Abstract
This manuscript surveys the literature on passive sampler methods (PSMs) used in contaminated sediments to assess the chemical activity of organic contaminants. The chemical activity in turn dictates the reactivity and bioavailability of contaminants in sediment. Approaches to measure specific binding of compounds to sediment components, for example, amorphous carbon or specific types of reduced carbon, and the associated partition coefficients are difficult to determine, particularly for native sediment. Thus, the development of PSMs that represent the chemical activity of complex compound–sediment interactions, expressed as the freely dissolved contaminant concentration in porewater (Cfree), offer a better proxy for endpoints of concern, such as reactivity, bioaccumulation, and toxicity. Passive sampling methods have estimated Cfree using both kinetic and equilibrium operating modes and used various polymers as the sorbing phase, for example, polydimethylsiloxane, polyethylene, and polyoxymethylene in various configurations, such as sheets, coated fibers, or vials containing thin films. These PSMs have been applied in laboratory exposures and field deployments covering a variety of spatial and temporal scales. A wide range of calibration conditions exist in the literature to estimate Cfree, but consensus values have not been established. The most critical criteria are the partition coefficient between water and the polymer phase and the equilibrium status of the sampler. In addition, the PSM must not appreciably deplete Cfree in the porewater. Some of the future challenges include establishing a standard approach for PSM measurements, correcting for nonequilibrium conditions, establishing guidance for selection and implementation of PSMs, and translating and applying data collected by PSMs. Integr Environ Assess Manag 2014;10:167–178. © 2014 The Authors. Integrated Environmental Assessment and Management published by Wiley Periodicals, Inc. on behalf of SETAC.
Keywords: Passive sampling methods, Organic contaminants, Freely dissolved aqueous concentration, Bioavailability, Sediment-associated contaminants
Editor's note
This paper represents 1 of 6 papers in the special series “Passive Sampling Methods for Contaminated Sediments,” which was generated from the SETAC Technical Workshop “Guidance on Passive Sampling Methods to Improve Management of Contaminated Sediments,” held November 2012 in Costa Mesa, California, USA. Recent advances in passive sampling methods (PSMs) offer an improvement in risk based decision making, since bioavailability of sediment contaminants can be directly quantified. Forty four experts, representing PSM developers, users, and decision makers from academia, government, and industry, convened to review the state of science to gain consensus on PSM applications in assessing and supporting management actions on contaminated sediments.
Introduction
Contaminated sediments pose a significant global challenge for environmental risk assessment and management. One formidable barrier was the reliable prediction of contaminant bioavailability using traditional analytical methods and contaminant normalization procedures, which were often not predictive of the field environment. Recent advances in passive sampling methods (PSMs) offer a promising alternative to support risk-based decision making, because the bioavailability of sediment contaminants can be directly quantified. The current paper represents a literature review on PSMs in contaminated sediment analysis for organic contaminants (Supplemental Data Table S1) and highlights potential applications as well as technical issues that are currently limiting widespread adoption and application by the user community. The details for the application and current limitations of PSMs are addressed in detail in companion papers for organics by Mayer et al. (this issue), Ghosh et al. (this issue), and Greenberg et al. (this issue), and for metals in Peijnenburg et al. (this issue).
For decades scientists have recognized that sediments serve as both a sink and source of contaminants in aquatic ecosystems. In addition, the fate, transport, and toxicity of sediment-associated contaminants are influenced by several biological, chemical, and physical processes. Initial efforts to evaluate the impact of sediment-associated contaminants and their role in environmental processes focused on sediment quality thresholds based on bulk or “total” sediment concentrations. These assessments were complicated by the varying compositions among sediments and the different interactions of contaminants with these components. For instance, toxicity of contaminants in sediment exhibited significantly different concentration–response curves for the same contaminant among various sediments because of differences in sediment composition (DiToro et al. 1991). Research over the past several decades continued to find better approaches to interpret sediment contamination so that factors influencing contaminant exposure were better understood.
Because bioaccumulation is proportional to chemical activity in the exposure environment, better assessments of the bioaccumulation and toxicity of sediment-associated contaminants lies in clearer determination of the chemical activity of contaminants in this complex matrix. An early approach to estimate chemical activity for hydrophobic organic contaminants (HOCs) used organic carbon normalization through an organic carbon porewater partition coefficient (DiToro et al. 1991). As proposed by this approach, the route of exposure is not important provided the compound of interest comes to equilibrium in all phases and thus represents the chemical activity of the exposure. Thus, measures of chemical activity would predict the maximum exposure for an organism at equilibrium. This approach reduced the variability in predictions of bioaccumulation and toxicity of HOCs among sediments (DiToro et al. 1991), suggesting that improved measures of chemical activity would lead to improved assessments. However, attempts to apply this approach revealed variability among sediments that could not be explained with simple normalization to total organic carbon content (USEPA 2012a, 2012b). This variability was controlled by the presence of different forms of organic carbon that had different binding coefficients for HOCs than those proposed for organic carbon in sediment. Included in these materials were fresh plant matter with much lower binding constants (Kukkonen et al. 2005) and to a greater extent carbon in more chemically reduced forms (Pignatello and Xing 1996; Luthy et al. 1997; Kupryianchyk et al. 2011), including soots, chars, and other forms of organic carbon, such as weathered oils with much higher binding constants (Jonker and Barendregt 2006). Because the type of organic matter varies in and among sediments and because it is quite challenging to differentiate between the individual organic carbon components that also cannot be uniquely defined (Ghosh et al. 2003; Jonker and Koelmans 2002) or separated, this complication leaves the state of the partitioning among components unknown. An alternative approach that could provide an estimate of the chemical activity measured as Cfree is the isolation of porewater and analytical measurement of the contaminant. For this approach, relatively large volumes of porewater are required to meet analytical detection limits for dissolved HOCs (ASTM 2008). The collection and analysis lead to the potential for disturbance of the equilibrium during the separation process (ASTM 2008), and a requirement to account for the binding to dissolved organic carbon in the porewater (Morehead et al. 1986) to obtain accurate determination of Cfree. As a result, a panel of experts, convened by the National Research Council, determined that finding improved methods that would lead to a better understanding of the bioavailability of the contaminants associated with sediment independent of its composition (NRC 2003) was important.
Multiple methods have been the focus of research and development over the past few decades to more simply describe the availability of sediment-associated contaminants (NRC 2003; Menzie and Driscoll 2013); these can be divided into selective depletive (accessibility-based) techniques and equilibrium (activity-based) techniques that are the basis for PSMs. In spite of great utility for accessibility-based techniques, such as the use of Tenax extraction (Pignatello 1991), for describing the bioaccumulation of organic contaminants across sediments (Landrum et al. 2007; Mackenbach et al. 2012; Harwood, Landrum, Weston, et al. 2013), the focus of this paper is on PSMs reflecting the objectives of the SETAC technical workshop, where participants limited the scope to passive samplers as devices that are placed in contact with sediment to assess the chemical activity of the contaminant within the sediment. Passive sampling methods integrate across a range of binding phases and when at equilibrium provide a measure of the chemical activity, which is related to the concentration of freely dissolved contaminant in sediment porewater (Cfree, which in turn is related to the other potential binding phases through specific partition coefficients [Figure 1]). The chemical activity, represented by Cfree, is the driving force for all chemical interactions with sediment-associated contaminants, because the bound forms cannot participate directly in the processes governing bioavailability, diffusive exchange, and environmental reactivity of the contaminant. The bound forms only participate in such chemical interactions as sources governed by the partition coefficients and the sizes of the specifically bound contaminant pools. Having direct measures that lead to estimates of Cfree eliminates the need for a detailed understanding of sediment composition, both the content and amount of each partitioning phase, and their respective partition coefficients to establish a link to processes, such as bioaccumulation and toxicity (Oen et al. 2006; Sun and Ghosh 2008; Zielke et al. 2011; Maruya et al. 2012; Mayer et al. this issue).
Figure 1.
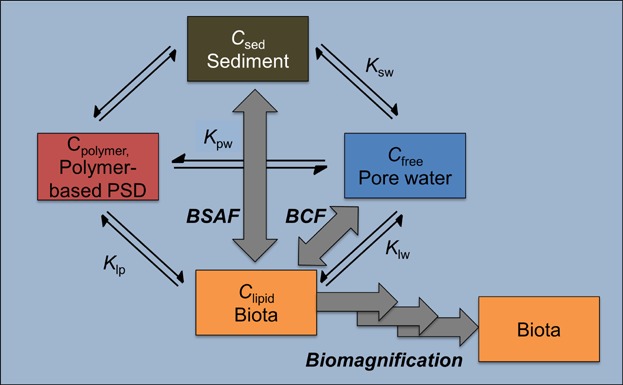
Relationship between sediment, water, and biota and the polymer-based passive sampling devices at chemical equilibrium. Toxicokinetic processes are denoted with gray arrows, and the associated partition coefficients are denoted with the equilibrium arrows. Ksw is the partition coefficient between the binding phases in sediment, usually organic carbon for hydrophobic organic contaminants, and the porewater, Kpw is the partition coefficient between the passive sampler polymer and the porewater, Klw is the partition coefficient between organism lipid and the porewater, and Klp is the partition coefficient between organism lipid and the polymer phase.
Through an extensive review of the available literature, the current paper examines the major approaches to assess the chemical activity of HOCs in sediments using passive samplers, summarizes various applications, particularly estimating bioavailability to, and highlights technical issues that are currently limiting widespread adoption of PSMs by the user community.
Results of literature survey
A summary of available PSM literature and their descriptions is listed in Supplemental Data Table S1. This summary includes 90 papers describing the application and performance of methods that use samplers of different configurations and materials for the assessment of sediment-associated organic contaminants. Of these papers, 82% were based on allowing the PSM to come to chemical equilibrium with the porewater, and 20% employed kinetic approaches generally using first-order accumulation models to estimate the equilibrium condition with the porewater (some studies used both or a combination thereof). Several experimental conditions have been evaluated, including ex situ (field sediment brought back to the laboratory and tests conducted, 61%), in situ (PSMs placed in the field, 20%), and laboratory-spiked sediment (41%), with some overlap between ex situ and in situ studies. These papers measured Cfree (84%), bioaccumulation (34%), toxicity (18%), degradation (5%), remediation (5%), or polymer–water partition coefficients (1%). Of these studies, 10% used performance reference compounds to correct for nonequilibrium conditions. Approximately half of the papers used sheets or films split between polyethylene and polyoxymethylene to make the measurements, and a similar fraction used coated fibers with the dominant polymer phase polydimethyl siloxane (PDMS). The papers were also about equally split between examining polycyclic aromatic hydrocarbons (PAHs) and chlorinated hydrocarbon contamination, with several other compound classes also included. The following sections address these studies in greater detail, and the paper concludes with a description of current technical limitations, which impede broader adoption for contaminated sediment assessment and management.
