anticoagulant agents | 6 (8.5) | 157 (8.0) | 0.884 |
| Proton pump inhibitors | 58 (81.7) | 1,592 (80.9) | 0.860 |
CHD, coronary heart disease; MI, myocardial infarction; PCI, percutaneous coronary intervention; RVD, reference vessel diameter; DAPT, dual antiplatelet therapy.
Figure 1.
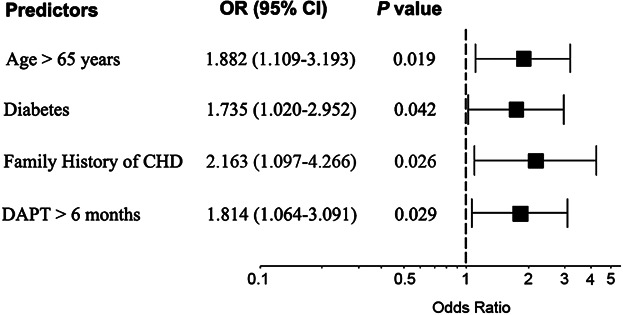
Independent predictors of bleeding between 6 and 12 months. CHD, coronary heart disease; DAPT, dual antiplatelet therapy; OR: odds ratio; CI: confidence interval.
Table 3.
Time of Bleeding, DAPT Discontinuation and Adverse Events in the 9 Patients with Bleeding-Related Events
| ID | Bleeding Classification | Bleeding Time (Days) | Time of DAPT Discontinuation (Days) | Adverse Events | Events Time (Days) |
|---|---|---|---|---|---|
| 3 | Major/minor (GI bleeding) | 196 | 197 | TLR | 307 |
| 348 | Major/minor (GI bleeding) | 219 | 219 | Sudden death/ST | 312 |
| 923 | Minimal (Gum bleeding) | 222 | 223 | TLR | 351 |
| 975 | Major/minor (GI bleeding) | 239 | 239 | Died of HF | 351 |
| 1,276 | Major/minor (GI bleeding) | 263 | 263 | Died of VA | 329 |
| 1,461 | Major/minor (ICH) | 247 | 247 | Died of ICH | 247 |
| 1,749 | Major/minor (GI bleeding) | 206 | 206 | Sudden death/ST | 227 |
| 1,780 | Major/minor (GI bleeding) | 224 | 225 | MI | 291 |
| 2,056 | Minimal (Gum bleeding) | 236 | 236 | TLR | 299 |
DAPT, dual antiplatelet therapy; TLR, target lesion revascularization; ST stent thrombosis; VA, ventricular arrhythmias; ICH, intracerebral hemorrhage; MI, myocardial infarction.
Bleeding-related Adverse Events
Case-based analysis showed that a total of 17 patients (23.9%) with bleeding suffered from major adverse cardiac events between 6 and 12 months, including 5 cardiac deaths, 1 MI, and 11 TLRs. Of those patients, 8 had TLRs before bleeding. The other 9 patients had events after bleeding, which were therefore defined as bleeding-related event. All those 9 patients had DAPT at the time of bleeding and clopidogrel was discontinued thereafter. Details of these events are listed in Table3. Even taking into account the 8 nonbleeding related TLRs, patients who had any bleeding event between 6 and 12 months had a significantly higher incidence of all events, which included all-cause death, cardiac death, nonfatal MI, TLR, and ST at follow-up (Table4). Survival curves demonstrated that bleeding events were predictive of significantly worse event-free survival rates (Fig. 2).
Table 4.
The Relationship between Bleeding and Clinical Outcomes
| Parameters | Any Bleeding between 6 and 12 Months | P-Values | |
|---|---|---|---|
| Yes (n = 71) | No (n = 1,969) | ||
| All events between 6 and 12 months | 17 (23.9) | 23 (1.2) | <0.001 |
| All-cause death | 5 (7.0) | 8 (0.4) | <0.001 |
| Cardiac death | 5 (7.0) | 0 (0.0) | <0.001 |
| Non-fatal MI | 1 (1.4) | 0 (0.0) | 0.035 |
| TLR | 11 (15.5) | 15 (0.7) | 0.023 |
| Stent thrombosis | 3 (4.2) | 0 (0.0) | 0.001 |
MI, myocardial infarction; TLR, target lesion revascularization.
Figure 2.
