Abstract
Evidences are accumulating that extract of Acanthopanax senticosus Harms (ASH; syn Eleutherococcus senticosus [Rupr. & Maxim.] Maxim), a shrub native to Northeastern Asia, has antiinflammatory effects. In this study, we examined prophylactic and therapeutic effects of ASH extract (ASHE) on rheumatoid arthritis using collagen-induced arthritis (CIA) mouse model. Acanthopanax senticosus Harms extract was administered before the onset of arthritis in the prophylaxis model. In the therapeutic model, ASHE was administered after the onset of arthritis with or without anti-TNF-α antibody. The ASHE treatment showed efficacy before onset of CIA but there was no effect after CIA was established. The ASHE treatment delayed the onset and decreased severity of CIA. In vitro examinations showed that ASHE is an antioxidant and that ASHE suppresses TNF-α and interleukin-6 production in human peripheral blood mononuclear cells. The combination therapy with ASHE and anti-TNF-α antibody reduced the severity of arthritis compared with anti-TNF-α antibody alone. The present study shows that ASHE has prophylactic effect against CIA and support therapeutic effect of anti-TNF-α antibody. © 2014 The Authors. Phytotherapy Research published by John Wiley & Sons Ltd.
Keywords: Acanthopanax senticosus Harms, collagen-induced arthritis, reactive oxygen species, inflammatory cytokines, anti-TNF-α antibody
INTRODUCTION
Acanthopanax senticosus Harms (ASH; syn Eleutherococcus senticosus [Rupr. & Maxim.] Maxim), also known as Siberian ginseng or eleuthero, is a shrub that belongs to the family Araliaceae. It grows in taiga of China, Korea, Russia, and Hokkaido island of Japan. For thousands of years, the extract of this herb has been used as oriental folk medicine and known to have beneficial effects on psychophysical stress, fatigue, chronic bronchitis, autoimmune diseases, hypertension, ischemic heart disease, gastric ulcer, and allergic responses (Davydov and Krikorian, 2000; Dowling et al., 1996; Fujikawa et al., 1996; Nishibe et al., 1990; Yi et al., 2002). ASH extracts can be obtained in the USA and numerous other countries, and are used as an herbal dietary supplement that exhibits nutritionally fortifying effects.
Rheumatoid arthritis (RA) is an inflammatory autoimmune disorder characterized by chronic, symmetric, and erosive synovitis caused by infiltration of activated T-cells and macrophages resulting in hyperproliferation of synovial cells (Feldmann et al., 1996). A number of cytokines, including TNF-α, interleukin 1β (IL-1β), and IL-6 are critical pathogenesis of RA; they are produced by synovial cells, macrophages, and T-cells (McInnes and Schett, 2007); and these cytokines are the targets to treat RA (Nam et al., 2010). Osteoclasts are differentiated and stimulated by IL-1β and TNF-α and are responsible for joint destruction (McInnes and Schett, 2007).
Reactive oxygen species (ROS) are another toxic factor in RA (Hitchon and El-Gabalawy, 2004). ROS are generated by various types of cells in the body, and they are involved in physiological as well as pathological processes (Valko et al., 2007). Cigarette smoking is a risk factor of developing RA (Heliovaara et al., 1993; Silman et al., 1996); it is a well-known source of ROS (Valavanidis et al., 2009). Oxidative damage to hyaluronate (Grootveld et al., 1991), lipoproteins (Dai et al., 2000), and DNA (Hajizadeh et al., 2003) is observed in synovial fluid of patients with RA. Many antioxidants including superoxide dismutase 3, an enzyme that scavenge superoxide, are shown to inhibit arthritis in rodent models (Cuzzocrea et al., 2000; Bandt et al., 2002; Iyama et al., 2001). Although, arthritis is enhanced in superoxide dismutase 3 knockout mice (Ross et al., 2004). Redox signaling is a critical regulator of transcription factors involved in RA (Michiels et al., 2002).
Recently, ASH was demonstrated to inhibit superoxide and hydrogen peroxide production in mouse peritoneal macrophages in vitro and in vivo (Lin et al., 2008). In this study, we investigated whether or not ASH extract (ASHE) exerts a therapeutic effect in a collagen-induced arthritis (CIA) mouse model and compared it with the effects of vitamin C (ascorbic acid), which is used as a simple, easily available well-known nutritional supplement, and is well-established.
MATERIALS AND METHODS
Reagents
Acanthopanax senticosus Harms powder was obtained from Yakuhan Pharmaceutical Co., LTD., (Kitahiroshima, Japan). They are dissolved in distilled water at 100 mg/mL to obtain ASHE solution. Ascorbic acid (AA), cytochrome c, phorbol 12-myristate 13-acetate, lipopolysaccharide (LPS, derived from Escherichia coli serotype 026:B26) were purchased from Sigma-Aldrich (St. Louis, MO, USA). Bovine type II collagen (CII) was purchased from Collagen Research Center (Tokyo, Japan). Freund’s incomplete adjuvant was purchased from Becton Dickinson (Franklin Lakes, NJ, USA). Goat anti-mouse TNF-α antibody was purchased from BioLegend, (San Diego, CA, USA).
