Introduction
Food allergy is a result of immune response-driven adverse health effects that occur reproducibly on exposure to a given food. Symptoms of food allergy emerge as immediate (within 2 hours) or delayed (within 6-72 hours) gastrointestinal, dermatological, respiratory, or cardiovascular reactions- potentially culminating into fatal or near-fatal incidents of anaphylaxis. The current cumulative prevalence for food allergy in adults and children is estimated to be 5% and 8% respectively, and is reportedly on continuous rise. Food allergy has thus become a serious public health concern, and invokes critical intervention [1,2,3].
Foods with most commonly encountered food-allergens include milk, eggs, peanuts, soy, wheat, tree-nuts, fish, and shellfish. Adverse reactions to a food allergen can either be IgE-mediated, or non-IgE mediated . The present review focuses on IgE-mediated food allergies.
Immune mechanism of IgE-mediated food allergy
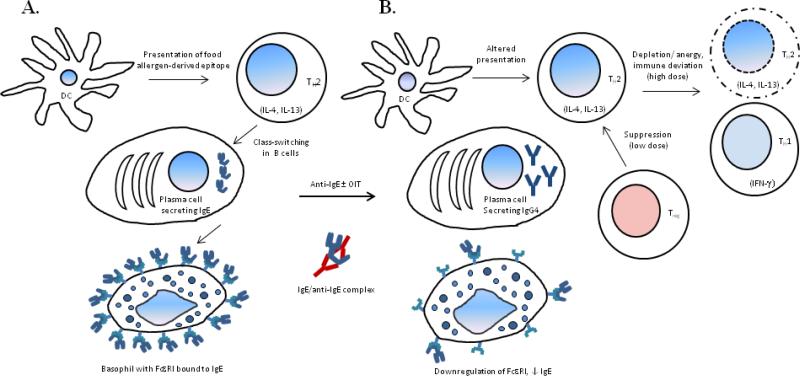
Under the IgE-mediated pathway, on primary exposure, antigen presenting cells of an individual capture, process, and present cognate food allergen/s to naïve T cells resulting in their polarization towards TH2 direction. These allergen-specific TH2 cells secrete large amounts of IL-4 and IL-13, which in turn promote antibody class switching and differentiation of B cells into plasma cells secreting IgE antibodies recognizing that food-allergen/s. Free IgE in plasma binds to its high affinity receptor- FcεRI expressed by basophils and mast cells (Fig. 1A). On re-exposure, the cognate food allergen is recognized by cell-bound IgE triggering downstream signaling cascade leading to degranulation of basophils and mast cells. Chemical mediators such as histamines, leukotrienes, prostaglandins released during degranulation process give rise to the aforementioned adverse health effects [2,4,5].
Figure 1. Immune mechanism of food allergy and post-treatment desensitization.
In IgE-mediated food allergy, primary exposure to a food leads to development high levels of food allergen-specific IgE, which binds to FcεRI on the surface of basophils (A). On re-exposure, degranulation of basophils ensuing recognition of cognate food allergen-derived epitope by IgE gives rise to allergic symptoms. Anti-IgE molecules form a biologically inert complex with IgE. Anti-IgE + OIT combination therapy has been shown to bring about depletion of, or anergy in TH2 cells, with concomitant repolarization/ immune deviation towards TH1 direction. While this effect is attributed to high dose administration during combination therapy, desensitization through OIT alone with relatively low doses is more likely driven by increase in suppression by Treg cells. Anti-IgE treatment leads to downmodulation of FcεRI expression on basophils. In addition, decrease in the levels of allergen-specific IgE, and significant increase in those of IgG4, tends to imply successful desensitization (B), with alleviation of allergy symptoms as the net clinical outcome.
Diagnosis
Double-blind placebo-controlled oral food challenge (DBPCFC) has been described as the current gold standard in clinical diagnosis of food allergy. In this method, the patient ingests gradually increasing amounts of food to which sensitization is suspected, or an unrelated food- a placebo. Elicitation of allergic symptoms on consumption of food allergen versus the lack of any such symptoms with placebo affirm food allergy.
Besides, immune response measurement through skin pick test and estimation of allergen-specific IgE, in conjunction with patient's medical history and physical examination can aid a physician in providing care to food allergy patients. In skin prick test, microscopic amount of food allergen is introduced into patient's skin through a pin-prick. The response is recorded in terms of diameter of wheal, which reflects food-allergen-induced mast cell degranulation, and thus demonstrates prior sensitization. Allergen-specific IgE levels are estimated through commercially available sandwich assays [6,7]. Although helpful in identifying foods that can potentially provoke IgE-mediated allergic reactions, skin prick test or allergen-specific IgE alone, or in combination, are not recommended to confirm diagnosis of food allergy [1].