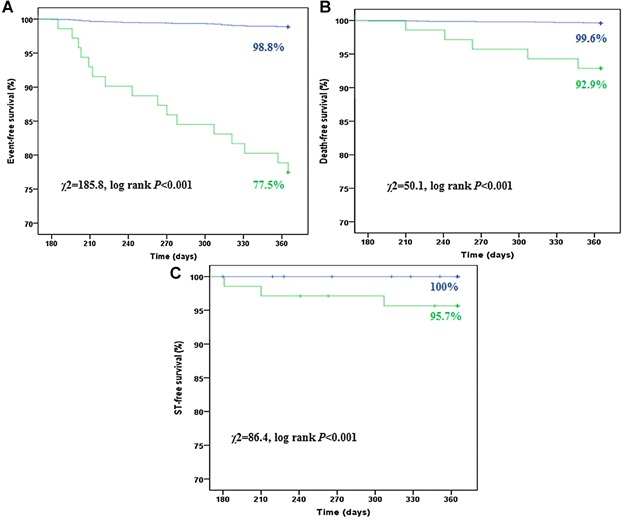
Kaplan–Meier curves of survival free from all events (A), all-cause death (B) and any ST (C) stratified by bleeding events between 6 and 12 months (green line: with bleeding; blue line: without bleeding).
Discussion
This is a post hoc analysis of the multicenter registry of the CREATE study. The main findings are as follows: (1) prolonged DAPT increased the risk of bleeding; (2) higher risk of bleeding could be predicted by female gender, older age, diabetes, family history of CAD, prior stroke, and longer duration of DAPT; (3) bleeding events occurring between 6 and 12 months were associated with a significant increase in MACEs at 1-year follow-up.
Literature Review
The advent of DES has dramatically reduced the rate of in-stent restenosis and repeat interventions. However, due to the delayed healing on the endovascular wall, the use of prolonged DAPT, ideally up to 12 months, has been strongly recommended.18 This prolonged DAPT, coupled with the development of more potent antiplatelet agents, prompted safety concerns of bleeding complications.23 DAPT has been previously associated with increased risk of bleeding with a hazard ratio of 19.8.24 However, safety data from real-world patients are limited, as most analyses are derived from randomized trials with stringent inclusion criteria, which may not reflect the true scope of problem. Moreover, most published studies address the incidence of bleeding during the acute phase of ACS, often reflecting the in-hospital utilization of powerful antithrombotic regimens, as opposed to the long-term risk related to prolonged DAPT. In a recent pooled analysis of the ACUITY and HORIZONS-AMI trials, non-CABG-related major bleeding occurred within 30 days of randomization in 520 (3.8%) of the 13,819 ACUITY patients and in 224 (6.2%) of the 3,602 HORIZONS-AMI patients.18 Data from the OASIS-Registry, OASIS-2, and CURE trials reported a 6-month major bleeding rate of 2%.25 Moreover, DES with biodegradable polymer have been associated a lower risk of too late stent thrombosis occurring >1 year after implantation. However, in that study, there was no difference in stent thrombosis at 1 year, while DAPT use was variable between 6 and 12 months.26
Bleeding Rate
Consistent with the results of previous studies, our study demonstrated that over 3.5% of patients experienced a bleeding event in the 12 months following DES implantation, with 2.1% experiencing major bleeding. Of note, the present population, although derived from a “real-world” scenario, may have a lower bleeding risk profile, as all patients were selected for DES implantation. The suggestion to patient for DES implantation often implies a careful prior stratification of patients for bleeding risk. Moreover, 10% of the patients in this study had stable coronary artery disease, a subpopulation associated with a lower risk of bleeding complications.
Predictors of Bleeding
Large-scale registry and clinical trial data have consistently identified elderly patients, women, and patients with impaired renal function, ACS, diabetes, and/or baseline anemia to be at increased risk for bleeding.15,26,27 In our study, bleeding can be predicted by female gender, older age, diabetes, family history of CAD, prior stroke, and prolonged duration of DAPT. To balance thrombotic prevention and bleeding risk, prediction of bleeding complications as well as thrombotic events would be crucial. Diabetes has been shown to be an independent predictor of stent thrombosis.7
Like other previous studies, our study has also shown diabetes to be a predictor of bleeding. The encouraging news from our study could be a potentially shorter duration of DAPT (6 months) after DES with biodegradable polymer resulting in a lower incidence of subsequent bleeding events without a higher incidence of stent thrombosis. Diener et al.14 showed that DAPT in patients with recent history of stroke is associated with a higher risk of major bleeding especially, intracranial hemorrhage, with the risk increasing consistently beyond the first 3 months of DAPT. With prior stroke being a predictor of increased bleeding events beyond 6 months of DAPT in our study, patients with prior stroke who need a DES could potentially benefit from 6-month-only DAPT, which could reduce risk of major bleeding subsequently.
