Abstract
Purpose/Objectives
To assess dietary supplement use and its association with demographic and health-related characteristics among cancer survivors, and to investigate differences in supplement use patterns by cancer site.
Design
A cross-sectional survey.
Sample
A sample of 1233 adult (30-69y) survivors participating in the Penn State Cancer Survivor Study who underwent an interviewer-administered questionnaire.
Methods
Descriptive statistics were primarily used, along with multivariate logistic regression to determine demographic, disease, and health-related predictors of supplement use.
Main Research Variables
Use of dietary supplements and types of supplements taken.
Findings
Supplement use ranged from 50% among blood cancer survivors to 85% among melanoma skin cancer survivors, with an overall prevalence rate of 73%. Multivariate logistic regression revealed statistically significant associations (P-values <0.05) between supplement use and older age (≥ 50 years), higher levels of education, higher levels of physical activity, female gender, lower body mass index (BMI), and white ethnicity.
Conclusions
Overall, a wide variety of supplements were reported, although multivitamins, calcium/vitamin D combinations, and antioxidant vitamin combinations were the most prevalent. Seventy-eight percent of supplement users took more than one supplement.
Implications for Nursing
Our findings support continued efforts by oncology nurses to identify the types of supplements cancer survivors are using. Nurses should caution against the use of individual supplements, as well as combinations of different supplements, containing nutrient quantities above daily recommended intake levels. Further, oncology nurses and other healthcare professionals should be receptive to questions and prepared to initiate conversations with patients about their use of dietary supplements.
Introduction
During the post-acute care stage, cancer survivors may initiate diet, physical activity, and other lifestyle changes in an attempt to prevent recurrence or other chronic disease, or improve overall health and quality of life (Demark-Wahnefried, Aziz, Rowland, & Pinto, 2005). Complications or consequences related to cancer treatment also may provide the impetus for health-related behavior change. Despite limited scientific data available regarding the role of dietary supplements in promoting health and preventing cancer recurrence or secondary cancers (Brown et al., 2003), one of the most common behavior changes among survivors is the use of new dietary supplements.
According to the United States Dietary Supplement and Health Education Act (DSHEA) (1994), dietary supplements include products containing not only vitamins and minerals but also herbs or other botanicals, amino acids, glandular extracts, or other non-nutrient ingredients. Under the DSHEA, dietary supplements are not required to undergo prescreening or safety and efficacy studies prior to production and marketing by manufacturers (Larsen & Berry, 2003). In addition, since the passage of the DSHEA, the use of dietary supplements has been increasing, with the sharpest growths noted in the non-nutrient and antioxidant sectors (Wold et al., 2005). The rise in dietary supplement use is of particular concern, as cancer survivors appear more likely to use dietary supplement use compared to the general U.S. adult population; in contrast to a prevalence rate of approximately 50% in the general population, prevalence rates ranging from 64% to 81% have been observed among cancer survivors (Velicer & Ulrich, 2008).
It is important to note that several potential beneficial roles for dietary supplements have been suggested. A daily multivitamin-multimineral (MVMM) supplement supplying nutrient doses at or below recommended intake levels (Institute of Medicine, 1997, 2000a, 2001, 2004) is generally considered safe by physicians, other healthcare professionals, and the research community (Norman et al., 2003). Further, guidelines recently issued by the World Cancer Research Fund and the American Institute for Cancer Research suggest that select vitamins and minerals from dietary supplements, such as calcium and selenium, may decrease risk of certain cancers (2007). At the other end of the spectrum, a growing body of evidence suggests that the use of antioxidant supplements, namely beta-carotene, vitamin A and vitamin E, may pose health-related risks (Bjelakovic, Nikolova, Gluud, Simonetti, & Gluud, 2007). Coupled with the increasing prevalence of fortified foods, supplemental intakes of vitamins and minerals could result in nutrient intakes surpassing the Tolerable Upper Intake Levels (ULs) established by the Institute of Medicine of the National Academy of Science (IOM/NAS). Overall, very little is known concerning the efficacy and safety of use of most dietary supplements (Mulholland & Benford, 2007).
Characterizing supplement use patterns is an important step in accurately evaluating the associated health benefits and risks of supplement use. The specific aims of this study were to assess dietary supplement use and its association with demographic and health-related characteristics among cancer survivors, and to investigate differences in supplement use patterns by cancer site. A clear understanding of supplement use by cancer site is essential for the guidance of scientific research and the development of firm guidelines for cancer survivors concerning the use of various dietary supplements. These findings will highlight the types of supplements most commonly used by survivors of various cancer types, as well as the characteristics of survivors more likely to use them. This information may be particularly valuable to oncology nurses, who are in excellent positions to initiate conversations with patients and provide education related to dietary supplement use (Lengacher et al., 2002).