Current treatment options
Despite being recognized as a major public health issue, there are no effective treatment options or curative strategies currently available for food allergy patients. The accepted standard of care is limited to strict avoidance of allergenic foods, nutritional counseling, and access to rapid emergency care (i.e. ready availability of epinephrine autoinjector) to alleviate acute symptoms in the event of accidental exposure. Although undeclared food allergens in commercial food preparations, lapse in awareness of food preparer or consumer about the ingredients serve as loopholes in strict allergen avoidance. Thus there always is a threat of accidental exposure and ensuing allergic reactions, which in turn has a significant negative impact on psychosocial wellbeing of susceptible individuals and their families [8,9].
The problem is being addressed by exploring novel therapeutic approaches aimed to induce desensitization, and eventually develop tolerance to allergenic food/s. These approaches can be classified as allergen-specific, and allergen-nonspecific.
Allergen-specific therapy
Allergen-specific therapy consists of administration of small amounts of food allergen to the patient through oral, sublingual, epicutaneous, or subcutaneous route. Of these methods, the results obtained so far through oral administration (, i.e. OIT,) have been most promising [10,11,12]. In general, an initial DBPCFC confirming the allergic status of a patient to the cognate food allergen precedes initiation of OIT. An ‘initial day dose escalation’ marks the first day of OIT, wherein the patient is administered increasing amounts of food allergen over 6-8 hours under clinical supervision to establish the highest tolerated dose. This dose is administered as the starting dose during the consecutive ‘build-up’ or ‘dose escalation’ phase, with gradual increase in dose occurring weekly or biweekly until a targeted maintenance dose is reached. The patient continues to consume the maintenance dose of the food allergen throughout the third and final ‘maintenance phase’ spanning over a few weeks to months. A post-treatment final oral food challenge (OFC) evaluates the efficacy of the OIT protocol, with success asserted by statistically significant increase in tolerated dose of the food allergen - one that would protect the patient on accidental exposure, or ideally allow the patient to incorporate normal amounts of that food to his/her daily diet.
Outcome of various OIT studies undertaken to desensitize patients allergic to milk, egg, and peanuts have previously been reviewed in detail [10,11,12]. Although objective comparative analysis of the results obtained so far is not feasible owing to the differences among study designs in terms of patient enrollment criteria, allergen doses and time duration for each phase, and reported readouts. Nonetheless, a commonly encountered pattern reveals that (i) although 50-75% patients are successfully desensitized, 10-25% of patients achieve only partial desensitization post-therapy. Presence of higher allergen-specific IgE likely compromises therapeutic desensitization in this subset of patients, (ii) majority of the patients experience at least one adverse reaction over the course of study- especially during the dose escalation phase. Severity of some of such reactions highlights the fact that patient safety remains the prime concern in any OIT protocol. Efforts to devise a more effective protocol that tackles these issues are underway.
Allergen-nonspecific therapy
Allergen-nonspecific approaches include treatment options such as administration of anti-IgE to neutralize IgE, or use of Food Allergy Herbal Formula-2 (FAHF-2), a modified version of herbal concoction suggested in Chinese traditional medicine [13,14]. We will focus on the former treatment strategy in this review.
Anti-IgE: development and mechanism of action
As described earlier, presence of food allergen-specific IgE in plasma implies sensitization to that food. Crosslinking of allergen-bound IgE being the first step in triggering the highly sensitive basophil signaling cascade, IgE- FcεRI interaction is an obvious target to block pathways leading to anaphylaxis.
As the other immunoglobulins, IgE molecular structure features Fab and Fc regions. Fab fragment is composed of a pair of light chains covalently bound to variable and proximal constant (Cε1) domains of heavy chain pair. Fc fragment comprises Cε2, Cε3, and Cε4 domains of heavy chain, of which, Cε3 docks into the α subunit of FcεRI [15].
With early clinical studies demonstrating efficacy of therapies aimed at reducing IgE load in ameliorating symptoms of asthma and allergy, efforts were undertaken to generate monoclonal anti-IgE antibodies, which would specifically interfere with binding of IgE to its receptor. Clones selected based on their binding specificity were humanized to downmodulate antigenicity, and thus enable their clinical application. Omalizumab (Xolair®; Genentech, South San Fransisco, CA, USA), and Talizumab (TNX-901; Tanox, Houston, TX, USA) are two such independently developed anti-IgE antibodies [16,17], which have demonstrated success in clinical trials. Although owing to multiple lawsuits surrounding patent infringement, Talizumab is yet to reach commercial market. Omalizumab on the other hand is commercially available, and has been approved by FDA to treat moderate to severe persistent asthma refractory to inhaled corticosteroids [18].
