Abstract
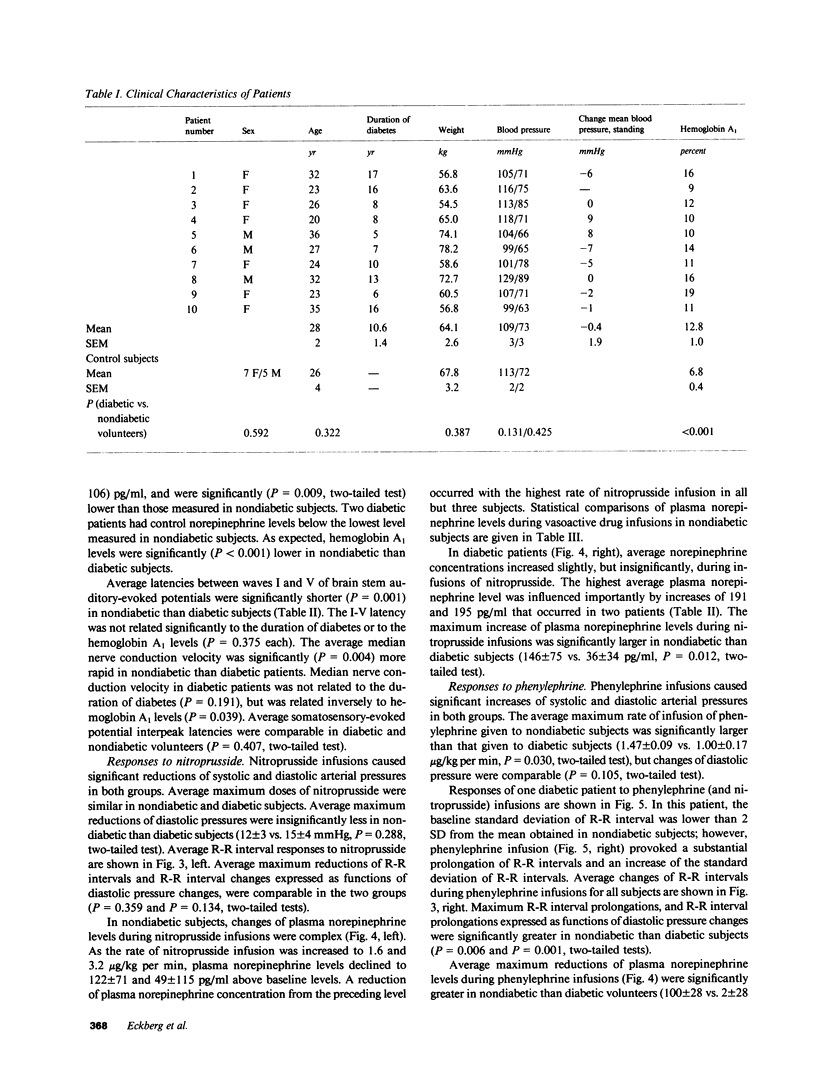
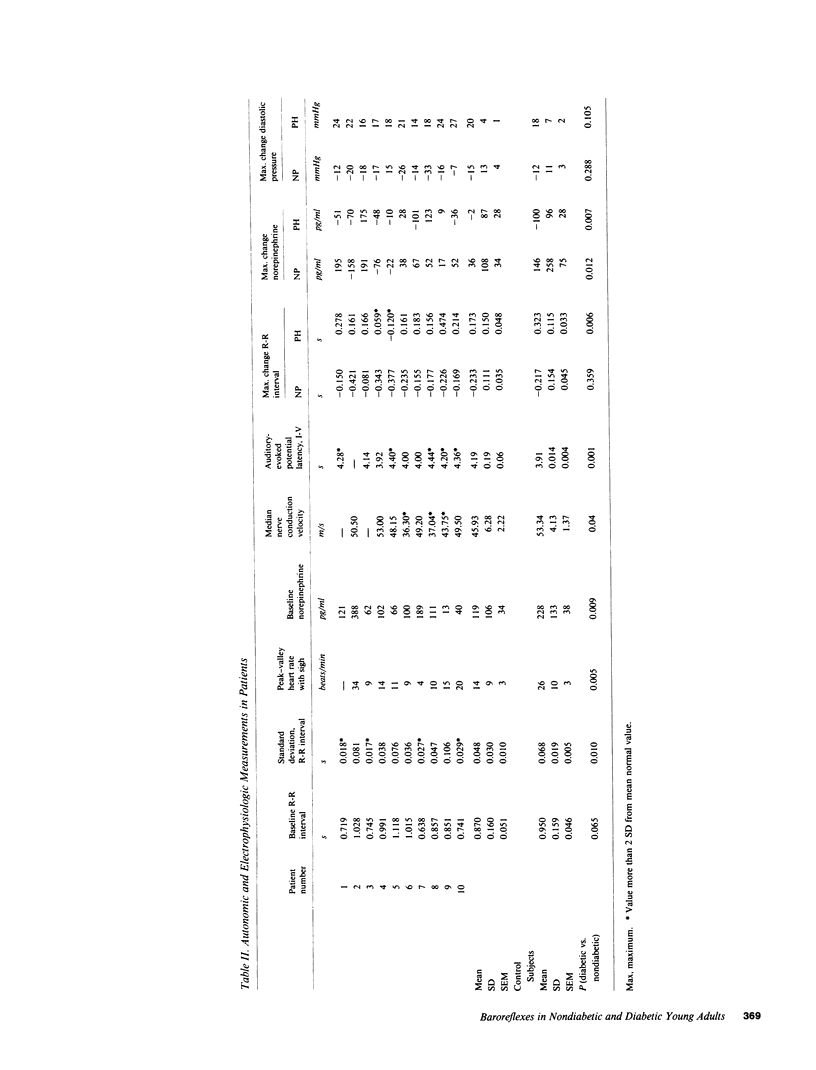
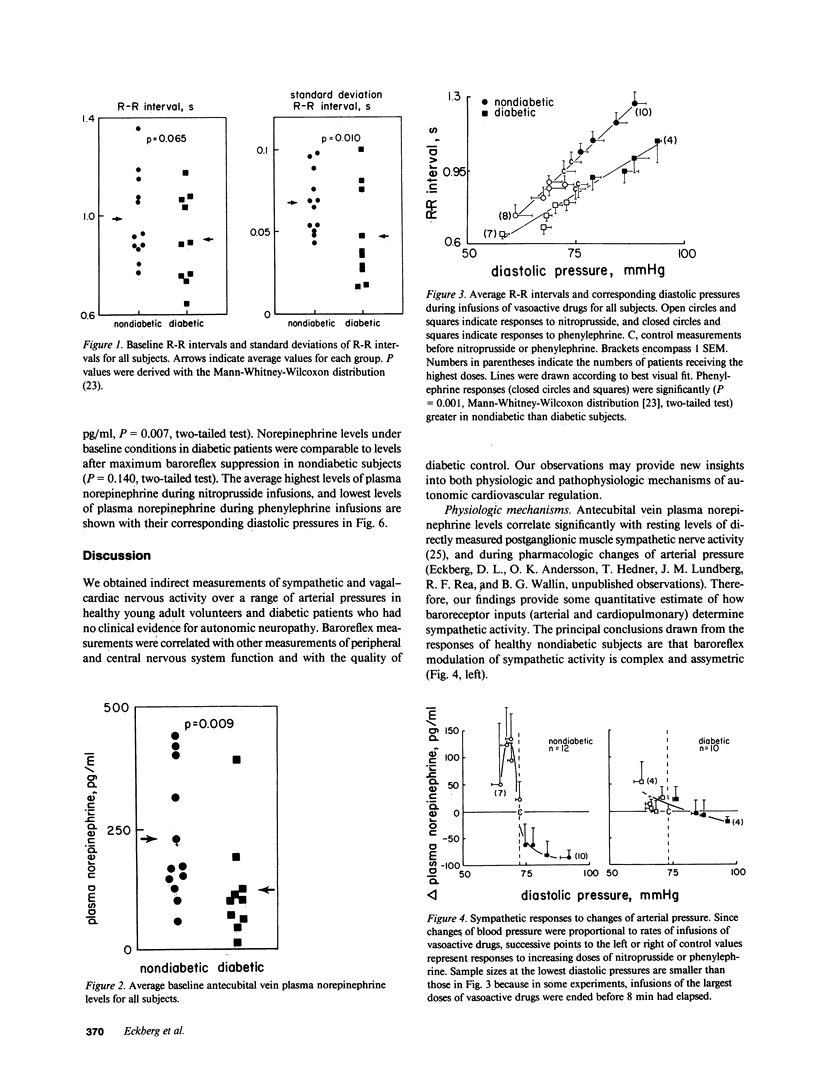
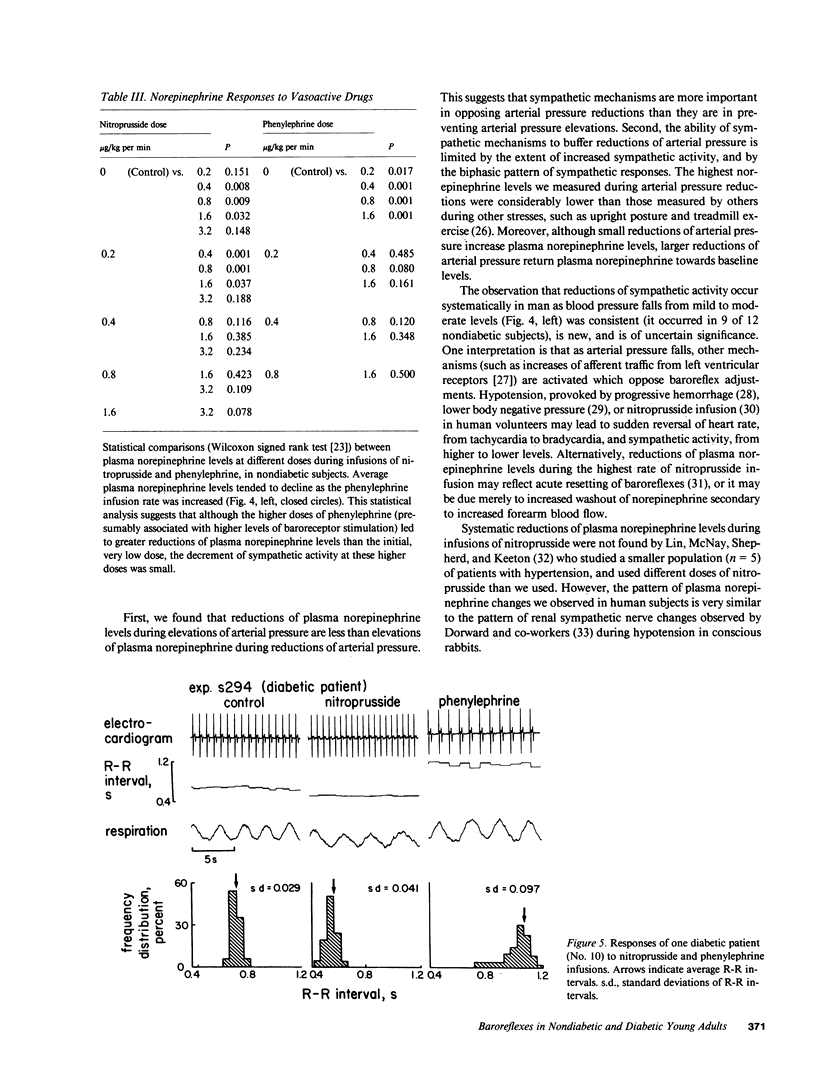
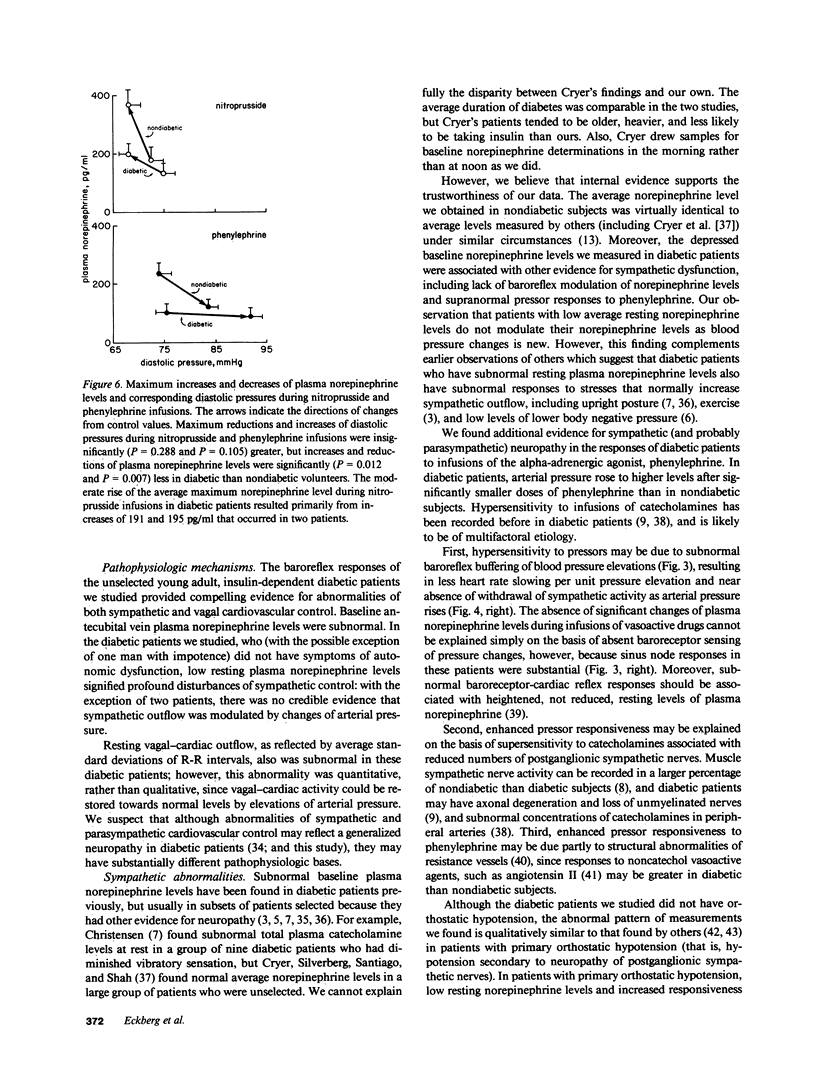
Resting diabetic patients may have excessively rapid heart rates, reduced heart rate variability, and subnormal plasma catecholamine levels. Although all of these abnormalities may relate in some way to baroreceptor reflex function, there have been surprisingly few attempts to evaluate systematically baroreflex mechanisms in diabetic patients. Accordingly, we studied autonomic responses over a range of pharmacologically induced arterial pressure changes in 10 unselected young adult insulin-dependent diabetic patients who had no symptoms of autonomic neuropathy, and 12 age-matched nondiabetic subjects. Sympathetic responses were estimated from antecubital vein plasma norepinephrine levels, and parasympathetic responses were estimated from electrocardiographic R-R intervals and their variability (standard deviation). Both were correlated with other noninvasive indexes of peripheral and central nervous system function. Multiple derangements of baroreflex function were found in the diabetic patients studied. Sympathetic abnormalities included subnormal baseline norepinephrine levels, virtual absence of changes of norepinephrine levels during changes of arterial pressure, and supranormal pressor responses to phenylephrine infusions. Parasympathetic abnormalities included subnormal baseline standard deviations of R-R intervals, and