Methods
Study Sample and Design
All cases diagnosed with a first cancer between 1997 and 1999 were identified from tumor registries in central and northeastern Pennsylvania (Geisinger Medical Center, Hershey Medical Center, and Lehigh Valley Hospital) and Baltimore, Maryland (Johns Hopkins Medical Center). Detailed descriptions of the Penn State Cancer Survivor Study have been published previously (Short, Vasey, & Belue, 2007). Because a main focus of the Penn State Cancer Survivor Study was employment and disability, selected cases were between the ages of 25 and 62 years at the time of diagnosis with primary cancer other than non-melanoma skin cancer. Cases with Stage IV tumors were excluded, with the exception of Stage IV leukemias, lymphomas, and plasma cell cancers due to the relatively favorable survival rates associated with these cancer sites. Male urological cancers (e.g. prostate and testicular cancer) at Johns Hopkins were outside the administrative control of the tumor registry, and thus were not available for contact using our recruitment tactics.
The Institutional Review Boards at the Pennsylvania State University and at each respective hospital approved the research protocol. Hospital employees were responsible for mailing recruitment packages to potential respondents (n = 5150) and obtaining formal consent; verbally at three sites and in writing at one site. Potential subjects were not identified to the research team until verbal or written consent was obtained. All consenting cases (n = 2076) were sent to the Survey and Epidemiology Services division of Social and Scientific Systems, Inc. of Silver Springs, Maryland, where trained interviewers conducted the computer-assisted telephone interviews (CATI). Eighty-eight percent of those who consented and survived (n = 1763) completed the first interview, and 1233 of these participants completed all four interviews through 2004. To address the low participation rate, extensive analyses were conducted to identify characteristics that were significantly different between participants and non-participants. These analyses are described in greater detail in a previous publication (Short et al., 2007) and included bivariate chi-square tests and a logistic regression model predicting participation from characteristics provided by the tumor registries for eligible cases (n = 5150).
Demographic, Disease and Health-Related Characteristics
Demographic and disease-related data including age, race, gender, date of diagnosis, cancer site, and cancer stage were obtained from the cancer registries. The CATI included questions concerning each subject at the time of interview, such as current employment, health status, health insurance, and quality of life, as well as retrospective questions ascertaining information prior to cancer diagnosis (e.g. employment, health, etc.). Body mass index (BMI) was calculated from self-reported height and weight [weight (kg) / height (m)2]. Participants were re-interviewed annually for the following three years. The fourth and final survey was administered in 2004 and included physical activity questions adapted from the National Health Information Survey (NHIS) (Centers for Disease Control and Prevention, National Center for Health Statistics, 1999). Data pertaining to frequency, intensity, and length of time spent performing various subcategories of exercise were collected. Light or moderate activities were described to participants as those resulting in light sweating or a slight to moderate increase in breathing or heart rate, while vigorous activities were those producing heavy sweating or large increases in breathing or heart rate.
Dietary Supplements
Questions pertaining to the use of dietary supplements were administered during the final survey in 2004 by a questionnaire adapted from the National Health and Nutrition Examination Survey (NHANES) (Centers for Disease Control and Prevention, National Center for Health Statistics, 2002). The questionnaire captured information on the use of 34 specific dietary supplements, with additional open-ended questions regarding the use of other combination vitamin or mineral supplements, other single ingredient vitamin or mineral supplements, and any other alternative preparation or herbal remedy not mentioned specifically in the questionnaire. Data pertaining to frequency of supplement use (e.g. at least once per week during the past month), duration of use (e.g. more or less than one year) and initiation of use (e.g. before or after cancer diagnosis) were obtained. For our analyses, certain individual supplements were collapsed into broader categories after the supplement data were collected. The category of antioxidants included any supplement containing one or more of the following antioxidant nutrients taken either in combination or alone: β-carotene, vitamins A, C or E, and any antioxidant vitamin combination supplements. The category of calcium/vitamin D included single calcium, calcium-containing antacids, calcium combination supplements, and single vitamin D.
Data Analysis
Participants who reported the use of one or more dietary supplements at least once per week during the past month were defined as supplement users. Differences between supplement users and nonusers with respect to demographic, disease and health-related characteristics first were evaluated using bivariate chi-square analyses. Characteristics significantly associated with supplement use (P<0.05) from bivariate chi-square analyses then were entered as candidate predictor variables in multivariate logistic regression analysis to determine significant predictors of use among cancer survivors. Logistic regression analysis was chosen because the outcome variable of supplement use was dichotomous (use/nonuse). Independent variables included in the final logistic regression model were age, body mass index (BMI), education, ethnicity, gender, and physical activity level (P-values <0.05). All data were analyzed using the Statistical Analysis Software Package 9.1.3 (SAS Institute Inc., Cary, NC); a two-tailed alpha level of 0.05 was employed for all statistical tests.