Through recognition of, and ensuing binding to Cε3 domain of free IgE, omalizumab and talizumab abrogate IgE-FcεRI interaction, hence preventing degranulation by basophils and mast cells. The IgE/anti-IgE complexes thus formed are biologically inert, and can safely be cleared from circulation without causing immune complex-related reactions. Also, since circulating IgE, and signaling cascade initiated by crosslinking of cell-bound IgE can induce the expression of FcεRI, blockade by anti-IgE antibodies interrupts this positive feedback loop thereby increasing the threshold allergen dose for basophil/mast cell activation [19]. In addition to basophils and mast cells, FcεRI is also expressed by dendritic cells (DCs), where it is thought to play a role in allergen presentation to T cells. By abrogating of FcεRI upregulation, omalizumab and talizumab are speculated to downmodulate allergen presentation by DCs, and consequent decrease in TH2 function, thus leading to alleviation of allergic symptoms [20,21,22].
So far, only a handful of clinical trials have tested the efficacy of anti-IgE in treating food allergies. Anti-IgE has been used either as a monotherapy, or as adjunctive treatment with OIT. Results from these studies are summarized as follows:
Anti-IgE as monotherapy
The first study showing the efficacy of anti-IgE treatment in food allergy patients was published by Leung and colleagues in 2003 [23]. In this multi-center, double-blind trial, 84 patients with the history of immediate hypersensitivity to peanut (confirmed pre-enrollment by oral food challenge) were randomly assigned in 3:1 ratio to receive four doses of either 150 / 300 / 450 mg TNX-901 or placebo subcutaneously over four weeks. The final oral food challenge conducted two to four weeks after the fourth dose indicated a trend of dose-dependent improvement in peanut tolerance. The group on 450 mg TNX-901 regimen could tolerate 2805 mg peanut flour vs. 1010 mg tolerated by subjects in placebo group. This increase in tolerated threshold amount of peanut was found to be statistically significant, and enough to confer substantial protection against accidental peanut ingestion. Although encouraging as findings from the first of its kind study, the observed improvement was suboptimal to induce desensitization. (This is the only trial examining efficacy of talizumab in food allergy therapy. With the development of TNX-901 on hold, further trials were carried out using omalizumab.)
A subsequent phase II parallel-group, double-blind, placebo-controlled study was designed to extend these findings by evaluating the efficacy of omalizumab in reducing the risk of peanut-induced allergic reactions [24]. Patients, who qualified through an initial screening DBPCFC were administered omalizumab every 2 to 4 weeks over 20 to 22 weeks. A second DBPCFC was performed at week 24 to evaluate treatment performance. Although this study intended to randomize 150 subjects, it was prematurely terminated due to 2 severe incidents of anaphylaxis during the initial qualifying oral food challenge. Nevertheless, the data from 14 subjects, who completed the therapy and underwent final oral food challenge showed an anti-IgE-mediated increase in peanut tolerability, with 44.4% patients among omalizumab-treated vs. 20% patients in placebo-treated group being able to tolerate ≥ 1000 mg of peanut flour. Though this trial could not conclusively assert the efficacy of omalizumab, the limited available data was consistent with the overall findings from TNX-901 trial, and justified pursuing anti-IgE as a treatment option for food allergy.
A recent open-label study by Savage et al. with peanut allergic patients addresses kinetic and mechanistic details behind omalizumab therapy. 14 subjects were enrolled in the study based on an initial DBPCFC, skin prick test titration (SPTT), and basophil histamine release (BHR). Omalizumab was administered every 2-4 weeks for 24 weeks. Repeat BHR, OFC, and SPTT were carried out at specified time points to evaluate efficacy of the treatment. All 10 subjects, who completed the study, showed statistically significant increase in the threshold tolerated dose of peanut; although only 4 were able to tolerate doses higher than 10,000 mg, hence desensitized [25]. More importantly, this study unravels a very interesting variation in the kinetics of (i) desensitization of subjects with low vs. high allergen-specific IgE, (ii) suppression of basophil vs. mast cell response. Further analysis of these clinical observations revealed increased intrinsic sensitivity of basophils to IgE-mediated stimulation as a factor possibly compromising efficacy of omalizumab [26]. Indeed, considering overall variability in the efficacy of anti-IgE as a monotherapy, more such studies focused on mechanism will be of great importance to identify ‘biomarkers’ that can help distinguish between potential responders and non-responders [27].
Anti-IgE as adjunctive therapy with OIT
The need for measures to reduce severity and frequency of adverse reactions during OIT from the viewpoint of patient safety has been elaborated earlier. A 2006 study with ragweed-induced allergic rhinitis first reported the beneficial effects of omalizumab pretreatment, which allowed administration of higher doses of allergen over a short period of time (i.e. rapid desensitization through rush immunotherapy), without compromising on patient safety [28]. The rationale from this study was implemented in food allergy therapy for the first time by Nadeau et al. [29].