R-R interval prolongations during elevations of arterial pressure which were unmistakably present, but subnormal. Our data suggest that in diabetic patients, subnormal baseline plasma norepinephrine levels may signify profound, possibly structural defects of sympathetic pathways. Subnormal resting levels of respiratory sinus arrhythmia may have different implications, however, since vagal, unlike sympathetic reflex abnormalities, can be reversed partly by arterial pressure elevations.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abraham E. C., Huff T. A., Cope N. D., Wilson J. B., Jr, Bransome E. D., Jr, Huisman T. H. Determination of the glycosylated hemoglobins (HB AI) with a new microcolumn procedure. Suitability of the technique for assessing the clinical management of diabetes mellitus. Diabetes. 1978 Sep;27(9):931–937. doi: 10.2337/diab.27.9.931. [DOI] [PubMed] [Google Scholar]
- Allison T., Wood C. C., Goff W. R. Brain stem auditory, pattern-reversal visual, and short-latency somatosensory evoked potentials: latencies in relation to age, sex, and brain and body size. Electroencephalogr Clin Neurophysiol. 1983 Jun;55(6):619–636. doi: 10.1016/0013-4694(83)90272-9. [DOI] [PubMed] [Google Scholar]
- Bennett T., Farquhar I. K., Hosking D. J., Hampton J. R. Assessment of methods for estimating autonomic nervous control of the heart in patients with diabetes mellitus. Diabetes. 1978 Dec;27(12):1167–1174. doi: 10.2337/diab.27.12.1167. [DOI] [PubMed] [Google Scholar]
