Abstract
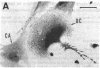
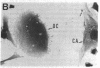
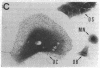
Calcitonin receptors have been characterized for the first time in isolated osteoclasts. These receptors have been demonstrated by autoradiographic and biochemical methods, and the cells have also been shown to respond to calcitonin with a dose-dependent increase in cyclic AMP. The receptors in rat osteoclasts are specific and of high affinity (dissociation constant, Kd, 1 to 6 X 10(-10) M), and are present in greater numbers than in any cell previously studied (greater than 10(6) per cell). Chemical cross-linking of 125I-labeled salmon calcitonin to osteoclasts using disuccinimidyl suberate resulted in identification of a receptor component with a relative molecular weight of 80,000-90,000. By counting grains in autoradiographic experiments, we found that greater than 80% of specifically bound radioactivity was associated with multinucleate osteoclasts and the remainder was associated with mononuclear cells that are not osteoblasts, but that may be osteoclast precursors.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chambers T. J., Athanasou N. A., Fuller K. Effect of parathyroid hormone and calcitonin on the cytoplasmic spreading of isolated osteoclasts. J Endocrinol. 1984 Sep;102(3):281–286. doi: 10.1677/joe.0.1020281. [DOI] [PubMed] [Google Scholar]
- Chambers T. J., Magnus C. J. Calcitonin alters behaviour of isolated osteoclasts. J Pathol. 1982 Jan;136(1):27–39. doi: 10.1002/path.1711360104. [DOI] [PubMed] [Google Scholar]
- Chausmer A. B., Stevens M. D., Severn C. Autoradiographic evidence for a calcitonin receptor on testicular Leydig cells. Science. 1982 May 14;216(4547):735–736. doi: 10.1126/science.6281881. [DOI] [PubMed] [Google Scholar]
- Findlay D. M., Martin T. J. Relationship between internalization and calcitonin-induced receptor loss in T 47D cells. Endocrinology. 1984 Jul;115(1):78–83. doi: 10.1210/endo-115-1-78. [DOI] [PubMed] [Google Scholar]
- Findlay D. M., Michelangeli V. P., Eisman J. A., Frampton R. J., Moseley J. M., MacIntyre I., Whitehead R., Martin T. J. Calcitonin and 1,25-dihydroxyvitamin D3 receptors in human breast cancer cell lines. Cancer Res. 1980 Dec;40(12):4764–4767. [PubMed] [Google Scholar]
- Findlay D. M., Michelangeli V. P., Orlowski R. C., Martin T. J. Biological activities and receptor interactions of des-Leu16 salmon and des-Phe16 human calcitonin. Endocrinology. 1983 Apr;112(4):1288–1291. doi: 10.1210/endo-112-4-1288. [DOI] [PubMed] [Google Scholar]
- Findlay D. M., Ng K. W., Niall M., Martin T. J. Processing of calcitonin and epidermal growth factor after binding to receptors in human breast cancer cells (T 47D). Biochem J. 1982 Aug 15;206(2):343–350. doi: 10.1042/bj2060343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Findlay D. M., deLuise M., Michelangeli V. P., Ellison M., Martin T. J. Properties of a calcitonin receptor and adenylate cyclase in BEN cells, a human cancer cell line. Cancer Res. 1980 Apr;40(4):1311–1317. [PubMed] [Google Scholar]
- Findlay D. M., deLuise M., Michelangeli V. P., Martin T. J. Independent down-regulation of insulin and calcitonin receptors on a human tumour cell line. J Endocrinol. 1981 Feb;88(2):271–281. doi: 10.1677/joe.0.0880271. [DOI] [PubMed] [Google Scholar]
- Forrest S. M., Ng K. W., Findlay D. M., Michelangeli V. P., Livesey S. A., Partridge N. C., Zajac J. D., Martin T. J. Characterization of an osteoblast-like clonal cell line which responds to both parathyroid hormone and calcitonin. Calcif Tissue Int. 1985 Jan;37(1):51–56. doi: 10.1007/BF02557679. [DOI] [PubMed] [Google Scholar]