In this phase I pilot study, 11 patients with the history of IgE-mediated milk allergy (median milk-specific IgE of 50 kUA/L) were enrolled at two sites. Omalizumab was administered for 16 weeks every 2 to 4 weeks. Oral milk desensitization was initiated at week 9 from the start of omalizumab therapy with rush desensitization on the first day, followed by dose escalation phase. During the rush oral desensitization, increasing doses of milk powder (starting with 0.1 mg to the maximum of 1000 mg) were administered every 30 minutes. During dose escalation phase, desensitization was continued with weekly increases in milk dose over the next 7 to 11 weeks. A DBPCFC at week 24 of the study showed that 9 out of 10 patients, who completed the study, were able to tolerate one full serving of milk. Mean frequency for total adverse reactions was as low as 1.6%, and most reactions were graded to be mild to moderate [29]. This study does have certain drawbacks such as small sample size, lack of placebo group, and lack of a baseline OFC. Nonetheless, given that the enrolled subjects had very high levels of milk-specific IgE and history of severe milk allergy, desensitization affording intake of normal amounts of milk in daily diet ( >8000 mg/d) within 4 months of OIT with only mild reactions is indeed a remarkable success.
An elegant follow-up study focused on analysis of immune cells of desensitized subjects provides valuable insights into the mechanism of anti-IgE+ OIT- induced tolerance [30]. The results show induction of anergy in, or depletion of milk-specific CD4+ T cells during rush desensitization. Interestingly, milk-specific CD4+ T cell response returned during maintenance phase, although was characterized as TH1-biased as opposed to TH2-biased pre-therapy. No changes were seen in frequency or function of regulatory T (Treg) cells. Significant reduction in milk-specific IgE, and concomitant increase in milk-specific IgG4 levels, together with downregulated basophil response reflected desensitization.
A recent study by Schneider et al. [31] has investigated the efficacy of this combination therapy in patients allergic to peanuts. 13 subjects (median peanut-specific IgE level of 229kUA/L), who failed the initial DBPCFC at peanut flour ≤100 mg, were enrolled in the study. Omalizumab was administered every 2-4 weeks over 20 weeks. Oral desensitization was initiated at week 12 of omalizumab therapy. During the rush desensitization on day 1 of OIT, all subjects reached a cumulative dose of 992 mg peanut flour with minimal or no symptoms. Through dose-escalation phase, 12 subjects reached a maximum maintenance dose of 4000 mg peanut flour per day in the median time of 8 weeks. In the final DBPCFC carried out between week 30-32 of therapy, these 12 subjects could tolerate 8000 mg peanut flour, and continued eating 10 to 20 peanuts daily without adverse health effects. This study too, was performed with small number of subjects, and lacks placebo control. However, with 92% of the highly susceptible patients desensitized over a very short duration of time with minimal symptoms, the findings consolidate the promise of anti-IgE + OIT combination.
The most recent addition to the reports on clinical trials of combination therapy describes the results of a single-center, phase I, open-label study that included children with allergies to multiple foods. Having confirmed the safety and feasibility of OIT to confer desensitization to up to 5 allergens simultaneously in an independent phase I study [32], the authors investigated whether using anti-IgE as an adjunctive therapy to ‘multi-OIT’ safely allows for a faster desensitization to multiple allergens simultaneously. 25 participants enrolled based on failure in an initial DBPCFC were administered omalizumab every 2 to 4 weeks for 16 weeks. A single day rush oral desensitization was carried out on the 9th week of omalizumab administration, wherein under clinical supervision, subjects consumed a mix of offending food allergens in increasing doses ranging from 5 mg to 1250 mg of total food allergen protein at defined time intervals. Out of 25, 19 participants tolerated the highest dose with minimal or no rescue therapy during this rush desensitization. All the participants were started on their highest tolerated dose as their initial daily home dose, which was escalated every 2 weeks, or at a latter, best-suited time point based on participant's allergic reactions and safety outcomes. With this protocol, the participants reached their maintenance dose of 4000 mg protein per allergen at a median of 18 weeks. The reported adverse reaction rate during home dosing was 5.3% with 94% reactions being mild [33]. Given that 30% of the children with food allergy are sensitized to multiple foods, and in their case if the desensitization to each allergen were to be achieved individually can take up to many years, the multi-OIT protocol certainly holds great promise, which is further accentuated with anti-IgE adjunctive therapy, whereby the target maintenance dose was reached 67 weeks earlier than multi-OIT alone [33].