Animal and induction of collagen-induced arthritis
The study protocol was approved by the Animal Ethics Committee of Sapporo Medical University (No. 09–089). Male 4–6 weeks old DBA/1J mice were purchased from Charles River Laboratories (Yokohama, Japan). The mice were acclimatized to our facility for at least 7 days before the experiment. They were housed in standard laboratory conditions (22 ± 3°C; relative humidity, 50–55%; 12 h light/dark cycle) and given free access to food and water.
Collagen-induced arthritis was induced as described, previously (Coutenay et al., 1980). Briefly, the mice were immunized with 100 µg of CII emulsified in Freund’s incomplete adjuvant by intradermal injection on day −21. The mice received booster immunization on day 0. After the secondary immunization, severity of arthritis was monitored according to a previously described clinical score (0, normal; 1, erythema and mild swelling confined to the ankle joint and toes; 2, erythema and mild swelling extending from the ankle to the midfoot; 3, erythema and severe swelling extended from the ankle to the metatarsal joints; and 4, ankylosing deformity with joint swelling) (Ji et al., 2012). In addition to the clinical score, thickness of hind paw of each mouse was measured using a foot pad thickness gauge (Ozaki Mfg. Co., Ltd., Tokyo, Japan).
Treatment protocol
Treatment protocols are designed as described in Fig.1. In the Experiment-1, prophylactic effect of ASHE and AA was compared. A single dose of ASHE (500 µg/g body weight/day) or AA (10 mg/g body weight/day) was administered to each mouse by gastric gavage, everyday from day −28. The doses of ASHE and AA administered to mice were determined with reference to previous studies (Huang et al., 2011a, 2011b; Craven et al., 1997). In the Experiment-2, whether ASHE enhances therapeutic effect of anti-TNF-α antibody was examined. Either 50 µg of anti-TNF-α antibody or its isotype control was administered intraperitoneally to each mouse on day 10, 13, 16, and 19. ASHE or distilled water was administered to each mouse by gastric gavage, everyday from day 10.
Figure 1.
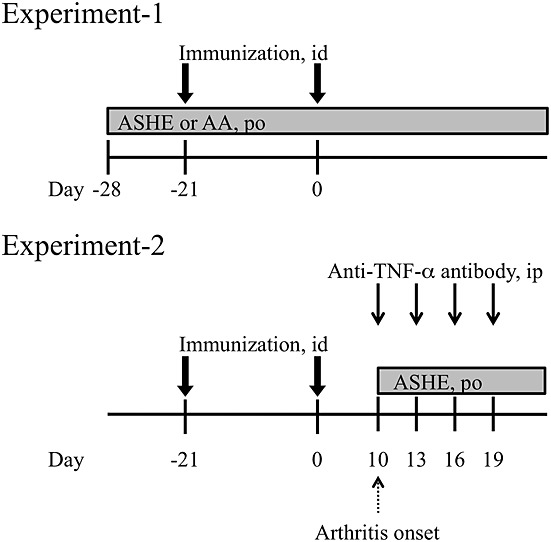
Experimental designs of collagen-induced arthritis in a mouse model. The detail is described in Materials and Methods.
Neutrophil and peripheral blood mononuclear cell isolation
Peripheral blood mononuclear cells (PBMCs) and neutrophils were purified from healthy volunteer using Ficoll-Paque (GE Healthcare, Piscataway, NJ), according to the manufacturer’s instructions. Briefly, whole blood sample was laid over Ficoll-Paque. After centrifugation, PBMCs were collected from the upper layer. Neutrophils and red blood cells were collected from the sediments. The red blood cells were lysed by hypotonic buffer, and neutrophils were purified. Purity of PBMCs and neutrophils were 96% and 93%, respectively.
Electron spin resonance spectroscopy
Radical scavenging activities of ASHE and AA were examined using electron spin resonance (ESR) spectroscopy, according to a method described (Nakayama et al., 2001). In brief, superoxide and hydroxyl radicals were generated by ultraviolet irradiation (200-W mercury arc RUF-203S, Radical Research, Inc., Tokyo, Japan) of a sodium phosphate buffer (0.1 M, pH 7.4) containing riboflavin (Sigma-Aldrich Biotechnology) or H2O2 (Sigma-Aldrich Biotechnology), respectively. The free radicals were trapped by 5-(2,2-dimethyl-1,3-propoxy cyclophosphoryl)-5-methyl-1- pyrroline-N-oxide) (Radical Research, Inc., Tokyo, Japan), and the ESR signals were recorded using JEOL FE3XG X-band spectrometer (Japan Electron Optics Laboratory, Tokyo, Japan).
