Abstract
Background
Bacteriuria in the form of symptomatic urinary tract infection (UTI) and asymptomatic bacteriuria (ASB) is common in the elderly. There is no clinical benefit obtained by treating elderly individuals with ASB. However, its high prevalence leads to the overdiagnosis of UTI and unnecessary antibiotic use, which can result in adverse events, including Clostridium difficile diarrhea and reinfection with antibiotic-resistant organisms.
Methods
This was a retrospective audit that assessed the management of nosocomial bacteriuria in elderly patients admitted to the over-65 years rehabilitation unit of a secondary level care hospital in New Zealand. Identified bacteriuria episodes had the timing of sample collection relative to admission, microbial etiology, antibiotic susceptibility profile, inflammatory marker level, and treatment determined. Episodes were classified into six different clinical groups based on the presence or absence of signs and symptoms, urinary catheter status, and systemic inflammatory response. The proportion of bacteriuria episodes by clinical grouping and the level of treatment by clinical group were determined, followed by assessment of the amount of overtreatment in terms of the number of unnecessary antibiotic courses and unnecessary antibiotic treatment days.
Results
Significant bacteriuria was identified in 30% of patients, with 35% of urine samples collected in the immediate postadmission period. Fifty-four percent of the bacteriuria episodes were ASB or catheter-associated ASB (CA-ASB) without an inflammatory response, 24% were ASB or CA-ASB with raised inflammatory markers, and 22% were UTI or CA-UTI. The most common cause of bacteriuria was Escherichia coli, although the etiology was diverse, especially after prolonged hospitalization or in catheterized patients. A large proportion of organisms were resistant to one or more of the commonly used oral antibiotics. Treatment of ASB and CA-ASB accounted for 43% of all antibiotic courses received. Furthermore, treatment of ASB and CA-ASB combined with unnecessarily prolonged treatment days for clinically relevant infections accounted for 55% of all antibiotic treatment days received.
Conclusion
The results suggest that inappropriate urine screening was occurring and that 43% of antibiotic courses and 55% of all antibiotic treatment days were unnecessary. Current practice is amenable to improvement by performing urine culture only when clinically indicated, focusing on clinical signs and symptoms to diagnose clinically significant UTI rather than a positive culture, and using, where possible, the ecologically least damaging antibiotic for the shortest duration required.
Keywords: asymptomatic bacteriuria, nosocomial urinary tract infection, elderly, antibiotic overuse
Introduction
Urinary tract infection (UTI) is a common infection in the elderly and accounts for substantial antibiotic use.1,2 Bacteriuria is the presence of bacteria in the urine and can be divided clinically into symptomatic UTI and asymptomatic bacteriuria (ASB) depending on the presence or absence of symptoms referable to the urinary tract.3,4
In the community setting, ASB is present in 15%–20% of females over the age of 70 years, and in 5%–10% of males over the age of 65 years, rising to 25%–50% and 25%–40%, respectively, in aged residential care facilities.5 The high prevalence of ASB in the elderly occurs in association with hypoestrogenic urogenital mucosal changes in postmenopausal women and varying degrees of prostatic hypertrophy-related urinary obstruction in older men.5 The risk of bacteriuria is also increased by age-related comorbidities such as cognitive impairment, incontinence, neurological disease, diabetes, and indwelling urinary catheter use.1,5 Individuals with short-term urinary catheters have a 3%–8% risk of bacteriuria each day the catheter is in place, and long-term urinary catheterization results in a prevalence of bacteriuria close to 100%.6 Escherichia coli is the leading cause of bacteriuria in the elderly.7 However, in comparison with the younger population, bacteriuria in the elderly is more frequently caused by other Enterobacteriaceae, Pseudomonas aeruginosa, or Enterococcus spp.7
The elderly population should not be screened or treated for ASB, as treatment does not affect the risk of symptomatic UTI, change chronic urinary symptoms, or alter overall survival.4,5 The exception to the rule is in individuals undergoing invasive urological procedures.4 Unnecessary screening and treatment of ASB promotes reinfection with antibiotic-resistant organisms and adverse drug events, including Clostridium difficile diarrhea, and generates avoidable laboratory and treatment costs.3–5 In hospitalized elderly patients, overdiagnosis of UTI is common.8 The identification of bacteriuria frequently triggers antibiotic treatment regardless of signs or symptoms and perhaps even clinical assessment.2,9 Treatment of bacteriuria is also prompted by nonspecific changes in the elderly, such as functional decline or lethargy, without clear evidence for an association.1,3 Furthermore, malodorous urine and pyuria may incorrectly prompt urine culture and antibiotic treatment, respectively.4,5 It is recognized, however, that it can be challenging to differentiate ASB from UTI in the elderly, as they may lack classical urinary tract symptoms and have restricted communication.1,3 In the absence of other inflammatory conditions, measuring blood inflammatory markers may assist management decisions regarding bacteriuria.3,10