Each of these studies employing combination therapy was carried out with children, as opposed to monotherapy studies, wherein participants were mostly adults.
Although all these open-label pilot trials are highly encouraging and novel in the domain of food allergy therapy, further multi-center, double-blind, placebo-controlled, phase II and III trials are needed to cement their findings, which can be translated into clinical practice. Indeed, two of such studies are underway at Mount Sinai School of Medicine, New York (sponsor: Dr. Hugh Sampson), and Duke University, North Carolina (sponsor: Dr. Wesley Burks) comparing safety and efficacy of omalizumab + OIT combination therapy in milk, and peanut allergic patients respectively (www.clinicaltrials.gov).
Emerging trends and future directions
Table 1 summarizes the outcome of clinical trials employing anti-IgE in treating food allergy. Existing data, though highly limited, does highlight superior performance of anti-IgE and OIT combination therapy over anti-IgE monotherapy, or OIT alone [10,12].
| Trial | Therapy | Allergen | No. of patients enrolled (in test group) | Allergen- specific IgE range(kUA/L) | Initial DBPCFC range (mg) | Final DBPCFC range (mg) | % desensitized |
|---|---|---|---|---|---|---|---|
| Leung (2003) | TNX-901 (450 | Peanut | 21 | 0.69-100 | 178 | 2803 | 24 |
| Sampson (2011) | Om alizum ab | Peanut | 9 | 7.1-323 | <5to 100 | 30-8000 | 11 |
| Savage (2012) | Om alizum ab | Peanut | 14 | 1.1-134 | 10-700 | 1830-10,000 | 29 |
| Nadeau (2011) | Om alizum ab +OIT | Milk | 11 | 41.6-342 | Not done | 8000 | 82 |
| Schneider (2013) | Om alizum ab +OIT | Peanut | 13 | 21-617 | ≤100 | 8000 | 92 |
| Begin (2014) | Om alizum ab + multi-OIT | Multiple (2 to 5) | 25 | 2-236 | ≤182 | 4000 per allergen (maintenance dose reached) | 100 |
Suppression of basophil activation afforded by anti-IgE pretreatment clearly protects subjects from acute adverse reactions on initiation of OIT. It thus enables administration of high doses of food allergen, and rapid dose escalation. This factor indeed gives combination therapy a distinct advantage over OIT alone, wherein desensitization has to be carried out at a slower rate with relatively small doses.
Understanding immunologic mechanism behind desensitization through each mode of desensitization is really important as the insight thus gained will serve as a guiding factor in designing the most effective protocol for therapy. Unfortunately though, current data in this regard is quite scarce. Certain studies on OIT have reported increase in frequency and suppressive ability of Treg post-therapy [34,35]. Study by Bedoret et al. mentioned previously is the only reference point at present to understand mechanistic details behind anti-IgE + OIT combination therapy. This study shows depletion of TH2-polarized cells and/or deviation towards TH1 direction rather than changes in Treg compartment [30]. Suppression of basophil activity, a trend of decrease in allergen-specific IgE, and significant increase in allergen-specific IgG4 are commonly observed findings irrespective of the therapeutic mode (Fig. 1B). An interesting consideration of contribution of high vs. low dose in driving T cell anergy vs. suppression put forth by Bedoret et al. invokes further in-depth investigation [36]. In addition, the possibility of generation of tolerogenic DCs and other changes among innate immune cells, induction of tolerance reflected by circulating immune cells vs. that in the gut-resident population are some of the interesting questions worth addressing through future research.
Clinical outcome and concomitant immunologic analysis of samples of subjects undergoing trials based on a uniform, well-designed protocol for omalizumab monotherapy, or OIT, vis-a-vis omalizumab + OIT combination therapy will be of great value to appraise relative efficacy.
The fact remains that as is the case with most OIT studies, the studies with combination therapy so far have focused on desensitization alone, and not on long-term tolerance, whereby a desensitized subject continues not to react to the previously offending allergen even after a phase of complete avoidance of potentially allergenic foods. Results from protocols, which follow desensitized subject post-avoidance phase are awaited, and would be truly conclusive in order to decide the ‘curative’ potential of oral immunotherapy.
An important consideration is, with the cost of a 150 mg vial of omalizumab being > $500, anti-IgE adjunctive therapy remains a pricy treatment option. Nevertheless, since desensitization through combination therapy is achieved at much faster rate, it would spare the cost of extra visits to be scheduled for slower dose escalation during OIT alone [33]. An in-depth pharmaco-economic analysis in this context will certainly be helpful for a concrete feasibility check.