Measurement of superoxide production
Superoxide production was determined by the cytochrome c reduction method using 96-well plates, according to a method described (Nagata et al., 1995). Briefly, neutrophils were suspended in PBS at a concentration of 2 × 107 cells/mL, and the reaction was initiated by mixing 200 μL of cell suspension with 1.5 mM cytochrome c and 25 µg/mL phorbol 12-myristate 13-acetate in the presence or absence of ASHE or AA. The absorbance of reduced cytochrome c was recorded at 37°C for 5 min using a spectrophotometer with a wavelength of 550 nm. Superoxide production was expressed in nmol/min/1 × 105 cells.
RNA extraction and reverse transcriptase-polymerase chain reaction
Peripheral blood mononuclear cells were plated in six-well plates at 1 × 105 cells/well and incubated for 1 h with or without 20 µg/mL LPS in the presence or absence of ASHE or AA in serum-free RPMI-1640. Total RNA was isolated using the RNeasyPlus Mini kit (Qiagen, Valencia, CA, USA) according to the manufacturer’s instructions. The cDNA was synthesized by TaqMan Reverse Transcription Reagents (Applied Biosystems, Branchburg, NJ). Polymerase chain reaction (PCR) amplification of the TNF-α, IL-6, and glyceraldehyde 3-phosphate dehydrogenase genes was performed using the following primers. For TNF-α mRNA, 5′-TCTCGAACCCCGAGTGACAA-3′ and 5′-GATGAGGGTCCAGGAGAAGT-3′ were used as forward and reverse primers, respectively. For IL-6 mRNA, 5′-ATGAACTCCTTCTCCACAAGCGC-3′ and 5′-GTCAGGTCGGACTCCCGAGAAG-3′ were used as forward and reverse primers, respectively. For internal control, glyceraldehyde 3-phosphate dehydrogenase was amplified using 5′-GCAGGGGGGAGCCAAAAGGG-3′ and 5′-TGCCAGCCCCAGCGTCAAAG-3′ were used as forward and reverse primer, respectively. The PCR products were subjected to polyacrylamide gel electrophoresis, and they were visualized and semiquantified using the ChemiDoc XRS System (Bio-Rad Laboratories, Hercules, CA, USA).
Cytokine detection by enzyme-linked immunosorbent assay
Peripheral blood mononuclear cells were plated in 96-well plates at 1 × 105 cells/well and incubated for 0, 12, or 24 h with or without 20 µg/mL LPS in the presence or absence of ASHE or AA in serum-free RPMI-1640. Protein levels of TNF-α and IL-6 of the culture supernatants were analyzed using the Human TNF-alpha/TNF-SF1A Quantikine HS enzyme-linked immunosorbent assay (ELISA) and Human IL-6 QuantiGlo ELISA kit (R&D System, Minneapolis, MN, USA), respectively, according to the manufacturer’s instructions.
Statistical analyses
Statistical analysis was performed using the Kruskall Wallis tests as appropriate. One-way analysis of variance followed by Dunnett’s post hoc test was used to compare the differences between three or more groups. The program used for the statistical analysis was the spss statistical software package, standard version 22.0 ( spss, Chicago, IL, USA). p-values <0.05 were considered statistically significant.
RESULTS
Acanthopanax senticosus Harms extract delays onset of collagen-induced arthritis and reduces arthritis severity
We first examined the effects of ASHE and AA on CIA (Fig.1, Experiment-1). In the control group, signs of arthritis appeared on day 11.3 ± 0.4 and worsened in a time-dependent manner (Tables1 and 2, Fig.2). On the other hand, mice treated with ASHE showed signs of arthritis from day 19.6 ± 0.7 (Table2). ASHE delayed development of CIA from the point of view of its onset and the peak (Tables1 and 2).
Table 1.
Day of onset and peak severity of collagen-induced arthritis evaluated by hind paw thickness
| Onset | Peak | ||
|---|---|---|---|
| DW | 12.3 ± 1.0 | 25.4 ± 0.9 | |
| AA | 11.9 ± 0.8 | 25.0 ± 1.2 | |
| ASHE | 25.4 ± 0.9 (p < 0.01) | 28.3 ± 1.0 (p < 0.01) | |
| (n = 8) |
DW, distilled water; AA, ascorbic acid; ASHE, Acanthopanax senticosus Harms extract solution.
Statistics analyses were performed between vehicle and administered groups, respectively.
Table 2.
Day of onset and peak severity of collagen-induced arthritis evaluated by clinical score
| Onset | Peak | ||
|---|---|---|---|
| DW | 11.3 ± 0.4 | 26.8 ± 1.3 | |
| AA | 9.8 ± 0.7 | 26.0 ± 1.1 | |
| ASHE | 19.6 ± 0.7 (p < 0.01) | 28.5 ± 1.1 (p < 0.01) | |
| (n = 8) |
DW, distilled water; AA, ascorbic acid; ASHE, Acanthopanax senticosus Harms extract solution.