In young women, the treatment of uncomplicated lower UTI with a short course (3 days) of antibiotics is well established.11 Conversely, in elderly women, uncomplicated lower UTI has traditionally been treated for 7 days or more. However, the available evidence and international guidelines now suggest that short course antibiotic therapy is also likely to be sufficient in elderly women, although the guidelines make recommendations only regarding therapy in the community.12,13 Complicated lower UTI, which includes UTI in males and those with catheters, and upper UTI should be treated for a minimum of 7 days.6,11,12 In catheter-associated UTI (CA-UTI), the catheter should be changed or, if possible, removed to accelerate the speed of symptom resolution.6 Allowing for microbial susceptibility and the site of infection, antibiotics with the lowest impact on microbial ecology should be used.11 At the time of the audit, the local hospital guidelines that covered empiric antibiotic therapy recommended quinolones for nonseptic UTI and gentamicin plus amoxicillin for urosepsis.
Optimization of treatment practices in the elderly is required. The aim of this audit was to assess the management of bacteriuria in elderly inpatients, in comparison with published guidelines, and to identify areas for improvement.
Methods
This was a retrospective audit of all significant bacteriuria episodes in patients admitted to the over-65 rehabilitation ward of a secondary level care hospital in New Zealand between July 1, 2011 and June 25, 2012. The over-65 rehabilitation unit receives medically stable patients from the acute medical and surgical services who require optimization of their functional status before returning home or to residential care.
The electronic laboratory results of all patients admitted during the audit time period were reviewed to identify episodes of significant bacteriuria, causative organism(s), drug susceptibility results, and neutrophil and C-reactive protein levels. The threshold for significant bacteriuria was defined as ≥1×103 colony forming units/milliliter (CFU/mL) if a single organism was isolated, or ≥1×105 CFU/mL for mixed growth. A threshold of ≥1×103 CFU/mL was used for single organism isolates to maximize the capture of bacteriuria cases that may prompt treatment. When multiple urine cultures were taken from the same patient within 3 days of each other, they were considered to be the same episode, and only the first significant bacteriuria result was included. The clinical records of individuals with significant bacteriuria were reviewed to identify any signs and symptoms, indwelling urinary catheter status, antimicrobial treatment, and total length of hospitalization. Bacteriuria episodes with available clinical records were then divided into clinical groups based on the presence or absence of signs and symptoms and urinary tract catheterization, with asymptomatic episodes further subdivided based on the presence or absence of raised inflammatory markers (RIM) (Table 1). Fever and flank pain or tenderness was considered an indicator of potential upper UTI.
Table 1.
Clinical grouping of bacteriuria episodes
| Asymptomatic bacteriuria (ASB) | Noncatheter-associated significant bacteriuria with no locala or systemicb signs or symptoms and no inflammatory marker elevation |
| ASB with raised inflammatory markers (ASB-RIM) | Noncatheter-associated significant bacteriuria with no local or systemic signs or symptoms but with raised inflammatory markersc |
| Urinary tract infection (UTI) | Noncatheter-associated significant bacteriuria with one or more local or systemic signs or symptoms not attributable to another cause |
| Catheter-associated asymptomatic bacteriuria (CA-ASB) | Catheter-associatedd significant bacteriuria with no local or systemic signs or symptoms and no inflammatory marker elevation |
| CA-ASB with raised inflammatory markers (CA-ASB-RIM) | Catheter-associated significant bacteriuria with no local or systemic signs or symptoms but with raised inflammatory markers |
| Catheter-associated urinary tract infection (CA-UTI) | Catheter-associated significant bacteriuria with one or more local or systemic signs or symptoms not attributable to another cause |
Notes:
Local signs and symptoms included as relevant were dysuria, frequency, hematuria, suprapubic pain or tendernessss, and flank pain or tenderness
systemic signs and symptoms included as relevant were fever ≥37.5°C, acute confusion, and urosepsis syndrome (urosepsis syndrome was defined as two or more of: T° ≥38.0°C, heart rate >90 per minute, respiratory rate >20 per minute, white cell count >12×109 cells/L, plus significant bacteriuria, and no other infectious source)
a neutrophil count of ≥7.5×109 cells/L or a C-reactive protein level of ≥30 mg/L was considered elevated
catheter-associated infection includes individuals who had a urinary catheter removed within the previous 48 hours.