Modes of Operation, Calibration, and Design Considerations
Passive sampling methods are applied in 2 operational modes: an equilibrium mode in which sufficient time is allowed for the sampler and sediment to reach an equilibrium distribution, and a kinetic mode that targets a time-specific concentration that must be corrected to the equilibrium condition. In the kinetic mode, the time frame for the sampler exposure, characteristics of the sampler, and the behavior of the contaminants determine the kinetic state of the contaminant in the sampler for a particular experimental condition. The more important characteristics that affect the operating mode include the time to equilibrium, which increases with the polymer–water partition coefficient (Kpw) and sampler thickness, which decreases with increased sediment mass contributing to the exposure (Smedes et al. 2013). In contrast, the ability to detect contaminants increases with increasing sampler mass. Thus, the optimum characteristics of the sampler represent a tradeoff between its ability to reach equilibrium in a given exposure time and the sensitivity of the method for HOCs at low Cfree concentrations. For applications that involve simultaneous measurement of multiple contaminants over a large range of hydrophobicity, such as polychlorinated biphenyl congeners (PCBs), individual compounds may come to complete equilibrium and for some equilibrium is not achieved within the time allowed for PSM exposure. Furthermore, the detectability of a given contaminant by a PSM will vary based on the physicochemical and partitioning properties of the target analytes.
To apply samplers in various applications, they must be precalibrated to determine the time required to reach equilibrium, or the kinetic parameters described by the sampler rate dynamics must be known. In addition, the distribution of the target HOCs between the sediment and passive sampler phase must be determined at equilibrium or a predefined exposure time (kinetic mode), while also insuring nondepletive conditions. This is necessary so that equilibrium concentrations can be estimated for the appropriate calculation of Cfree (Mayer et al. 2003; Vrana et al. 2005; Ouyang and Pawliszyn 2008; You et al. 2011). To reduce equilibration times in laboratory exposures, agitation or shaking has been incorporated to enhance mass transport within the aqueous phase and increase mass transfer across the aqueous–polymer interface (Zeng et al. 2005; Yang et al. 2006; You et al. 2007; Hunter et al. 2009; Harwood et al. 2012a).
Additionally, the efficiency of the sampling method can vary in relation to the limits of detection and sampling equilibration time. For example, because of the relatively small volume of the sampling phase, solid phase microextractions (SPMEs) can exhibit relatively high limits of detection, especially for the more water-soluble compounds (Bao and Zeng 2011; You et al. 2011). Highly hydrophobic compounds have larger partition coefficients to the PDMS sampling phase than more water-soluble compounds, which results in larger mass uptake at equilibrium and thus comparatively lower limits of detection. An effective approach to lower the limits of detection is to rely on thermal desorption rather than solvent extraction to increase analytical sensitivity or use passive samplers with larger volumes of sampling phase, such as solid-phase microextraction fibers with a relatively thick PDMS coating. However, increasing the thickness (and thus volume) of the sampling phase lowers the surface to volume ratio, resulting in longer equilibration times. Although not always the case, many currently available passive samplers could take weeks to months for HOCs to reach equilibrium, particularly for highly hydrophobic compounds and under low-energy, field conditions (Mayer et al. 2000; Gschwend et al. 2011).
Passive sampling strategies at equilibrium
Equilibrium partitioning was proposed by DiToro et al. (1991) to describe the distribution of contaminants among sediment phases (e.g., sediment organic carbon, sediment porewater, and biota lipid), where the chemical activity of the target compound was equal among the 3 phases. At equilibrium, an expression relating the chemical activity in the aqueous phase, Cfree, to the activity (expressed as concentration) on the sampler phase (polymer, Cp) can be made using Equation 1:
| (1) |
where Kpw is the polymer–water partition coefficient.
Two requirements are needed for equilibrium sampling with passive samplers (Mayer et al. 2000, 2003). First, equilibrium must be reached among the different phases. Second, the sorption capacity of the sampler should be negligible compared with the exposure environment, commonly referred to as a “nondepletive” condition. This will assure accurate measurements of Cfree in the porewater, with no significant disturbance of the original sediment–porewater equilibrium condition (Yang et al. 2007). Guidance for ensuring the equilibrium and negligible depletion criteria are discussed further by Mayer et al. (this issue).
Passive samplers used in the kinetic phase
Although determining the equilibrium condition of the PSM is critical to attain good estimates of Cfree, PSMs do not always reach equilibrium. Thus, a method was needed to determine the equilibrium condition and provide an estimate of Cfree. A 2-compartment model, which describes the flux into and out of the sampler, can serve as a method to estimate equilibrium (Equation 2). In a nondepletive equilibration, the difference in chemical activity of the target compounds between the 2 phases, the source and passive sampler, drives the transport of the compound to the receiving phase (the sampler). In a well-mixed system, the chemical flux into the sampler is controlled by a static aqueous diffusion layer at the interface of the 2 phases (Bayen et al. 2009), diffusion into the polymer controlled by polymer thickness and surface area, and relies on desorption from the sediment being sufficiently fast to maintain Cfree at or near the original value. The exchange rate constant that reflects these processes (ke) dictates the time required for equilibrium to be achieved. The solubility capacity of the sampler relative to the water is described by the partition coefficient at equilibrium and is a function of the characteristics of the polymer and the target contaminant (Kpw; see Ghosh et al. this issue). The sampler dynamics can be modeled empirically using an apparent-first-order model:
| (2) |
where ke is the exchange constant, which depends on the sampler and target HOC characteristics as well as the experimental (mixing) conditions.
However, when such samplers are placed in sediments without mixing, contaminant desorption and diffusion within the sediment may effectively reduce the flux into the sampler, requiring longer exposures for the system to return to the original equilibrium state. Such conditions can slow the kinetics affecting the estimates based on kinetics determined from a well-mixed system. To correct for this condition, performance reference compounds (PRCs) that mimic the behavior of target analytes can be added to field-deployed polyethylene samplers to correct for potential nonequilibrium conditions encountered in situ (Booij et al. 2003; Tomaszewski and Luthy 2008; Fernandez, Harvey, et al. 2009). Further discussion of PRCs can be found in Ghosh et al. (this issue).
Configurations and Materials
The 2 major characteristics that define passive samplers used for measurement of Cfree are the physical size and shape of the sampler (or “configuration”) and the type of polymeric sorbent material used to construct the sampler. Essentially 2 configurations are used: 1) thin films or membranes cut into sheets or strips, and 2) coatings. Sheets and strips are homogeneous samplers that vary in thickness and physical dimensions to accommodate the experimental design, whereas coatings (also of different thicknesses) can be applied to solid supports including glass fibers and glass jars. The polymer sorbing “phase” dictates the sorption affinity (partition coefficient), whereas the phase volume in combination with the partition coefficient determines the sorption capacity for the device. Some phases including PDMS are commonly employed as coatings, whereas others, such as polyethylene (PE) and polyoxymethylene (POM), are used in sheet form. Typical phase thicknesses range between 25 and 50 μm, but they can extend from less than 10 µm to as thick as 100 μm. Supplemental Data Table S1 provides a summary of the literature organized by configuration and polymer type and provides the details for the subsequent sections.
Sheet configurations
Polyethylene
Passive samplers based on triolein-filled low-density PE tubes, also known as semipermeable membrane devices (Huckins et al. 1993; Leppänen and Kukkonen 2006; Lyytikäinen et al. 2003), were first developed for overlying water, but they have been used in sediments to a limited extent. One of the main reasons for the limited use of semipermeable membrane devices in solid matrices was the relatively long exposure time required for target HOCs to reach equilibrium between the triolein-filled PE tube and the matrix, which led to other issues, such as biofouling. To overcome this shortcoming, PE devices without a lipid reservoir (e.g., triolein) were designed to measure Cfree (Lohmann et al. 2005; Tomaszewski and Luthy 2008). Compared with semipermeable membrane devices, PE devices have the advantages of shorter equilibrium times and easier cleanup procedures. Finally, PE devices are constructed of inexpensive, commercially available sheets that can be purchased in bulk in many thicknesses (e.g., 25 and 50 μm), and they are easily fabricated in sheet or strip form to maximize surface area to volume for any sorbent mass. These features result in their ability to potentially measure ultra-low contaminant concentrations of Cfree (pg/L or lower) (Adams et al. 2007; Tomaszewski and Luthy 2008; Gschwend 2009, 2010).
Polyoxymethylene
Similar to PE, POM readily sorbs HOCs with partition coefficients similar to PE (Jonker and Koelmans 2001; Janssen et al. 2011). A distinguishing feature of POM is that sorption is likely limited to the surface, as it has been shown that sorbate diffusion coefficients are orders of magnitude lower than PDMS materials (see below), and also much lower than in PE (see below) (Ahn et al. 2005; Rusina et al. 2007; Rusina et al. 2010). The low diffusion coefficients correspond to the higher partition coefficient values observed for thinner materials (Cornelissen, Pettersen et al. 2008), further supporting the adsorption hypothesis. Thus, PSMs involving POM would in principle need to be calibrated for each thickness of material used because of differences in equilibration times among the different thicknesses. Like PE, a relatively large mass of POM can be applied in sheet configuration, resulting in estimated porewater concentrations of HOCs at pg/L levels. Compared with PE, POM has a smoother yet harder surface, which reduces the likelihood of trapping particles, such as soots, as well as reducing biofouling (Jonker and Koelmans 2001). This polymer also has a repeating ether group (-CH2-O-CH2-), resulting in a better affinity for polar compounds compared with PE and PDMS (Endo et al. 2011). The oxygen-containing groups in POM may result in nonequilibrium passive sampling (e.g., adsorption rather than absorption), which could complicate the interpretation of POM data.
Coatings
Coated fibers (“Solid phase microextraction”)
Solid-phase microextraction (SPME), developed by Arthur and Pawliszyn (1990), uses a fused silica fiber with an external polymer coating, typically PDMS, for measuring HOCs. Different coating thicknesses are available, as well as other polymer coating phases (e.g., polyacrylate), allowing the user to select a composition and/or configuration based on the target analyte(s), the operational characteristics, and the desired performance (Lambropoulou et al. 2002; ter Laak et al. 2008; Geiger 2010; Reible and Lotufo 2012).