Statistics analyses were performed between vehicle and administered groups, respectively.
Figure 2.
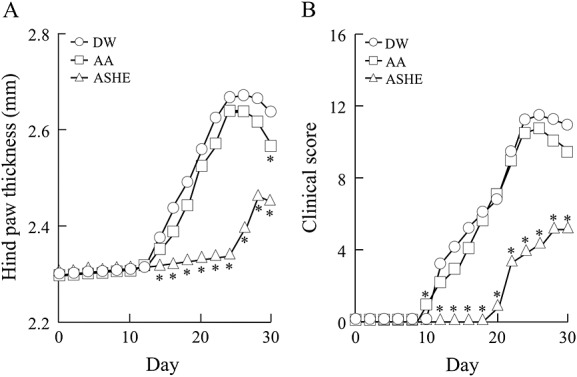
Prophylactic effects of ASHE in collagen-induced arthritis mice. Experiment-1 is carried out as described in Materials and Methods. The figure shows dot plot of hind paw thickness (A) and clinical scores (B). DW, distilled water; AA, ascorbic acid; ASHE, Acanthopanax senticosus Harms extract solution.*, statistically significant (p < 0.05) compared with the values of DW treated mice. (n = 8).
The worst clinical score and maximum hind paw thickness were better in mice treated with ASHE than the control group (Clinical score 11.8 ± 2.1 vs. 5.6 ± 0.8, p = 3.81 × 10−4; hind paw thickness 2.68 ± 0.09 mm vs. 2.47 ± 0.05 mm, p = 8.29 × 10−4) (Fig.2). Mice treated with AA had no significant differences compared with the control group in terms of arthritis development and the severity (Fig.2, Tables1 and 2).
Acanthopanax senticosus Harms extract and ascorbic acid show antioxidant activities in vitro
Next, we examined whether ASHE and AA inhibit ROS production or scavenge free radicals by cytochrome c reduction assay and ESR spectroscopy, respectively. ASHE and AA eliminated superoxide (Fig.3A) and hydroxyl radical (Fig.3B), dose-dependently. In these concentrations, ASHE and AA inhibited the superoxide production in a dose-dependent manner (Fig.3C), and the viability of neutrophils was maintained within concentrations (data not shown). These results show that both ASHE and AA have antioxidant activities in vitro.
Figure 3.
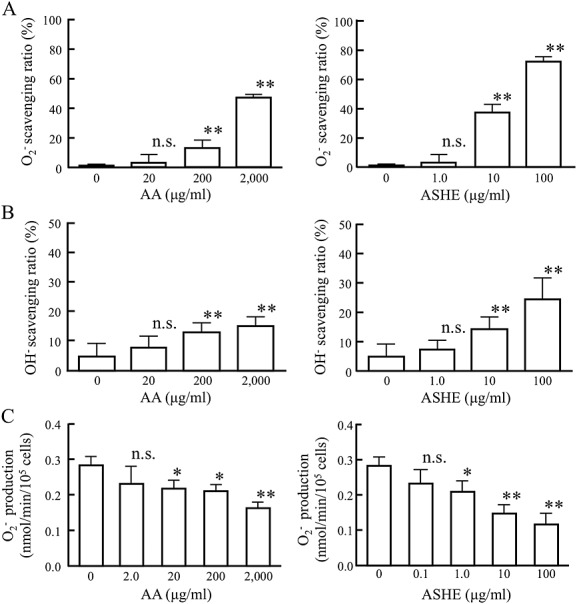
Ascorbic acid (AA) and Acanthopanax senticosus Harms extract (ASHE) scavenge free radicals and inhibits the production of superoxide in human neutrophils in vitro. Radical scavenging activities of ASHE and AA were measured as described in Materials and Methods. (A) Superoxide scavenging activity were measured by electron spin resonance spectroscopy. (B) Hydroxyl radical scavenging activity was measured by electron spin resonance spectroscopy. (C) Superoxide produced by neutrophils was measured in the presence or absence of AA or ASHE. Error bars denote +2 standard errors of the mean (n = 9). *, statistically significant (p < 0.05) compared with the values of control. **, statistically significant (p < 0.01) compared with the values of control. n.s., statistically not significant compared with the values of control.
Acanthopanax senticosus Harms extract suppresses production of inflammatory cytokines
The effect of ASHE and AA on TNF-α and IL-6 synthesis was examined by measuring mRNA levels of TNF and IL-6 of human PBMCs after LPS stimulation. Incubation of PBMCs with ASHE resulted in a concentration-dependent suppression of TNF and IL-6 mRNA expression (Fig.4A and B). Meanwhile, AA showed small effect on TNF and IL-6 mRNA production (Fig.4A and B).
Figure 4.