It should be mentioned that the high affinity of IgE-FcεRI interaction (Kd ~ 1 nM) limits action of omalizumab, since it can bind only to free IgE, and cannot effectively block activation through IgE pre-bound to FcεRI. Improving upon the existing monoclone, finding natural inhibitors or designing synthetic ones that can disrupt IgE-FcεRI binding has been a challenge, although some of the ongoing endeavors have yielded exciting results [37,38]. Candidate inhibitors of particular interest are ‘Designed ankyrin repeat proteins (DARPins)’; especially since one among these engineered proteins- DARPin E2_79 has been show to disrupt IgE-FcεRI interaction through facilitated dissociation [39]. Whether such inhibitors outperform anti-IgE in vivo, and can eventually enter clinics needs to be tested through further experimentation.
Taken together, exploring the potential of anti-IgE in food allergy therapy is an active area of research. Especially results from trials with anti-IgE+ OIT combination therapy, though limited and with certain drawbacks, show great promise, concomitantly raising many questions and possibilities, which have opened new arenas for investigators.
Opinion statement.
Inappropriate immune response to certain food components leads to food allergy. With its increasing prevalence over the past two decades, and potentially fatal consequences, food allergy has become a formidable public health issue. Currently, there is no effective therapy to treat food allergy, hence patients have to resort to strictly avoiding allergenic foods, and need to have quick access to emergency care in the event of accidental exposure. There is thus an urgent need for treatment options. Allergen-specific and allergen-nonspecific therapeutic measures are being actively explored through ongoing research. The data so far has pointed out the promise of oral immunotherapy (OIT) among allergen-specific, and anti-IgE administration among allergennonspecific treatment modes. In addition, results from three recent trials employing anti-IgE as an adjunctive therapy with OIT indeed show an outstanding potential to safely and rapidly desensitize patients with severe food allergies. The overall existing data however, is very limited, and derived from diverse study designs, which in turn have certain individual shortcomings. Readouts from current and proposed multi-center clinical trials following a well-designed, uniform treatment protocol will thus be highly valuable to carry out thorough comparative analysis, and draw concrete inferences, which will pave the way for an approved food allergy therapy.
Footnotes
Conflict of Interest
Monali Manohar and Kari C. Nadeau declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
References and Recommended Reading
Papers of particular interest, published recently, have been highlighted as:
• Of importance
•• Of major importance
- 1.Boyce JA, Assa'ad A, Burks AW, Jones SM, Sampson HA, et al. Guidelines for the Diagnosis and Management of Food Allergy in the United States: Summary of the NIAID-Sponsored Expert Panel Report. Nutrition Research. 2011;31:61–75. doi: 10.1016/j.nutres.2011.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Holgate ST, Polosa R. Treatment strategies for allergy and asthma. Nat Rev Immunol. 2008;8:218–230. doi: 10.1038/nri2262. [DOI] [PubMed] [Google Scholar]
- 3.Sicherer SH, Sampson HA. Food allergy: Epidemiology, pathogenesis, diagnosis, and treatment. The Journal of allergy and clinical immunology. 2014;133:291–307. e295. doi: 10.1016/j.jaci.2013.11.020. [DOI] [PubMed] [Google Scholar]
- 4.Galli SJ, Tsai M. IgE and mast cells in allergic disease. Nat Med. 2012;18:693–704. doi: 10.1038/nm.2755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.MacGlashan DW. IgE-dependent signaling as a therapeutic target for allergies. Trends in pharmacological sciences. 2012;33:502–509. doi: 10.1016/j.tips.2012.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Syed A, Kohli A, Nadeau KC. Food allergy diagnosis and therapy: where are we now? Immunotherapy. 2013;5:931–944. doi: 10.2217/imt.13.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bégin P, Nadeau KC. Diagnosis of Food Allergy. Pediatr Ann. 2013;42:102–109. doi: 10.3928/00904481-20130522-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Henson M, Burks AW. The future of food allergy therapeutics. Seminars in Immunopathology. 2012;34:703–714. doi: 10.1007/s00281-012-0319-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gupta R, Holdford D, Bilaver L, Dyer A, Holl JL, et al. THe economic impact of childhood food allergy in the united states. JAMA Pediatrics. 2013;167:1026–1031. doi: 10.1001/jamapediatrics.2013.2376. [DOI] [PubMed] [Google Scholar]