- Bennett T., Fentem P. H., Fitton D., Hampton J. R., Hosking D. J., Riggott P. A. Assessment of vagal control of the heart in diabetes. Measures of R-R interval variation under different conditions. Br Heart J. 1977 Jan;39(1):25–28. doi: 10.1136/hrt.39.1.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett T., Hosking D. J., Hampton J. R. Baroreflex sensitivity and responses to the Valsalva manoeuvre in subjects with diabetes mellitus. J Neurol Neurosurg Psychiatry. 1976 Feb;39(2):178–183. doi: 10.1136/jnnp.39.2.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett T., Hosking D. J., Hampton J. R. Cardiovascular responses to graded reductions of central blood volume in normal subjects and in patients with diabetes mellitus. Clin Sci (Lond) 1980 Mar;58(3):193–200. doi: 10.1042/cs0580193. [DOI] [PubMed] [Google Scholar]
- Chen R. Y., Matteo R. S., Fan F. C., Schuessler G. B., Chien S. Resetting of baroreflex sensitivity after induced hypotension. Anesthesiology. 1982 Jan;56(1):29–35. doi: 10.1097/00000542-198201000-00007. [DOI] [PubMed] [Google Scholar]
- Christensen N. J. Plasma catecholamines in long-term diabetics with and without neuropathy and in hypophysectomized subjects. J Clin Invest. 1972 Apr;51(4):779–787. doi: 10.1172/JCI106872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christlieb A. R., Janka H. U., Kraus B., Gleason R. E., Icasas-Cabral E. A., Aiello L. M., Cabral B. V., Solano A. Vascular reactivity to angiotensin II and to norepinephrine in diabetic subjects. Diabetes. 1976 Apr;25(4):268–274. doi: 10.2337/diab.25.4.268. [DOI] [PubMed] [Google Scholar]