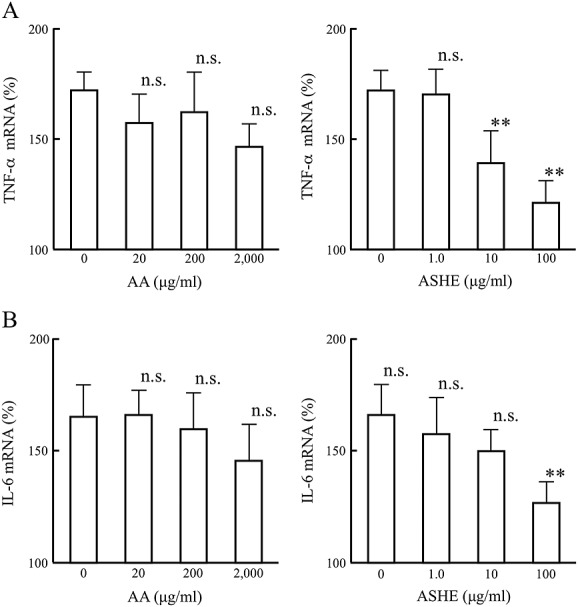
Acanthopanax senticosus Harms extract (ASHE) suppresses inflammatory cytokine production in human peripheral blood mononuclear cells. Levels of TNF-α(A) and IL-6 (B) mRNA were quantified by reverse transcriptase-polymerase chain reaction (n = 9). Levels of target mRNAs were normalized relative to those of GAPDH transcripts. Error bars denote two standard deviations. **, statistically significant (p < 0.01) compared with the values of control. n.s., statistically not significant compared with the values of control.
Enzyme-linked immunosorbent assay confirmed that production of TNF-α and IL-6 was remarkably reduced with 100 µg/mL ASHE treatment (Table3). Consistent to reverse transcription polymerase chain reaction analysis, AA showed smaller effect on TNF-α and IL-6 production, compared with ASHE.
Table 3.
Effect of ascorbic acid and Acanthopanax senticosus Harms extract on cytokine production in human peripheral blood mononuclear cells in vitro
| TNF-α (pg/mL) | IL-6 (pg/mL) | ||
|---|---|---|---|
| DW | 31.5 ± 6.2 | 57.0 ± 12.4 | |
| AA | |||
| 20 µg/mL | 28.8 ± 6.2 | 51.3 ± 5.6 | |
| 200 µg/mL | 29.8 ± 5.1 | 41.8 ± 4.0 | |
| 2,000 µg/mL | 25.2 ± 6.5 | 33.0 ± 6.3 | |
| ASHE | |||
| 1 µg/mL | 28.6 ± 5.7 | 50.0 ± 6.9 | |
| 10 µg/mL | 25.1 ± 4.0 | 38.7 ± 8.8 | |
| 100 µg/mL | 7.3 ± 1.9 (P < 0.05) | 12.2 ± 4.7 (p < 0.05) | |
| (n = 6) | |||
PBMCs, peripheral blood mononuclear cells; DW. distilled water; AA, ascorbic acid; ASHE, Acanthopanax senticosus Harms extract solution.
Statistics analyses were performed between vehicle and treatment groups, respectively.
Acanthopanax senticosus Harms extract enhances therapeutic effect of anti-TNF-α antibody
Next, we checked whether ASHE enhances therapeutic efficacy of anti-TNF-α antibody in CIA mice (Fig.1, animal experiment-2). As shown in Fig.5, combination of ASHE and anti-TNF-α antibody significantly reduced arthritis compared with anti-TNF-α antibody alone, as demonstrated by the hind paw thickness (Fig.5A) and clinical score (Fig.5B).
Figure 5.
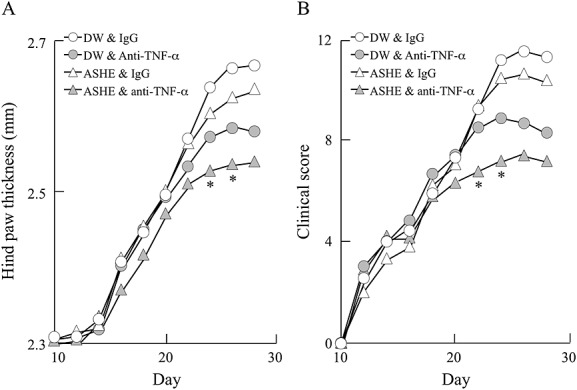
Acanthopanax senticosus Harms extract enhances the therapeutic effect of anti-TNF-α antibody. Experiment-2 is carried out as described in Materials and Methods. The figure shows the dot plot of hind paw thickness (A) and clinical scores (B). DW, distilled water; AA, ascorbic acid; ASHE, Acanthopanax senticosus Harms extract solution. *, statistically significant (p < 0.05) compared with the value of mice treated anti-TNF-α antibody alone. (n = 6).