Treatment analysis was performed if clinical records were available, if the patient was not on antibiotics for another infection, and if the patient was still in hospital when bacteriuria was identified. Treatment of ASB and CA-ASB without an inflammatory marker rise was considered unwarranted. Unnecessarily prolonged treatment for clinically significant infections was also determined. A 3-day antibiotic course was used as the standard for uncomplicated lower UTI in females, except when nitrofurantoin was used, where 5 days was accepted. A 7-day course was used as the standard for lower UTI in males and CA infection. Extended antibiotic courses were accepted as prescribed in cases of upper UTI or urosepsis.
Data were collated and analyzed using SPSS software (IBM Corporation, Armonk, NY, USA) to generate descriptive statistics and were graphically presented using Microsoft Excel (Microsoft Corporation, Redmond, WA, USA). Percentage values were rounded to the nearest whole number. This audit was conducted for the purpose of local quality assurance in accordance with the National Health and Disability Ethics Committee guidelines.14
Results
During the 12-month time period, 396 individuals were admitted to the over-65 rehabilitation unit, with 166 episodes of significant bacteriuria identified from 120 individuals. This equated to significant bacteriuria being identified in 30% of admissions. One hundred and sixty-two out of 166 bacteriuria episodes had ≥1×105 CFU/mL. Of the 166 bacteriuria episodes, 109 (66%) were in female patients and 110 (66%) were in patients aged ≥80 years. The urine sample was collected within 24 hours of admission in 35% of cases.
Admission records were available for 161 of the 166 episodes. Of these 161 episodes, there were 53 cases of ASB (33%), 18 cases of ASB-RIM (11%), 26 cases of UTI (16%), 34 cases of CA-ASB (21%), 20 cases of CA-ASB-RIM (12%), and ten cases of CA-UTI (6%) (Figure 1). Asymptomatic infection (ASB and CA-ASB) accounted for a larger proportion of the bacteriuria episodes in females (62%) compared with males (38%). Ten febrile bacteriuria episodes were potential upper UTIs, four of which had systemic derangements suggesting urosepsis.
Figure 1.
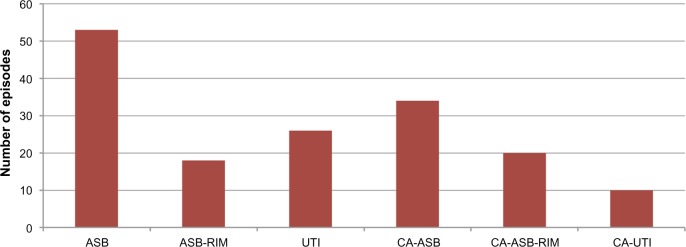
Number of bacteriuria episodes by clinical classification.
Abbreviations: ASB, asymptomatic bacteriuria; ASB-RIM, ASB with raised inflammatory markers; CA-ASB, catheter-associated ASB; CA-ASB-RIM, CA-ASB with raised inflammatory markers; CA-UTI, catheter-associated UTI; UTI, urinary tract infection.
The most commonly identified organism(s) was E. coli (55, 33%), followed by “mixed growth” (47, 28%), P. aeruginosa (16, 10%), Klebsiella pneumoniae (15, 9%), Proteus mirabilis (12, 7%), Enterococcus spp. (9, 5%), Citrobacter spp. (3, 2%), Staphylococcus aureus (3, 2%), Enterobacter spp. (2, 1%), Morganella morganii (2, 1%), and non- S. saprophyticus coagulase-negative Staphylococcus (2, 1%). Episodes reported as mixed growth were not further identified to the species level. The etiology of bacteriuria changed with catheter use, duration of hospitalization, and in symptomatic versus asymptomatic infection (Tables 2 and 3).
Table 2.