Regrettably, most research of prediction models for bleeding events have been confined to the short term.18,28–30 Moreover, some risk factors of bleeding such as ACS, diabetes mellitus, and renal insufficiency31,32 were, at the same time, reported as risk factors of ST.33,34 This observation is reflective of the common paradox in clinical practice whereby, for a specific therapy, there is often concurrence of maximal benefit and maximal risk in the same population.
Stent Thrombosis after Bleeding
Major bleeding is the Achilles heel of prolonged DAPT. Aside from the obvious hemorrhagic sequelae, bleeding has been associated with MI, stroke, ST, and death, in patients with ACS and in those undergoing PCI35–40 in both short- and long-term antithrombotic settings.16,41 There is a seemingly paradoxical association between bleeding and thrombotic/ischemic risk. One possible explanation for this observation may be the obligatory withdrawal of crucial antithrombotic medications, mainly P2Y12 inhibitors during acute bleeding. In our study, case-based analysis of the bleeding events occurring between 6 and 12 months showed that all 9 bleeding-related events happened after discontinuation of DAPT. This observation questions the appearance of ST after routine discontinuation of P2Y12 inhibitor versus the abrupt stopping of P2 Y12 inhibitors in a patient with increasing thrombotic tendency during acute bleed. Thus, balancing the anti-ischemic benefits against the bleeding risk of antitplatelet agents and interventions is of paramount importance in assessing new therapies and in managing patients. One of the speculative solutions to reduce the length of required DAPT is to use a stent with biodegradable polymer because biodegradable polymer produces better endothelialization and tissue coverage to the stent struts, as in the Optical Coherence Tomographic substudy on the Limus-Eluted from a Durable versus Erodable Stent Coating (LEADERS) Trial.42
In conclusion, current guidelines regarding the duration of DAPT may not be reflective of the latest stent technologies, such as biodegradable polymers and possibly the second-generation DES such as everolimus and Zotarolimus durable polymer stents. Current data suggest that 6-month DAPT may be safe for some lower-risk patients, while ACS or high-risk patients might require a longer duration of DAPT, up to 12 months. The significance of other risk factors in balancing ST and bleeding risks remains to be determined. With developing technology, newer DES like EXCEL may permit shorter DAPT duration, as compared with the first-generation DES. So there is a need for further study in a randomized fashion with longer follow-up.
Limitations
This study has several limitations shared by most nonrandomized trials. Follow-up information, including BARC-defined bleeding events and DAPT adherence rates, was based on patient self-reports. The number of events was small, due to the low frequency of the adverse events. Patients at a higher risk of bleeding could have been excluded from this study. The risk of bleeding with aspirin and clopidogrel would undoubtedly be higher in unselected patients. Furthermore, the causal effect of bleeding on MACE is not clear as it may be related to comorbidities not addressed by the statistical analysis. Finally, despite multivariable adjustment of important confounders in all of the models, there remains the possibility of unmeasured confounders. The results of this study should not be generalized to patients unlike those enrolled.
Acknowledgments
This study was supported by the National Key Technology R&D Program in the 12th Five-Year Plan of China (No. 2011BAI11B07), Major High-Tech Clinical Army Projects (No. 2010GXJS001), Key Project of National 12th Five-Year Research Program of China (No. 2012ZX093016-002).