The use of SPME fibers with an integrated syringe assembly allow for direct injection into analytical instruments, combining sampling and isolation in 1 step (Ouyang and Pawliszyn 2008), which greatly decreases the sample preparation time and requires less consumable materials (e.g., extraction solvents) than other sorbents. Disposable SPME fibers can be cut from bulk rolls of fiber optic material into custom lengths and offer the same measurement advantages as manufactured SPME fibers, at a fraction of the material cost. The use of disposable SPME is exemplified by Mayer et al. (2000) to calculate Cfree for organic contaminants in sediment porewater. Injection equipment that allows for direct thermal desorption of disposable SPME fibers followed by gas chromatography–flame ionization detection or gas chromatography mass spectroscopy quantification is commercially available and has been applied in design of PSMs (Woods et al. 2007).
Polymer-coated vials and jars
Glass vials with the interior surfaces coated with a polymer, often PDMS, were developed as an alternative chemical activity–based PSM (Minha et al. 2006; Reichenberg et al. 2008; Maenpaa et al. 2011). With high surface area and relatively thin (0.05–16 µm) polymer coatings, the time to achieve equilibrium is reduced compared with thicker passive sampler configurations. Provided exposures are nondepletive, and equilibrium can be confirmed by demonstrating that equal concentrations of target analytes are found in the polymer using multiple vials with different coating thicknesses (Reichenberg et al. 2008; Maenpaa et al. 2011). Coated vials can be used similarly to other polymer-based equilibrium ex situ methods as a means of estimating bioaccumulation (Reichenberg et al. 2008).
Glass jars coated with ethylene vinyl acetate co-polymer have also been used as a passive sampler. Results of equilibration with laboratory-spiked and field sediments were used to estimate Cfree as a measure of bioavailability of HOCs (Golding et al. 2007, 2008; Meloche et al. 2009). As with other coatings, ethylene vinyl acetate co-polymer worked well to estimate the bioavailability of PAHs and PCBs to marine species (Golding et al. 2007; Meloche et al. 2009).
Polysiloxane (i.e., silicone rubber) is unique as a passive sampler phase because it has been used both in both coating and sheet formats to estimate Cfree and bioavailability in marine sediments (Yates et al. 2011; Smedes et al. 2013). The use of silicone rubber sheets allowed determination of Cfree for a range of sampler to sediment conditions, resulting in an estimate of the Koc for the accessible fraction (Smedes et al. 2013). This study showed that for selected PAHs, only a fraction of the total concentration in field sediment was susceptible to desorption and available to contribute to Cfree.
Application to contaminated sediments
Historically, the application of passive samplers for detecting environmental contaminants in the sediment porewater began in the laboratory with ex situ applications of PSMs (Lohmann et al. 2005; Fernandez, MacFarlane, et al. 2009). Freely dissolved porewater concentrations of HOCs have been determined by placing the passive samplers directly into the sediment (Mayer et al. 2000; Conder et al. 2003; Vinturella et al. 2004; Fernandez, MacFarlane, et al. 2009; Maruya et al. 2009) or through equilibrium solid-phase extractions of sediment slurries with samplers (Kraaij et al. 2003; Mayer et al. 2003; ter Laak et al. 2006; Friedman et al. 2009; Witt et al. 2009; Friedman et al. 2011). Such laboratory-based contaminant porewater data can be used to determine spatial characterization of sediment porewater, including vertical porewater profiles for sediment core samples taken from the field to the laboratory and investigating contaminant bioavailability (Arp et al. 2011).
Passive samplers are useful for examining the variables that alter the chemical activity in sediments and can be applied to various matrices and in logistically and technically “difficult” sampling environments (Cornelissen, Arp et al. 2008; Cornelissen, Pettersen et al. 2008). As such, these samplers can be applied at all geospatial scales, using very fine resolution, as demonstrated with the multisectional PSMs capable of synchronous measurement in a sediment column (Reible and Lotufo 2012), and across the sediment–water and water–air interfaces (Bao and Zeng 2011). Ultimately broad implementation of PSMs depends on their reliability in assessing bioavailability, contaminant mobility, and risk of exposure more effectively than current approaches, for example, the use of bulk sediment concentrations or simple equilibrium partitioning models.
Furthermore, passive samplers can be placed in the top layer of sediment to calculate composite porewater concentrations (Supplemental Data Table S1; Ghosh et al. 2000; Tomaszewski and Luthy 2008; Fernandez, MacFarlane, et al. 2009; van der Heijden and Jonker 2009). Measures of vertical porewater concentration profiles also reflect the effectiveness of in situ remediation, for example, when assessing sediment capping (Eek et al. 2008; Lampert et al. 2011; Gidley et al. 2012) and amendment treatments (Hale et al. 2010; Oen et al. 2011). Incorporating vertical porewater concentrations with in situ treatment provides information on the effectiveness of remediation with depth. This application is very useful for assessing sediment capping and amendment remediation within the biologically active layer (Hale et al. 2010; Oen et al. 2011).
When using PSMs in any matrix, one must understand their capability to detect chemicals with short residency times. With respect to varying concentrations caused by variable emission sources and abiotic or biotic losses, a PSM integrates the concentration over time in accordance with the sampler kinetics. When degradation occurs in sediments, chemical activity-based approaches may not be able to assess the status of the compound. For instance, DDT was not detectable with a larger SPME type sampler compared with a smaller one at low concentrations, perhaps because of the time required to reach equilibrium and the relative stability of DDT in sediment (Maruya et al. 2009). The time to equilibrium was also thought to influence the SPME concentrations for fipronil degradation products (Brennan et al. 2009). Thus one must carefully consider and, if warranted, account for the instability of the chemical targeted when estimating calibration parameters for PSM applications (Lao et al. 2012). This concern can be partly addressed by suppressing biological activity in ex situ applications, with the addition of a biocide, such as mercuric chloride (You et al. 2011), or by using techniques that rely on kinetic measures, although kinetic approaches can have limited accuracy depending on the system.
Bioaccumulation assessments
Because they are designed to measure the chemical activity of contaminants in sediment, PSMs are appropriate for evaluating the exposure of organisms, usually expressed in terms of bioaccumulation. For instance, the ability of PSM measures of Cfree to predict bioaccumulation across a range of compounds of differing log Kow demonstrates the potential to address the complex binding that occurs in sediments, which influences bioavailability (Figure 2). Although PSMs can serve as surrogate estimates of exposure, they are limited in that some important bioaccumulation processes, such as digestive and active processes, including biotransformation, cannot be addressed. Thus, the passive sampler may not provide the same magnitude of response as observed in the organism when these conditions dominate bioaccumulation. However, passive sampler concentrations will be proportional to the observed bioaccumulated contaminant in most cases (Conder and La Point 2005; Bowen et al. 2006; You et al. 2006; You et al. 2007; Hunter et al. 2008; Trimble et al. 2008; Harwood et al. 2012b). Finally, for nondegrading target contaminants (e.g., PCBs), PSM concentrations on a polymer basis will be similar to organism concentrations on a lipid basis at equilibrium.
Figure 2.
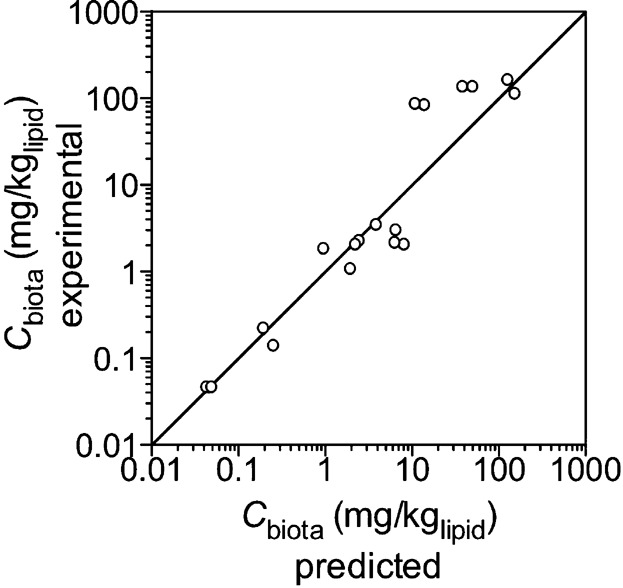
Measured versus predicted steady-state lipid normalized concentrations (Cbiota) in Tubificidae. The estimated steady-state concentrations were calculated from Cbiota = BCF * Cpore water, where the BCF is the bioconcentration factor, which was based on literature values that provided a Log Kow model from which BCF values were estimated and the Cpore water was determined with passive samplers. The solid line represents the 1:1 line for 16 organic chemicals, with log Kow values ranging from 4.6 to 7.5. Figure was adapted with permission from Kraaij et al. (2003).
Both POM and SPMEs have been effectively used to assess bioaccumulation of sediment-dwelling invertebrates. Exposing Lumbriculus variegatus to laboratory-spiked compounds in sediments from 3 river basins demonstrated that POM was a successful technique for estimating bioavailability of sediment-associated contaminants, including those with log Kow values greater than 6 (Sormunen et al. 2010). Furthermore, in a comparison of several methods, POM and SPME provided excellent estimates of bioavailability for field-exposed L. variegatus. These samplers yielded a 1:1 relationship between equilibrium-predicted bioaccumulation based on Cfree and bioaccumulation measured in the field (van der Heijden and Jonker 2009). The bioavailability of PAHs in Norwegian sediments was investigated using both polychaetes (Nereis diversicolor) and gastropods (Hinia reticulata) with POM to determine concentrations of PAHs in porewater (Cornelissen, Breedveld, NæS, et al. 2006; Ruus et al. 2010, 2013). These studies observed better correlations between Cfree as determined by POM and tissue concentrations in these species compared with tissue concentrations calculated from total sediment concentrations.
The impact of spatial placement of the sampler to capture bioaccumulation can also be detected with PSMs. A difference between in situ and ex situ uptake into passive sampling devices and biota for POM samplers depended on the sediment depth represented by the experimental design. Samplers deployed in the top 0.5 cm in situ correlated with in situ bioaccumulation data for Neanthes arenaceodentata but were not as well correlated to laboratory bioaccumulation, because the samples used in the laboratory exposures represented an integrated depth sample (Janssen et al. 2011). In addition, the passive sampling device was better able to estimate exposure than either exhaustive extraction or mild solvent extraction of bulk sediments, but the variation in predictability was approximately a factor of 10 (Janssen et al. 2011).
