Abstract
Background
Long-term mortality and causes of death in patients with pulmonary tuberculosis (PTB) and extrapulmonary tuberculosis (EPTB) are poorly documented. In this study, long-term mortality and causes of death in PTB and EPTB patients were compared with the background population and it was investigated whether mortality was associated with family-related risk factors.
Methods
A nationwide cohort study was conducted including: all adult Danes notified with PTB or EPTB from 1977 to 2008 and alive 1 year after diagnosis; a randomly selected comparison cohort matched on birth date and sex; adult siblings of PTB patients; and population controls. Data were extracted from national registries. All-cause and cause-specific mortality rate ratios were calculated for patients and siblings and compared with their respective control cohorts. A total of 8,291 patients (6,402 PTB and 1,889 EPTB), 24,873 population controls, 1,990 siblings of PTB patients and 11,679 siblings of PTB population controls were included.
Results
Overall, the mortality rate ratio was 1.86 (95% confidence interval [CI] 1.77–1.96) for PTB patients and 1.24 (95% CI 1.12–1.37) for EPTB patients. Both patient cohorts had significantly increased risk of death due to infectious diseases and diabetes. Further, the PTB patients had increased mortality due to cancers (mainly respiratory and gastrointestinal tract), liver and respiratory system diseases, and alcohol and drug abuse. The PTB patients had increased mortality compared with their siblings (mortality rate ratio 3.55; 95% CI 2.57–4.91) as did the siblings of the PTB patients compared with the siblings of population controls (mortality rate ratio 2.16; 95% CI 1.62–2.87).
Conclusion
We conclude that adult PTB patients have an almost two-fold increased long-term mortality whereas EPTB patients have a slightly increased long-term mortality compared with the background population. The increased long-term mortality in PTB patients stems from diseases associated with alcohol, tobacco, and drug abuse as well as immune suppression, and family-related factors.
Keywords: pulmonary tuberculosis, extrapulmonary tuberculosis, mortality, causes of death, siblings, cohort study
Introduction
Tuberculosis (TB) causes more than 1.4 million deaths annually and is the second leading cause of death due to an infectious disease after human immunodeficiency virus (HIV).1 The morbidity and mortality associated with TB is especially pronounced in low-income developing countries where malnutrition, crowded living conditions, and lack of TB control measures makes the disease a serious public health burden.1–3 In high-income industrialized nations, the epidemiology of TB has changed, with immigrants from endemic countries now constituting a major proportion of new TB cases.4,5 Several studies have established specific risk factors and predictors of short-term case fatality rates, but the long-term prognosis of patients with TB is still poorly described.6–8
In order to assess whether long-term mortality is affected in patients who have survived a TB episode compared with the background population, we conducted a nationwide population-based cohort study to determine long-term mortality and causes of death in patients diagnosed with pulmonary (PTB) or extrapulmonary tuberculosis (EPTB). To investigate potential family-related risk factors, we also estimated mortality in siblings of the patients with TB.
Materials and methods
Ethics statement
This study is a registry-based cohort study involving data from various Danish national registries. All data were anonymized before any statistical analysis was performed and the study was approved by the Danish Data Protection Agency.
Study design
This study was conducted as a population-based nationwide cohort study. The study populations were all PTB and EPTB patients registered in Denmark in the period 1977–2008 and a cohort from the background population individually matched on age and sex. Outcomes were time to death and to cause-specific death. The risk of these outcomes was further estimated in the siblings of the patients with TB and compared with that of the siblings of the comparison cohort.
Setting
The population of Denmark was 5.08 million on January 1, 1977 and 5.48 million on January 1, 2008.9 The incidence of TB decreased in the study period from 12/100.000 in 1977 to 6/100.000 in 2008.10,11 Denmark has an estimated HIV prevalence of 0.07%.12 Throughout the study period, Danish citizens had access to free health care and antituberculosis medication. The Bacille Calmette Guerin vaccine was phased out of the Danish national childhood vaccination program in the period 1976–1980. All culture-based TB diagnostics are centralized at the International Reference Laboratory of Mycobacteriology at Statens Serum Institut, Copenhagen, Denmark.
Data sources
The unique 10-digit personal identification number assigned to all Danish citizens at birth or immigration was used to avoid multiple registrations and to track individuals in the following registries.
Danish Tuberculosis Registry
All TB patients diagnosed in Denmark are notified to the Danish Tuberculosis Registry by the treating physician and by the International Reference Laboratory of Mycobacteriology. This notification is mandatory for all patients initiating anti-TB treatment. The Danish Tuberculosis Registry contains data on anatomical localization of TB, ethnic origin, date of diagnosis, residence, and microbiologic data.
Danish National Patient Registry
The Danish National Patient Registry (DNPR) contains information on all patients discharged from Danish somatic hospitals since 1977.13 Data includes dates of admission and discharge diagnosis coded by the International Classification of Diseases, Eighth Revision (ICD-8), until the end of 1993 and the Tenth Revision (ICD-10) thereafter. From this registry, date of first admission with PTB or EPTB was extracted.
Danish Civil Registration System
The Danish National Patient Registry (DCRS) was established in 1968 and contains information on date and place of birth and death, sex, immigration and/or emigration for all Danish residents.14
Danish Registry of Causes of Death
The Danish Registry of Causes of Death contains data from all Danish death certificates since 1943 and is updated to December 31, 2009.15 Causes of death are coded according to ICD-8 until the end of 1993 and according to ICD-10 thereafter. The physician can code one immediate cause of death followed by secondary and/or tertiary causes of death and finally one underlying (main) cause of death.
Study population
PTB and EPTB patients
We included all individuals who fulfilled the following five criteria: notified to the Danish Tuberculosis Registry from January 1, 1977 until December 31, 2008; registered in the DNPR with a diagnosis of PTB (ICD-8 codes 011.00–013.00 and ICD-10 codes A15.0–17.0) or EPTB (ICD-8 codes 014.00–019.00 and ICD-10 codes A 18.0–20.0); notified to the Danish Tuberculosis Registry within 1 year of DNPR date of discharge; ≥16 years of age at time of diagnosis; and registered in the DCRS. EPTB was defined as TB of all anatomical localizations other than lungs and pleura, with the exception of tuberculous meningitis and intracerebral tuberculoma (a detailed study of this patient category has recently been published by Christensen et al)16 and patients diagnosed with miliary TB (to ensure uniform patient populations with sole organ involvement). Diagnosis was either microbiologically verified by positive culture, nucleic acid amplification, or direct microscopy, or based on clinical symptoms and/or radiology. The date of TB diagnosis was defined as the date of first admission for TB according to DNPR, and the index date as 1 year after TB diagnosis. Patients were excluded from the study population if they died, emigrated, or were lost to follow-up within the first year of TB diagnosis.
Population comparison cohorts
For each TB patient, we identified all Danish citizens in the DCRS who were born in Denmark with the same birth date and sex as the patient and were alive and not diagnosed with TB on the index date of the respective patient. From this population, we randomly identified three individuals per TB patient who constituted the comparison cohort. The index date for the population controls was therefore the same as for the TB patient to whom they were matched.
Siblings
From the DCRS, all siblings of the PTB patients and PTB population controls were identified who fulfilled the following criteria: full siblings; born after January 1, 1952, and alive and not younger than 16 years of age at the time of the patient’s or control’s index date. Less than 10% of siblings of individuals born before 1952 could be identified in DCRS, rising to 43% in the 1952 birth cohort and 96% for individuals born after 1958.17 EPTB siblings were not included due to inadequate study population size which did not allow for sibling mortality studies.
Comorbidity
A modified Charlson Comorbidity Index (CCI) score was used as a measurement of comorbidity and calculated from DNPR diagnoses prior to the first TB diagnosis.18 Three levels of comorbidity were defined: none (CCI score 0), low (CCI score 1–2), and high (CCI score >3). As an alternative measure of comorbidity, data on in-hospital patient admissions in the 5 years prior to the date of TB diagnosis was extracted from the DNPR for all patients, population controls, and siblings. These data were subcategorized according to diagnostic categories based on discharge diagnosis.
Statistical analysis
Time was calculated from index date to the date of death, emigration, loss to follow-up or December 31, 2009, whichever came first. Kaplan–Meier analyses were used to construct survival curves. All-cause mortality rate ratios (MRR), representing relative risk of death, and 95% confidence intervals (CIs) were calculated and stratified according to sex, age at time of diagnosis, ethnicity, and level of CCI score of the TB patient. Cause-specific MRRs were based on the underlying cause of death and categorized as listed in the Supplementary materials.
For siblings we computed time from January 1, 1977, or the index date of the respective patient or population control, whichever came last, until date of death, emigration, loss to follow-up, or December 31, 2009, whichever came first. Poisson regression analyses were used to calculate all-cause and cause-specific MRRs (adjusted for age and sex) for siblings of PTB patients compared with siblings of the PTB population controls and for PTB patients born after 1 January, 1952 compared with their respective siblings. Statistical Package for the Social Sciences version 15.0 for Windows (SPSS Inc., Chicago, IL, USA), R version 2.13.0 (R Foundation for Statistical Computing, Vienna, Austria), and Stata version 11 (StataCorp, College Station, TX, USA) were used for data analysis.