Results
Sample Characteristics
A total of 1233 cancer survivors completed the final survey that included dietary supplement and physical activity questions. Results from bivariate chi-square analyses revealed a greater proportion of the remaining sample to be white and to have higher reported incomes compared to the 530 individuals who did not complete the entire study. No other significant differences were found with respect to the demographic, disease, and health-related characteristics investigated. Findings from the logistic regression model predicting participation revealed significant differences in cancer site, facility, gender, and race between participants and non-participants (Short & Mallonee, 2006; Short et al., 2007). Therefore, female Caucasian breast cancer survivors comprised approximately one-third of our total sample.
The following cancer sites were represented in the final sample: blood (n = 58), breast (n = 406), central nervous system (n = 35), colorectal (n = 70), head and neck (n = 41), lymphoma (n = 31), prostate (n = 115), respiratory (n = 42), melanoma skin (n = 42), thyroid (n = 94), urinary (n = 42), uterus (n = 84), other (n = 135), and unknown (n = 38). Age at the last interview in 2004 ranged from 30 to 69 years, with a mean respondent age of 55 years. Study participants were predominately white (94%) and female (67%). Time since primary cancer diagnosis ranged from 4 to 8 years, with a mean of 6 years. Within this sample, 44% had a stage I tumor at diagnosis, 32% a stage II tumor, 13% a stage III tumor, and 7% a stage IV tumor; the remaining 3% of cases had an unknown or unrecorded tumor stage at diagnosis.
While 90% of survivors reported performing at least ten minutes of light, moderate or vigorous activity each week, slightly more than one-half reported no activity resulting in heavy sweating or large increases in breathing or heart rate. Over two-thirds of our sample were overweight (BMI ≥25 kg/m2) and 28% were obese (BMI ≥ 30 kg/m2). Additional demographic, disease and health-related characteristics of our sample are provided in Table 1.
Table 1.
Demographic Characteristics, Disease Stage and Health-Related Indicators of Supplement Users Compared to Nonusers
| Characteristic | All Respondents n = 1233 |
Users of Supplements n = 900 |
|---|---|---|
| n (%) | n (%) | |
| Age | ||
| ≤50 yr | 339 (27) | 219 (65)* |
| >50 yr | 894 (73) | 681 (76)* |
| Gender | ||
| Female | 822 (67) | 631 (77)* |
| Male | 411 (33) | 269 (65)* |
| Ethnicity | ||
| White | 1148 (94) | 848 (74)** |
| Nonwhite | 82 (6) | 49 (60)** |
| Education | ||
| <High school | 58 (5) | 31 (53)* |
| High school | 384 (31) | 271 (71)* |
| Some college or bachelor’s degree | 528 (43) | 390 (74)* |
| Graduate or professional degree | 261 (21) | 206 (79)* |
| Annual Household Income | ||
| <100% FPL | 36 (3) | 17 (47)** |
| >100-400 FPL | 257 (21) | 179 (70)** |
| >400 FPL | 690 (56) | 518 (75)** |
| Unreported | 250 (20) | 186 (74)** |
| Tumor Stage | ||
| I | 545 (44) | 406 (75)*** |
| II | 400 (32) | 296 (74)*** |
| III | 163 (13) | 126 (77)*** |
| IVa | 87 (7) | 53 (60)*** |
| Unknown | 36 (3) | 19 (53)*** |
| Body Mass Index | ||
| <25 kg/m2 | 424 (34) | 332 (78)** |
| 25-30 kg/m2 | 454 (37) | 327 (72)** |
| ≥30 kg/m2 | 355 (29) | 241 (68)** |
| Perceived Health | ||
| Excellent/Good | 1050 (85) | 783 (75)** |
| Fair/Poor | 180 (15) | 114 (63)** |
| Exercise | ||
| No weekly exercise | 115 (9) | 74 (64)*** |
| 0-1 hour/weekb | 751 (61) | 542 (72)*** |
| > 1 hour/weekb | 367 (30) | 284 (77)*** |
| Years Since Diagnosis (mean ± SD) | 6.03 ± 0.97 | 6.06 ± 0.99 |
P < 0.001;
P < 0.01;
P < 0.05
Includes Stage IV leukemias, lymphomas, and plasma cell cancers only
Refers to exercise producing heavy sweating or large increases in breathing or heart rate
Supplement Use
Dietary supplement use was common in our study, with 900 cancer survivors (73%) reporting the use of at least one supplement once per week during the previous month. Nearly one-half of participants began the use of a new supplement after cancer diagnosis. Prevalence of use ranged greatly by cancer site, from a low of 50% among blood cancer survivors to a high of 85% among melanoma skin cancer survivors. Among individuals who reported the use of dietary supplements, 78% took more than one supplement and 20% used more than five, with a range from one to 17 different supplements taken at least once or more each week.