Microbial etiology of bacteriuria: overall, by IDC status, and by length of hospitalization
| Organism(s) | Frequency of isolation, n (%)
|
|||||
|---|---|---|---|---|---|---|
| Overall | Non-CA bacteriuriaa | CA bacteriuriaa | ≤2 weeks of hospitalization | 2–4 weeks of hospitalization | >4 weeks of hospitalization | |
| Escherichia coli | 55 (33) | 39 (40) | 12 (19) | 21 (58) | 16 (26) | 18 (26) |
| Mixed growth | 47 (28) | 28 (29) | 18 (28) | 10 (28) | 17 (28) | 20 (29) |
| Pseudomonas aeruginosa | 16 (10) | 7 (7) | 9 (14) | 3 (8) | 7 (11) | 6 (9) |
| Klebsiella pneumoniae | 15 (9) | 10 (10) | 5 (8) | 0 (0) | 6 (10) | 9f (13) |
| Proteus mirabilis | 12 (7) | 3 (3) | 9 (14) | 1 (3) | 6 (10) | 5 (7) |
| Enterococcus spp.b | 9 (5) | 4 (4) | 5 (8) | 0 (0) | 1 (2) | 8 (12) |
| Citrobacter spp.c | 3 (2) | 1 (1) | 2 (3) | 0 (0) | 2 (3) | 1 (1) |
| Staphylococcus aureus | 3 (2) | 2 (2) | 1 (2) | 1 (3) | 2 (3) | 0 (0) |
| Enterobacter spp.d | 2 (1) | 0 (0) | 2 (3) | 0 (0) | 0 (0) | 2 (3) |
| Morganella morganii | 2 (1) | 2 (2) | 0 (0) | 0 (0) | 2 (3) | 0 (0) |
| CNSe | 2 (1) | 1 (1) | 1 (2) | 0 (0) | 2 (3) | 0 (0) |
| Total | 166 | 97 | 64 | 36 | 61 | 69 |
Notes:
Only 161 bacteriuria episodes were assessed for the presence of an IDC, as clinical notes were not available for five episodes
Enterococci were not identified to species level
two Citrobacter freundii and one C. koseri were identified
one Enterobacter cloacae and one E. aerogenes were identified
novobiocin-sensitive CNS (“not S. saprophyticus”) were not further identified
including three extended-spectrum β-lactamase-producing strains. Percentages rounded to nearest whole number.
Abbreviations: CA, catheter associated; CNS, coagulase-negative Staphylococcus; IDC, indwelling urinary catheter; n, number of isolates of respective organism(s); %, percentage of bacteriuria episodes in respective group due to respective organism(s).
Table 3.
Microbial etiology of bacteriuria: by clinical classificationa
| Organism(s) | Frequency of isolation, n (%)
|
|||||
|---|---|---|---|---|---|---|
| ASB | ASB-RIM | UTI | CA-ASB | CA-ASB-RIM | CA-UTI | |
| Escherichia coli | 23 (43) | 7 (39) | 9 (35) | 5 (15) | 4 (20) | 3 (30) |
| Mixed growth | 16 (30) | 8 (44) | 4 (15) | 13 (38) | 3 (15) | 2 (20) |
| Pseudomonas aeruginosa | 3 (6) | 1 (6) | 3 (12) | 5 (15) | 2 (10) | 2 (20) |
| Klebsiella pneumoniae | 7 (13) | 0 (0) | 3 (12) | 1 (3) | 4 (20) | 0 (0) |
| Proteus mirabilis | 1 (2) | 2 (11) | 0 (0) | 7 (21) | 2 (10) | 0 (0) |
| Enterococcus spp.b | 0 (0) | 0 (0) | 4 (15) | 0 (0) | 3 (15) | 2 (20) |
| Citrobacter spp.c | 1 (2) | 0 (0) | 0 (0) | 1 (3) | 0 (0) | 1 (10) |
| Staphylococcus aureus | 1 (2) | 0 (0) | 1 (4) | 1 (3) | 0 (0) | 0 (0) |
| Enterobacter spp.d | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 2 (10) | 0 (0) |
| Morganella morganii | 1 (2) | 0 (0) | 1 (4) | 0 (0) | 0 (0) | 0 (0) |
| CNSe | 0 (0) | 0 (0) | 1 (4) | 1 (3) | 0 (0) | 0 (0) |
| Total | 53 | 18 | 26 | 34 | 20 | 10 |
Notes:
Only 161 bacteriuria episodes were grouped, as clinical notes were not available for five episodes
Enterococci were not identified to species level
two Citrobacter freundii and one C. koseri were identified
one Enterobacter cloacae and one E. aerogenes were identified
novobiocin-sensitive CNS (“not S. saprophyticus”) were not further identified. Percentages rounded to nearest whole number.