Results
Characteristics of TB patient cohorts
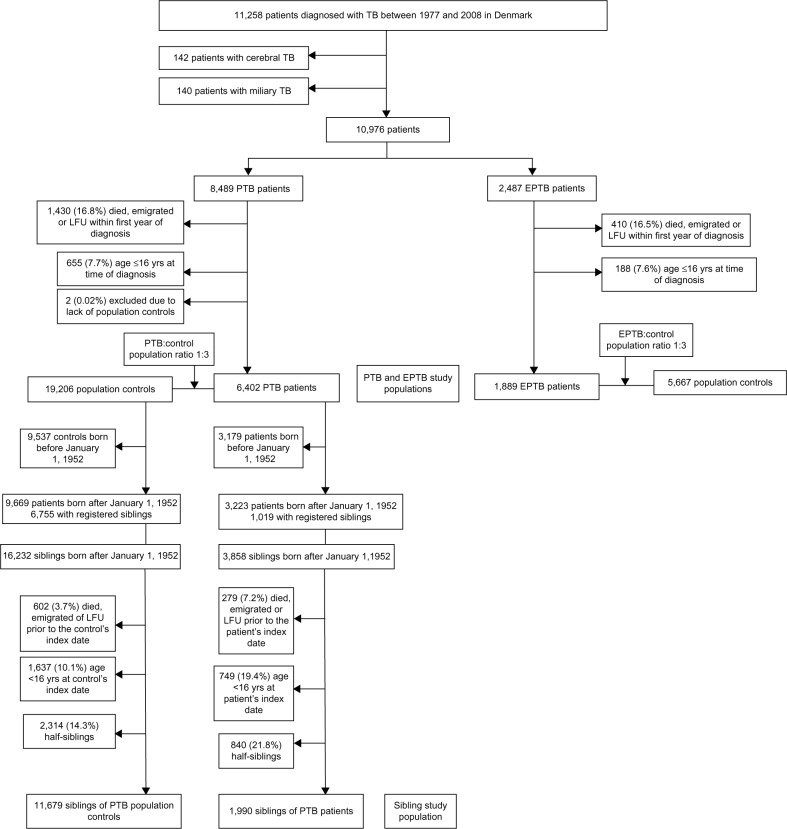
We identified 11,258 TB patients of whom 6,402 PTB patients and 1,889 EPTB patients fulfilled the inclusion criteria (Figure 1). A total of 5,290 (82.6%) PTB patients and 1,713 (90.7%) EPTB patients were microbiologically verified. For both cohorts, the proportion of immigrants increased with calendar time (Table 1). Due to the study design, the control cohorts were well matched with respect to age and sex, but PTB and EPTB patients had more comorbidity and had been hospitalized more frequently than their respective population control cohorts (Table 1).
Figure 1.
Summary of study design.
Notes: Nationwide cohort study of long-term mortality in patients with TB, Denmark, 1977–2008.
Abbreviations: EPTB, extrapulmonary tuberculosis; LFU, lost to follow-up; PTB, pulmonary tuberculosis; TB, tuberculosis; yrs, years.
Table 1.
Characteristics of PTB and EPTB patient cohorts, population comparison cohorts, and sibling cohorts
| PTB patients | PTB population controls | Siblings of PTB patients | Siblings of PTB population controls | EPTB patients | EPTB population controls | |
|---|---|---|---|---|---|---|
| Total number, n (%) | 6,402 (25.0) | 19,206 (75.0) | 1,990 (14.6) | 11,679 (85.4) | 1,889 (25.0) | 5,667 (75.0) |
| Males, n (%) | 3,855 (60.2) | 11,565 (60.2) | 1,055 (53.0) | 6,244 (53.5) | 816 (43.2) | 2,448 (43.2) |
| Immigrants, n (%) | 2,326 (36.3) | 0 | 405 (20.4) | 0 | 1,242 (65.7) | 0 |
| Observation time (total), years | 64,212 | 234,484 | 19,268 | 12,449 | 20,339 | 67,272 |
| Observation time, median (IQR), years | 8.1 (3.7–14.1) | 10.5 (5.5–17.2) | 8.3 (4.1–13.3) | 9.7 (5.4–14.2) | 9.3 (4.8–14.7) | 10.4 (5.9–15.8) |
| Observation time (total), years, Danes | 40,398 | 7,291 | ||||
| Observation time, median (IQR), years, Danes | 7.8 (3.4–14.2) | 9.1 (4.6–16.4) | ||||
| Observation time (total), years, immigrants | 23,814 | 13,047 | ||||
| Observation time, median (IQR), years, immigrants | 8.7 (4.3–13.8) | 9.4 (5.0–13.8) | ||||
| Lost to follow-up during study period, n (%) | 79 (1.2) | 4 (0.01) | 7 (0.4) | 5 (0.01) | 34 (1.8) | 2 (0.001) |
| Emigrated during study period, n (%) | 281 (4.4) | 204 (1.1) | 54 (2.7) | 198 (1.7) | 158 (8.4) | 62 (1.1) |
| Age at diagnosis, median (IQR), years | 42.8 (30.1–58.6) | 42.8 (30.1–58.6) | 31.7 (25.3–38.4) | 31.5 (24.0–38.2) | 38.8 (28.9–60.2) | 38.8 (28.9–60.2) |
| Age at diagnosis by age intervals, n (%) | ||||||
| 16–30 years | 1,570 (24.5) | 4,710 (24.5) | 889 (44.7) | 5,043 (43.2) | 540 (28.6) | 1,620 (28.6) |
| 31–60 years | 3,357 (52.4) | 10,071 (52.4) | 1,101 (55.3) | 6,636 (56.8) | 874 (46.3) | 2,622 (46.3) |
| >61 years | 1,475 (23.0) | 4,425 (23.0) | 0 | 0 | 475 (25.1) | 1,425 (25.1) |
| Patients diagnosed by decade, n (%) | ||||||
| 1977–1986 | 2,026 (31.6) | 6,078 (31.6) | 548 (29.0) | 1,644 (29.0) | ||
| Immigrants, n (%) | 383 (18.9) | 0 | 168 (30.7) | 0 | ||
| 1987–1996 | 1,500 (23.4) | 4,500 (23.4) | 463 (24.5) | 1,389 (24.5) | ||
| Immigrants, n (%) | 605 (40.3) | 0 | 326 (70.4) | 0 | ||
| 1997–2008 | 2,876 (44.9) | 8,628 (44.9) | 878 (46.5) | 2,634 (46.5) | ||
| Immigrants, n (%) | 1,338 (46.5) | 0 | 748 (85.2) | 0 | ||
| Age at diagnosis by decade, median (IQR), years | ||||||
| 1977–1986 | 52.6 (34.7–65.9) | 52.6 (34.7–65.9) | 57.6 (36.3–70.4) | 57.6 (36.3–70.4) | ||
| 1987–1996 | 41.2 (29.7–57.6) | 41.2 (29.7–57.6) | 36.3 (27.2–60.6) | 36.3 (27.2–60.6) | ||
| 1997–2008 | 39.5 (28.9–51.5) | 39.5 (28.9–51.5) | 34.6 (28.1–46.0) | 34.6 (28.1–46.0) | ||
| Modified Charlson Comorbidity Index, n (%) | ||||||
| None (score 0) | 5,498 (85.9) | 17,744 (92.4) | 1,897 (95.3) | 11,254 (96.4) | 1,680 (88.9) | 5,231 (92.3) |
| Low (score 1–2) | 791 (12.4) | 1,340 (7.0) | 85 (4.3) | 385 (3.3) | 177 (9.4) | 408 (7.2) |
| High (score≥3) | 113 (1.8) | 122 (0.6) | 8 (0.4) | 40 (0.3) | 32 (1.7) | 28 (0.5) |
| Hospital admission in 5 years prior to TB | ||||||
| Total patients, n (%) | 2,677 (41.8) | 5,589 (29.1) | 660 (33.2) | 3,695 (31.6) | 871 (46.1) | 1,854 (32.7) |
| Infectious diseases | 176 (2.7) | 171 (0.9) | 55 (2.8) | 116 (1.0) | 58 (3.1) | 59 (1.0) |
| Pulmonology | 426 (6.7) | 403 (2.1) | 50 (2.5) | 255 (2.2) | 61 (3.2) | 131 (2.3) |
| Endocrinology | 133 (2.1) | 192 (1.0) | 16 (0.8) | 82 (0.7) | 43 (2.3) | 51 (0.9) |
| Gastroenterology | 491 (7.7) | 834 (4.3) | 77 (3.9) | 369 (3.2) | 134 (7.1) | 246 (4.3) |
| Psychiatry | 225 (3.5) | 116 (0.6) | 29 (1.5) | 91 (0.8) | 9 (0.5) | 37 (0.7) |
| Cardiovascular | 320 (5.0) | 824 (4.3) | 38 (1.9) | 166 (1.4) | 88 (4.7) | 227 (4.0) |
| Alcohol-related | 152 (2.4) | 51 (0.3) | 10 (0.5) | 24 (0.2) | 2 (0.1) | 9 (0.2) |
| Injury or poisoning | 677 (10.6) | 1,105 (5.8) | 158 (7.9) | 704 (6.0) | 106 (5.6) | 306 (5.4) |
| Neurology | 90 (1.4) | 167 (0.9) | 20 (1.0) | 97 (0.8) | 20 (1.1) | 56 (1.0) |
| Malignant neoplasm prior to study inclusion, n (%) | 248 (3.9) | 565 (2.9) | 17 (0.9) | 97 (0.8) | 61 (3.2) | 190 (3.4) |
| HIV diagnosis before or at time of TB diagnosis, n (%) | 107 (1.7) | 28 (0.001) | 9 (0.5) | 14 (0.1) | 18 (1.2) | 6 (0.001) |
| Medical history of TB before study inclusion, n (%) | 0 | 0 | 163 (8.2) | 17 (0.1) | 0 | 0 |
Abbreviations: EPTB, extrapulmonary tuberculosis; HIV, human immunodeficiency virus; IQR, interquartile range; PTB, pulmonary tuberculosis; TB, tuberculosis.