- Clarke B. F., Ewing D. J., Campbell I. W. Diabetic autonomic neuropathy. Diabetologia. 1979 Oct;17(4):195–212. doi: 10.1007/BF01235856. [DOI] [PubMed] [Google Scholar]
- Cryer P. E., Silverberg A. B., Santiago J. V., Shah S. D. Plasma catecholamines in diabetes. The syndromes of hypoadrenergic and hyperadrenergic postural hypotension. Am J Med. 1978 Mar;64(3):407–416. doi: 10.1016/0002-9343(78)90220-6. [DOI] [PubMed] [Google Scholar]
- Donald M. W., Erdahl D. L., Surridge D. H., Monga T. N., Lawson J. S., Bird C. E., Letemendia F. J. Functional correlates of reduced central conduction velocity in diabetic subjects. Diabetes. 1984 Jul;33(7):627–633. doi: 10.2337/diab.33.7.627. [DOI] [PubMed] [Google Scholar]
- Dorward P. K., Riedel W., Burke S. L., Gipps J., Korner P. I. The renal sympathetic baroreflex in the rabbit. Arterial and cardiac baroreceptor influences, resetting, and effect of anesthesia. Circ Res. 1985 Oct;57(4):618–633. doi: 10.1161/01.res.57.4.618. [DOI] [PubMed] [Google Scholar]
- Duchen L. W., Anjorin A., Watkins P. J., Mackay J. D. Pathology of autonomic neuropathy in diabetes mellitus. Ann Intern Med. 1980 Feb;92(2 Pt 2):301–303. doi: 10.7326/0003-4819-92-2-301. [DOI] [PubMed] [Google Scholar]
- Eckberg D. L. Human sinus arrhythmia as an index of vagal cardiac outflow. J Appl Physiol Respir Environ Exerc Physiol. 1983 Apr;54(4):961–966. doi: 10.1152/jappl.1983.54.4.961. [DOI] [PubMed] [Google Scholar]
- Ewing D. J., Borsey D. Q., Bellavere F., Clarke B. F. Cardiac autonomic neuropathy in diabetes: comparison of measures of R-R interval variation. Diabetologia. 1981 Jul;21(1):18–24. doi: 10.1007/BF03216217. [DOI] [PubMed] [Google Scholar]