Solid-phase microextraction was also found to provide reliable assessments of bioaccumulation (Conder and La Point 2005; Hawthorne et al. 2007; Sormunen et al. 2008; Wang et al. 2011; You et al. 2011; Reible and Lotufo 2012). Although most applications have been performed in the laboratory, a recent study with L. variegatus and SPME exposed in situ at several sites contaminated with PAHs found that SPME effectively predicted the bioaccumulation of the worms within a factor of 4 by accounting for site-specific bioavailability (Muijs and Jonker 2012). This confirms a previous set of in situ exposures with L. variegatus and SPME in sediments (van der Heijden and Jonker 2009). However, because of analytical detection limitations, the SPME as deployed was limited in providing measured values for some PAHs, which were found in worms and sediment from the same system (van der Heijden and Jonker 2009). In a proof of concept demonstration, Burton et al. (2012) deployed benthic organisms and passive samplers in a marine setting, and qualitative comparisons were made between the samplers and accumulation in mussels. Similar patterns and concentrations of selected PAHs were found in both media.
Two concerns directly relate to active processes in organisms. First is biotransformation, where the prediction by the PSM may overestimate bioaccumulation (Echols et al. 2000), and the second is when feeding behavior can be modified, affecting the flux into the organism (Lyytikäinen et al. 2003). However, studies have determined that PSMs can estimate bioaccumulation and toxicity for biotransformed and toxic compounds (Ding et al. 2012a, 2012b; Harwood et al. 2012b; Harwood, Landrum, and Lydy 2013; Harwood, Landrum, Weston et al., 2013). Data collected suggest a need for a more complete examination of compounds and conditions once standardized methods are established, as discussed in Ghosh et al. (this issue).
Strong evidence suggests that the SPME approach can produce consistent results with laboratory-exposed organisms regardless of whether they are exposed to field-contaminated or laboratory-spiked sediments (Wang et al. 2011; You et al. 2011). A relationship between SPME concentrations or the corresponding Cfree has been correlated to Lumbriculus bioaccumulation from sediment for several compounds, including PCBs, PAHs, and some pesticides (You et al. 2006; You, Pehkonen, et al. 2007; Trimble et al. 2008; Hunter et al. 2009; Harwood et al. 2012b). Some limitations for the intercomparison of SPME data from these studies may exist, in part, from an absence of a standard approach for evaluating the data, because some studies focused on bioconcentration factors (Vinturella et al. 2004; Muijs and Jonker 2012), whereas others reported lipid-normalized body residues (You et al. 2006). These methods, which link bioaccumulation to exposure in terms of the chemical activity, are expected to produce consistent predictive relationships. However, standardization and comparative studies would lead to improved confidence in the method for management applications (see Greenberg et al. this issue).
Toxicity assessments
Although fewer in number compared with studies that have addressed bioaccumulation, (Supplemental Data Table S1), examples in the literature illustrate that toxicity assessments using PSMs can be accomplished through several approaches. For example, the sampler can provide a measure of Cfree in porewater that can be compared with water quality criteria (Maruya et al. 2012). This approach was employed for evaluating the toxicity of sediment-associated PAHs and comparing the toxic response of Hyalella azteca with the number of toxic units calculated from the SPME-determined Cfree, which were based on the US Environmental Protection Agency (USEPA) sum toxic unit model (Kreitinger et al. 2007). Similarly, the toxicity of pyrethroids was found to be independent of sediment characteristics when the toxic response was based on Cfree, once again determined using SPME (Xu et al. 2007; Harwood, Landrum, and Lydy 2013). Finally, one may develop a direct application of the passive sampler concentration by determining the relationship between the sampler concentration and the response endpoint, such as mortality for individual species (Conder, La Point, et al. 2004; Conder, Lotufo, et al. 2004; Zielke et al. 2011; Rosen et al. 2012; Harwood, Landrum, and Lydy 2013). Evidence that these approaches are interchangeable has been demonstrated in 2 papers, in which chironomids and amphipods were exposed in water-only solutions (Ding et al. 2012a, 2012b) and the ability to apply the values to sediment was demonstrated (Ding et al. 2013).
Comparative and Integrated studies
Relatively few studies compare Cfree measurements in sediment porewater using multiple PSMs. Most of these studies reported agreement in estimates of Cfree to within a factor of 3. When POM and PDMS tubing were compared to assess bioaccumulation of native PAHs in N. virens for marine sediments and L. variegatus for freshwater sediments, biota sediment accumulation factors calculated for the worms were compared with biota sediment accumulation factors calculated based on the values of Cfree; the resulting values were found to vary within a factor of 2 for POM and by a factor of 20 for the PDMS tubing (Barthe et al. 2008). A second study compared SPME-PDMS and POM to assess bioaccumulation of PAHs in L. variegatus in both laboratory and field exposure scenarios (Jonker and van der Heijden 2007). Bioconcentration factors (BCFs) derived from field and laboratory exposures with SPME were similar in magnitude; in addition, BCF values derived from POM and SPME were also comparable. In a subsequent study, van der Heijden and Jonker (2009) investigated the bioavailability of sediment-associated PAHs to L. variegatus exposed in situ at 3 locations, using POM and SPME to determine Cfree. The best agreement was observed for in situ SPME and laboratory-based POM estimates of Cfree. A more recent study compared in situ bioaccumulation in aquatic worms and application of SPME at several sites contaminated with petroleum hydrocarbons with ex situ application of POM on samples collected from these sites (Muijs and Jonker 2011). The in situ SPME appeared less suitable for predicting bioaccumulation of oil constituents than the laboratory-based POM application, which allowed prediction to within a factor of 3 of measured worm tissue concentrations.
One of the first interlaboratory exercises focused on analytical variability between 13 laboratories and 1 reference laboratory. For each tested sample, participants were instructed to expose field-collected sediment using silicone rubber passive sampling device (10-µm coated inside bottles), followed by determination of Cfree for PAHs and PCBs (Smedes, Davies, et al. 2007; Smedes, van der Zande, et al. 2007). The resulting PAH water concentrations were within a factor of 2 between the participating laboratories and the reference laboratory; however, because of the lower concentrations used, higher variability was observed in the more limited data set for PCBs (Smedes, van der Zande, et al. 2007). A subsequent exercise compared 3 different PSMs (SPME-PDMS, POM, and PE) applied to a single PCB-contaminated sediment in not-mixed and well-mixed laboratory exposure modes (Gschwend et al. 2011). Both PE and SPME-PDMS were evaluated in both exposure modes, whereas POM was applied only in the well-mixed exposure mode. In addition, PSM measurements of Cfree (for the sum of targeted PCB congeners) were compared with aqueous concentrations determined using an air bridge, an apparatus that produces a water sample with uniform Cfree reflecting the “true” activity of the sediment sample investigated. The PSM-derived and air bridge Cfree measurements obtained by conventional extraction methods agreed within 20% for the well-mixed exposures, whereas the PSMs applied in the static mode agreed within a factor of 2. The authors also found good correlations (r2 values ranged from 0.64 to 0.91) for total PCB tissue concentrations in the polychaete Neanthes arenaceodentata and PSM data (Gschwend et al. 2011).
Passive samplers have also been applied in the static mode to investigate the remedial efficacy of activated carbon–amended sediment (Cho et al. 2009; Oen et al. 2011). Polyethylene passive samplers pre-impregnated with PRCs were employed to measure Cfree for PCBs in this study, and produced good correlations between PCB uptake into co-deployed samplers (Cho et al. 2009). This work was expanded by comparing PCB interstitial water profile concentrations determined using PE and POM (both impregnated with PRCs) (Oen et al. 2011). Similar to previous studies, measured and calculated values of Cfree were within a factor of 2 in this study.
Passive samplers have also been incorporated into platforms that gather multiple lines of evidence on sediment quality in situ. One such platform is the “SEA ring” (Burton et al. 2012; Rosen et al. 2012), a polycarbonate carousel that houses various chambers for toxicity and bioaccumulation exposures, PSDs, and other water quality instruments. The SEA ring chamber design allows for exposures in the water column, at the sediment–water interface and in surficial sediment. These platforms were deployed at selected stations in San Diego Bay (Burton et al. 2012) with test organisms to assess sediment toxicity, including amphipods (E. estuarius), polychaetes (N. arenaceodentata), mysid shrimp (A. bahia), and mussels (M. galloprovincialis) to test for toxicity at the sediment–water interface. Bioaccumulation was assessed using mussels (Musculista senhousia) and polychaetes (N. arenaceodentata), and Cfree for PAHs were determined using SPME-PDMS. Integration of multiple lines of evidence provided by platforms, such as the SEA ring, allow for linkages between field exposure and effects to be investigated and established (Rosen et al. 2012).
A large-scale program focusing on validation of PSM systems has highlighted consistency between passive sampling of water in the field, native and deployed mussels, and sediment PSMs in the laboratory. Between 2006 and 2007, the International Council for the Exploration of the Sea orchestrated an extensive Passive Sampling Trial Survey involving 12 European and 1 Australian laboratory. The goal was to investigate sources of uncertainty associated with PSM application and chemical analyses, using marine bivalves to compare HOC uptake by samplers deployed in water and sediment across 31 estuarine and coastal marine locations (Smedes, Davies, et al. 2007; Smedes, van der Zande, et al. 2007). The Passive Sampling Trial Survey was able to confirm environmental validation of passive sampling in water and sediment through multiple replicated parallel analyses of uptake in mussels versus passive samplers, and concentrations in sediments reflecting known contaminant levels. The project also validated the application potential of passive samplers across a wide geospatial scale from cold-temperate to sub-tropical regions.
Research and Application needs
Drawing on this review, workshop participants identified 3 primary focus areas to enable widespread utilization of PSMs for contaminated sediment assessment and management: 1) establish a unifying science-based theoretical framework for applying PSMs that target Cfree in sediment assessment, addressed in Mayer et al. (this issue) and Peijnenburg et al. (this issue) for organics and metals, respectively; 2) provide practical guidance for selection and implementation of PSMs, including standardization of calibration parameters (e.g., partition coefficients), establishment of equilibrium (or correction for non-attainment thereof), and nondepletion and quality assurance or quality control provisions for reliable estimation of Cfree, addressed in Ghosh et al. (this issue); and 3) describe application of Cfree measurements using PSMs in sediment risk management decisions, addressed in Greenberg et al. (this issue).
Despite progress to date, uncertainties still remain relative to PSM selection, equilibration times, fouling impact, and linkage to specific biological endpoints. The most critical criteria to establish the use of chemical activity-based PSMs to provide good estimates of pore water concentrations is the availability of accurate values of Kpw. Currently there is a shortage of high quality Kpw data available, which represents a current challenge for the application of PSMs (see Ghosh et al. this issue). For organics, the use of PRCs to correct for nonequilibrium conditions attributable to abbreviated exposure times or biofouling clearly requires additional coverage. Limited discussion has been focused on whether the use of PRCs is necessary to compensate for realistic environmental concentrations in aquatic environments. This is an area in which current standard approaches are missing, and guidance should reflect the ever-changing state-of-the-art (Mayer et al. this issue; Ghosh et al. this issue). Furthermore, limited comparisons are available in the literature among samplers, environmental phases, and laboratories; therefore, improvement in this area is also required before widespread implementation of PSMs for regulatory use (see Ghosh et al. this issue).