Characteristics of sibling cohorts
A total of 1,990 siblings of PTB patients and 11,679 siblings of the PTB control cohort were identified. The PTB patients’ siblings had slightly increased comorbidity, including TB, and more were HIV-positive compared with the siblings of the population control cohort (Table 1).
Mortality in PTB patient population versus matched population controls
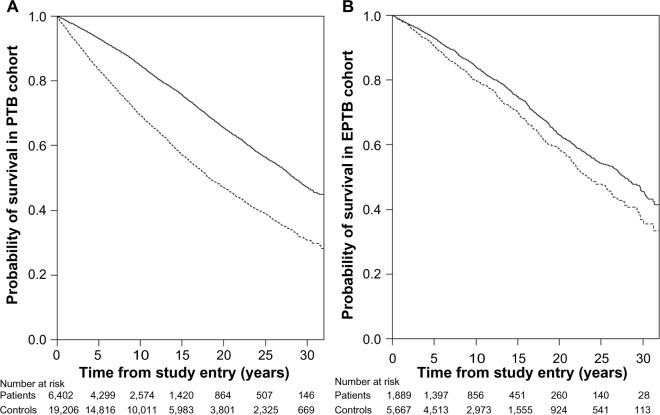
A total of 2,388 (37.3%) PTB patients and 4,683 (24.4%) PTB population controls died during the study period. A Kaplan–Meier survival curve for the PTB cohort and their population control cohort is shown in Figure 2. An overall MRR of 1.86 (95% CI 1.77–1.96) was found for the PTB cohort (Table 2).
Figure 2.
Kaplan–Meier survival curves.
Notes: Graphs depict long-term mortality in patients (dashed line) with PTB (A), and EPTB (B), compared with population controls (solid line), Denmark, 1977–2008.
Abbreviations: EPTB, extrapulmonary tuberculosis; PTB, pulmonary tuberculosis.
Table 2.
All-cause mortality rate ratios in PTB and EPTB patients, population controls, and siblings, Denmark, 1977–2008
| PTB cohort
|
EPTB cohort
|
Siblings (n=1,990) of PTB patients versus siblings (n=11,679) of PTB controls; all born after January 1, 1952
|
PTB patients (n=1,019) versus their respective siblings (n=1,990); all born after January 1, 1952
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Number of patients who died | Number of controls who died | MRR (95% CI) | Number of patients who died | Number of controls who died | MRR (95% CI) | Number of siblings of patients who died (%) | Number of siblings of controls who died (%) | MRR (95% CI)a | Number of patients who died (%) | Number of siblings of patients who died (%) | MRR (95% CI)a | |
| Overall | 2,388 (37.3%) | 4,683 (24.4%) | 1.86 (1.77–1.96) | 515 (27.3%) | 1,379 (24.3%) | 1.24 (1.12–1.37) | 74 (3.7) | 206(1.8) | 2.16(1.62-2.87) | 110(10.8) | 74 (3.7) | 3.55(2.57-4.91) |
| Overall for individuals born after January 1, 1952 | 271 (8.4) | 193 (2.0) | 4.62 (3.85–5.57) | |||||||||
| MRR stratified on individual risk factors | ||||||||||||
| Sex | ||||||||||||
| Females | 812 | 1,757 | 1.61 (1.48–1.75) | 299 | 789 | 1.24 (1.09–1.42) | 16 | 65 | 1.53 (0.86-2.73) | 30 | 16 | 6.95(3.46-13.94) |
| Males | 1,576 | 2,926 | 2.04 (1.92–2.17) | 216 | 590 | 1.23 (1.05–1.43) | 58 | 141 | 2.45(1.76-3.40) | 80 | 58 | 2.86(1.98-4.12) |
| Age at diagnosis (years) | ||||||||||||
| 16–30 | 87 | 106 | 2.71 (2.04–3.59) | 16 | 20 | 2.61 (1.35–5.04) | 21 | 68 | 2.09(1.27-3.44) | 29 | 21 | 2.93 (1.63-5.28) |
| 0–10 years observation time | 12 | 18 | 2.19 (1.05–4.55) | 5 | 4 | 4.09 (1.10–15.25) | ||||||
| > 10 years observation time | 0 | 1 | n/a | 0 | 0 | n/a | ||||||
| 31–60 | 1,104 | 1,464 | 2.80 (2.59–3.03) | 140 | 310 | 1.50 (1.23–1.83) | 53 | 138 | 2.17(1.54-3.08) | 81 | 53 | 3.88 (2.63-5.72) |
| 0–10 years observation time | 500 | 306 | 5.87 (5.09–6.77) | 36 | 73 | 1.64 (1.10–2.44) | ||||||
| >10 years observation time | 184 | 198 | 3.44 (2.81–4.20) | 20 | 29 | 2.39 (1.35–4.22) | ||||||
| >60 | 1,197 | 3,113 | 1.57 (1.47–1.68) | 359 | 1,049 | 1.15 (1.02–1.29) | 0 | 0 | n/a | 0 | 0 | n/a |
| 0–10 years | 1,079 | 2,032 | 2.23 (2.07–2.40) | 266 | 680 | 1.32 (1.14–1.52) | ||||||
| observation time | ||||||||||||
| >10 years observation time | 613 | 2,128 | 1.38 (1.26–1.50) | 188 | 593 | 1.15 (0.98–1.36) | ||||||
| Ethnicity | ||||||||||||
| Native Danes | 2,119 | 4,147 | 1.94 (1.84–2.04) | 429 | 1,158 | 1.27 (1.13–1.42) | 72 | 206 | 2.38(1.78-3.18) | 105 | 72 | 3.45 (2.48^.80) |
| Immigrantsb | 269 | 536 | 1.71 (1.48–1.98) | 86 | 221 | 1.26 (0.98–1.62) | 2 | 0 | n/a | 5 | 2 | 5.85(1.12-30.61) |
| Modified Charlson | ||||||||||||
| Comorbidity Index | ||||||||||||
| Score 0 | 1,857 | 3,689 | 1.80 (1.70–1.90) | 407 | 1,119 | 1.18 (1.06–1.33) | ||||||
| Score 1 | 460 | 902 | 2.20 (1.96–2.46) | 90 | 234 | 1.46 (1.15–1.86) | ||||||
| Score 2 | 71 | 92 | 4.22 (3.09–5.75) | 18 | 26 | 3.11 (1.70–5.67) | ||||||
Notes:
Adjusted for sex and age
immigrant patients compared with their Danish population controls.
Abbreviations: CI, confidence interval; EPTB, extrapulmonary tuberculosis; MRR, mortality rate ratio; n/a, not applicable; PTB, pulmonary tuberculosis.
When stratified by individual risk factors, a higher mortality was found in the PTB cohort for males (MRR 2.04; 95% CI 1.92–2.17) compared with females (MRR 1.61; 95% CI 1.48–1.75). The MRR decreased with increasing age at time of TB diagnosis and the relative risk of death increased with increased comorbidity (Table 2). Danish-born PTB patients had a slightly higher risk of death (MRR 1.94; 95% CI 1.84–2.04) than immigrants (MRR 1.71; 95% CI 1.48–1.98).
The PTB patients had an increased risk of death from natural causes (infectious, malignant, respiratory, rheumatic, liver/pancreatic diseases, and diabetes) and also from unnatural causes of death (alcohol and drug abuse, injury, and poisoning; Table 3 and Table S1). The increased risk of malignant diseases in PTB patients was mainly driven by cancers of the respiratory tract, esophagus, breast, and hematologic system.
Table 3.