References
- Bhatt DL, Fox KA, Hacke W. Clopidogrel and aspirin versus aspirin alone for the prevention of atherothrombotic events. N Engl J Med. 2006;354:1706–1717. doi: 10.1056/NEJMoa060989. [DOI] [PubMed] [Google Scholar]
- Mehta SR, Yusuf S, Peters RJ. Effects of pretreatment with clopidogrel and aspirin followed by long-term therapy in patients undergoing percutaneous coronary intervention: The PCI-CURE study. Lancet. 2001;358:527–533. doi: 10.1016/s0140-6736(01)05701-4. [DOI] [PubMed] [Google Scholar]
- Eisenstein EL, Anstrom KJ, Kong DF. Clopidogrel use and long-term clinical outcomes after drug-eluting stent implantation. J Am Med Assoc. 2007;297:159–168. doi: 10.1001/jama.297.2.joc60179. [DOI] [PubMed] [Google Scholar]
- Stabile E, Cheneau E, Kinnaird T. Late thrombosis incypher stents after the discontinuation of antiplatelet therapy. Cardiovasc Radiat Med. 2004;5:173–176. doi: 10.1016/j.carrad.2004.11.001. [DOI] [PubMed] [Google Scholar]
- Spertus JA, Kettelkamp R, Vance C. Prevalence, predictors, and outcomes of premature discontinuation of thienopyridine therapy after drug-eluting stent placement: Results from the PREMIER registry. Circulation. 2006;113:2803–2809. doi: 10.1161/CIRCULATIONAHA.106.618066. [DOI] [PubMed] [Google Scholar]
- McFadden EP, Stabile E, Regar E. Late thrombosis in drug-eluting coronary stents after discontinuation of antiplatelet therapy. Lancet. 2004;364:1519–1521. doi: 10.1016/S0140-6736(04)17275-9. [DOI] [PubMed] [Google Scholar]
- Iakovou I, Schmidt T, Bonizzoni E. Incidence, predictors, and outcome of thrombosis after successful implantation of drug-eluting stents. JAMA. 2005;293:2126–2130. doi: 10.1001/jama.293.17.2126. [DOI] [PubMed] [Google Scholar]
- Rossini R, Capodanno D, Lettieri C. Prevalence, predictors, and long-term prognosis of premature discontinuation of oral antiplatelet therapy after drug eluting stent implantation. Am J Cardiol. 2011;107:186–194. doi: 10.1016/j.amjcard.2010.08.067. [DOI] [PubMed] [Google Scholar]
- Park S-J, Park D-W, Kim Y-H. Duration of dual antiplatelet therapy after implantation of drug-eluting stents. N Engl J Med. 2010;362:1374–1382. doi: 10.1056/NEJMoa1001266. [DOI] [PubMed] [Google Scholar]
- Kandzari DE, Angiolillo DJ, Price MJ. Identifying the “optimal” duration of dual antiplatelet therapy after drug eluting stent revascularization. J Am Coll Cardiol Cardiovasc Interv. 2009;2:1279–1285. doi: 10.1016/j.jcin.2009.09.012. [DOI] [PubMed] [Google Scholar]
- Airoldi F, Colombo A, Morici N. Incidence and predictors of drug-eluting stent thrombosis during and after discontinuation of thienopyridine treatment. Circulation. 2007;116:745–754. doi: 10.1161/CIRCULATIONAHA.106.686048. [DOI] [PubMed] [Google Scholar]
- Schulz S, Schuster T, Mehilli J. Stent thrombosis after drug-eluting stent implantation: Incidence, timing, and relation to discontinuation of clopidogrel therapy over a 4-year period. Eur Heart J. 2009;30:2714–2721. doi: 10.1093/eurheartj/ehp275. [DOI] [PubMed] [Google Scholar]
- Bhatt DL, Flather MD, Hacke W. Patients with prior myocardial infarction, stroke, or symptomatic peripheral arterial disease in the CHARISMA trial. J Am Coll Cardiol. 2007;49:1982–1988. doi: 10.1016/j.jacc.2007.03.025. [DOI] [PubMed] [Google Scholar]
- Diener HC, Bogousslavsky J, Brass LM. Aspirin and clopidogrel compared with clopidogrel alone after recent ischaemic stroke or transient ischaemic attack in high-risk patients (MATCH): Randomised, double-blind, placebo-controlled trial. Lancet. 2004;364:331–337. doi: 10.1016/S0140-6736(04)16721-4. [DOI] [PubMed] [Google Scholar]
- Feit F, Voeltz MD, Attubato MJ. Predictors and impact of major hemorrhage on mortality following percutaneous coronary intervention from the REPLACE-2 trial. Am J Cardiol. 2007;100:1364–1369. doi: 10.1016/j.amjcard.2007.06.026. [DOI] [PubMed] [Google Scholar]