Abbreviations: ASB, asymptomatic bacteriuria; ASB-RIM, ASB with raised inflammatory markers; CA-ASB, catheter-associated ASB; CA-ASB-RIM, CA-ASB with raised inflammatory markers; CA-UTI, catheter-associated UTI; CNS, coagulase-negative Staphylococcus; n, number of isolates of respective organism(s); UTI, urinary tract infection; %, percentage of bacteriuria episodes in respective clinical group due to respective organism(s).
One hundred and fifty-six of the 166 bacteriuria episodes were eligible for analysis of treatment. Eighty-eight of the 156 eligible bacteriuria episodes (56%) were treated with antibiotics. The proportion that received antimicrobial treatment was highest for UTI (89%), followed by CA-UTI (80%), ASB-RIM (59%), CA-ASB-RIM (50%), CA-ASB (46%), and ASB (44%) (Figure 2). In absolute terms, antibiotics were given for 23 episodes of ASB, 23 episodes of UTI, 15 episodes of CA-ASB, ten episodes of ASB-RIM, nine episodes of CA-ASB-RIM, and eight episodes of CA-UTI (Figure 3). This meant that of all antibiotic courses given, 43% were for ASB and CA-ASB, 35% were for UTI and CA-UTI, and 22% were for ASB-RIM and CA-ASB-RIM.
Figure 2.
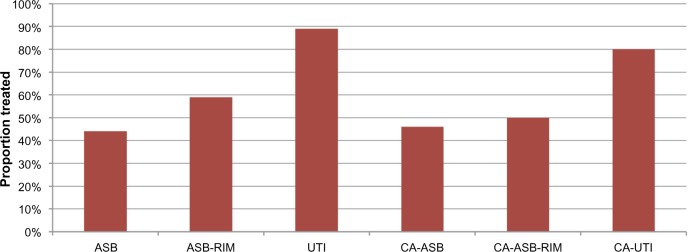
Level of antibiotic treatment of bacteriuria by clinical classification.
Abbreviations: ASB, asymptomatic bacteriuria; ASB-RIM, ASB with raised inflammatory markers; CA-ASB, catheter-associated ASB; CA-ASB-RIM, CA-ASB with raised inflammatory markers; CA-UTI, catheter-associated UTI; UTI, urinary tract infection.
Figure 3.
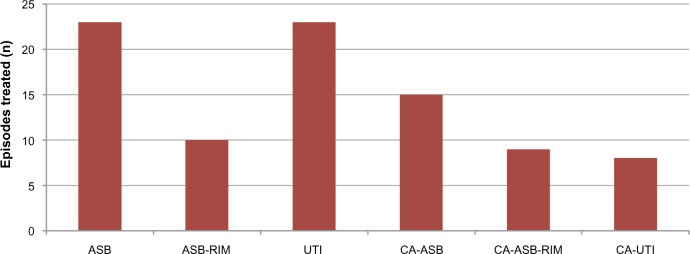
Number of antibiotic-treated bacteriuria episodes by clinical classification.
Abbreviations: ASB, asymptomatic bacteriuria; ASB-RIM, ASB with raised inflammatory markers; CA-ASB, catheter-associated ASB; CA-ASB-RIM, CA-ASB with raised inflammatory markers; CA-UTI, catheter-associated UTI; n, number of respective bacteriuria episodes treated with antibiotics; UTI, urinary tract infection.
Antibiotics were given by the oral route in 95% of treated episodes, with quinolones the most frequently used class (57%). There was a high prevalence of antibiotic resistance, particularly in association with catheterization and prolonged admission (Table 4). In 67% of treated episodes, antibiotics were started 24 hours or more after the sample was collected. In these cases, antibiotic selection is presumed to be largely based on susceptibility results, with the organisms sensitive to the respective prescribed antibiotic in 96% of cases. In 33% of treated episodes, antibiotics were started on the same day as the sample was collected. In these cases, it is presumed that treatment was empiric, with the subsequently identified organism sensitive to the prescribed antibiotic in 67% of cases.
Table 4.