- Fouchereau-Peron M., Moukhtar M. S., Benson A. A., Milhaud G. Characterization of specific receptors for calcitonin in porcine lung. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3973–3975. doi: 10.1073/pnas.78.6.3973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman J., Raisz L. G. Thyrocalcitonin: inhibitor of bone resorption in tissue culture. Science. 1965 Dec 10;150(3702):1465–1467. doi: 10.1126/science.150.3702.1465. [DOI] [PubMed] [Google Scholar]
- Goldring S. R., Dayer J. M., Ausiello D. A., Krane S. M. A cell strain cultured from porcine kidney increases cyclic AMP content upon exposure to calcitonin or vasopressin. Biochem Biophys Res Commun. 1978 Jul 28;83(2):434–440. doi: 10.1016/0006-291x(78)91009-4. [DOI] [PubMed] [Google Scholar]
- Horton M. A., Rimmer E. F., Moore A., Chambers T. J. On the origin of the osteoclast: the cell surface phenotype of rodent osteoclasts. Calcif Tissue Int. 1985 Jan;37(1):46–50. doi: 10.1007/BF02557678. [DOI] [PubMed] [Google Scholar]
- Hunt N. H., Ellison M., Underwood J. C., Martin T. J. Calcitonin-responsive adenylate cyclase in a calcitonin-producing human cancer cell line. Br J Cancer. 1977 Jun;35(6):777–784. doi: 10.1038/bjc.1977.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt N. H., Smith B., Pembrey R. Cyclic nucleotide excretion in human malignancies. Clin Sci (Lond) 1980 Jun;58(6):463–467. doi: 10.1042/cs0580463. [DOI] [PubMed] [Google Scholar]
- Ito M. B., Schraer H., Gay C. V. The effects of calcitonin, parathyroid hormone and prostaglandin E2 on cyclic AMP levels of isolated osteoclasts. Comp Biochem Physiol A Comp Physiol. 1985;81(3):653–657. doi: 10.1016/0300-9629(85)91043-6. [DOI] [PubMed] [Google Scholar]
- Lamp S. J., Findlay D. M., Moseley J. M., Martin T. J. Calcitonin induction of a persistent activated state of adenylate cyclase in human breast cancer cells (T 47D). J Biol Chem. 1981 Dec 10;256(23):12269–12274. [PubMed] [Google Scholar]
- Loutit J. F., Nisbet N. W. The origin of osteoclasts. Immunobiology. 1982 Apr;161(3-4):193–203. doi: 10.1016/S0171-2985(82)80074-0. [DOI] [PubMed] [Google Scholar]
- Luben R. A., Wong G. L., Cohn D. V. Biochemical characterization with parathormone and calcitonin of isolated bone cells: provisional identification of osteoclasts and osteoblasts. Endocrinology. 1976 Aug;99(2):526–534. doi: 10.1210/endo-99-2-526. [DOI] [PubMed] [Google Scholar]
- Maier R., Kamber B., Riniker B., Rittel W. Analogues of human calcitonin. IV. Influence of leucine substitutions in positions 12, 16 and 19 on hypocalcaemic activity in the rat. Clin Endocrinol (Oxf) 1976;5 (Suppl):327S–332S. doi: 10.1111/j.1365-2265.1976.tb03841.x. [DOI] [PubMed] [Google Scholar]
- Martin T. J., Findlay D. M., MacIntyre I., Eisman J. A., Michelangeli V. P., Moseley J. M., Partridge N. C. Calcitonin receptors in a cloned human breast cancer cell line (MCF 7). Biochem Biophys Res Commun. 1980 Sep 16;96(1):150–156. doi: 10.1016/0006-291x(80)91193-6. [DOI] [PubMed] [Google Scholar]
- Marx S. J., Aurbach G. D., Gavin J. R., 3rd, Buell D. W. Calcitonin receptors on cultured human lymphocytes. J Biol Chem. 1974 Nov 10;249(21):6812–6816. [PubMed] [Google Scholar]
- Marx S. J., Woodward C. J., Aurbach G. D. Calcitonin receptors of kidney and bone. Science. 1972 Dec 1;178(4064):999–1001. doi: 10.1126/science.178.4064.999. [DOI] [PubMed] [Google Scholar]