Specific Dietary Supplements Reported
Participants in our study reported a wide variety of dietary supplements. Figure 1 presents the frequency of use of specific dietary supplements according to cancer site. The most prevalent supplements were MVMM products (62%), antioxidants (40%), calcium/vitamin D (40%), and herbal preparations (21%). Prevalence rates of specific supplements reported varied greatly by cancer site; for instance, 22% of blood cancer survivors reported the use of an antioxidant supplement compared to 51% of colorectal cancer survivors, while prevalence of calcium/vitamin D use ranged from 17% of blood cancer survivors to 56% of breast cancer survivors. Use of any herbal preparation ranged from 9% of central nervous system cancer survivors to 33% of urinary cancer survivors, with an overall prevalence rate of 21% among all survivors. The most commonly reported single mineral, vitamin and herbal supplements were calcium (20%), vitamin E (22%), and glucosamine (8%), respectively (data not shown).
Figure 1.
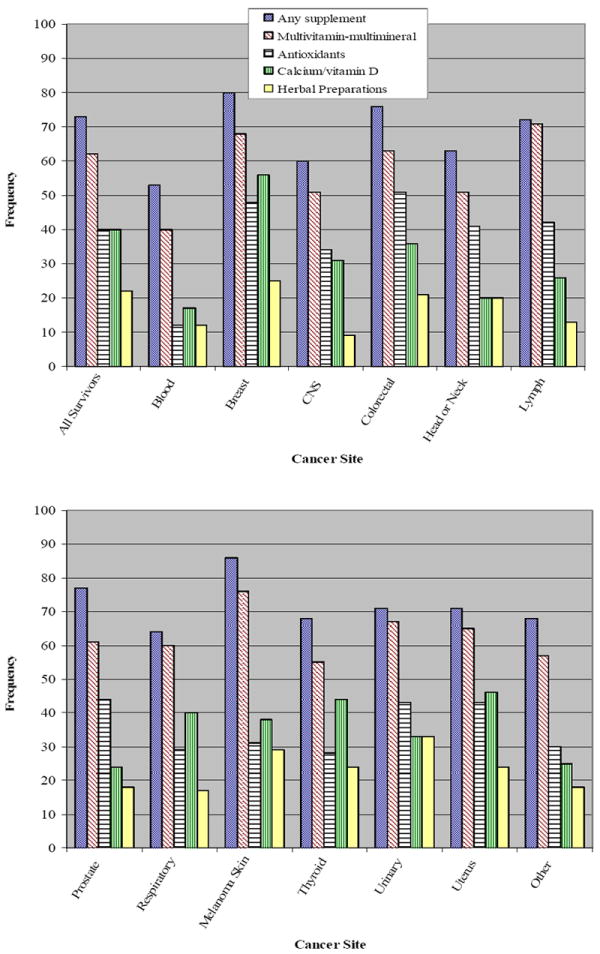
Most Commonly Reported Dietary Supplements According to Specific Cancer Site
Characteristics Associated with Supplement Use
Bivariate chi-square analyses revealed statistically significant differences between dietary supplement users and nonusers with regard to age, BMI, cancer site, education, ethnicity, gender, measures of income, perceived health, physical activity level, and tumor stage (Table 1). Subjects with higher levels of educational attainment, income, and vigorous physical activity were progressively more likely to use dietary supplements, as were those with a lower BMI. Older age (>50y), female gender, and white ethnicity were positively associated with dietary supplement use. A total of 79% of cancer survivors with a professional or graduate degree used dietary supplements compared to 53% of individuals with less than a high school education (P<0.001).
As shown in Table 2, multivariate logistic regression revealed older age, lower BMI, white ethnicity, female gender, higher levels of educational attainment, and higher levels of physical activity as statistically significant positive predictors of dietary supplement use. Our study found survivors 50 years of age or older to be nearly twice as likely to use supplements compared to younger survivors (95% CI 1.48-2.61). Females were 1.87 times (95% CI 1.41-2.47) as likely to use supplements, and nonwhites compared with whites were 40% less likely (95% CI 1.11-2.87). Individuals who were obese were 35% less likely to take supplements compared to those who were normal weight (95% CI 0.46-0.91). With less than a high school education as the reference, a high school degree (OR=1.81, 95% CI 1.00-3.25), some college education and/or a bachelor’s degree (OR=2.11, 95%CI 1.18-3.77) or graduate or professional education (OR=2.68, 95%CI 1.43-5.03) were statistically significant predictors of supplement use. Additionally, individuals reporting greater than one hour of vigorous physical activity each week were 1.73 times more likely to use supplements compared to individuals reporting no weekly physical activity (95% CI 1.07-2.80).