Antibiotic usage and antibiotic sensitivity of urinary isolates
| Antibiotic
|
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Norfloxacina | Amoxicillin–clavulanatea | Trimethoprima | Ciprofloxacina | Nitrofurantoina | Amoxicillina | Ceftriaxoneb,c | Gentamicinb | Piperacillin–tazobactamb,c | ||
| First-line use, n (%) | 45 (51) | 13 (15) | 13 (15) | 5 (6) | 4 (5) | 4 (5) | 2 (2) | 1 (1) | 1 (1) | |
| Antibiotic sensitivityd | ||||||||||
| All isolates | 81% | 67% | 61% | 81% | 63% | 35% | 85% | |||
| Non-CA isolates | 84% | 67% | 68% | 84% | 74% | 28% | 87% | |||
| CA isolates | 79% | 65% | 47% | 79% | 42% | 42% | 81% | |||
| ≤2 weeks of hospitalization | 92% | 58% | 73% | 92% | 81% | 35% | 100% | |||
| 2–4 weeks of hospitalization | 88% | 74% | 63% | 88% | 54% | 33% | 91% | |||
| 4 weeks of hospitalization | 68% | 66% | 53% | 68% | 61% | 36% | 72% | |||
Notes:
These antibiotics were given by the oral route
these antibiotics were given by the intravenous route
susceptibility testing not routinely performed for ceftriaxone and piperacillin–tazobactam
drug susceptibility data includes only episodes where single pathogens were identified. Percentages rounded to nearest whole number.
Abbreviations: CA, catheter associated; n, number of first-line uses.
All bacteriuria cases identified over the audit time period received a combined total of 553 antibiotic treatment days. Appropriate length treatment of UTI and CA-UTI accounted for 156 of 553 antibiotic treatment days (28%), and appropriate length treatment of ASB-RIM and CA-ASB-RIM accounted for 90 of 553 antibiotic treatment days (16%). Conversely, unnecessary treatment of ASB and CA-ASB accounted for 228 of 553 antibiotic treatment days (41%), and unnecessarily prolonged treatment days for lower UTI, CA lower UTI, ASB-RIM, and CA-ASB-RIM accounted for 79 of 553 antibiotic treatment days (14%) (Figure 4).
Figure 4.
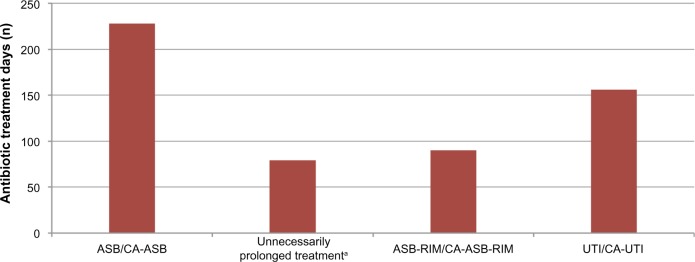
Breakdown of all antibiotic treatment days received for bacteriuria.
Note: aUnnecessarily prolonged treatment of lower UTI, CA lower UTI, ASB-RIM, and CA-ASB-RIM. A 3-day antibiotic course was used as the standard in females, except when nitrofurantoin was used, where 5 days was accepted. A 7-day course was used as the standard in males and CA infection.
Abbreviations: ASB, asymptomatic bacteriuria; ASB-RIM, ASB with raised inflammatory markers; CA-ASB, catheter-associated ASB; CA-ASB-RIM, CA-ASB with raised inflammatory markers; CA-UTI, catheter-associated UTI; n, number of antibiotic treatment days; UTI, urinary tract infection.
Discussion
This audit assessed the management of nosocomial bacteriuria in elderly inpatients to identify areas for improvement in practice. The high prevalence of bacteriuria identified, in particular asymptomatic infection, is consistent with that reported in community dwelling and institutionalized elderly.5 This high pretest probability of finding bacteriuria regardless of the clinical presentation illustrates the limitations of using urine culture to diagnose clinically relevant UTI in hospitalized elderly individuals.3,5 However, despite its limitation for diagnosing UTI, urine culture is required to facilitate antibiotic susceptibility testing and appropriate antibiotic selection.3 This is particularly important in nosocomial infections where there are high levels of microbial diversity and antimicrobial resistance. The requirement for culture and antibiotic susceptibility testing was illustrated by the one-third of empirically treated episodes that subsequently had organisms identified that were resistant to the empiric antibiotic.