DISCUSSION
In the present study, ASHE demonstrated prophylactic and therapeutic effects in CIA mouse model (Figs2 and 5). Moreover, ASHE showed antioxidant activity and suppressed TNF-α and IL-6 production in vitro (Figs3, and 4). To the best of our knowledge, this is the first study to show beneficial effects of ASHE on arthritis in vivo. The results, reduction of ROS and suppression of inflammatory cytokine production, are consistent with the observations reported in previous studies (Lin et al., 2008; Yi et al., 2001; Yokozawa et al., 2003; Chen et al., 2010).
Acanthopanax senticosus Harms extract and AA both had antioxidant activity in vitro (Fig.3); however, only ASHE was beneficial to CIA (Fig.2). This discrepancy could probably be attributed to: (i) AA was not able to decrease ROS in vivo to the level, which is sufficient to prevent arthritis, (ii) in addition to the reduction of ROS, inhibition of cytokine production is required to prevent arthritis, or (iii) there may be a difference between AA and ASH in terms of body clearance. Anti-CII antibody triggers arthritis in CIA (Cho et al., 2007). To gain an insight into the mechanism how ASHE delayed onset of CIA, we have monitored titers of antibody against CII in mice treated with vehicle and ASHE. As shown in Figure S1, expression of anti-CII antibody in mice treated with ASHE delayed compared to that of mice treated with vehicle. TNF-α and IL-6 play central role in immune responses including humoral immunity (Hehlgans and Pfeffer, 2005; Kishimoto et al., 1995). This suggests that ASHE, which inhibits inflammatory cytokine production, may therefore also affect the production of anti-CII antibody, thus exerting a prophylactic effect on CIA mice. These observations indicate that inhibition of cytokine production may be the key feature of ASHE to delay CIA in mouse model.
To date, 88 chemical constituents are isolated from ASHE, including volatile compounds, triterpenoid saponins, lignans, coumarins, and flavones (Huang et al., 2011a, 2011b). Among the components, chlorogenic acid, eleutheroside B (syringin), and (+)-syringaresinol-O-β-D-glucoside are able to inhibit free radical and cytokine production (Huang et al., 2011a, 2011b), the compositions of these major compounds in ASHE are shown in Table S1; therefore, these are the candidate components of ASHE that inhibit CIA.
Acanthopanax senticosus Harms extract extract is commercially available in many countries, and several clinical trials are conducted; no obvious adverse effect is reported, when it is administered as single agent (Huang et al., 2011a, 2011b). In this study, we have monitored body weight and performed blood examination every week until day 70 in mice treated with ASHE and found no unfavorable event. ASHE is a safe drug that can be used over a relatively long period.
Acanthopanax senticosus Harms extract had prophylactic effect when it was administered before primary CII immunization in CIA mouse model (Fig.2). Although, it did no reduce arthritis when it was administered as single agent after arthritis is established (Fig.5). From these observations, ASHE should be combined with nonbiological disease-modifying antirheumatic drugs or biological agents targeting inflammatory cytokines to treat active rheumatoid arthritis. Otherwise, it may be administered to patients in remission to prevent reactivation of RA.
According to a study reported by Williams et al., 50 µg of anti-TNF-α antibody per mouse is a dose that shows minimum therapeutic effect in CIA mouse model (Williams et al., 1992). In the experiment-2, we have chosen this dose of anti-TNF-α antibody to test therapeutic effects of ASHE. It is reasonable to speculate that greater therapeutic response can be obtained in CIA, if dose of anti-TNF-α antibody is increased.
In this study, we investigated the prophylactic and therapeutic effects of the supplements AA and ASHE in CIA model mice. ASHE delayed RA onset and augmented the effect of anti-TNF-α antibody therapy in CIA mouse model. Consequently, ASHE was shown to have potential to lead prophylactic effect or to support therapeutic effect of anti-TNF-α antibody. As ASHE can be commercially purchased as a supplement, it is readily available and immediately accessible to the patients.
Acknowledgments
We would like to thank Dr Hirotada Fujii (Center of Medical Education, School of Medicine, Sapporo Medical University) for helping us in the ESR spectroscopic measurement of ROS scavenging activity.
Conflict of Interest
The authors have declared that there is no conflict of interest.