Conclusions
The workshop focused on passive sampling methods that lead to measuring and/or estimating the chemical activity of contaminants in sediment, which is expressed as Cfree. Multiple applications can be identified in the literature for PSM use, including determining spatial gradients, bioaccumulation, toxicity, and evaluating remedial efficacy. Studies that illustrate the utility of PSMs for these applications have used samplers composed of various polymeric sorbents (e.g., polysiloxanes, polyethylene, polyoxymethylene) configured as thin films (sheets or strips) or coated onto solid supports (e.g., fibers, glass jars). The literature also illustrates that passive samplers have been employed for examining conditions in laboratory-dosed, field-collected, and in situ field sediments. The development of these methods has improved the detection of HOCs to the nanograms per liter range and in some cases at pictogram per liter concentrations. For simplicity and robustness of Cfree measurements, most published studies have employed PSMs in the equilibrium operating mode, as opposed to kinetic phase measurement, which requires correction to attain the equilibrium condition or precalibration (i.e., time-dependent) of samplers that are system dependent. The ideal application is to expose samplers until equilibrium is achieved. The design of PSMs represents a tradeoff between desired sensitivity (which influences selection of sampler mass and/or thickness), the practical need to achieve equilibrium within a finite time frame, and the pros and cons associated with ex situ and in situ applications. Whereas the use of PSMs in sediments has been reported in a wide range of environments from fresh water to marine conditions as well as across different regions, further needs for advancing broader use of PSMs are addressed in companion papers in this series (Peijnenburg et al. this issue; Mayer et al. this issue; Ghosh et al. this issue; Greenberg et al. this issue).
Acknowledgments
Support for the workshop is gratefully acknowledged from ExxonMobil Corporation, Southern California Coastal Water Research Project (SCCWRP), US Department of Defense Strategic Environmental Research and Development Program, Department of Environmental Sciences, University of California, Riverside, and Society of Environmental Toxicology and Chemistry (SETAC). The participants thank Nikki Mayo and Greg Schiefer (SETAC), and Angelica Bajza, Maribel Gonzalez, and Stephen Weisberg (SCCWRP) for assistance in workshop planning. We also thank Wenjian Lao, Abigail Joyce, Mallory Pirogovsky, and Kai Zhang for their assistance during the workshop.
Supplemental data
All Supplemental Data may be found in the online version of this article.
Application of passive sampling methods that target the freely dissolved concentration (Cfree) in sediment.
References
- Adams RG, Lohmann R, Fernandez LA, MacFarlane JK, Gschwend PM. Polyethylene devices: Passive samplers for measuring dissolved hydrophobic organic compounds in aquatic environments. Environ Sci Technol. 2007;41:1317–1323. doi: 10.1021/es0621593. [DOI] [PubMed] [Google Scholar]
- Ahn S, Werner D, Werner D, Karapanagioti HK, McGlothlin DR, Zare RN, Luthy RG. Phenanthrene and pyrene sorption and intra-particle diffusion in polyoxymethylene, coke, and activated carbon. Environ Sci Technol. 2005;39:6516–6526. doi: 10.1021/es050113o. [DOI] [PubMed] [Google Scholar]
- Arp HPH, Viller F, Lepland A, Kalaitzidis S, Christanis K, Oen AMP, Breedveld GD, Cornelissen G. Influence of historical industrial epochs on pore water and partitioning profiles of polycyclic aromatic hydrocarbons and polychlorinated biphenyls in Oslo Harbor, Norway, sediment cores. Environ Toxicol Chem. 2011;30:843–851. doi: 10.1002/etc.466. [DOI] [PubMed] [Google Scholar]
- Arthur CL, Pawliszyn J. Solid phase microextraction with thermal desorption using fused silica optical fibers. Anal Chem. 1990;62:2145–2148. [Google Scholar]
- American Society for Testing and Materials (ASTM) 2008. Standard test method for measuring the toxicity of sediment-associated contaminants with estuarine and marine invertebrates. E1367.
- Bao L, Zeng EY. Passive sampling techniques for sensing freely dissolved hydrophobic organic chemicals in sediment porewater. Trends Anal Chem. 2011;30:1422–1428. [Google Scholar]
- Barthe M, Pelletier E, Breedveld GD, Cornelissen G. Passive samplers versus surfactant extraction for the evaluation of PAH availability in sediments with variable levels of contamination. Chemosphere. 2008;71:1486–1493. doi: 10.1016/j.chemosphere.2007.12.015. [DOI] [PubMed] [Google Scholar]
- Bayen S, ter Laak TL, Buffle J, Hermens JLM. Dynamic exposure of organisms and passive samplers to hydrophobic chemicals. Environ Sci Technol. 2009;43:2206–2215. doi: 10.1021/es8029895. [DOI] [PubMed] [Google Scholar]
- Booij K, Hoedemaker JR, Bakker JF. Dissolved PCBs, PAHs and HCB in pore waters and overlying waters of contaminated harbor sediments. Environ Sci Technol. 2003;37:4213–4220. doi: 10.1021/es034147c. [DOI] [PubMed] [Google Scholar]
- Bowen AT, Conder JM, La Point TW. Solid phase microextraction of aminodinitrotoluenes in tissue. Chemosphere. 2006;63:58–63. doi: 10.1016/j.chemosphere.2005.07.057. [DOI] [PubMed] [Google Scholar]
- Brennan AA, Harwood AD, You J, Landrum PF, Lydy MJ. Degradation of fipronil in anaerobic sediments and the effect on pore water concentrations. Chemosphere. 2009;77:22–28. doi: 10.1016/j.chemosphere.2009.06.019. [DOI] [PubMed] [Google Scholar]
- Burton GA, Jr, Rosen G, Chadwick DB, Greenberg MS, Taulbee WK, Lotufo GR, Reible DD. A sediment ecotoxicity assessment platform for in situ measures of chemistry, bioaccumulation and toxicity. Part 1: System description and proof of concept. Environ Pollut. 2012;162:449–456. doi: 10.1016/j.envpol.2011.11.018. [DOI] [PubMed] [Google Scholar]
- Cho YM, Ghosh U, Kennedy AJ, Grossman A, Ray G, Tomaszewski JE, Smithenry DW, Bridges TS, Luthy RG. Field application of activated carbon amendment for in-situ stabilization of polychlorinated biphenyls in marine sediment. Environ Sci Technol. 2009;43:3815–3823. doi: 10.1021/es802931c. [DOI] [PubMed] [Google Scholar]
- Conder JM, La Point TW, Lotufo GR, Steevens JA. Nondestructive, minimal-disturbance, direct-burial solid-phase microextraction fiber technique for measuring TNT in sediment. Environ Sci Technol. 2003;37:1625–1632. doi: 10.1021/es0260770. [DOI] [PubMed] [Google Scholar]
- Conder JM, La Point TW, Steevens JA, Lotufo GR. Recommendations for the assessment of TNT toxicity in sediment. Environ Toxicol Chem. 2004;23:141–149. doi: 10.1897/03-137. [DOI] [PubMed] [Google Scholar]
- Conder JM, Lotufo GR, Bowen AT, Turner PK, La Point TW, Steevens JA. Solid phase microextraction fibers for estimating the toxicity of nitroaromatic compounds. Aquat Ecosys Health Manage. 2004;7:387–397. [Google Scholar]
- Conder JM, La Point TW. Solid-phase microextraction for predicting the bioavailability of 2,4,6-trinitrotoluene and its primary transformation products in sediment and water. Environ Toxicol Chem. 2005;24:1059–1066. doi: 10.1897/04-484r.1. [DOI] [PubMed] [Google Scholar]
- Cornelissen G, Breedveld GD, Næs K, Oen AMP, Ruus A. Bioaccumulation of native polycyclic aromatic hydrocarbons from sediment by a polychaete and a gastropod: Freely dissolved concentrations and activated carbon amendment. Environ Toxicol Chem. 2006;25:2349–2355. doi: 10.1897/06-026r.1. [DOI] [PubMed] [Google Scholar]
- Cornelissen G, Arp HPA, Pettersen A, Hauge A, Breedveld GD. Assessing PAH and PCB emissions from the relocation of harbor sediments using equilibrium passive samplers. Chemosphere. 2008;72:1581–1587. doi: 10.1016/j.chemosphere.2008.04.041. [DOI] [PubMed] [Google Scholar]
- Cornelissen G, Pettersen A, Broman D, Mayer P, Breedveld GD. Field testing of equilibrium passive samplers to determine freely dissolved native polycyclic aromatic hydrocarbon concentrations. Environ Toxicol Chem. 2008;27:499–508. doi: 10.1897/07-253.1. [DOI] [PubMed] [Google Scholar]
- Cornelissen G, Wiberg K, Broman D, Arp HPH, Persson Y, Sundqvist K, Jonsson P. Freely dissolved concentrations and sediment-water activity ratios of PCDD/Fs and PCBs in the open Baltic Sea. Environ Sci Technol. 2008;42:8733–8739. doi: 10.1021/es8018379. [DOI] [PubMed] [Google Scholar]
- Ding Y, Landrum PF, You J, Harwood AD, Lydy MJ. Use of solid phase microextraction to estimate toxicity: Relating fiber concentrations to toxicity—part 1. Environ Toxicol Chem. 2012a;31:2159–2167. doi: 10.1002/etc.1935. [DOI] [PubMed] [Google Scholar]
- Ding Y, Landrum PF, You J, Harwood AD, Lydy MJ. Use of solid phase microextraction to estimate toxicity: Relating fiber concentrations to body residues-part 2. Environ Toxicol Chem. 2012b;31:2168–2174. doi: 10.1002/etc.1936. [DOI] [PubMed] [Google Scholar]
- Ding Y, Landrum PF, You J, Lydy MJ. Assessing bioavailability and toxicity of permethrin and DDT in sediment using matrix solid phase microextraction. Ecotoxicology. 2013;22:109–117. doi: 10.1007/s10646-012-1007-z. [DOI] [PubMed] [Google Scholar]
- DiToro DM, Zarba CS, Hansen DJ, Berry WJ, Swartz RC, Cowan CE, Pavlou SP, Allen HE, Thomas NA, Paquin PR. Technical basis for establishing sediment quality criteria for nonionic organic chemicals using equlibrium partitioning. Environ Toxicol Chem. 1991;10:1541–1583. [Google Scholar]
- Echols KR, Gale RW, Schwartz TR, Huckins JN, Williams LL, Meadows JC, Morse D, Petty JD, Orazio CE, Tillitt DE. Comparing polychlorinated biphenyl concentrations and patterns in the Saginaw River using sediment, caged fish, and semipermeable membrane devices. Environ Sci Technol. 2000;34:4095–4102. [Google Scholar]
- Eek E, Cornelissen G, Kibsgaard A, Breedveld GD. Diffusion of PAH and PCB from contaminated sediments with and without mineral capping: Measurement and modelling. Chemosphere. 2008;71:1629–1638. doi: 10.1016/j.chemosphere.2008.01.051. [DOI] [PubMed] [Google Scholar]
- Endo S, Hale SE, Goss KU, Arp HPH. Equilibrium partition coefficients of diverse polar and nonpolar organic compounds to polyoxymethylene (POM) passive sampling devices. Environ Sci Technol. 2011;45:10124–10132. doi: 10.1021/es202894k. [DOI] [PubMed] [Google Scholar]
- Fernandez LA, Harvey CF, Gschwend PM. Using performance reference compounds in polyethylene passive samplers to deduce sediment porewater concentrations for numerous target chemicals. Environ Sci Technol. 2009;43:8888–8894. doi: 10.1021/es901877a. [DOI] [PubMed] [Google Scholar]
- Fernandez LA, MacFarlane JK, Tcaciuc AP, Gschwend PM. Measurement of freely dissolved PAH concentrations in sediment beds using passive sampling with low-density polyethylene strips. Environ Sci Technol. 2009;43:1430–1436. doi: 10.1021/es802288w. [DOI] [PubMed] [Google Scholar]
- Friedman CL, Burgess RM, Perron MM, Cantwell MG, Ho KT, Lohmann R. Comparing polychaete and polyethylene uptake to assess sediment resuspension effects on PCB bioavailability. Environ Sci Technol. 2009;43:2865–2870. doi: 10.1021/es803695n. [DOI] [PubMed] [Google Scholar]
- Friedman CL, Lohmann R, Burgess RM, Perron MM, Cantwell MG. Resuspension of polychlorinated biphenyl-contaminated field sediment: Release to the water column and determination of site-specific Kdoc. Environ Toxicol Chem. 2011;30:377–384. doi: 10.1002/etc.408. [DOI] [PubMed] [Google Scholar]
- Geiger SC. 2010. Final report: The determination of sediment polycyclic aromatic hydrocarbon (PAH) bioavailability using direct pore water analysis by solid-phase microextraction (SPME). ESTCP Project No. ER-200709. ESTCP, Alexandria, VA. [cited 2012 November 12]. http://serdp-estcp.org/content/download/14052/165034/file/ER-200709-FR.pdf.