Table 2.
Factors Predicting Dietary Supplement Use in Multivariate Logistic Regression
| Odds Ratioa (95% CI) | |
|---|---|
| Age | |
| ≤50 yr | 1.00 |
| >50 yr | 1.97 (1.48, 2.61) |
| Gender | |
| Male | 1.00 |
| Female | 1.87 (1.41, 2.47) |
| Ethnicity | |
| White | 1.00 |
| Nonwhite | 0.60 (0.40, 0.98) |
| Education | |
| <High school | 1.00 |
| High school | 1.81 (1.00, 3.25) |
| Some college or bachelor’s degree | 2.11 (1.18, 3.77) |
| Graduate or professional degree | 2.68 (1.43, 5.03) |
| ptrend | 0.001 |
| BMI | |
| <25 kg/m2 | 1.00 |
| 25-30 kg/m2 | 0.84 (0.60, 1.16) |
| ≥30 kg/m2 | 0.65 (0.46, 0.91) |
| ptrend | 0.002 |
| Exercise | |
| No weekly exercise | 1.00 |
| 0-1 hour/weekb | 1.35 (0.87, 2.08) |
| >1 hour/weekb | 1.73 (1.07, 2.80) |
| ptrend | 0.026 |
Risk estimates adjust for all other covariates presented in table
Refers to exercise producing heavy sweating or large increases in breathing or heart rate
Discussion
In this sample of 1233 cancer survivors, a majority (73%) reported taking dietary supplements, ranging from 50% among blood cancer survivors to 85% among melanoma skin cancer survivors. The prevalence rate observed is comparable to other estimates of supplement use among cancer survivors (Velicer & Ulrich, 2008) but considerably higher than estimates of supplement use among the general population. Radimer and colleagues found that only 52% of healthy adults aged 20 years and above reported the use of dietary supplements (1999-2000 NHANES) (2004). The observation of greater prevalence of use among cancer survivors has been reported previously (Rock, 2007); however, few studies have investigated supplement use within a large cohort of survivors of various cancer sites.
The volume of supplements taken by participants in our study was highly variable, ranging from one to 17 formulations, with a mean value of three. Seventy-eight percent of supplement users reported taking more than one formulation, which is considerably greater than estimates among healthy adult supplement users aged 20 years and above (47%) (Radimer et al., 2004). Further, 28% of supplement users in the present study reported the regular use of at least five unique dietary supplements.
Similar to findings from other surveys and studies (Velicer & Ulrich, 2008), our data suggest that supplement use is associated with several demographic, disease, and health-related characteristics. Therefore, supplement use may serve as a marker for other health-related behaviors, evidenced by higher levels of physical activity and lower prevalence rates of obesity among supplement users. This clustering of healthier lifestyle behavior observed in our study and others should be considered in future studies as it may confound findings supporting a beneficial role of dietary supplements in promoting health and preventing cancer recurrence, secondary cancers and other chronic disease. The observed associations between demographic and lifestyle characteristics and dietary supplement use highlight the need to use caution when interpreting results of observational studies relating dietary supplement use to health outcomes.
In our sample, MVMM supplements were the most prevalent dietary supplements, while calcium, vitamin E and glucosamine were the most common single mineral, vitamin and herbal products reported, respectively. Our findings parallel those from prior investigations (Kishiyama et al., 2005; McDavid, Breslow, & Radimer, 2001; Radimer et al., 2004). Additionally, the prevalence rate of herb or other botanical supplement use in our study (21%) is comparable to other reports in the literature (Marinac et al., 2007; Norred, 2002). Despite the many differences in supplement use assessment tools as well as study samples, a consistently high and increasing prevalence of supplement use over time remains clear.
It is important to consider potential health-related risks associated with dietary supplement use. Coupled with the increasing prevalence of fortified foods, supplement use may result in nutrient intakes surpassing the Tolerable Upper Intake Levels (ULs) established by the IOM/NAS. Findings from a recent meta-analysis suggest that supplementation with antioxidant vitamin and minerals, namely beta-carotene, vitamin A, and vitamin E, may increase mortality (Bjelakovic et al., 2007). Although a clearer understanding of the association between antioxidant supplement use and increased mortality is needed, a few mechanisms of action have been suggested. Antioxidant supplements are synthetic, similar to pharmaceutical agents, yet are not subjected to comparably rigorous toxicity studies; further, the use of antioxidant supplements may interfere with natural defense mechanisms (e.g. apoptosis and phagocytosis) (Bjelakovic et al., 2007). The use of multiple supplements containing some of the same vitamins and minerals is of particular concern; twenty-eight percent of supplement users in our sample reported the regular use of at least five different supplements and more than 47% reported the use of both a MVMM supplement and at least one of the following antioxidant supplements: β-carotene, vitamins A, C, or E, or any antioxidant vitamin combination supplement. Notably, nearly 80% of cancer survivors in our study who reported taking single vitamin E also reported the use of a MVMM supplement.