- 10.Nadeau KC, Kohli A, Iyengar S, DeKruyff RH, Umetsu DT. Oral Immunotherapy and Anti-IgE Antibody-Adjunctive Treatment for Food Allergy. Immunology and Allergy Clinics of North America. 2012;32:111–133. doi: 10.1016/j.iac.2011.11.004. [DOI] [PubMed] [Google Scholar]
- 11.Khoriaty E, Umetsu DT. Oral Immunotherapy for Food Allergy: Towards a New Horizon. Allergy Asthma Immunol Res. 2013;5:3–15. doi: 10.4168/aair.2013.5.1.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang J, Sampson H. Oral and sublingual immunotherapy for food allergy. Asian Pac J Allergy Immunol. 2013;31:198–209. [PubMed] [Google Scholar]
- 13.Wang J, Patil SP, Yang N, Ko J, Lee J, et al. Safety, tolerability, and immunologic effects of a food allergy herbal formula in food allergic individuals: a randomized, double-blinded, placebo-controlled, dose escalation, phase 1 study. Annals of allergy, asthma & immunology : official publication of the American College of Allergy, Asthma, & Immunology. 2010;105:75–84. doi: 10.1016/j.anai.2010.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Patil SP, Wang J, Song Y, Noone S, Yang N, et al. Clinical safety of Food Allergy Herbal Formula-2 (FAHF-2) and inhibitory effect on basophils from patients with food allergy: Extended phase I study. The Journal of allergy and clinical immunology. 2011;128:1259–1265. doi: 10.1016/j.jaci.2011.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gould HJ, Sutton BJ. IgE in allergy and asthma today. Nat Rev Immunol. 2008;8:205–217. doi: 10.1038/nri2273. [DOI] [PubMed] [Google Scholar]
- 16.Shields R, Whether W, Zioncheck K, O'Connell L, Fendly B, et al. Inhibition of allergic reactions with antibodies to IgE. Int Arch Allergy Immunol. 1995;107:308–312. doi: 10.1159/000237010. [DOI] [PubMed] [Google Scholar]
- 17.Kolbinger F, Saldanha J, Hardman N, Bendig MM. Humanization of a mouse anti-human IgE antibody: a potential therapeutic for IgE-mediated allergies. Protein Engineering. 1993;6:971–980. doi: 10.1093/protein/6.8.971. [DOI] [PubMed] [Google Scholar]
- 18.Vichyanond P. Omalizumab in allergic diseases, a recent review. Asian Pac J Allergy Immunol. 2011;29:209–219. [PubMed] [Google Scholar]
- 19.MacGlashan DW, Bochner BS, Adelman DC, Jardieu PM, Togias A, et al. Down-regulation of Fc(epsilon)RI expression on human basophils during in vivo treatment of atopic patients with anti-IgE antibody. The Journal of Immunology. 1997;158:1438–1445. [PubMed] [Google Scholar]
- 20.Holgate S, Smith N, Massanari M, Jimenez P. Effects of omalizumab on markers of inflammation in patients with allergic asthma. Allergy. 2009;64:1728–1736. doi: 10.1111/j.1398-9995.2009.02201.x. [DOI] [PubMed] [Google Scholar]
- 21.van Neerven RJJ, van Roomen CPAA, Thomas WR, de Boer M, Knol EF, et al. Humanized Anti-IgE mAb Hu-901 Prevents the Activation of Allergen-Specific T Cells. International Archives of Allergy and Immunology. 2001;124:400–402. doi: 10.1159/000053770. [DOI] [PubMed] [Google Scholar]
- 22.Segal M, Stokes JR, Casale TB. Anti-Immunoglobulin E therapy. World Allergy Organization Journal. 2008;1:174–183. doi: 10.1097/WOX.0b013e318187a310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23•.Leung DYM, Sampson HA, Yunginger JW, Burks AW, Schneider LC, et al. Effect of Anti-IgE Therapy in Patients with Peanut Allergy. New England Journal of Medicine. 2003;348:986–993. doi: 10.1056/NEJMoa022613. [This is the first study reporting efficacy of anti-IgE (talizumab) in the treatment of food allergy.] [DOI] [PubMed] [Google Scholar]
- 24.Sampson HA, Leung DYM, Burks AW, Lack G, Bahna SL, et al. A phase II, randomized, double-blind, parallel-group, placebo-controlled oral food challenge trial of Xolair (omalizumab) in peanut allergy. Journal of Allergy and Clinical Immunology. 2011;127:1309–1310. doi: 10.1016/j.jaci.2011.01.051. [Although this study has limited data owing to early termination, it serves as only referene point for a placebo-controlled omalizumab monotherapy trial in treating patients with peanut allergy.] [DOI] [PubMed] [Google Scholar]