- Moscucci M, Fox KA, Cannon CP. Predictors of major bleeding in acute coronary syndromes: The Global Registry of Acute Coronary Events (GRACE) Eur Heart J. 2003;24:1815–1823. doi: 10.1016/s0195-668x(03)00485-8. [DOI] [PubMed] [Google Scholar]
- Mehran R, Pocock SJ, Stone GW. Associations of major bleeding and myocardial infarction with the incidence and timing of mortality in patients presenting with non-ST-elevation acute coronary syndromes: A risk model from the ACUITY trial. Eur Heart J. 2009;30:1457–1466. doi: 10.1093/eurheartj/ehp110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehran R, Pocock SJ, Nikolsky E. A risk score to predict bleeding in patients with acute coronary syndromes. J Am Coll Cardiol. 2010;55:2556–2566. doi: 10.1016/j.jacc.2009.09.076. [DOI] [PubMed] [Google Scholar]
- Han Y, Jing Q, Xu B. Safety and efficacy of biodegradable polymer-coated sirolimus-eluting stents in daily practice: 18-month clinical and 9-month angiographic outcomes. JACC Cardiovasc Interv. 2009;2:209–303. doi: 10.1016/j.jcin.2008.12.013. [DOI] [PubMed] [Google Scholar]
- Han Y, Jing Q, Li Y. Sustained clinical safety and efficacy of a biodegradable-polymer coated sirolimus-eluting stent in “real-world” practice: Three-year outcomes of the CREATE (Multi-Center Registry of EXCEL Biodegradable Polymer Drug Eluting Stents) study. Catheter Cardiovasc Interv. 2012;79:211–216. doi: 10.1002/ccd.23113. [DOI] [PubMed] [Google Scholar]
- Mehran R, Rao SV, Bhatt DL. Standardized bleeding definitions for cardiovascular clinical trials: A consensus report from the bleeding academic research consortium. Circulation. 2011;123:2736–2747. doi: 10.1161/CIRCULATIONAHA.110.009449. [DOI] [PubMed] [Google Scholar]
- Cutlip DE, Windecker S, Mehran R. Clinical end points in coronary stent trials: A case for standardized definitions. Circulation. 2007;115:2344–2351. doi: 10.1161/CIRCULATIONAHA.106.685313. [DOI] [PubMed] [Google Scholar]
- Wiviott SD, Braunwald E, McCabe CH. Prasugrel versus clopidogrel in patients with acute coronary syndromes. N Engl J Med. 2007;357:2001–2015. doi: 10.1056/NEJMoa0706482. [DOI] [PubMed] [Google Scholar]
- Latib A, Morici N, Cosgrave J. Incidence of bleeding and compliance on prolonged dual antiplatelet therapy (aspirin thienopyridine) following drug-eluting stent implantation. Am J Cardiol. 2008;102:1477–1481. doi: 10.1016/j.amjcard.2008.07.038. [DOI] [PubMed] [Google Scholar]
- Eikelboom JW, Mehta SR, Anand SS. Adverse impact of bleeding on prognosis in patients with acute coronary syndromes. Circulation. 2006;114:774–882. doi: 10.1161/CIRCULATIONAHA.106.612812. [DOI] [PubMed] [Google Scholar]
- Stefanini GG, Byrne RA, Serruys PW. Biodegradable polymer drug-eluting stents reduce the risk of stent thrombosis at 4 years in patients undergoing percutaneous coronary intervention: A pooled analysis of individual patient data from the ISAR-TEST 3, ISAR-TEST 4, and LEADERS randomized trials. Eur Heart J. 2012;33(10):1214–1222. doi: 10.1093/eurheartj/ehs086. doi: 10.1093/eurheartj/ehs086. Epub 2012 Mar. 24. [DOI] [PubMed] [Google Scholar]
- Manoukian SV, Feit F, Mehran R. Impact of major bleeding on 30-day mortality and clinical outcomes in patients with acute coronary syndromes: An analysis from the ACUITY Trial. J Am Coll Cardiol. 2007;49:1362–1368. doi: 10.1016/j.jacc.2007.02.027. [DOI] [PubMed] [Google Scholar]
- Subherwal S, Bach RG, Chen AY. Baseline risk of major bleeding in non-ST-segment-elevation myocardial infarction: The CRUSADE (Can Rapid risk stratification of Unstable angina patients Suppress Adverse outcomes with Early implementation of the ACC/AHA Guidelines) Bleeding Score. Circulation. 2009;119:873–1882. doi: 10.1161/CIRCULATIONAHA.108.828541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta SK, Frutkin AD, Lindsey JB. Bleeding in patients undergoing percutaneous coronary intervention: The development of a clinical risk algorithm from the National Cardiovascular Data Registry. Circ Cardiovasc Interv. 2009;2:222–229. doi: 10.1161/CIRCINTERVENTIONS.108.846741. [DOI] [PubMed] [Google Scholar]