There are several strengths of the present study. The size of our sample and the distribution of survivors among multiple cancer types are two notable strengths compared to previous investigations of supplement use patterns among survivors, which tend to be limited to breast cancer survivors or are comprised of smaller samples (Boon et al., 2000; Fleischauer, Simonsen, & Arab, 2003; Newman et al., 1998; Patterson et al., 2002, Patterson et al., 2003; Rock et al., 1997; Stolzenberg-Solomon et al., 2006). The use of cancer registry data is an additional strength of our study, as self-reported data may underestimate cancer prevalence (McDavid et al., 2001). Further, our survey contained a broad range of questions aimed at capturing the use of a variety of dietary supplements, including supplements recently suggested as having potentially adverse effects on health outcomes, including beta-carotene, vitamin A, and vitamin E.
As in all studies, there are several limitations with the current investigation. Subjects were asked questions pertaining to dietary supplement use and physical activity during the last of four annual interviews. Therefore, change in health behavior cannot be assessed as dietary supplement and physical activity data are not available for all 1753 subjects who completed the baseline survey. Results from bivariate chi-square tests revealed that a greater proportion of the remaining sample were white, with higher reported annual incomes compared to the 530 individuals who did not complete the entire study. There was no significant gender difference in attrition rate. Findings from the logistic regression model predicting participation in our study revealed significant differences in cancer site, facility, gender, and race between participants and non-participants (Short & Mallonee, 2006; Short et al., 2007); therefore, female Caucasian breast cancer survivors comprised approximately one-third of our total sample. As a recent review of the literature highlights (Velicer & Ulrich, 2008), female gender is one of the most consistent factors associated with supplement use; a more even distribution of participants by gender may have resulted in a lower overall prevalence rate of supplement use in our study, or possibly different patterns of use. Twenty percent of the 1233 participants who completed the final interview reported a cancer recurrence or secondary cancer (n=250). Individuals with recurrent or secondary cancer were similar to cancer-free survivors with regards to age, education, ethnicity, and gender but were less likely to exercise vigorously more than one hour per week and were more likely to use dietary supplements. Lastly, caution should be used in the interpretation of these results, as the present study was not designed to assess benefits or risks of dietary supplement use or the potential association of supplements and cancer recurrence or secondary cancer.
Conclusions and Implications for Nursing Practice
Dietary supplement use is prevalent among cancer survivors. Further, nearly one-half of participants began the use of a new supplement after cancer diagnosis and a vast majority of supplement users took more than one supplement. Therefore, oncology nurses should be both receptive to questions and prepared to initiate conversations with patients about their use of dietary supplements. In addition, findings from our study suggest that supplement use may serve as a marker for a range of other health-related behaviors, evidenced by higher levels of physical activity and lower levels of overweight and obesity among supplement users in our sample. It is important for future researchers to consider the potential confounding effects of this observed clustering of behaviors when investigating the impact of dietary supplement use on various health outcomes, including cancer recurrence among survivors. Similar to findings from other studies, supplement use was more prevalent among females, individuals with higher levels of educational attainment, and those further removed from diagnosis. Lastly, continued efforts to evaluate potentially adverse effects associated with chronically high intakes of vitamins and minerals from dietary supplements among cancer survivor populations are warranted. Oncology nurses and other healthcare professionals should strongly caution against the use of individual supplements, as well as combinations of different supplements, containing nutrient quantities above daily recommended intake levels.
Key Points.
An estimated 10.8 million cancer survivors live in the United States today; this population is at increased risk for cancer recurrence, second primaries, obesity, osteoporosis, cardiovascular disease, diabetes, and functional decline.
Survivors may initiate health-related behavior change in an effort to improve health outcomes after cancer diagnosis, and one of the most common behavior changes is the use of new dietary supplements.
The role of supplements in promoting health and preventing cancer recurrence, secondary cancers, and other chronic diseases is still unclear.
A vast majority of cancer survivors in our sample took dietary supplements (73%); further, 78% of supplement users took more than one supplement and 20% used more than five.
Assessment of dietary supplement use among survivors is an important consideration for oncology nurses.
Acknowledgments
The present study was supported by the National Institutes of Health Grant RO1 CA82619.