- Ghosh U, Luthy RG, Gillette JS, Zare RN. Micro-scale location, characterization, and association of polycyclic aromatic hydrocarbons on harbor sediment particles. Environ Sci Technol. 2000;34:1729–1736. [Google Scholar]
- Ghosh U, Zimmerman J, Luthy RG. PCB and PAH speciation among particle types in contaminated sediments and effects on PAH bioavailability. Environ Sci Technol. 2003;37:2209–2217. doi: 10.1021/es020833k. [DOI] [PubMed] [Google Scholar]
- Ghosh U, Kane Driscoll S, Burgess R, Gobas FAPC, Maruya K, Jonker C, Gala W, Choi Y, Beegan C, Apitz S. Passive sampling methods for contaminated sediments: Practical guidance for selection, calibration and implementation. Integr Environ Assess Manag. 2014;10:210–223. doi: 10.1002/ieam.1507. et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gidley PT, Kwon S, Yakirevich A, Magar VS, Ghosh U. Advection dominated transport of polycyclic aromatic hydrocarbons in amended sediment caps. Environ Sci Technol. 2012;46:5032–5039. doi: 10.1021/es202910c. [DOI] [PubMed] [Google Scholar]
- Golding CJ, Gobas FAPC, Birch G. Characterization of polycyclic aromatic hydrocarbon bioavailability in estuarine sediments using thin-film extraction. Environ Toxicol Chem. 2007;26:829–836. doi: 10.1897/06-378r.1. [DOI] [PubMed] [Google Scholar]
- Golding CJ, Gobas FAPC, Birch G. A fugacity approach for assessing the bioaccumulation of hydrophobic organic compounds from estuarine sediment. Environ Toxicol Chem. 2008;27:1047–1054. doi: 10.1897/07-457.1. [DOI] [PubMed] [Google Scholar]
- Greenberg MS, Chapman PM, Allan IJ, Anderson KA, Apitz SE, Beegan C, Bridges TS, Brown SS, Cargill JG, IV, McCulloch MC. Passive sampling methods for contaminated sediments: Risk assessment and management. Integr Environ Assess Manag. 2014;10:224–236. doi: 10.1002/ieam.1511. et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gschwend PM. 2009. Passive polyethylene sampling in support of in situ remediation of contaminated sediments. ESTCP Project No. ER-200915. ESTCP, Alexandria, VA. [cited 2012 November 12]. Available from. http://serdp.org/Program-Areas/Environmental-Restoration/Contaminated-Sediments/ER-200915/ER-200915.
- Gschwend PM. 2010. Final report: Using passive polyethylene samplers to evaluate chemical activities controlling fluxes and bioaccumulation of organic contaminants in bed sediments. SERDP Project No. ER-1496. SERDP, Alexandria, VA. [cited 2012 November 12]. Available from: http://serdp.org/Program-Areas/Environmental-Restoration/Contaminated-Sediments/ER-1496/ER-1496/(language)/eng-US.
- Gschwend PM, MacFarlane JK, Reible DD, Lu X, Hawthorne SB, Nakles DV, Thompson T. Comparison of polymeric samplers for accurately assessing PCBs in pore waters. Environ Toxicol Chem. 2011;30:1288–1296. doi: 10.1002/etc.510. [DOI] [PubMed] [Google Scholar]
- Hale SE, Tomaszewski JE, Luthy RG, Werner D. Sorption of dichlorodiphenyltrichloroethane (DDT) and its metabolites by activated carbon in clean water and sediment slurries. Water Res. 2009;43:4336–4346. doi: 10.1016/j.watres.2009.06.031. [DOI] [PubMed] [Google Scholar]
- Hale SE, Meynet P, Davenport RJ, Jones DM, Werner D. Changes in polycyclic aromatic hydrocarbon availability in River Tyne sediment following bioremediation treatments or activated carbon amendment. Water Res. 2010;44:4529–4536. doi: 10.1016/j.watres.2010.06.027. [DOI] [PubMed] [Google Scholar]
- Harwood AD, Landrum PF, Lydy MJ. A comparison of exposure methods for SPME-based bioavailability estimates. Chemosphere. 2012a;86:506–511. doi: 10.1016/j.chemosphere.2011.10.015. [DOI] [PubMed] [Google Scholar]
- Harwood AD, Landrum PF, Lydy MJ. Can SPME fiber and Tenax methods predict the bioavailability of biotransformed insecticides. Environ Sci Technol. 2012b;46:2413–2419. doi: 10.1021/es2035174. [DOI] [PubMed] [Google Scholar]
- Harwood AD, Landrum PF, Lydy MJ. Bioavailability-based toxicity endpoints of bifenthrin for Hyalella azteca and Chironomus dilutus. Chemosphere. 2013;90:1117–1122. doi: 10.1016/j.chemosphere.2012.09.017. [DOI] [PubMed] [Google Scholar]
- Harwood AD, Landrum PF, Weston DP, Lydy MJ. Using SPME fibers and Tenax to predict the bioavailability of pyrethroids and chlorpyrifos in field sediments. Environ Pollut. 2013;173:47–51. doi: 10.1016/j.envpol.2012.09.012. [DOI] [PubMed] [Google Scholar]
- Hawthorne SB, Azzolina NA, Neuhauser EF, Kreitinger JP. Predicting bioavailability of sediment polycyclic aromatic hydrocarbons to Hyalella azteca using equilibrium partitioning, supercritical fluid extraction, and pore water concentrations. Environ Sci Technol. 2007;41:6297–6304. doi: 10.1021/es0702162. [DOI] [PubMed] [Google Scholar]
- Huckins JN, Manuweera GK, Petty JD, Mackay D, Lebo JA. Lipid-containinig semipermeable membrane devices for monitoring organic contaminants in water. Environ Sci Technol. 1993;27:2489–2496. [Google Scholar]
- Hunter W, Xu Y, Spurlock F, Gan J. Using disposable polydimethylsiloxane fibers to assess the bioavailability of permethrin in sediment. Environ Toxicol Chem. 2008;27:568–575. doi: 10.1897/07-335.1. [DOI] [PubMed] [Google Scholar]
- Hunter W, Yang Y, Reichenberg F, Mayer P, Gan J. Measuring pyrethroids in sediment pore water using matrix-solid phase microextraction. Environ Toxicol Chem. 2009;28:36–43. doi: 10.1897/08-209.1. [DOI] [PubMed] [Google Scholar]
- Janssen EML, Oen AMP, Luoma SN, Luthy RG. Assessment of field-related influences on polychlorinated biphenyl exposures and sorbent amendment using polychaete bioassays and passive sampler measurements. Environ Toxicol Chem. 2011;30:173–180. doi: 10.1002/etc.367. [DOI] [PubMed] [Google Scholar]
- Jonker MO, Koelmans AA. Polyoxymethylene solid phase extraction as a partitioning method for hydrophobic organic chemicals in sediment and soot. Environ Sci Technol. 2001;35:3742–3748. doi: 10.1021/es0100470. [DOI] [PubMed] [Google Scholar]
- Jonker MTO, Koelmans AA. Sorption of polycyclic aromatic hydrocarbons and polychlorinated biphenyls to soot and soot-like materials in the aqueous environment: Mechanistic considerations. Environ Sci Technol. 2002;36:3725–3734. doi: 10.1021/es020019x. [DOI] [PubMed] [Google Scholar]
- Jonker MTO, Barendregt A. Oil is a sedimentary super super-sorbent for polychlorinated biphenyls. Environ Sci Technol. 2006;40:3829–3835. doi: 10.1021/es0601080. [DOI] [PubMed] [Google Scholar]
- Jonker MTO, van der Heijden SA. Bioconcentration factor hydrophobicity cutoff: An artificial phenomenon reconstructed. Environ Sci Technol. 2007;41:7363–7369. doi: 10.1021/es0709977. [DOI] [PubMed] [Google Scholar]
- Kraaij R, Mayer P, Busser FJM, van het Bolscher M, Seinen W, Tolls J. Measured pore-water concentrations make equilibrium partitioning work: A data analysis. Environ Sci Technol. 2003;37:268–274. doi: 10.1021/es020116q. [DOI] [PubMed] [Google Scholar]
- Kreitinger JP, Neuhauser EF, Doherty FG, Hawthorne SB. Greatly reduced bioavailability and toxicity of polycyclic aromatic hydrocarbons to Hyalella azteca in sediments from manufactured-gas plant sites. Environ Toxicol Chem. 2007;26:1146–1157. doi: 10.1897/06-207r.1. [DOI] [PubMed] [Google Scholar]
- Kukkonen JVK, Mitra S, Landrum PF, Gossiaux DC, Gunnarsson J, Weston D. The contrasting roles of sedimentary plant-derived carbon and black carbon on sediment-spiked hydrophobic organic contaminant bioavailability to Diporeia species and Lumbriculus variegatus. Environ Toxicol Chem. 2005;24:877–885. doi: 10.1897/04-171r.1. [DOI] [PubMed] [Google Scholar]
- Kupryianchyk D, Reichman EP, Rakowska MI, Peeters ETHM, Grotenhuis JTC, Koelmans AA. Ecotoxicological effects of activated carbon amendments on macroinvertebrates in non-polluted and polluted sediments. Environ Sci Technol. 2011;45:8567–8574. doi: 10.1021/es2014538. [DOI] [PubMed] [Google Scholar]