Supporting Information
REFERENCES
- Bandt MD, Grossin M, Driss F, Pincemail J, Babin-Chevaye C, Pasquier C. Vitamin E uncouples joint destruction and clinical inflammation in a transgenic mouse model of rheumatoid arthritis. Arthritis Rheum. 2002;46:522–532. doi: 10.1002/art.10085. [DOI] [PubMed] [Google Scholar]
- Chen CYO, Ribaya-Mercado JD, McKay DL, Croom E, Blumberg JB. Differential antioxidant and quinone reductase inducing activity of American, Asian, and Siberian ginseng. Food Chem. 2010;119:445–451. [Google Scholar]
- Cho YG, Cho ML, Min SY, Kim HY. Type II collagen autoimmunity in a mouse model of human rheumatoid arthritis. Autoimmun Rev. 2007;7:65–70. doi: 10.1016/j.autrev.2007.08.001. [DOI] [PubMed] [Google Scholar]
- Coutenay JS, Dallman MJ, Dayan AD, Martin A, Mosedale B. Immunisation against heterologous type II collagen induces arthritis in mice. Nature. 1980;283:666–668. doi: 10.1038/283666a0. [DOI] [PubMed] [Google Scholar]
- Craven P, Derubertis F, Kagan V, Melhem M, Studer R. Effects of supplementation with vitamin C or E on albuminuria, glomerular TGF-b, and glomerular size in diabetes. J Am Soc Nephrol. 1997;8:1405–1414. doi: 10.1681/ASN.V891405. [DOI] [PubMed] [Google Scholar]
- Cuzzocrea S, McDonald MC, Mota-Filipe H, et al. Beneficial effects of tempol, a membrane-permeable radical scavenger, in a rodent model of collagen-induced arthritis. Arthritis Rheum. 2000;43:320–328. doi: 10.1002/1529-0131(200002)43:2<320::AID-ANR11>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- Dai L, Lamb DJ, Leake DS, et al. Evidence for oxidised low density lipoprotein in synovial fluid from rheumatoid arthritis patients. Free Radic Res. 2000;32:479–486. doi: 10.1080/10715760000300481. [DOI] [PubMed] [Google Scholar]
- Davydov M, Krikorian AD. Eleutherococcus senticosus (Rupr. & Maxim.) Maxim. (Araliaceae) as an adaptogen: a closer look. J Ethnopharmacol. 2000;72:345–393. doi: 10.1016/s0378-8741(00)00181-1. [DOI] [PubMed] [Google Scholar]
- Dowling EA, Redondo DR, Branch JD, Jones S, McNabb G, Williams MH. Effect of Eleutherococcus senticosus on submaximal and maximal exercise performance. Med Sci Sports Exer. 1996;28:482–489. doi: 10.1097/00005768-199604000-00013. [DOI] [PubMed] [Google Scholar]
- Feldmann M, Brennan FM, Maini RN. Rheumatoid arthritis. Cell. 1996;85:307–310. doi: 10.1016/s0092-8674(00)81109-5. [DOI] [PubMed] [Google Scholar]
- Fujikawa T, Yamaguchi A, Morita I, Takeda H, Nishibe S. Protective effects of Acanthopanax senticosus Harms from Hokkaido and its components on gastric ulcer in restrained cold water stressed rats. Biol Pharm Bull. 1996;19:1227–1230. doi: 10.1248/bpb.19.1227. [DOI] [PubMed] [Google Scholar]
- Grootveld M, Henderson EB, Farrell A, Blake DR, Parkes HG, Haycock P. Oxidative damage to hyaluronate and glucose in synovial fluid during exercise of the inflamed rheumatoid joint. Detection of abnormal low-molecular-mass metabolites by proton-N.M.R. spectroscopy. Biochem J. 1991;273:459–467. doi: 10.1042/bj2730459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajizadeh S, DeGroot J, TeKoppele JM, Tarkowski A, Collins LV. Extracellular mitochondrial DNA and oxidatively damaged DNA in synovial fluid of patients with rheumatoid arthritis. Arthritis Res Ther. 2003;5:234–240. doi: 10.1186/ar787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hehlgans T, Pfeffer K. The intriguing biology of the tumour necrosis factor/tumour necrosis factor receptor superfamily: players, rules and the games. Immunology. 2005;115:1–20. doi: 10.1111/j.1365-2567.2005.02143.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heliovaara M, Aho K, Aromaa A, Knekt P, Reunanen A. Smoking and risk of rheumatoid arthritis. J Rheumatol. 1993;20:1830–1835. [PubMed] [Google Scholar]
- Hitchon CA, El-Gabalawy HS. Oxidation in rheumatoid arthritis. Arthritis Res Ther. 2004;6:265–278. doi: 10.1186/ar1447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang L, Zhao H, Huang B, Zheng C, Peng W, Qin L. Acanthopanax senticosus: review of botany, chemistry and pharmacology. Pharmazie. 2011a;66:83–97. [PubMed] [Google Scholar]
- Huang L, Huang B, Liang J, et al. Antifatigue activity of the liposoluble fraction from Acanthopanax senticosus. Phytother Res: PTR. 2011b;25:940–943. doi: 10.1002/ptr.3346. [DOI] [PubMed] [Google Scholar]