Cause-specific mortality rate ratios in PTB and EPTB patients, population controls, and siblings, Denmark, 1977–2008
| PTB cohort
|
EPTB cohort
|
Siblings of PTB patents versus siblings of PTB controls
|
PTB patients versus their respective siblings
|
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Number of patients who died (n=2,388) | Number of controls who died (n=4,683) | MRR | 95% CI | Number of patients who died (n=515) | Number of controls who died (n=1,379) | MRR | 95% CI | Number of siblings of patients who died (n=74 total) | Number of siblings of controls who died (n=206 total) | MRRa | 95% CI | Number of patients who died (n=110 total) | Number of siblings of patients who died (n=74 total) | MRRa | 95% CI | |
| Infectious diseases | 156 | 31 | 18.38 | 12.50–27.02 | 14 | 8 | 5.79 | 2.43–13.80 | 7 | 2 | 10.53 | 1.76–63.00 | 11 | 7 | 10.56 | 2.33–17.83 |
| Neoplasmsb | 530 | 1,242 | 1.56 | 1.41–1.73 | 118 | 334 | 1.17 | 0.95–1.44 | 13 | 54 | 1.32 | 0.67–2.61 | 13 | 13 | 2.71 | 1.15–6.37 |
| Malignant neoplasms of lip, oral cavity, and pharynx | 28 | 22 | 4.65 | 2.66–8.12 | 0 | 7 | ||||||||||
| Malignant neoplasms of digestive organs | 128 | 349 | 1.34 | 1.10–1.65 | 34 | 72 | 1.56 | 1.04–2.35 | ||||||||
| Malignant neoplasms of respiratory and intrathoracic organs | 191 | 303 | 2.30 | 1.92–2.76 | 25 | 72 | 1.15 | 0.73–1.81 | ||||||||
| Malignant neoplasms of bone and articular cartilage | 1 | 3 | 1.22 | 0.13–11.70 | 1 | 1 | ||||||||||
| Melanoma and other malignant neoplasms of skin | 3 | 17 | 0.64 | 0.19–2.20 | 1 | 7 | 0.47 | 0.06–3.84 | ||||||||
| Malignant neoplasms of breast | 27 | 63 | 1.57 | 1.00–2.46 | 10 | 23 | 1.44 | 0.68–3.02 | ||||||||
| Malignant neoplasms of female genital organs | 13 | 57 | 0.83 | 0.46–1.52 | 5 | 31 | 0.53 | 0.21–1.37 | ||||||||
| Malignant neoplasms of male genital organs | 24 | 100 | 0.88 | 0.56–1.37 | 7 | 20 | 1.16 | 0.49–2.74 | ||||||||
| Malignant neoplasms of urinary tract | 21 | 74 | 1.04 | 0.64–1.68 | 5 | 28 | 0.59 | 0.23–1.53 | ||||||||
| Malignant neoplasms of central nervous system | 7 | 32 | 0.80 | 0.35–1.81 | 2 | 4 | ||||||||||
| Malignant neoplasms of thyroid and other endocrine organs | 0 | 3 | 1 | 0 | ||||||||||||
| Malignant hematologic neoplasms | 37 | 87 | 1.55 | 1.06–2.28 | 15 | 25 | 1.98 | 1.05–3.76 | ||||||||
| Malignant neoplasm of other and ill-defined sites | 50 | 125 | 1.46 | 1.05–2.03 | 10 | 43 | 0.75 | 0.38–1.49 | ||||||||
| Benign neoplasms | 0 | 7 | 2 | 1 | ||||||||||||
| Hematologic diseases | 10 | 15 | 2.43 | 1.09–5.42 | 1 | 3 | 1.10 | 0.11–10.60 | 0 | 3 | 1 | 0 | ||||
| Endocrine diseases | 67 | 123 | 1.99 | 1.48–2.68 | 17 | 35 | 1.61 | 0.90–2.87 | 1 | 4 | 1.76 | 0.20–15.75 | 3 | 1 | 5.41 | 0.56–52.29 |
| Psychiatric disorders (including those caused by alcohol and/or drug abuse) | 108 | 142 | 2.78 | 2.16–3.57 | 8 | 27 | 0.98 | 0.45–2.16 | 12 | 15 | 4.66 | 2.09–10.37 | 11 | 12 | 2.44 | 0.97–6.09 |
| Neurologic diseases | 21 | 73 | 1.05 | 0.65–1.71 | 9 | 14 | 2.13 | 0.92^.91 | 0 | 6 | 1 | 0 | ||||
| Cardiovascular diseases | 573 | 1,761 | 1.19 | 1.08–1.3 1 | 191 | 582 | 1.09 | 0.92–1.28 | 5 | 26 | 1.35 | 0.52–3.52 | II | 5 | 3.87 | 1.34–11.21 |
| Respiratory diseases | 353 | 432 | 2.98 | 2.59–3.43 | 40 | 141 | 0.94 | 0.66–1.33 | 3 | 5 | 1.40 | 0.16–11.95 | 7 | 3 | 15.37 | 1.87–126.61 |
| Gastrointestinal diseases | 178 | 210 | 3.10 | 2.53–3.78 | 27 | 43 | 2.08 | 1.28–3.36 | 10 | 17 | 4.17 | 1.82–9.53 | 25 | 10 | 6.49 | 2.80–15.05 |
| Dermatologic diseases | 0 | 2 | 2 | 2 | 3.31 | 0.47–23.48 | 0 | 0 | 0 | 0 | ||||||
| Rheumatologic diseases | 12 | 17 | 2.58 | 1.23–5.40 | 1 | 8 | 0.41 | 0.05–3.31 | 0 | 0 | 1 | 0 | ||||
| Genitourinary diseases | 22 | 78 | 1.03 | 0.64–1.65 | 12 | 16 | 2.48 | 1.17–5.24 | 0 | 1 | 0 | 0 | ||||
| Injury or poisoning | 150 | 219 | 2.50 | 2.03–3.08 | 27 | 72 | 1.24 | 0.80–1.93 | 12 | 54 | 1.57 | 0.84–2.93 | 14 | 12 | 2.00 | 0.92–4.32 |
| Not classified elsewhere | 155 | 244 | 2.32 | 1.90–2.84) | 32 | 79 | 1.34 | 0.89–2.02 | 0 | 9 | 8 | 0 | ||||
| Unknown | 51 | 89 | 2.09 | 1.48–2.95 | 14 | 15 | 3.09 | 1.49–6.40 | 11 | 7 | 11.06 | 4.29–28.52 | 3 | 11 | 0.47 | 0.13–1.70 |
| Congenital cause of death | 2 | 5 | 1 | 0 | 0 | 3 | 1 | 0 | ||||||||
| Pregnancy-related death | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | ||||||||
Notes:
Adjusted for sex and age.
Detailed neoplasm subanalysis not performed for sibling study populations due to small case numbers.
Abbreviations: CI, confidence interval; EPTB, extra-pulmonary tuberculosis; MRR, mortality rate ratio; PTB, pulmonary tuberculosis.
Mortality in EPTB patient population versus matched population controls
A total of 515 (27.3%) EPTB patients and 1,379 (24.3%) EPTB population controls died during the study period. A Kaplan–Meier survival curve for the EPTB cohort and their respective population control cohort is shown in Figure 2. An overall MRR of 1.24 (95% CI 1.12–1.37) was found for the EPTB cohort (Table 2). When stratified by individual risk factors, the MRR was found to decrease with increasing age at time of TB diagnosis. Also, the MRR increased with higher levels of comorbidity. The relative risk of death was not found to differ significantly when data were stratified by sex and ethnicity. The EPTB patients had an increased risk of death due to infectious, gastrointestinal, and genitourinary diseases, diabetes, and gastrointestinal and hematologic neoplasms (Table 3 and Table S1).
Mortality in PTB patient population versus their siblings
Compared with their respective siblings, the PTB patients had a significantly increased relative risk of death (MRR 3.55; 95% CI 2.57–4.91). In the stratified analyses we observed an increased risk of death in all subgroups (Table 2). As all siblings were born after January 1, 1952, for comparison we calculated MRR for PTB patients born in the same period compared with their respective control cohort (MRR 4.62; 95% CI 3.85–5.57). Compared with their siblings, the PTB patients were at increased risk of death from infectious diseases (MRR 10.56; 95% CI 2.33–47.83), malignant neoplasms (MRR 2.71; 95% CI 1.15–6.37), and cardiovascular, respiratory, and gastrointestinal diseases (Table 3).
Mortality in siblings of PTB patient population versus siblings of PTB control population
The siblings of the PTB patients had a more than twofold excess mortality compared with siblings of the PTB control cohort (Table 2), and this was not substantially reduced after exclusion of siblings with a history of TB (MRR 1.99; 95% CI 1.48–2.69), data not shown in tables. In the stratified analyses, an increased relative risk of death (MRR 2.45; 95% CI 1.76–3.40) was found for male siblings of PTB patients compared with male siblings of PTB controls.
Compared with siblings of the population controls, the siblings of the PTB patients had a significantly increased risk of death from infectious diseases (MRR 10.53; 95% CI 1.76–63.00), mental disorders, including alcohol and/or drug abuse (MRR 4.66; 95% CI 2.90–10.37), and gastrointestinal diseases (MRR 4.17; 95% CI 1.82–9.53, Table 3).
Discussion
In this nationwide, population-based cohort study, we observed an almost two-fold increased long-term mortality in PTB patients compared with an age-matched and sex-matched population comparison cohort. Increased relative risk of death was mainly predicted by male sex, younger age, native citizenship, comorbidity, and HIV coinfection. Further, an increased mortality was observed in the siblings of PTB patients. An increased risk of death was also identified in EPTB patients, although smaller than for the PTB patients.
To our knowledge, this is the first study to investigate long-term mortality in TB patients in a nationwide, population-based, cohort study design including estimates of mortality in siblings of TB patients. Our study has several strengths. First, the centralized registration of TB patients in Denmark enabled us to identify a large cohort of TB patients over a more than 30-year period. Second, having a purely native Danish population control cohort excludes the possible confounding related to a mixed ethnic control cohort. Third, the high proportion of microbiologically verified TB cases minimized risk of misclassification. Fourth, the extracted data originated from Danish national registries with high coverage and long-term and complete follow-up, thus providing high validity. Finally, access to the Danish registries allowed us to generate well matched, population-based comparison cohorts and identify siblings of the patients and population comparison cohorts. We were not able to adjust for potential confounding from smoking, alcohol abuse, and socioeconomic factors which may partly explain the observed excess mortality. We did not have access to patient files and were therefore not able to account for clinical parameters and treatment regimes. Also, our results are derived from a high-income setting with a low incidence of TB and HIV, and may not be comparable with settings of developing nations with a high incidence of HIV and drug-resistant TB.