The high proportion of urine samples collected in the immediate postadmission period suggests that “admission urine screening” was occurring. We also know that cloudy or malodorous urine incorrectly prompts urine culture.5 Ward staff need to limit these incorrect screening practices and erroneous treatment prompts by culturing urine only when a UTI is suspected on appropriate clinical grounds. This requires greater awareness of the nature of ASB in the elderly. Educational interventions targeted at urine screening practices and ASB management can decrease the number of urine cultures performed.15
The most frequent cause of bacteriuria was E. coli. Mixed growth was also common, particularly in asymptomatic infection. This likely reflects anterior urethral and periurethral contamination, associated with frequent inability to obtain midstream urine samples in hospitalized elderly patients, and the high proportion of catheter samples, which are prone to polymicrobial infection. The diversity of species increased in catheterized patients and those with a longer duration of hospitalization, reflecting the breakdown of normal physiological barriers, exposure to nosocomial pathogens, and increased likelihood of prior antibiotic exposure, and highlights the importance of broad-spectrum antibiotic coverage when empirically treating nosocomial urosepsis. The microbial differences by clinical group are not distinct enough to dictate empiric antibiotic choice or predict the clinical significance of an individual isolate.
The local treatment recommendation of quinolones for nonseptic UTI was reflected in the results. However, the proportion of isolates that were quinolone resistant was still large, especially in the isolates obtained after 4 weeks of hospitalization. This was associated with the increased frequency of isolation of intrinsically resistant Enterococcus spp. and multidrug-resistant K. pneumoniae during this time. Minimizing the selection of antibiotic-resistant organisms is required through prudent use of antibiotics for all indications.1 Additionally, when antibiotics are required for UTI, agents associated with a lesser effect on gut flora and a lower risk of reinfection with resistant organisms, such as nitrofurantoin or trimethoprim, should be used where possible.11 This could be promoted by treatment guidelines that differentiate between empiric and drug susceptibility directed antibiotic use.
The proportion of ASB (44%) and CA-ASB (46%) episodes that were unnecessarily treated is comparable with that found in other preintervention studies.15 ASB and CA-ASB were treated less frequently than symptomatic infections; however, because they were the most commonly identified clinical groups, they still accounted for 43% of all antibiotic courses. This suggests that 43% of all antibiotic courses for bacteriuria were unwarranted. The diagnosis of a clinically relevant UTI should be based primarily on signs and symptoms, but in elderly individuals this can be difficult, with some degree of overtreatment probably unavoidable. However, the current level of overtreatment of ASB and CA-ASB can be decreased through quality improvement interventions, including treatment algorithms and staff education to address the correct management of bacteriuria.15,16
The episodes of ASB-RIM/CA-ASB-RIM illustrate the limitation of the UTI versus ASB dichotomy in elderly patients who may have a clinically relevant infection without typical signs or symptoms.3 Inflammatory markers may assist in elucidating the significance of bacteriuria.3 However, the best management of these patients is uncertain, and they require a full evaluation before attributing the inflammatory response to the bacteriuria.
Antibiotic treatment of ASB and CA-ASB, combined with the days of unnecessarily prolonged treatment of lower UTI, CA lower UTI, ASB-RIM, and CA-ASB-RIM, meant that 55% of all antibiotic treatment days appeared to be unwarranted. The majority stemmed from the treatment of ASB and CA-ASB. However, an excessive duration of treatment, largely for lower UTI in women, accounted for an important proportion of overuse. There is a lack of evidence to support either short or long course treatment for nosocomial UTI in elderly women. However, it would be sensible to minimize exposure if possible. Short course treatment in appropriate cases, with measures that ensure that antibiotics are stopped promptly, is needed to decrease antibiotic overuse.
There are methodological limitations with a retrospective audit, with the clinical information available determined by the quality of documentation, potentially leading to misclassification of cases. Additionally, a single author (MB) extracted the data, which is a potential source of bias.