- 25•.Savage JH, Courneya J-P, Sterba PM, MacGlashan DW, Saini SS, et al. Kinetics of mast cell, basophil, and oral food challenge responses in omalizumab-treated adults with peanut allergy. Journal of Allergy and Clinical Immunology. 2012;130:1123–1129. doi: 10.1016/j.jaci.2012.05.039. [This report uncovers very interesting kinetic and mechanistic details behind desensitization through omalizumab monotherapy.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.MacGlashan DW, Jr, Savage JH, Wood RA, Saini SS. Suppression of the basophil response to allergen during treatment with omalizumab is dependent on 2 competing factors. Journal of Allergy and Clinical Immunology. 2012;130:1130–1135. doi: 10.1016/j.jaci.2012.05.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gernez Y, Tirouvanziam R, Yu G, Ghosn EEB, Reshamwala N, et al. Basophil CD203c Levels Are Increased at Baseline and Can Be Used to Monitor Omalizumab Treatment in Subjects with Nut Allergy. International Archives of Allergy and Immunology. 2011;154:318–327. doi: 10.1159/000321824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Casale TB, Busse WW, Kline JN, Ballas ZK, Moss MH, et al. Omalizumab pretreatment decreases acute reactions after rush immunotherapy for ragweed-induced seasonal allergic rhinitis. The Journal of allergy and clinical immunology. 2006;117:134–140. doi: 10.1016/j.jaci.2005.09.036. [DOI] [PubMed] [Google Scholar]
- 29••.Nadeau KC, Schneider LC, Hoyte L, Borras I, Umetsu DT. Rapid oral desensitization in combination with omalizumab therapy in patients with cow's milk allergy. The Journal of allergy and clinical immunology. 2011;127:1622–1624. doi: 10.1016/j.jaci.2011.04.009. [This is the first open-label pilot study demonstrating efficacy of anti-IgE as an adjunctive therapy with OIT in children with milk allergy.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30••.Bedoret D, Singh AK, Shaw V, Hoyte EG, Hamilton R, et al. Changes in antigen-specific T-cell number and function during oral desensitization in cow's milk allergy enabled with omalizumab. Mucosal Immunol. 2012;5:267–276. doi: 10.1038/mi.2012.5. [This report represents an excellent reference to understand immune mechanism behind anti-IgE + OIT combination therapy.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31••.Schneider LC, Rachid R, LeBovidge J, Blood E, Mittal M, et al. A pilot study of omalizumab to facilitate rapid oral desensitization in high-risk peanut-allergic patients. The Journal of allergy and clinical immunology. 2013;132:1368–1374. doi: 10.1016/j.jaci.2013.09.046. [This report demonstrates efficacy of anti-IgE + OIT combination therapy in treating peanut allergic children, and thus endorses previous findings from milk study.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bégin P, Winterroth LC, Dominguez T, Wilson S, Bacal L, et al. Safety and feasibility of oral immunotherapy to multiple allergens for food allergy. Allergy Asthma Clin Immunol. 2014;10:1. doi: 10.1186/1710-1492-10-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33••.Bégin P, Dominguez T, Wilson SP, Bacal L, Mehrotra A, et al. Phase 1 results of safety and tolerability in a rush oral immunotherapy protocol to multiple foods using omalizumab. Allergy Asthma Clin Immunol. 2014;10:7. doi: 10.1186/1710-1492-10-7. [This recent open label, phase I study is the first one to demonstrate safe and efficeint administration of anti-IgE+ OIT combination therapy in desensitization to multiple food allergens simultenously.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jones SM, Pons L, Roberts JL, Scurlock AM, Perry TT, et al. Clinical efficacy and immune regulation with peanut oral immunotherapy. The Journal of allergy and clinical immunology. 2009;124:292–300. doi: 10.1016/j.jaci.2009.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Varshney P, Jones SM, Scurlock AM, Perry TT, Kemper A, et al. A randomized controlled study of peanut oral immunotherapy: Clinical desensitization and modulation of the allergic response. The Journal of allergy and clinical immunology. 2011;127:654–660. doi: 10.1016/j.jaci.2010.12.1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rachid R, Umetsu D. Immunological mechanisms for desensitization and tolerance in food allergy. Seminars in Immunopathology. 2012;34:689–702. doi: 10.1007/s00281-012-0333-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Smith LD, Leatherbarrow RJ, Spivey AC. Development of small molecules to target the IgE:FcεRI protein–protein interaction in allergies. Future Medicinal Chemistry. 2013;5:1423–1435. doi: 10.4155/fmc.13.112. [DOI] [PubMed] [Google Scholar]
- 38.Jackman J, Chen Y, Huang A, Moffat B, Scheer JM, et al. Development of a Two-part Strategy to Identify a Therapeutic Human Bispecific Antibody That Inhibits IgE Receptor Signaling. Journal of Biological Chemistry. 2010;285:20850–20859. doi: 10.1074/jbc.M110.113910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kim B, Eggel A, Tarchevskaya SS, Vogel M, Prinz H, et al. Accelerated disassembly of IgE-receptor complexes by a disruptive macromolecular inhibitor. Nature. 2012;491:613–617. doi: 10.1038/nature11546. [DOI] [PMC free article] [PubMed] [Google Scholar]