Several studies have identified age, male sex, comorbidity, diabetes, HIV, and native citizenship as risk factors for active TB and short-term mortality in TB patients.19,20 We identified the same factors, except age, to be risk factors for increased long-term relative risk of death. The relative risk of death was highest in the younger age categories which may seem counterintuitive, but reflects the fact that the absolute risk of death is highest in the older background population, and increased mortality in the older TB patients thereby leads to a minor impact on relative risk of death.
Both PTB and EPTB patients were at increased risk of death due to TB, which is in accordance with a study from the UK which found TB to be responsible for 21% of all deaths during a 14-year follow-up.20 Both our cohorts were also at increased risk of death due to diabetes, a disease associated with not only an increased risk of TB but also increased mortality in TB patients; two retrospective US cohort studies both reported more than a six-fold increased mortality in diabetic TB patients compared with nondiabetic controls.21,22 With increasing use of biologic disease-modifying antirheumatic drugs, patients with rheumatic diseases are at increased risk of TB infection;23 this is reflected in our results, with PTB patients exhibiting more than a two-fold increased risk of death due to underlying rheumatic disease.
The epidemiologic evidence that alcohol abuse and exposure to cigarette smoke increase the risk of active TB is strong.24,25 The prevalence of alcohol abuse disorders among TB patients in developed countries ranges from 10% to 50%,26–30 and it has been estimated that almost one third of Danish PTB patients suffer from alcohol abuse and one in ten from drug abuse.31,32 This is supported in our data on in-hospital admissions in the 5 years prior to TB diagnosis, where admissions linked to alcohol abuse were significantly more frequent among the PTB patients compared with population controls. Further, lower socioeconomic status is associated with active transmission of TB among native Danes.33 We observed an increased risk of long-term mortality in PTB patients from chronic lung diseases and lung cancer, alcohol and drug abuse, and gastrointestinal diseases, particularly liver-related. Also, the risk of death was increased in the siblings of these patients. Our data thereby underlines the fact that conditions related to tobacco, alcohol and drug abuse, immune suppression, and socioeconomic factors are not only risk factors for contracting TB and short-term mortality, but also are main determinants of long-term mortality.
Compared with PTB patients, we observed only minor increased risk of death in the EPTB patients, which supports previous results of lower case fatality rates among patients with EPTB.19 Also, the findings point toward less impact from tobacco, alcohol, and drug abuse.
We found a significantly increased risk of death due to malignant neoplasms in the PTB patient group compared with population controls. This excess mortality was primarily driven by an increased prevalence of deaths due to cancers of the airways, esophagus, breast and hematopoietic system. The majority of these subcategories have been strongly associated with smoking, alcohol abuse, and immunodeficiency.34–39 A recent population-based cohort study from Taiwan that included 5,657 TB patients and 23,984 controls found a statistically significant increased incidence of lung cancer in the years after TB infection (269 per 100,000 person-years) compared with population controls (153 per 100,000 person-years).40
The siblings of the PTB patients were at markedly increased risk of death compared with the siblings of the population controls and appeared to be at increased risk of death due to infectious and gastrointestinal diseases and alcohol and drug abuse. This high excess mortality among the siblings of PTB patients indicates that the excess long-term mortality among PTB patients cannot solely be attributed to an effect of TB infection, but must also be influenced by other family-associated, behavioral, and/or socioeconomic-related factors. We presume that these associations are explained by the fact that alcohol and drug abuse clusters in families and also leads to increased risk of TB as well as death due to infectious and gastrointestinal diseases. However, the MRR of PTB patients born after January 1, 1952 was almost twice that of their siblings, indicating that specific risk factors are also present in the PTB patient cohort and increase their mortality, although part of this excess mortality can be reflected in the younger age of the siblings. Studies have shown that growing up in an environment of low socioeconomic status increases the risk of death later in life;41,42 hence, one explanation might be that the patients and siblings share the same low socioeconomic status during childhood and are thus at increased risk of death in adulthood. Genetic predisposition to death from infectious diseases43 has been proposed along with evidence of increased genetic host susceptibility to acquiring infectious diseases such as TB.44–47
Our study has several clinical implications. It emphasizes that, in high-income settings, PTB patients suffer from substantially increased long-term mortality compared with EPTB patients and compared with the background population. Furthermore, it establishes that classical risk factors for active TB and short-term mortality are also the main risk factors for long-term mortality, and that PTB patients are at a significantly increased risk of dying from conditions related to tobacco, alcohol, and drug abuse and immune suppression. The study underlines the importance of addressing lifestyle and socioeconomic risk factors to substantially reduce long-term mortality in PTB patients.
Supplementary materials
Detailed subcategorization of causes of death according to ICD-8 and ICD-10 diagnosis codes
The 18 categories of specific underlying causes of death were specified by the International Classification of Diseases, Eighth Revision (ICD-8), codes for the years 1977–1993 and the International Classification of Diseases, Tenth Revision (ICD-10), codes for 1994–2006. For infectious diseases, the codes were 000–134.99/A00–B99; cancer 140–209/C00–C96; blood/immune diseases 280–289.99/D50–D89; endocrine diseases 240–279.09/E00–E90; mental and behavioral disorders, including those due to psychoactive substance use and inebriation 290–315/F00–F99; nervous system diseases 320–358.09/G00–G99; diseases of the sensory organs 360–389.99/H00–H59; cardiovascular diseases 390–458.99/I00–I99; respiratory diseases 460–519.99/J00–J99; digestive system diseases 520–577.99/K00–K93; skin diseases 680–709.99/L00–L99; rheumatic diseases 710–738.09/M00–M99; genitourinary diseases 580–629.99/N00–N99; neonatal/congenital disorders 740–779.99/P00–Q99; pregnancy-related diseases 630–678.09/O00–O99; injury/poisoning 800–999/S00–T98, V, W, X, Y; ill-defined causes 780–796.99/R00–R99; and no cause of death reported.
Table S1.
Detailed cause-specific mortality rate ratios in PTB and EPTB patients compared with population controls, Denmark, 1977–2008
| PTB cohort
|
EPTB cohort
|
|||||||
|---|---|---|---|---|---|---|---|---|
| Number of patients who died (n=2,388) | Number of controls who died (n=4,683) | MRR | 95% CI | Number of patients who died (n=515) | Number of controls who died (n=1,379) | MRR | 95% CI | |
| Infectious diseases | 156 | 31 | 18.38 | 12.50–27.02 | 14 | 8 | 5.79 | 2.43–13.80 |
| Tuberculosis and HIV/AIDS | 135 | 3 | 164.33 | 52.34–515.92 | 10 | 0 | ||
| Other infectious diseases | 21 | 28 | 2.95 | 1.66–5.24 | 4 | 8 | 1.89 | 0.55–6.46 |