- Lambropoulou DA, Sakkas VA, Albanis TA. Validation of an SPME method, using PDMS, PA, PDMS-DVB, and CW-DVB SPME fiber coatings, for analysis of organophosphorus insecticides in natural waters. Anal Bioanal Chem. 2002;374:932–941. doi: 10.1007/s00216-002-1549-7. [DOI] [PubMed] [Google Scholar]
- Lampert DJ, Sarchet WV, Reible DD. Assessing the effectiveness of thin-layer sand caps for contaminated sediment management through passive sampling. Environ Sci Technol. 2011;45:8437–8443. doi: 10.1021/es200406a. [DOI] [PubMed] [Google Scholar]
- Landrum PF, Robinson SD, Gossiaux DC, You J, Lydy MJ, Mitra S, ten Hulscher TEM. Predicting bioavailability of sediment-associated organic contaminants for Diporeia spp. and oligochaetes. Environ Sci Technol. 2007;41:6442–6447. doi: 10.1021/es0706807. [DOI] [PubMed] [Google Scholar]
- Lao W, Tsukada D, Maruya KA. A two-component mass balance model for calibration of solid-phase microextraction fibers for pyrethroids in seawater. Anal Chem. 2012;84:9362–9369. doi: 10.1021/ac302120m. [DOI] [PubMed] [Google Scholar]
- Leppänen MT, Kukkonen JVK. Evaluating the role of desorption in bioavailability of sediment-associated contaminants using oligochaetes, semipermeable membrane devices and Tenax extraction. Environ Pollut. 2006;140:150–163. doi: 10.1016/j.envpol.2005.06.010. [DOI] [PubMed] [Google Scholar]
- Lohmann R, Macfarlane JK, Gschwend PM. Importance of black carbon to sorption of native PAHs, PCBs, and PCDDs in Boston and New York Harbor sediments. Environ Sci Technol. 2005;39:141–148. doi: 10.1021/es049424+. [DOI] [PubMed] [Google Scholar]
- Luthy RG, Aiken GR, Brusseau ML, Cunningham SD, Gschwend PM, Pignatello JJ, Reinhard M, Traina SJ, Weber WJ, Westall JC. Sequestration of hydrophobic organic contaminants by geosorbents. Environ Sci Technol. 1997;31:3341–3347. [Google Scholar]
- Lyytikäinen M, Rantalainen AL, Paasivirta J, Hamalainen H, Kukkonen JVK. Similarities in bioaccumulation patterns of polychlorinated dibenzo-p-dioxins and furans and polychlorinated diphenyl ethers in laboratory-exposured oligochaetes and semipermeable membrane devices and in field-contaminated chironomids. Environ Toxicol Chem. 2003;22:2405–2415. doi: 10.1897/02-433. [DOI] [PubMed] [Google Scholar]
- Maenpaa K, Leppanen MT, Reichenberg F, Figueiredo K, Mayer P. Equilibrium sampling of persistent and bioaccumulative compounds in soil and sediment: Comparison of two approaches to determine equilibrium partitioning concentrations in lipids. Environ Sci Technol. 2011;45:1041–1047. doi: 10.1021/es1029969. [DOI] [PubMed] [Google Scholar]
- Mackenbach EM, You J, Mills MA, Landrum PF, Lydy MJ. A Tenax model to predict bioavailability of PCBs in field sediments. Environ Toxicol Chem. 2012;31:2210–2216. doi: 10.1002/etc.1943. [DOI] [PubMed] [Google Scholar]
- Maruya KA, Zeng EY, Tsukada D, Bay SM. A passive sampler based on solid-phase microextraction for quantifying hydrophobic organic contaminants in sediment pore water. Environ Toxicol Chem. 2009;28:733–740. doi: 10.1897/08-322R.1. [DOI] [PubMed] [Google Scholar]
- Maruya KA, Landrum PF, Burgess RM, Shine JP. Incorporating contaminant bioavailability into sediment quality assessment frameworks. Integr Environ Assess Manag. 2012;8:659–673. doi: 10.1002/ieam.135. [DOI] [PubMed] [Google Scholar]
- Mayer P, Vaes WHJ, Wijnke F, Legierse KCHM, Kraaij RH, Tolls J, Hermens JLM. Sensing dissolved sediment porewater concentrations of persistent and bioaccumulative pollutants using disposable solid-phase microextraction fibers. Environ Sci Technol. 2000;34:5177–5183. [Google Scholar]
- Mayer P, Tolls J, Hermens JLM, Mackay D. Equilibrium sampling devices. Environ Sci Technol. 2003;37:184–191. doi: 10.1021/es032433i. [DOI] [PubMed] [Google Scholar]
- Mayer P, Parkerton TF, Adams RG, Cargill JG, Gan J, Gouin T, Gschwend PM, Hawthorne SB, Helm P, Witt G. Passive sampling methods for contaminated sediments: Scientific rationale supporting use of freely dissolved concentrations. Integr Environ Assess Manag. 2014;10:197–209. doi: 10.1002/ieam.1508. et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meloche LM, deBruyn AMH, Otton SV, Ikonomou MG, Gobas FAPC. Assessing exposure of sediment biota to organic contaminants by thin-film solid phase extraction. Environ Toxicol Chem. 2009;28:247–253. doi: 10.1897/08-081.1. [DOI] [PubMed] [Google Scholar]
- Menzie C, Driscoll SK. 2013. Sediment bioavailability initiative (SBI): Development of standard methods and approaches for the use of passive samplers in assessment and management of contaminated sediment, ESTCP Project No. ER-201216. ESTCP, Alexandria, VA. [cited 2012 November 12]. Available from: http://www.serdp.org/Program-Areas/Environmental-Restoration/Contaminated-Sediments/ER-201216/ER-201216.
- Minha JK, Vasiluk L, Pinto LJ, Gobas FAPC, Moore MM. Mobilization of chrysene from soil in a model digestive system. Environ Toxicol Chem. 2006;25:1729–1737. doi: 10.1897/05-345r1.1. [DOI] [PubMed] [Google Scholar]
- Morehead NR, Eadie BJ, Berner DA, Landrum PF. The sorption of PAH onto dissolved organic matter in Lake Michigan waters. Chemosphere. 1986;15:403–402. [Google Scholar]
- Muijs B, Jonker MTO. Assessing the bioavailability of complex petroleum hydrocarbon mixtures in sediments. Environ Sci Technol. 2011;45:3554–3561. doi: 10.1021/es103855a. [DOI] [PubMed] [Google Scholar]
- Muijs B, Jonker MTO. Does equilibrium passive sampling reflect actual in situ bioaccumulation of PAHs and petroleum hydrocarbon mixtures in aquatic worms. Environ Sci Technol. 2012;46:937–944. doi: 10.1021/es202951w. [DOI] [PubMed] [Google Scholar]
- [NRC] National Research Council. Bioavailability of contaminants in soils and sediments: Processes, tools and applications. National Research Council Committee on Bioavailability of Contaminants in Soils and Sediments. Washington, DC: National Academies Press; 2003. p. 420. p. [Google Scholar]
- Oen AMP, Schaanning M, Ruus A, Cornelissen G, Kallqvist T, Breedveld GD. Predicting low biota to sediment accumulation factors of PAHs by using infinite-sink and equilibrium extraction methods as well as BC-inclusive modeling. Chemosphere. 2006;64:1412–1420. doi: 10.1016/j.chemosphere.2005.12.028. [DOI] [PubMed] [Google Scholar]
- Oen AMP, Janssen EML, Cornelissen G, Breedveld GD, Eek E, Luthy RG. In situ measurement of PCB pore water concentration profiles in activated carbon-amended sediment using passive samplers. Environ Sci Technol. 2011;45:4053–4059. doi: 10.1021/es200174v. [DOI] [PubMed] [Google Scholar]
- Ouyang G, Pawliszyn J. A critical review in calibration methods for solid-phase microextraction. Anal Chimica Acta. 2008;627:184–197. doi: 10.1016/j.aca.2008.08.015. [DOI] [PubMed] [Google Scholar]
- Peijnenburg WJGM, Teasdale PR, Reible D, Mondon J, Bennett WH, Campbell PGC. Passive sampling methods for contaminated sediments: State of the science for metals. Integr Environ Assess Manag. 2014;10:179–196. doi: 10.1002/ieam.1502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pignatello JJ. Desorption of tetrachloroethene and 1, 2-dibromo-3-chloropropane from aquifer sediments. Environ Toxicol Chem. 1991;10:1399–1404. [Google Scholar]
- Pignatello JJ, Xing B. Mechanisms of slow sorption of organic chemicals to natural particles. Environ Sci Technol. 1996;30:1–11. [Google Scholar]
- Reible D, Lotufo G. 2012. Demonstration and evaluation of solid phase microextraction for the assessment of bioavailability and contaminant mobility. Alexandria (VA): Environmental Restoration Project ER-0624. Final Report. SERDP-ESTCP.