- Marx S. J., Woodward C., Aurbach G. D., Glossmann H., Keutmann H. T. Renal receptors for calcitonin. Binding and degradation of hormone. J Biol Chem. 1973 Jul 10;248(13):4797–4802. [PubMed] [Google Scholar]
- Michelangeli V. P., Findlay D. M., Moseley J. M., Martin T. J. Mechanisms of calcitonin induction of prolonged activation of adenylate cyclase in human cancer cells. J Cyclic Nucleotide Protein Phosphor Res. 1983;9(2):129–141. [PubMed] [Google Scholar]
- Moseley J. M., Findlay D. M., Gorman J. J., Michelangeli V. P., Martin T. J. The calcitonin receptor on T 47D breast cancer cells. Evidence for glycosylation. Biochem J. 1983 Jun 15;212(3):609–616. doi: 10.1042/bj2120609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moseley J. M., Findlay D. M., Martin T. J., Gorman J. J. Covalent cross-linking of a photoactive derivative of calcitonin to human breast cancer cell receptors. J Biol Chem. 1982 May 25;257(10):5846–5851. [PubMed] [Google Scholar]
- Ng K. W., Livesey S. A., Larkins R. G., Martin T. J. Calcitonin effects on growth and on selective activation of type II isoenzyme of cyclic adenosine 3':5'-monophosphate-dependent protein kinase in T 47D human breast cancer cells. Cancer Res. 1983 Feb;43(2):794–800. [PubMed] [Google Scholar]
- Osdoby P., Martini M. C., Caplan A. I. Isolated osteoclasts and their presumed progenitor cells, the monocyte, in culture. J Exp Zool. 1982 Dec 30;224(3):331–344. doi: 10.1002/jez.1402240306. [DOI] [PubMed] [Google Scholar]
- Partridge N. C., Alcorn D., Michelangeli V. P., Kemp B. E., Ryan G. B., Martin T. J. Functional properties of hormonally responsive cultured normal and malignant rat osteoblastic cells. Endocrinology. 1981 Jan;108(1):213–219. doi: 10.1210/endo-108-1-213. [DOI] [PubMed] [Google Scholar]
- Rizzo A. J., Goltzman D. Calcitonin receptors in the central nervous system of the rat. Endocrinology. 1981 May;108(5):1672–1677. doi: 10.1210/endo-108-5-1672. [DOI] [PubMed] [Google Scholar]
- Singer F. R., Melvin K. E., Mills B. G. Acute effects of calcitonin on osteoclasts in man. Clin Endocrinol (Oxf) 1976;5 (Suppl):333S–340S. doi: 10.1111/j.1365-2265.1976.tb03842.x. [DOI] [PubMed] [Google Scholar]
- Steiner A. L., Wehmann R. E., Parker C. W., Kipnis D. M. Radioimmunoassay for the measurement of cyclic nucleotides. Adv Cyclic Nucleotide Res. 1972;2:51–61. [PubMed] [Google Scholar]
- Tashjian A. H., Jr, Wright D. R., Ivey J. L., Pont A. Calcitonin binding sites in bone: relationships to biological response and "escape". Recent Prog Horm Res. 1978;34:285–334. doi: 10.1016/b978-0-12-571134-0.50012-0. [DOI] [PubMed] [Google Scholar]
- Testa N. G., Allen T. D., Lajtha L. G., Onions D., Jarret O. Generation of osteoclasts in vitro. J Cell Sci. 1981 Feb;47:127–137. doi: 10.1242/jcs.47.1.127. [DOI] [PubMed] [Google Scholar]
- Tschopp F. A., Henke H., Petermann J. B., Tobler P. H., Janzer R., Hökfelt T., Lundberg J. M., Cuello C., Fischer J. A. Calcitonin gene-related peptide and its binding sites in the human central nervous system and pituitary. Proc Natl Acad Sci U S A. 1985 Jan;82(1):248–252. doi: 10.1073/pnas.82.1.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warshawsky H., Goltzman D., Rouleau M. F., Bergeron J. J. Direct in vivo demonstration by radioautography of specific binding sites for calcitonin in skeletal and renal tissues of the rat. J Cell Biol. 1980 Jun;85(3):682–694. doi: 10.1083/jcb.85.3.682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wrann M. M., Fox C. F. Identification of epidermal growth factor receptors in a hyperproducing human epidermoid carcinoma cell line. J Biol Chem. 1979 Sep 10;254(17):8083–8086. [PubMed] [Google Scholar]