| Malignant neoplasms | 530 | 1,242 | 1.56 | 1.41–1.73 | 118 | 334 | 1.17 | 0.95–1.44 |
| Malignant neoplasms of lip, oral cavity, and pharynx | 28 | 22 | 4.65 | 2.66–8.12 | 0 | 7 | ||
| Lip, oral cavity | 0 | 0 | ||||||
| Tongue, palate, parotid gland | 18 | 14 | 4.70 | 2.34–9.44 | ||||
| Tonsils and oropharynx | 4 | 4 | 3.65 | 0.91–14.60 | ||||
| Nasopharynx | 0 | 0 | ||||||
| Hypopharynx | 2 | 3 | 2.43 | 0.41–14.57 | ||||
| Unspecified cancer of the ear, nose, throat | 4 | 1 | 14.61 | 1.63–130.69 | ||||
| Malignant neoplasms of digestive organs | 128 | 349 | 1.34 | 1.10–1.65 | 34 | 72 | 1.56 | 1.04–2.35 |
| Esophagus | 29 | 31 | 3.42 | 2.06–5.67 | 1 | 12 | 0.28 | 0.04–2.12 |
| Stomach | 17 | 44 | 1.41 | 0.81–2.47 | 6 | 6 | 3.31 | 1.07–10.26 |
| Small intestine | 1 | 3 | 1.22 | 0.13–11.70 | 0 | 1 | ||
| Colon | 33 | 126 | 0.96 | 0.65–1.40 | 10 | 29 | 1.14 | 0.56–2.34 |
| Rectum | 17 | 54 | 1.15 | 0.67–1.98 | 7 | 6 | 3.86 | 1.30–11.48 |
| Liver and gallbladder | 13 | 30 | 1.58 | 0.83–3.03 | 3 | 3 | 3.31 | 0.67–16.39 |
| Pancreas | 16 | 55 | 1.06 | 0.61–1.85 | 6 | 14 | 1.42 | 0.54–3.69 |
| Unspecified gastrointestinal cancer | 2 | 6 | 1.22 | 0.25–6.03 | 1 | 1 | ||
| Malignant neoplasms of respiratory and intra-thoracic organs | 191 | 303 | 2.30 | 1.92–2.76 | 25 | 72 | 1.15 | 0.73–1.81 |
| Nasal cavity and middle ear | 0 | 1 | ||||||
| Larynx | 12 | 11 | 3.98 | 1.76–9.03 | ||||
| Lungs (including trachea) | 171 | 278 | 2.25 | 1.86–2.72 | ||||
| Pleura, mediastinum and other ill-defined sites | 8 | 13 | 2.25 | 0.93–5.42 | ||||
| Malignant neoplasms of bone and articular cartilage | 1 | 3 | 1.22 | 0.13–11.70 | 1 | 1 | 3.31 | 0.21–52.88 |
| Melanoma and other malignant neoplasms of skin | 3 | 17 | 0.64 | 0.19–2.20 | 1 | 7 | 0.47 | 0.06–3.84 |
| Malignant neoplasms of breast | 27 | 63 | 1.57 | 1.00–2.46 | 10 | 23 | 1.44 | 0.68–3.02 |
| Malignant neoplasms of female genital organs | 13 | 57 | 0.83 | 0.46–1.52 | 5 | 31 | 0.53 | 0.21–1.37 |
| Malignant neoplasms of male genital organs | 24 | 100 | 0.88 | 0.56–1.37 | 7 | 20 | 1.16 | 0.49–2.74 |
| Malignant neoplasms of urinary tract | 21 | 74 | 1.04 | 0.64–1.68 | 5 | 28 | 0.59 | 0.23–1.53 |
| Malignant neoplasms of central nervous system | 7 | 32 | 0.80 | 0.35–1.81 | 2 | 4 | 1.65 | 0.30–9.03 |
| Malignant hematologic neoplasms | 37 | 87 | 1.55 | 1.06–2.28 | 15 | 25 | 1.98 | 1.05–3.76 |
| Myeloma | 3 | 16 | 0.68 | 0.20–2.35 | 0 | 4 | ||
| Lymphoma | 11 | 21 | 1.91 | 0.92–3.97 | 4 | 10 | 1.32 | 0.41–4.22 |
| Leukemia | 23 | 50 | 1.68 | 1.03–2.75 | 11 | 11 | 3.31 | 1.43–7.63 |
| Malignant neoplasms of other and ill-defined sites | 50 | 125 | 1.46 | 1.05–2.03 | 10 | 43 | 0.75 | 0.38–1.49 |
| Benign neoplasms | 0 | 7 | 2 | 1 | ||||
| Hematologic diseases | 10 | 15 | 2.43 | 1.09–5.42 | 1 | 3 | 1.10 | 0.11–10.60 |
| Endocrine diseases | 67 | 123 | 1.99 | 1.48–2.68 | 17 | 35 | 1.61 | 0.90–2.87 |
| Diabetes mellitus | 56 | 92 | 2.22 | 1.59–3.10 | 15 | 25 | 1.98 | 1.05–3.76 |
| Psychiatric disorders | 108 | 142 | 2.78 | 2.16, 3.57 | 8 | 27 | 0.98 | 0.45, 2.16 |
| Caused by alcohol and/or drugs | 87 | 41 | 7.75 | 5.35, 11.23 | ||||
| Other psychiatric disorders | 21 | 101 | 0.76 | 0.47, 1.21 | ||||
| Neurologic diseases | 21 | 73 | 1.05 | 0.65, 1.71 | 9 | 14 | 2.13 | 0.92, 4.91 |
| Cardiovascular diseases | 573 | 1761 | 1.19 | 1.08, 1.31 | 191 | 582 | 1.09 | 0.92, 1.28 |
| Respiratory diseases | 353 | 432 | 2.98 | 2.59, 3.43 | 40 | 141 | 0.94 | 0.66, 1.33 |
| Acute infections of the upper respiratory tract | 5 | 7 | 2.61 | 0.83, 8.22 | 0 | 0 | ||
| Pneumonia and bronchitis | 79 | 135 | 2.14 | 1.62, 2.82 | 14 | 48 | 0.96 | 0.53, 1.75 |
| Chronic illness of the lower respiratory tract | 241 | 260 | 3.38 | 2.84, 4.03 | 23 | 84 | 0.91 | 0.57, 1.44 |
| Diseases involving pleura, lung abscesses or edema | 28 | 30 | 3.41 | 2.04, 5.70 | 3 | 9 | 1.10 | 0.30, 4.07 |
| Gastrointestinal diseases | 178 | 210 | 3.10 | 2.53, 3.78 | 27 | 43 | 2.08 | 1.28, 3.36 |
| Liver disease | 113 | 62 | 6.66 | 4.88, 9.07 | 6 | 17 | 1.17 | 0.46, 2.96 |
| Esophagus, stomach, duodenum | 14 | 64 | 0.80 | 0.45, 1.42 | 7 | 14 | 1.65 | 0.67, 4.10 |
| Inflammatory bowel disease | 0 | 4 | 0 | 0 | ||||
| Ileus, diverticulitis, abscess, peritonitis | 25 | 41 | 2.23 | 1.35, 3.66 | 8 | 8 | 3.31 | 1.24, 8.81 |
| Pancreas and gall-bladder disease | 22 | 22 | 3.65 | 2.02, 6.59 | 3 | 1 | 9.92 | 1.03, 95.39 |
| Other gastrointestinal disease, including hemorrhage | 4 | 17 | 0.86 | 0.29, 2.55 | 3 | 3 | 3.31 | 0.67, 16.39 |
| Dermatologic diseases | 0 | 2 | 2 | 2 | 3.31 | 0.47, 23.48 | ||
| Rheumatic diseases | 12 | 17 | 2.58 | 1.23, 5.40 | 1 | 8 | 0.41 | 0.05, 3.31 |
| Genitourinary diseases | 22 | 78 | 1.03 | 0.64, 1.65 | 12 | 16 | 2.48 | 1.17, 5.24 |
| Injury or poisoning | 150 | 219 | 2.50 | 2.03, 3.08 | 27 | 72 | 1.24 | 0.80, 1.93 |
| Not classified elsewhere | 155 | 244 | 2.32 | 1.90, 2.84 | 32 | 79 | 1.34 | 0.89, 2.02 |
| Unknown | 51 | 89 | 2.09 | 1.48, 2.95 | 14 | 15 | 3.09 | 1.49, 6.40 |
| Congenital cause of death | 2 | 5 | 1 | 0 | ||||
| Pregnancy related death | 0 | 0 | 1 | 0 | ||||
Abbreviations: CI, confidence interval; EPTB, extrapulmonary tuberculosis; MRR, mortality rate ratio; PTB, pulmonary tuberculosis; HIV, human immunodeficiency virus; AIDS, acquired immune deficiency syndrome.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.World Health Organization Global Tuberculosis Control: WHO Report 2011. [Accessed May 7, 2013]. Available from: http://www.who.int/tb/publications/global_report/2011/gtbr11_full.pdf.
- 2.Lonnroth K, Jaramillo E, Williams BG, Dye C, Raviglione M. Drivers of tuberculosis epidemics: the role of risk factors and social determinants. Soc Sci Med. 2009;68(12):2240–2246. doi: 10.1016/j.socscimed.2009.03.041. [DOI] [PubMed] [Google Scholar]
- 3.Lienhardt C, Fielding K, Sillah JS, et al. Investigation of the risk factors for tuberculosis: a case-control study in three countries in West Africa. Int J Epidemiol. 2005;34(4):914–923. doi: 10.1093/ije/dyi100. [DOI] [PubMed] [Google Scholar]
- 4.European Centre for Disease Prevention and Control Migrant Health Background note to the ECDC report on migration and infectious diseases in the EU. Jul, 2009. [Accessed August 15, 2013]. Available from: http://www.ecdc.europa.eu/en/publications/publications/0907_ter_migrant_health_background_note.pdf.
- 5.Statens Serum Institut, Department of Infectious Disease Epidemiology Epi-News No 49, 2011. Tuberculosis 2010 Part 1. [Accessed November 27, 2012]. Available from: http://www.ssi.dk/English/News/EPI-NEWS/2011/No%2049%20-%202011.aspx.
- 6.Pablos-Mendez A, Sterling TR, Frieden TR. The relationship between delayed or incomplete treatment and all-cause mortality in patients with tuberculosis. JAMA. 1996;276(15):1223–1228. doi: 10.1001/jama.1996.03540150025026. [DOI] [PubMed] [Google Scholar]
- 7.Lefebvre N, Falzon D. Risk factors for death among tuberculosis cases: analysis of European surveillance data. Eur Respir J. 2008;31(6):1256–1260. doi: 10.1183/09031936.00131107. [DOI] [PubMed] [Google Scholar]
- 8.Straetemans M, Glaziou P, Bierrenbach AL, Sismanidis C, van der Werf MJ. Assessing tuberculosis case fatality ratio: a meta-analysis. PLoS One. 2011;6(6):e20755. doi: 10.1371/journal.pone.0020755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Denmark’s Statistics Populations and elections: population in Denmark. [Accessed November 27, 2012]. Available from: http://www.statistikbanken.dk/statbank5a/default.asp?w=1600.
- 10.Statens Serum Institut, Department of Infectious Disease Epidemiology Epi-News No 50, 2009. Tuberculosis 2008 Part 1. [Accessed November 27, 2012]. Available from: http://www.ssi.dk/English/News/EPI-NEWS/~/media/Indhold/EN%20-%20engelsk/EPI-NEWS/2009/pdf/EPI-NEWS%20-%202009%20-%20No%2050.ashx.