- Fagius J., Wallin B. G. Sympathetic reflex latencies and conduction velocities in patients with polyneuropathy. J Neurol Sci. 1980 Sep;47(3):449–461. doi: 10.1016/0022-510x(80)90099-4. [DOI] [PubMed] [Google Scholar]
- Fouad F. M., Tarazi R. C., Ferrario C. M., Fighaly S., Alicandri C. Assessment of parasympathetic control of heart rate by a noninvasive method. Am J Physiol. 1984 Jun;246(6 Pt 2):H838–H842. doi: 10.1152/ajpheart.1984.246.6.H838. [DOI] [PubMed] [Google Scholar]
- Goldstein D. S. Arterial baroreflex sensitivity, plasma catecholamines, and pressor responsiveness in essential hypertension. Circulation. 1983 Aug;68(2):234–240. doi: 10.1161/01.cir.68.2.234. [DOI] [PubMed] [Google Scholar]
- Goldstein D. S., McCarty R., Polinsky R. J., Kopin I. J. Relationship between plasma norepinephrine and sympathetic neural activity. Hypertension. 1983 Jul-Aug;5(4):552–559. doi: 10.1161/01.hyp.5.4.552. [DOI] [PubMed] [Google Scholar]
- Grossman S. H., Davis D., Gunnells J. C., Shand D. G. Plasma norepinephrine in the evaluation of baroreceptor function in humans. Hypertension. 1982 Jul-Aug;4(4):566–571. doi: 10.1161/01.hyp.4.4.566. [DOI] [PubMed] [Google Scholar]
- Grubeck-Loebenstein B., Vierhapper H., Waldhäusl W., Korn A., Graf M., Panzer S. Adrenergic mechanisms and blood pressure regulation in diabetes mellitus. Klin Wochenschr. 1982 Aug 16;60(16):823–828. doi: 10.1007/BF01728348. [DOI] [PubMed] [Google Scholar]