References
- Bjelakovic G, Nikolova D, Gluud LL, Simonetti RG, Gluud C. Mortality in randomized trials of antioxidant supplements for primary and secondary prevention: systematic review and meta-analysis. Journal of the American Medical Association. 2007;297(8):842–857. doi: 10.1001/jama.297.8.842. [DOI] [PubMed] [Google Scholar]
- Boon H, Stewart M, Kennard MA, Gray R, Sawka C, Brown JB, et al. Use of complementary/alternative medicine by breast cancer survivors in Ontario: prevalence and perceptions. Journal of Clinical Oncology. 2000;18(13):2515–2521. doi: 10.1200/JCO.2000.18.13.2515. [DOI] [PubMed] [Google Scholar]
- Brown JK, Byers T, Doyle C, Coumeya KS, Demark-Wahnefried W, Kushi LH, et al. Nutrition and physical activity during and after cancer treatment: an American Cancer Society guide for informed choices. CA-A Cancer Journal for Clinicians. 2003;53(5):268–291. doi: 10.3322/canjclin.53.5.268. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention, National Center for Health Statistics. National Health Interview Survey, 1999. Hyattsville, MD: 1999. Retrieved December 1, 2007, from http://www.cdc.gov/nchs/nhis.htm. [Google Scholar]
- Centers for Disease Control and Prevention, National Center for Health Statistics. National Health and Nutrition Examination Survey Questionnaire 2001-2002. Hyattsville, MD: 2002. Retrieved December 1, 2007, from http://www.cdc.gov/nchs/nhanes.htm. [Google Scholar]
- Demark-Wahnefried W, Aziz NM, Rowland JH, Pinto BM. Riding the crest of the teachable moment: promoting long-term health after the diagnosis of cancer. Journal of Clinical Oncology. 2005;23(24):5814–5830. doi: 10.1200/JCO.2005.01.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietary Supplement Health and Education Act of 1994, Pub L No.103-417, 108 Stat 4325 (codified at 21 USC 301).
- Fleischauer AT, Simonsen N, Arab L. Antioxidant supplements and risk of breast cancer recurrence and breast cancer-related mortality among postmenopausal women. Nutrition and Cancer. 2003;46(1):15–22. doi: 10.1207/S15327914NC4601_02. [DOI] [PubMed] [Google Scholar]
- Institute of Medicine, Food and Nutrition Board. Dietary Reference Intakes for calcium, phosphorus, magnesium, vitamin D, and fluoride. Washington, DC: National Academies Press; 1997. [PubMed] [Google Scholar]
- Institute of Medicine, Food and Nutrition Board. Dietary Reference Intakes for thiamin, riboflavin, niacin, vitamin B6, folate, vitamin B12, pantothenic acid, biotin, and choline. Washington, DC: National Academies Press; 2000. [PubMed] [Google Scholar]
- Institute of Medicine, Food and Nutrition Board. Dietary Reference Intakes for vitamin C, vitamin E, selenium and carotenoids. Washington, DC: National Academies Press; 2000. [PubMed] [Google Scholar]
- Institute of Medicine, Food and Nutrition Board. Dietary Reference Intakes for vitamin A, vitamin K, arsenic, boron, chromium, copper, iodine, iron, manganese, molybdenum, nickel, silicon, vanadium, and zinc. Washington, DC: National Academies Press; 2001. [PubMed] [Google Scholar]
- Institute of Medicine, Food and Nutrition Board. Dietary Reference Intakes for water, potassium, sodium, chloride, and sulfate. Washington, DC: National Academies Press; 2004. [Google Scholar]
- Kishiyama SS, Leahy MJ, Zitzelberger TA, Guariglia R, Zajdel DP, Calvert JF, Jr, et al. Patterns of dietary supplement usage in demographically diverse older people. Alternative Therapies in Health and Medicine. 2005;11(3):48–53. [PMC free article] [PubMed] [Google Scholar]
- Larsen LL, Berry JA. The regulation of dietary supplements. Journal of the American Academy of Nurse Practitioners. 2003;15(9):410–414. doi: 10.1111/j.1745-7599.2003.tb00415.x. [DOI] [PubMed] [Google Scholar]
- Lengacher CA, Bennett MP, Kip KE, Keller R, LaVance MS, Smith LS, et al. Frequency of use of complementary and alternative medicine in women with breast cancer. Oncology Nursing Forum. 2002;29(10):1445–1452. doi: 10.1188/02.ONF.1445-1452. [DOI] [PubMed] [Google Scholar]
- Marinac JS, Buchinger CL, Godfrey LA, Wooten JM, Sun C, Willsie SK. Herbal products and dietary supplements: a survey of use, attitudes, and knowledge among older adults. Journal of the American Osteopathic Association. 2007;107(1):13–20. quiz 21-13. [PubMed] [Google Scholar]