Conclusion
Unnecessary antibiotic treatment of bacteriuria exposes patients to avoidable drug side effects, increases the risk of C. difficile diarrhea, increases the risk of reinfection with resistant organisms, and generates unnecessary treatment costs.3–5 This audit demonstrated that nosocomial bacteriuria in the elderly can lead to substantial antibiotic overuse, and illustrated three broad areas of clinical practice that can be improved upon. If ASB is not identified in the first place, erroneous treatment cannot be prompted. Therefore, reducing inappropriate urine culture is important. Clinically relevant UTI in the elderly should be diagnosed primarily by signs and symptoms, not just by the presence of a positive culture. Improving the interpretation of ASB and CA-ASB results is required to reduce overtreatment. Lastly, management can be optimized in bacteriuria episodes requiring treatment by using the ecologically least damaging antibiotics for the shortest duration required.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Beveridge L, Davey P, Phillips G, McMurdo M. Optimal management of urinary tract infections in older people. Clin Interv Aging. 2011;6:173–180. doi: 10.2147/CIA.S13423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McMurdo M, Gillespie N. Urinary tract infection in old age: over-diagnosed and over-treated. Age Ageing. 2000;29:297–298. doi: 10.1093/ageing/29.4.297. [DOI] [PubMed] [Google Scholar]
- 3.Woodfood H, George J. Diagnosis and management of urinary infections in older people. Clin Med. 2011;11(1):80–83. doi: 10.7861/clinmedicine.11-1-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nicolle L, Bradley S, Colgan R, Rice J, Schaeffer A, Hooton T. Infectious Diseases Society of America guidelines for the diagnosis and treatment of asymptomatic bacteriuria in adults. Clin Infect Dis. 2005;40:643–654. doi: 10.1086/427507. [DOI] [PubMed] [Google Scholar]
- 5.Nicolle L. Asymptomatic bacteriuria in older adults. Geriatr Aging. 2003;6(9):24–28. [Google Scholar]
- 6.Hooton T, Bradley S, Cardenas D, et al. Diagnosis, prevention, and treatment of catheter-associated urinary tract infection in adults: 2009 international clinical practice guidelines from the Infectious Diseases Society of America. Clin Infect Dis. 2010;50:624–663. doi: 10.1086/650482. [DOI] [PubMed] [Google Scholar]
- 7.Matthews J, Lancaster J. Urinary tract infections in the elderly population. Am J Geriatr Pharmacother. 2011;9(5):286–309. doi: 10.1016/j.amjopharm.2011.07.002. [DOI] [PubMed] [Google Scholar]
- 8.Woodfood H, George J. Diagnosis and management of urinary tract infection in hospitalised older people. J Am Geriatr Soc. 2009;57(1):107–114. doi: 10.1111/j.1532-5415.2008.02073.x. [DOI] [PubMed] [Google Scholar]
- 9.Walker S, McGeer A, Simor A, Armstrong-Evans M, Loeb M. Why are antibiotics prescribed for asymptomatic bacteriuria in institutionalized elderly people? Can Med Assoc J. 2000;163(3):273–277. [PMC free article] [PubMed] [Google Scholar]
- 10.Van Duin D. Diagnostic challenges and opportunities in older adults with infectious diseases. Clin Infect Dis. 2012;54(7):973–978. doi: 10.1093/cid/cir927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gupta K, Hooton T, Naber K, et al. International clinical practice guidelines for the treatment of acute uncomplicated cystitis and pyelonephritis in women: a 2010 update by the Infectious Diseases Society of America and the European Society for Microbiology and Infectious Diseases. Clin Infect Dis. 2011;52:103–120. doi: 10.1093/cid/ciq257. [DOI] [PubMed] [Google Scholar]
- 12.Scottish Intercollegiate Guidance Network (SIGN) Management of Suspected Bacterial Urinary Tract Infection in Adults. Edinburgh, Scotland: Scottish Intercollegiate Guidance Network; 2012. [Accessed August 5, 2014]. Available from: http://www.sign.ac.uk/pdf/sign88.pdf. [Google Scholar]
- 13.Lutters M, Vogt-Ferrier N. Antibiotic duration for treating uncomplicated, symptomatic lower urinary tract infection in elderly women. Cochrane Database Syst Rev. 2008;3 doi: 10.1002/14651858.CD001535.pub2. [DOI] [PubMed] [Google Scholar]
- 14.National Ethics Advisory Committee . Ethical Guidelines for Observational Studies: Observational Research, Audits and Related Activities. Wellington, New Zealand: National Ethics Advisory Committee; 2012. [Accessed August 5, 2014]. Revised edition. Available from: http://neac.health.govt.nz/streamlined-ethical-guidelines-health-and-disability-research. [Google Scholar]
- 15.Chowdhury F, Sarkar K, Branche A, et al. Preventing the inappropriate treatment of asymptomatic bacteriuria at a community teaching hospital. J Community Hosp Intern Med Perspect. 2012;2(2) doi: 10.3402/jchimp.v2i2.17814. 10.3402/jchimp.v2i2.17814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Egger M, Balmer F, Friedli-Wuthrich H, Muhlemann K. Reduction of urinary catheter use and prescription of antibiotics for asymptomatic bacteriuria in hospitalised patients in internal medicine. Swiss Med Wkly. 2013;143:w13796. doi: 10.4414/smw.2013.13796. [DOI] [PubMed] [Google Scholar]