- 11.Andersen PH, Kamper-Jorgensen Z, Stenz F, Soborg B. Various strategies for elimination of tuberculosis. Ugeskr Laeger. 2011;173(12):872–875. Danish. [PubMed] [Google Scholar]
- 12.Lohse N, Hansen AB, Jensen-Fangel S, et al. Demographics of HIV-1 infection in Denmark: results from the Danish HIV Cohort Study. Scand J Infect Dis. 2005;37(5):338–343. doi: 10.1080/00365540510031692. [DOI] [PubMed] [Google Scholar]
- 13.Andersen TF, Madsen M, Jorgensen J, Mellemkjoer L, Olsen JH. The Danish National Hospital Register. A valuable source of data for modern health sciences. Dan Med Bull. 1999;46(3):263–268. [PubMed] [Google Scholar]
- 14.Frank L. Epidemiology. When an entire country is a cohort. Science. 2000;287(5462):2398–2399. doi: 10.1126/science.287.5462.2398. [DOI] [PubMed] [Google Scholar]
- 15.Juel K, Helweg-Larsen K. The Danish registers of causes of death. Dan Med Bull. 1999;46(4):354–357. [PubMed] [Google Scholar]
- 16.Christensen AS, Roed C, Omland LH, Andersen PH, Obel N, Andersen AB. Long-term mortality in patients with tuberculous meningitis: a Danish nationwide cohort study. PLoS One. 2011;6(11):e27900. doi: 10.1371/journal.pone.0027900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Christensen K, Schmidt MM, Vaeth M, Olsen J. Absence of an environmental effect on the recurrence of facial-cleft defects. N Engl J Med. 1995;333(3):161–164. doi: 10.1056/NEJM199507203330305. [DOI] [PubMed] [Google Scholar]
- 18.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 19.Anyama N, Bracebridge S, Black C, Niggebrugge A, Griffin SJ. What happens to people diagnosed with tuberculosis? A population-based cohort. Epidemiol Infect. 2007;135(7):1069–1076. doi: 10.1017/S0950268807007996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Horne DJ, Hubbard R, Narita M, et al. Factors associated with mortality in patients with tuberculosis. BMC Infect Dis. 2010;10:258. doi: 10.1186/1471-2334-10-258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dooley KE, Tang T, Golub JE, Dorman SE, Cronin W. Impact of diabetes mellitus on treatment outcomes of patients with active tuberculosis. Am J Trop Med Hyg. 2009;80(4):634–639. [PMC free article] [PubMed] [Google Scholar]
- 22.Oursler KK, Moore RD, Bishai WR, et al. Survival of patients with pulmonary tuberculosis: clinical and molecular epidemiologic factors. Clin Infect Dis. 2002;34(6):752–759. doi: 10.1086/338784. [DOI] [PubMed] [Google Scholar]
- 23.Brassard P, Kezouh A, Suissa S. Antirheumatic drugs and the risk of tuberculosis. Clin Infect Dis. 2006;43(6):717–722. doi: 10.1086/506935. [DOI] [PubMed] [Google Scholar]
- 24.Lonnroth K, Williams BG, Stadlin S, Jaramillo E, Dye C. Alcohol use as a risk factor for tuberculosis – a systematic review. BMC Public Health. 2008;8:289. doi: 10.1186/1471-2458-8-289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shang S, Ordway D, Henao-Tamayo M, et al. Cigarette smoke increases susceptibility to tuberculosis – evidence from in vivo and in vitro models. J Infect Dis. 2011;203(9):1240–1248. doi: 10.1093/infdis/jir009. [DOI] [PubMed] [Google Scholar]
- 26.Brudney K, Dobkin J. Resurgent tuberculosis in New York City. Human immunodeficiency virus, homelessness, and the decline of tuberculosis control programs. Am Rev Respir Dis. 1991;144(4):745–749. doi: 10.1164/ajrccm/144.4.745. [DOI] [PubMed] [Google Scholar]
- 27.Enarson DA, Wang JS, Dirks JM. The incidence of active tuberculosis in a large urban area. Am J Epidemiol. 1989;129(6):1268–1276. doi: 10.1093/oxfordjournals.aje.a115246. [DOI] [PubMed] [Google Scholar]
- 28.Laifer G, Widmer AF, Simcock M, et al. TB in a low-incidence country: differences between new immigrants, foreign-born residents and native residents. Am J Med. 2007;120(4):350–356. doi: 10.1016/j.amjmed.2006.10.025. [DOI] [PubMed] [Google Scholar]
- 29.Milne RC. Alcoholism and tuberculosis in Victoria. Med J Aust. 1970;2(21):955–960. doi: 10.5694/j.1326-5377.1970.tb63286.x. [DOI] [PubMed] [Google Scholar]
- 30.Pincock TA. Alcoholism in tuberculosis patients. Can Med Assoc J. 1964;91:851–854. [PMC free article] [PubMed] [Google Scholar]
- 31.Lillebaek T, Poulsen S, Kok-Jensen A. Tuberculosis treatment in Denmark: treatment outcome for all Danish patients in 1992. Int J Tuberc Lung Dis. 1999;3(7):603–612. [PubMed] [Google Scholar]
- 32.Andersen RM, Bjorn-Praest SO, Gradel KO, Nielsen C, Nielsen HI. Epidemiology, diagnostic delay and outcome of tuberculosis in North Jutland, Denmark. Dan Med Bull. 2011;58(3):A4256. [PubMed] [Google Scholar]
- 33.Kamper-Jorgensen Z, Andersen AB, Kok-Jensen A, et al. Clustered tuberculosis in a low-burden country: nationwide genotyping through 15 years. J Clin Microbiol. 2012;50(8):2660–2667. doi: 10.1128/JCM.06358-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rodriguez T, Altieri A, Chatenoud L, et al. Risk factors for oral and pharyngeal cancer in young adults. Oral Oncol. 2004;40(2):207–213. doi: 10.1016/j.oraloncology.2003.08.014. [DOI] [PubMed] [Google Scholar]
- 35.Kreuzer M, Kreienbrock L, Gerken M, et al. Risk factors for lung cancer in young adults. Am J Epidemiol. 1998;147(11):1028–1037. doi: 10.1093/oxfordjournals.aje.a009396. [DOI] [PubMed] [Google Scholar]
- 36.Xue F, Willett WC, Rosner BA, Hankinson SE, Michels KB. Cigarette smoking and the incidence of breast cancer. Arch Intern Med. 2011;171(2):125–133. doi: 10.1001/archinternmed.2010.503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sandler DP, Shore DL, Anderson JR, et al. Cigarette smoking and risk of acute leukemia: associations with morphology and cytogenetic abnormalities in bone marrow. J Natl Cancer Inst. 1993;85(24):1994–03. doi: 10.1093/jnci/85.24.1994. [DOI] [PubMed] [Google Scholar]
- 38.Thomas X, Chelghoum Y. Cigarette smoking and acute leukemia. Leuk Lymphoma. 2004;45(6):1103–1109. doi: 10.1080/10428190310001638904. [DOI] [PubMed] [Google Scholar]
- 39.Franceschi S, Talamini R, Barra S, et al. Smoking and drinking in relation to cancers of the oral cavity, pharynx, larynx, and esophagus in northern Italy. Cancer Res. 1990;50(20):6502–6507. [PubMed] [Google Scholar]
- 40.Wu CY, Hu HY, Pu CY, et al. Pulmonary tuberculosis increases the risk of lung cancer: a population-based cohort study. Cancer. 2011;117(3):618–624. doi: 10.1002/cncr.25616. [DOI] [PubMed] [Google Scholar]
- 41.Gallo V, Mackenbach JP, Ezzati M, et al. Social inequalities and mortality in Europe – results from a large multi-national cohort. PLoS One. 2012;7(7):e39013. doi: 10.1371/journal.pone.0039013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Strand BH, Groholt EK, Steingrimsdottir OA, et al. Educational inequalities in mortality over four decades in Norway: prospective study of middle aged men and women followed for cause specific mortality, 1960–2000. BMJ. 2010;340:c654. doi: 10.1136/bmj.c654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Obel N, Christensen K, Petersen I, Sorensen TI, Skytthe A. Genetic and environmental influences on risk of death due to infections assessed in Danish twins, 1943–2001. Am J Epidemiol. 2010;171(9):1007–1013. doi: 10.1093/aje/kwq037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Storgaard M, Varming K, Herlin T, Obel N. Novel mutation in the interferon-gamma-receptor gene and susceptibility to mycobacterial infections. Scand J Immunol. 2006;64(2):137–139. doi: 10.1111/j.1365-3083.2006.01775.x. [DOI] [PubMed] [Google Scholar]
- 45.Baghdadi JE, Orlova M, Alter A, et al. An autosomal dominant major gene confers predisposition to pulmonary tuberculosis in adults. J Exp Med. 2006;203(7):1679–1684. doi: 10.1084/jem.20060269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Motsinger-Reif AA, Antas PR, Oki NO, et al. Polymorphisms in IL1-beta, vitamin D receptor Fok1, and Toll-like receptor 2 are associated with extrapulmonary tuberculosis. BMC Med Genet. 2010;11:37. doi: 10.1186/1471-2350-11-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gao L, Tao Y, Zhang L, Jin Q. Vitamin D receptor genetic polymorphisms and tuberculosis: updated systematic review and meta-analysis. Int J Tuberc Lung Dis. 2010;14(1):15–23. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1.