- McDavid K, Breslow RA, Radimer K. Vitamin/mineral supplementation among cancer survivors: 1987 and 1992 National Health Interview Surveys. Nutr Cancer. 2001;41(1-2):29–32. doi: 10.1080/01635581.2001.9680608. [DOI] [PubMed] [Google Scholar]
- Mulholland CA, Benford DJ. What is known about the safety of multivitamin-multimineral supplements for the generally healthy population? Theoretical basis for harm. Am J Clin Nutr. 2007;85(1):318S–322S. doi: 10.1093/ajcn/85.1.318S. [DOI] [PubMed] [Google Scholar]
- Newman V, Rock CL, Faerber S, Flatt SW, Wright FA, Pierce JP. Dietary supplement use by women at risk for breast cancer recurrence. The Women’s Healthy Eating and Living Study Group. Journal of the American Dietetic Association. 1998;98(3):285–292. doi: 10.1016/s0002-8223(98)00068-6. [DOI] [PubMed] [Google Scholar]
- Norman HA, Butrum RR, Feldman E, Heber D, Nixon D, Picciano MF, et al. The role of dietary supplements during cancer therapy. Journal of Nutrition. 2003;133(11 Suppl 1):3794S–3799S. doi: 10.1093/jn/133.11.3794S. [DOI] [PubMed] [Google Scholar]
- Norred CL. Complementary and alternative medicine use by surgical patients. AORN Journal. 2002;76(6):1013–1021. doi: 10.1016/s0001-2092(06)61003-x. [DOI] [PubMed] [Google Scholar]
- Patterson RE, Neuhouser ML, Hedderson MM, Schwartz SM, Standish LJ, Bowen DJ. Changes in diet, physical activity, and supplement use among adults diagnosed with cancer. Journal of the American Dietetic Association. 2003;103(3):323–328. doi: 10.1053/jada.2003.50045. [DOI] [PubMed] [Google Scholar]
- Patterson RE, Neuhouser ML, Hedderson MM, Schwartz SM, Standish LJ, Bowen DJ, et al. Types of alternative medicine used by patients with breast, colon, or prostate cancer: predictors, motives, and costs. Journal of Alternative and Complementary Medicine. 2002;8(4):477–485. doi: 10.1089/107555302760253676. [DOI] [PubMed] [Google Scholar]
- Radimer K, Bindewald B, Hughes J, Ervin B, Swanson C, Picciano MF. Dietary supplement use by US adults: data from the National Health and Nutrition Examination Survey 1999-2000. American Journal of Epidemiology. 2004;160(4):339–349. doi: 10.1093/aje/kwh207. [DOI] [PubMed] [Google Scholar]
- Rock CL. Multivitamin-multimineral supplements: who uses them? American Journal of Clinical Nutrition. 2007;85(1):277S–279S. doi: 10.1093/ajcn/85.1.277S. [DOI] [PubMed] [Google Scholar]
- Rock CL, Newman V, Flatt SW, Faerber S, Wright FA, Pierce JP. Nutrient intakes from foods and dietary supplements in women at risk for breast cancer recurrence. The Women’s Healthy Eating and Living Study Group. Nutrition and Cancer. 1997;29(2):133–139. doi: 10.1080/01635589709514614. [DOI] [PubMed] [Google Scholar]
- Short PF, Mallonee EL. Income disparities in the quality of life of cancer survivors. Medical Care. 2006;44(1):16–23. doi: 10.1097/01.mlr.0000188986.84819.3a. [DOI] [PubMed] [Google Scholar]
- Short PF, Vasey JJ, Belue R. Work disability associated with cancer survivorship and other chronic conditions. Psychooncology. 2008;17(1):91–97. doi: 10.1002/pon.1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stolzenberg-Solomon RZ, Chang SC, Leitzmann MF, Johnson KA, Johnson C, Buys SS, et al. Folate intake, alcohol use, and postmenopausal breast cancer risk in the Prostate, Lung, Colorectal, and Ovarian Cancer Screening Trial. American Journal of Clinical Nutrition. 2006;83(4):895–904. doi: 10.1093/ajcn/83.4.895. [DOI] [PubMed] [Google Scholar]
- Velicer CM, Ulrich CM. Vitamin and mineral supplement use among US adults after cancer diagnosis: a systematic review. Journal of Clinical Oncology. 2008;26(4):665–673. doi: 10.1200/JCO.2007.13.5905. [DOI] [PubMed] [Google Scholar]
- Wold RS, Lopez ST, Yau CL, Butler LM, Pareo-Tubbeh SL, Waters DL, et al. Increasing trends in elderly persons’ use of nonvitamin, nonmineral dietary supplements and concurrent use of medications. Journal of the American Dietetic Association. 2005;105(1):54–63. doi: 10.1016/j.jada.2004.11.002. [DOI] [PubMed] [Google Scholar]
- World Cancer Research Fund, American Institute for Cancer Research. Food, Nutrition, and Physical Activity, and the Prevention of Cancer: a Global Perspective. Washington, DC: American Institute for Cancer Research; 2007. [Google Scholar]