- Reichenberg F, Smedes F, Jönsson JÅ, Mayer P. Determining the chemical activity of hydrophobic organic compounds in soil using polymer coated vials. Chem Cent J. 2008;2:8. doi: 10.1186/1752-153X-2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen G, Chadwick DB, Burton GA, Taulbee WK, Greenberg MS, Lotufo GR, Reible DD. A sediment ecotoxicity assessment platform for in situ measures of chemistry, bioaccumulation, and toxicity. Part 2: Integrated application to a shallow estuary. Environ Pollut. 2012;162:457–465. doi: 10.1016/j.envpol.2011.11.013. [DOI] [PubMed] [Google Scholar]
- Rusina TP, Smedes F, Klanova J, Booij K, Holoubek I. Polymer selection for passive sampling: A comparison of critical properties. Chemosphere. 2007;68:1344–1351. doi: 10.1016/j.chemosphere.2007.01.025. [DOI] [PubMed] [Google Scholar]
- Rusina TP, Smedes F, Koblizkova M, Klanova J. Calibration of silicone rubber passive samplers: Experimental and modeled relations between sampling rate and compound properties. Environ Sci Technol. 2010;44:362–367. doi: 10.1021/es900938r. [DOI] [PubMed] [Google Scholar]
- Ruus A, Bøyum O, Grung M, Næs K. Bioavailability of PAHs in aluminum smelter affected sediments: Evaluation through assessment of pore water concentrations and in vivo bioaccumulation. Environ Sci Technol. 2010;44:9291–9297. doi: 10.1021/es103020e. [DOI] [PubMed] [Google Scholar]
- Ruus A, Allan IJ, Øxnevad S, Schaanning MT, Borgå K, Bakke T, Næs K. In vivo bioaccumulation of contaminants from historically polluted sediments: Relation to bioavailability estimates. Sci Total Environ. 2013;442:336–343. doi: 10.1016/j.scitotenv.2012.10.060. [DOI] [PubMed] [Google Scholar]
- Smedes F, Davies IM, Tronczynski J. 2007. ICES Passive sampling trial survey for water and sediment (PSTS) 2006–2007. Part 1: Objectives, design and realization. ICES CM 2007/J:02.
- Smedes F, van der Zande AE, Tixier C, Davies IM. 2007. ICES Passive sampling trial survey for water and sediment (PSTS) 2006–2007. Part 2: Laboratory inter-comparison, analytical issues and lessons learned. ICES CM 2007/J:03.
- Smedes F, van Vliet LA, Booij K. Multi-ratio equilibrium passive sampling method to estimate accessible and pore water concentrations of polycyclic aromatic hydrocarbons and polychlorinated biphenyls in sediment. Environ Sci Technol. 2013;47:510–517. doi: 10.1021/es3040945. [DOI] [PubMed] [Google Scholar]
- Sormunen AJ, Leppänen M, Kukkonen J. Influence of sediment ingestion and exposure concentration on the bioavailable fraction of sediment-associated tetrachlorobiphenyl in oligochaetes. Environ Toxicol Chem. 2008;27:854–863. doi: 10.1897/07-334.1. [DOI] [PubMed] [Google Scholar]
- Sormunen AJ, Tuikka AI, Akkanen J, Leppanen MT, Kukkonen JVK. Predicting the bioavailability of sediment-associated spiked compounds by using the polyoxymethylene passive sampling and Tenax extraction methods in sediments from three river basins in Europe. Arch Environ Contam Toxicol. 2010;59:80–90. doi: 10.1007/s00244-009-9453-1. [DOI] [PubMed] [Google Scholar]
- Sun X, Ghosh U. The effect of activated carbon on partitioning, desorption, and biouptake of native PCBs in four freshwater sediments. Environ Toxicol Chem. 2008;27:2287–2295. doi: 10.1897/08-020.1. [DOI] [PubMed] [Google Scholar]
- ter Laak TL, Barendregt A, Hermens JLM. Freely dissolved pore water concentrations and sorption coefficients of PAHs in spiked, aged, and field-contaminated soils. Environ Sci Technol. 2006;40:2184–2190. doi: 10.1021/es0524548. [DOI] [PubMed] [Google Scholar]
- ter Laak TL, Busser FJM, Hermens JLM. Poly(dimethylsiloxane) as passive sampler material for hydrophobic chemicals: Effect of chemical properties and sampler characteristics on partitioning and equilibration times. Anal Chem. 2008;80:3859–3866. doi: 10.1021/ac800258j. [DOI] [PubMed] [Google Scholar]
- Tomaszewski JE, Luthy RG. Field deployment of polyethylene devices to measure PCB concentrations in pore water of contaminated sediment. Environ Sci Technol. 2008;42:6086–6091. doi: 10.1021/es800582a. [DOI] [PubMed] [Google Scholar]
- Trimble TA, You J, Lydy MJ. Bioavailability of PCBs from field-collected sediments: Application of Tenax extraction and matrix-SPME techniques. Chemosphere. 2008;71:337–344. doi: 10.1016/j.chemosphere.2007.09.001. [DOI] [PubMed] [Google Scholar]
- [USEPA] US Environmental Protection Agency. 2012a. Guidelines for using passive samplers to monitor organic contaminants at Superfund sites. [cited 2012 November 12]. Available from: http://www.epa.gov/superfund/health/conmedia/sediment/pdfs/Passive_ Sampler_SAMS_Final_Camera_Ready_-_Jan_2013.pdf. [PubMed]
- [USEPA] US Environmental Protection Agency. 2012b. Equilibrium partitioning sediment benchmarks (ESBs) for the protection of benthic organisms: Procedures for the determination of the freely dissolved interstitial water concentrations of nonionic organics. [cited 2012 November 12]. Available from: http://www.epa.gov/nheerl/download_files/publications/RB%20ESB%202012final_2.pdf. [PubMed]
- van der Heijden SA, Jonker MTO. PAH bioavailability in field sediments: Comparing different methods for predicting in situ bioaccumulation. Environ Sci Technol. 2009;43:3757–3763. doi: 10.1021/es803329p. [DOI] [PubMed] [Google Scholar]
- Vinturella AE, Burgess RM, Coull BA, Thompson KM, Shine JP. Use of passive samplers to mimic uptake of polycyclic aromatic hydrocarbons by benthic polychaetes. Environ Sci Technol. 2004;38:1154–1160. doi: 10.1021/es034706f. [DOI] [PubMed] [Google Scholar]
- Vrana B, Mills GA, Allan IJ, Dominiak E, Svensson K, Knutsson J, Morrison G, Greenwood R. Passive sampling techniques for monitoring pollutants in water. Trends Anal Chem. 2005;24:845–868. [Google Scholar]
- Wang F, Bu Q, Xia X Shen. Contrasting effects of black carbon amendments on PAH bioaccumulation by Chironomus plumosus larvae in two distinct sediments: Role of water absorption and particle ingestion. Environ Pollut. 2011;159:1905–1913. doi: 10.1016/j.envpol.2011.03.033. [DOI] [PubMed] [Google Scholar]
- Witt G, Liehr GA, Borck D, Mayer P. Matrix solid-phase microextraction for measuring freely dissolved concentrations and chemical activities of PAHs in sediment cores from the western Baltic Sea. Chemosphere. 2009;74:522–529. doi: 10.1016/j.chemosphere.2008.09.073. [DOI] [PubMed] [Google Scholar]
- Woods RW, Letinski DJ, Febbo EJ, Dzamba CL, Connelly MJ, Parkerton TF. Assessing the aquatic hazard of commercial hydrocarbon resins. Ecotoxicol Environ Safety. 2007;66:159–168. doi: 10.1016/j.ecoenv.2005.11.004. [DOI] [PubMed] [Google Scholar]
- Xu Y, Spurlock F, Wang Z, Gan J. Comparison of five methods for measuring sediment toxicity of hydrophobic contaminants. Environ Sci Technol. 2007;41:8394–8399. doi: 10.1021/es071911c. [DOI] [PubMed] [Google Scholar]
- Yang ZY, Zeng EY, Xia H, Wang JZ, Mai BX, Maruya KA. Application of a static solid-phase microextraction procedure combined with liquid-liquid extraction to determine poly (dimethyl) siloxane-water partition coefficients for selected polychlorinated biphenyls. J Chromatogr A. 2006;1116:240–247. doi: 10.1016/j.chroma.2006.03.029. [DOI] [PubMed] [Google Scholar]
- Yang ZY, Zeng EY, Maruya KA, Mai BX, Ran Y. Predicting organic contaminant concentrations in sediment porewater using solid phase microextraction. Chemosphere. 2007;66:1408–1414. doi: 10.1016/j.chemosphere.2006.09.050. [DOI] [PubMed] [Google Scholar]
- Yates K, Pollard P, Davies IM, Webster L, Moffat CF. Application of silicone rubber passive samplers to investigate the bioaccumulation of PAHs by Nereis virens from marine sediments. Environ Pollut. 2011;159:3351–3356. doi: 10.1016/j.envpol.2011.08.038. [DOI] [PubMed] [Google Scholar]
- You J, Landrum PF, Lydy MJ. Comparison of chemical approaches for assessing bioavailability of sediment-associated contaminants. Environ Sci Technol. 2006;40:6348–6353. doi: 10.1021/es060830y. [DOI] [PubMed] [Google Scholar]
- You J, Landrum PF, Trimble TA, Lydy MJ. Availability of polychlorinated biphenyls in field contaminated sediment. Environ Toxicol Chem. 2007;26:1940–1948. doi: 10.1897/07-029R.1. [DOI] [PubMed] [Google Scholar]
- You J, Pehkonen S, Landrum PF, Lydy MJ. Desorption of hydrophobic compounds from laboratory-spiked sediments measured by Tenax absorbent and matrix-solid phase microextraction. Environ Sci Technol. 2007;41:5672–5678. doi: 10.1021/es0700395. [DOI] [PubMed] [Google Scholar]
- You J, Harwood AD, Li H, Lydy MJ. Chemical techniques for assessing bioavailability of sediment-associated contaminants: SPME versus Tenax extraction. J Environ Monit. 2011;13:792–800. doi: 10.1039/c0em00587h. [DOI] [PubMed] [Google Scholar]
- Zeng EY, Tsukada D, Noblet JA, Peng J. Determination of polydimethylsiloxane (PDMS)-seawater distribution coefficients for polychlorinated biphenyls and chlorinated pesticides by solid phase microextraction. J Chromatogr A. 2005;1066:165–175. doi: 10.1016/j.chroma.2005.01.023. [DOI] [PubMed] [Google Scholar]
- Zielke H, Seiler TB, Niebergall S, Leist E, Brinkmann M, Spira D, Streck G, Brack W, Feiler U, Braunbeck T. The impact of extraction methodologies on the toxicity of sediments in the zebrafish (Danio rerio) embryo test. J Soils Sediments. 2011;11:352–363. et al. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Application of passive sampling methods that target the freely dissolved concentration (Cfree) in sediment.