- Iyama S, Okamoto T, Sato T, et al. Treatment of murine collagen-induced arthritis by ex vivo extracellular superoxide dismutase gene transfer. Arthritis Rheum. 2001;44:2160–2167. doi: 10.1002/1529-0131(200109)44:9<2160::aid-art369>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- Ji YR, Kim HJ, Yu DH, et al. Enforced expression of roquin protein in T cells exacerbates the incidence and severity of experimental arthritis. J Biol Chem. 2012;287:42269–42277. doi: 10.1074/jbc.M112.374835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kishimoto T, Akira S, Narazaki M, Taga T. Interleukin-6 family of cytokines and gp130. Blood. 1995;86:1243–1254. [PubMed] [Google Scholar]
- Lin QY, Jin LJ, Cao ZH, Lu YN, Xue HY, Xu YP. Acanthopanax senticosus suppresses reactive oxygen species production by mouse peritoneal macrophages in vitro and in vivo. Phytother Res: PTR. 2008;22:740–745. doi: 10.1002/ptr.2341. [DOI] [PubMed] [Google Scholar]
- McInnes IB, Schett G. Cytokines in the pathogenesis of rheumatoid arthritis. Nat Rev Immunol. 2007;7:429–442. doi: 10.1038/nri2094. [DOI] [PubMed] [Google Scholar]
- Michiels C, Minet E, Mottet D, Raes M. Regulation of gene expression by oxygen: NF-kappaB and HIF-1, two extremes. Free Radic Bio Med. 2002;33:1231–1242. doi: 10.1016/s0891-5849(02)01045-6. [DOI] [PubMed] [Google Scholar]
- Nagata M, Sedgwick JB, Busse WW. Differential effects of granulocyte-macrophage colony-stimulating factor on eosinophil and neutrophil superoxide anion generation. J Immunol. 1995;155:4948–4954. [PubMed] [Google Scholar]
- Nakayama H, Akiyama S, Shiotani S, Gotoh H, Inagaki M, Oguchi K. Evaluation of superoxide dismutase activity in dialyzed patients by electron spin resonance spectroscopy. Am J Nephrol. 2001;22:6–10. doi: 10.1159/000046667. [DOI] [PubMed] [Google Scholar]
- Nam JL, Winthrop KL, van Vollenhoven RF, et al. Current evidence for the management of rheumatoid arthritis with biological disease-modifying antirheumatic drugs: a systematic literature review informing the EULAR recommendations for the management of RA. Ann Rheum Dis. 2010;69:976–986. doi: 10.1136/ard.2009.126573. [DOI] [PubMed] [Google Scholar]
- Nishibe S, Kinoshita H, Takeda H, Okano G. Phenolic compounds from stem bark of Acanthopanax senticosus and their pharmacological effect in chronic swimming stressed rats. Chem Pharm Bull. 1990;38:1763–1765. doi: 10.1248/cpb.38.1763. [DOI] [PubMed] [Google Scholar]
- Ross AD, Banda NK, Muggli M, Arend WP. Enhancement of collagen-induced arthritis in mice genetically deficient in extracellular superoxide dismutase. Arthritis Rheum. 2004;50:3702–3711. doi: 10.1002/art.20593. [DOI] [PubMed] [Google Scholar]
- Silman AJ, Newman J, MacGregor AJ. Cigarette smoking increases the risk of rheumatoid arthritis. Results from a nationwide study of disease-discordant twins. Arthritis Rheum. 1996;39:732–735. doi: 10.1002/art.1780390504. [DOI] [PubMed] [Google Scholar]
- Valavanidis A, Vlachogianni T, Fiotakis K. Tobacco smoke: involvement of reactive oxygen species and stable free radicals in mechanisms of oxidative damage, carcinogenesis and synergistic effects with other respirable particles. Int J Environ Res Public Health. 2009;6:445–462. doi: 10.3390/ijerph6020445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valko M, Leibfritz D, Moncol J, Cronin MT, Mazur M, Telser J. Free radicals and antioxidants in normal physiological functions and human disease. Int J Biochem Cell Biol. 2007;39:44–84. doi: 10.1016/j.biocel.2006.07.001. [DOI] [PubMed] [Google Scholar]
- Williams RO, Feldmann M, Maini RN. Antitumor necrosis factor ameliorates joint disease in murine collagen-induced arthritis. Proc Natl Acad Sci U S A. 1992;89:9784–9788. doi: 10.1073/pnas.89.20.9784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi JM, Kim MS, Seo SW, Lee KN, Yook CS, Kim HM. Acanthopanax senticosus root inhibits mast cell-dependent anaphylaxis. Clin Chim Acta. 2001;312:163–168. doi: 10.1016/s0009-8981(01)00613-1. [DOI] [PubMed] [Google Scholar]
- Yi JM, Hong SH, Kim JH, Kim HK, Song HJ, Kim HM. Effect of Acanthopanax senticosus stem on mast cell-dependent anaphylaxis. J Ethnopharmacol. 2002;79:347–352. doi: 10.1016/s0378-8741(01)00403-2. [DOI] [PubMed] [Google Scholar]
- Yokozawa T, Rhyu DY, Chen CP. Protective effects of Acanthopanax Radix extract against endotoxemia induced by lipopolysaccharide. Phytother Res: PTR. 2003;17:353–357. doi: 10.1002/ptr.1145. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