- Dauerman HL, Rao SV, Resnic FS. Bleeding avoidance strategies. Consensus and controversy. J Am Coll Cardiol. 2011;58:1–10. doi: 10.1016/j.jacc.2011.02.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montalescot G, Salette G, Steg G. Development and validation of a bleeding risk model for patients undergoing elective percutaneous coronary intervention. Int J Cardiol. 2011;150:79–83. doi: 10.1016/j.ijcard.2010.02.077. [DOI] [PubMed] [Google Scholar]
- Nikolsky E, Stone GW, Kirtane AJ. Gastrointestinal bleeding in patients with acute coronary syndromes: Incidence, predictors, and clinical implications: Analysis from the ACUITY (Acute Catheterization and Urgent Intervention Triage Strategy) trial. J Am Coll Cardiol. 2009;54:1293–1302. doi: 10.1016/j.jacc.2009.07.019. [DOI] [PubMed] [Google Scholar]
- Tsai TT, Messenger JC, Brennan JM. Safety and efficacy of drug-eluting stents in older patients with chronic kidney disease: A report from the linked CathPCI Registry-CMS claims database. J Am Coll Cardiol. 2011;58:1859–1869. doi: 10.1016/j.jacc.2011.06.056. [DOI] [PubMed] [Google Scholar]
- Cayla G, Hulot JS, O’Connor SA. Clinical, angiographic, and genetic factors associated with early coronary stent thrombosis. JAMA. 2011;306:1765–1774. doi: 10.1001/jama.2011.1529. [DOI] [PubMed] [Google Scholar]
- Kuchulakanti PK, Chu WW, Torguson R. Correlates and long-term outcomes of angiographically proven stent thrombosis with sirolimus- and paclitaxel eluting stents. Circulation. 2006;113:1108–1113. doi: 10.1161/CIRCULATIONAHA.105.600155. [DOI] [PubMed] [Google Scholar]
- Eikelboom JW, Mehta SR, Anand SS. Adverse impact of bleeding on prognosis in patients with acute coronary syndromes. Circulation. 2006;114:774–782. doi: 10.1161/CIRCULATIONAHA.106.612812. [DOI] [PubMed] [Google Scholar]
- Rao SV, O’Grady K, Pieper KS. Impact of bleeding severity on clinical outcomes among patients with acute coronary syndromes. Am J Cardiol. 2005;96:1200–1206. doi: 10.1016/j.amjcard.2005.06.056. [DOI] [PubMed] [Google Scholar]
- Kinnaird TD, Stabile E, Mintz GS. Incidence, predictors, and prognostic implications of bleeding and blood transfusion following percutaneous coronary interventions. Am J Cardiol. 2003;92:930–935. doi: 10.1016/s0002-9149(03)00972-x. [DOI] [PubMed] [Google Scholar]
- Kirtane AJ, Piazza G, Murphy SA. Correlates of bleeding events among moderate- to high-risk patients undergoing percutaneous coronary intervention and treated with eptifibatide: Observations from the PROTECT-TIMI-30 trial. J Am Coll Cardiol. 2006;47:2374–2379. doi: 10.1016/j.jacc.2005.09.080. [DOI] [PubMed] [Google Scholar]
- Manoukian SV, Feit F, Mehran R. Impact of major bleeding on 30-day mortality and clinical outcomes in patients with acute coronary syndromes: An analysis from the ACUITY trial. J Am Coll Cardiol. 2007;49:1362–1368. doi: 10.1016/j.jacc.2007.02.027. [DOI] [PubMed] [Google Scholar]
- Berger PB, Bhatt DL, Fuster V. Bleeding complications with dual antiplatelet therapy among patients with stable vascular disease or risk factors for vascular disease: Results from the Clopidogrel for High Atherothrombotic Risk and Ischemic Stabilization, Management, and Avoidance (CHARISMA) Trial. Circulation. 2010;121:2575–2583. doi: 10.1161/CIRCULATIONAHA.109.895342. [DOI] [PubMed] [Google Scholar]
- Ducrocq G, Wallace JS, Baron G. Risk score to predict serious bleeding in stable outpatients with or at risk of atherothrombosis. Eur Heart J. 2010;31:1257–1265. doi: 10.1093/eurheartj/ehq021. [DOI] [PMC free article] [PubMed] [Google Scholar]