Detailed cause-specific mortality rate ratios in PTB and EPTB patients compared with population controls, Denmark, 1977–2008
| PTB cohort
|
EPTB cohort
|
|||||||
|---|---|---|---|---|---|---|---|---|
| Number of patients who died (n=2,388) | Number of controls who died (n=4,683) | MRR | 95% CI | Number of patients who died (n=515) | Number of controls who died (n=1,379) | MRR | 95% CI | |
| Infectious diseases | 156 | 31 | 18.38 | 12.50–27.02 | 14 | 8 | 5.79 | 2.43–13.80 |
| Tuberculosis and HIV/AIDS | 135 | 3 | 164.33 | 52.34–515.92 | 10 | 0 | ||
| Other infectious diseases | 21 | 28 | 2.95 | 1.66–5.24 | 4 | 8 | 1.89 | 0.55–6.46 |
| Malignant neoplasms | 530 | 1,242 | 1.56 | 1.41–1.73 | 118 | 334 | 1.17 | 0.95–1.44 |
| Malignant neoplasms of lip, oral cavity, and pharynx | 28 | 22 | 4.65 | 2.66–8.12 | 0 | 7 | ||
| Lip, oral cavity | 0 | 0 | ||||||
| Tongue, palate, parotid gland | 18 | 14 | 4.70 | 2.34–9.44 | ||||
| Tonsils and oropharynx | 4 | 4 | 3.65 | 0.91–14.60 | ||||
| Nasopharynx | 0 | 0 | ||||||
| Hypopharynx | 2 | 3 | 2.43 | 0.41–14.57 | ||||
| Unspecified cancer of the ear, nose, throat | 4 | 1 | 14.61 | 1.63–130.69 | ||||
| Malignant neoplasms of digestive organs | 128 | 349 | 1.34 | 1.10–1.65 | 34 | 72 | 1.56 | 1.04–2.35 |
| Esophagus | 29 | 31 | 3.42 | 2.06–5.67 | 1 | 12 | 0.28 | 0.04–2.12 |
| Stomach | 17 | 44 | 1.41 | 0.81–2.47 | 6 | 6 | 3.31 | 1.07–10.26 |
| Small intestine | 1 | 3 | 1.22 | 0.13–11.70 | 0 | 1 | ||
| Colon | 33 | 126 | 0.96 | 0.65–1.40 | 10 | 29 | 1.14 | 0.56–2.34 |
| Rectum | 17 | 54 | 1.15 | 0.67–1.98 | 7 | 6 | 3.86 | 1.30–11.48 |
| Liver and gallbladder | 13 | 30 | 1.58 | 0.83–3.03 | 3 | 3 | 3.31 | 0.67–16.39 |
| Pancreas | 16 | 55 | 1.06 | 0.61–1.85 | 6 | 14 | 1.42 | 0.54–3.69 |
| Unspecified gastrointestinal cancer | 2 | 6 | 1.22 | 0.25–6.03 | 1 | 1 | ||
| Malignant neoplasms of respiratory and intra-thoracic organs | 191 | 303 | 2.30 | 1.92–2.76 | 25 | 72 | 1.15 | 0.73–1.81 |
| Nasal cavity and middle ear | 0 | 1 | ||||||
| Larynx | 12 | 11 | 3.98 | 1.76–9.03 | ||||
| Lungs (including trachea) | 171 | 278 | 2.25 | 1.86–2.72 | ||||
| Pleura, mediastinum and other ill-defined sites | 8 | 13 | 2.25 | 0.93–5.42 | ||||
| Malignant neoplasms of bone and articular cartilage | 1 | 3 | 1.22 | 0.13–11.70 | 1 | 1 | 3.31 | 0.21–52.88 |
| Melanoma and other malignant neoplasms of skin | 3 | 17 | 0.64 | 0.19–2.20 | 1 | 7 | 0.47 | 0.06–3.84 |
| Malignant neoplasms of breast | 27 | 63 | 1.57 | 1.00–2.46 | 10 | 23 | 1.44 | 0.68–3.02 |
| Malignant neoplasms of female genital organs | 13 | 57 | 0.83 | 0.46–1.52 | 5 | 31 | 0.53 | 0.21–1.37 |
| Malignant neoplasms of male genital organs | 24 | 100 | 0.88 | 0.56–1.37 | 7 | 20 | 1.16 | 0.49–2.74 |
| Malignant neoplasms of urinary tract | 21 | 74 | 1.04 | 0.64–1.68 | 5 | 28 | 0.59 | 0.23–1.53 |
| Malignant neoplasms of central nervous system | 7 | 32 | 0.80 | 0.35–1.81 | 2 | 4 | 1.65 | 0.30–9.03 |
| Malignant hematologic neoplasms | 37 | 87 | 1.55 | 1.06–2.28 | 15 | 25 | 1.98 | 1.05–3.76 |
| Myeloma | 3 | 16 | 0.68 | 0.20–2.35 | 0 | 4 | ||
| Lymphoma | 11 | 21 | 1.91 | 0.92–3.97 | 4 | 10 | 1.32 | 0.41–4.22 |
| Leukemia | 23 | 50 | 1.68 | 1.03–2.75 | 11 | 11 | 3.31 | 1.43–7.63 |
| Malignant neoplasms of other and ill-defined sites | 50 | 125 | 1.46 | 1.05–2.03 | 10 | 43 | 0.75 | 0.38–1.49 |
| Benign neoplasms | 0 | 7 | 2 | 1 | ||||
| Hematologic diseases | 10 | 15 | 2.43 | 1.09–5.42 | 1 | 3 | 1.10 | 0.11–10.60 |
| Endocrine diseases | 67 | 123 | 1.99 | 1.48–2.68 | 17 | 35 | 1.61 | 0.90–2.87 |
| Diabetes mellitus | 56 | 92 | 2.22 | 1.59–3.10 | 15 | 25 | 1.98 | 1.05–3.76 |
| Psychiatric disorders | 108 | 142 | 2.78 | 2.16, 3.57 | 8 | 27 | 0.98 | 0.45, 2.16 |
| Caused by alcohol and/or drugs | 87 | 41 | 7.75 | 5.35, 11.23 | ||||
| Other psychiatric disorders | 21 | 101 | 0.76 | 0.47, 1.21 | ||||
| Neurologic diseases | 21 | 73 | 1.05 | 0.65, 1.71 | 9 | 14 | 2.13 | 0.92, 4.91 |
| Cardiovascular diseases | 573 | 1761 | 1.19 | 1.08, 1.31 | 191 | 582 | 1.09 | 0.92, 1.28 |
| Respiratory diseases | 353 | 432 | 2.98 | 2.59, 3.43 | 40 | 141 | 0.94 | 0.66, 1.33 |
| Acute infections of the upper respiratory tract | 5 | 7 | 2.61 | 0.83, 8.22 | 0 | 0 | ||
| Pneumonia and bronchitis | 79 | 135 | 2.14 | 1.62, 2.82 | 14 | 48 | 0.96 | 0.53, 1.75 |
| Chronic illness of the lower respiratory tract | 241 | 260 | 3.38 | 2.84, 4.03 | 23 | 84 | 0.91 | 0.57, 1.44 |
| Diseases involving pleura, lung abscesses or edema | 28 | 30 | 3.41 | 2.04, 5.70 | 3 | 9 | 1.10 | 0.30, 4.07 |
| Gastrointestinal diseases | 178 | 210 | 3.10 | 2.53, 3.78 | 27 | 43 | 2.08 | 1.28, 3.36 |
| Liver disease | 113 | 62 | 6.66 | 4.88, 9.07 | 6 | 17 | 1.17 | 0.46, 2.96 |
| Esophagus, stomach, duodenum | 14 | 64 | 0.80 | 0.45, 1.42 | 7 | 14 | 1.65 | 0.67, 4.10 |
| Inflammatory bowel disease | 0 | 4 | 0 | 0 | ||||
| Ileus, diverticulitis, abscess, peritonitis | 25 | 41 | 2.23 | 1.35, 3.66 | 8 | 8 | 3.31 | 1.24, 8.81 |
| Pancreas and gall-bladder disease | 22 | 22 | 3.65 | 2.02, 6.59 | 3 | 1 | 9.92 | 1.03, 95.39 |
| Other gastrointestinal disease, including hemorrhage | 4 | 17 | 0.86 | 0.29, 2.55 | 3 | 3 | 3.31 | 0.67, 16.39 |
| Dermatologic diseases | 0 | 2 | 2 | 2 | 3.31 | 0.47, 23.48 | ||
| Rheumatic diseases | 12 | 17 | 2.58 | 1.23, 5.40 | 1 | 8 | 0.41 | 0.05, 3.31 |
| Genitourinary diseases | 22 | 78 | 1.03 | 0.64, 1.65 | 12 | 16 | 2.48 | 1.17, 5.24 |
| Injury or poisoning | 150 | 219 | 2.50 | 2.03, 3.08 | 27 | 72 | 1.24 | 0.80, 1.93 |
| Not classified elsewhere | 155 | 244 | 2.32 | 1.90, 2.84 | 32 | 79 | 1.34 | 0.89, 2.02 |
| Unknown | 51 | 89 | 2.09 | 1.48, 2.95 | 14 | 15 | 3.09 | 1.49, 6.40 |
| Congenital cause of death | 2 | 5 | 1 | 0 | ||||
| Pregnancy related death | 0 | 0 | 1 | 0 | ||||
Abbreviations: CI, confidence interval; EPTB, extrapulmonary tuberculosis; MRR, mortality rate ratio; PTB, pulmonary tuberculosis; HIV, human immunodeficiency virus; AIDS, acquired immune deficiency syndrome.