- Hilsted J., Galbo H., Christensen N. J. Impaired responses of catecholamines, growth hormone, and cortisol to graded exercise in diabetic autonomic neuropathy. Diabetes. 1980 Apr;29(4):257–262. doi: 10.2337/diab.29.4.257. [DOI] [PubMed] [Google Scholar]
- Hjemdahl P., Daleskog M., Kahan T. Determination of plasma catecholamines by high performance liquid chromatography with electrochemical detection: comparison with a radioenzymatic method. Life Sci. 1979 Jul 9;25(2):131–138. doi: 10.1016/0024-3205(79)90384-9. [DOI] [PubMed] [Google Scholar]
- Katona P. G., Jih F. Respiratory sinus arrhythmia: noninvasive measure of parasympathetic cardiac control. J Appl Physiol. 1975 Nov;39(5):801–805. doi: 10.1152/jappl.1975.39.5.801. [DOI] [PubMed] [Google Scholar]
- Kontos H. A., Richardson D. W., Norvell J. E. Norepinephrine depletion in idiopathic orthostatic hypotension. Ann Intern Med. 1975 Mar;82(3):336–341. doi: 10.7326/0003-4819-82-3-336. [DOI] [PubMed] [Google Scholar]
- Lake C. R., Chernow B., Goldstein D. S., Glass D. G., Coleman M., Ziegler M. G. Plasma catecholamine levels in normal subjects and in patients with secondary hypertension. Fed Proc. 1984 Jan;43(1):52–56. [PubMed] [Google Scholar]
- Lin M. S., McNay J. L., Shepherd A. M., Keeton T. K. Effects of hydralazine and sodium nitroprusside on plasma catecholamines and heart rate. Clin Pharmacol Ther. 1983 Oct;34(4):474–480. doi: 10.1038/clpt.1983.200. [DOI] [PubMed] [Google Scholar]
- Low P. A., Walsh J. C., Huang C. Y., McLeod J. G. The sympathetic nervous system in diabetic neuropathy. A clinical and pathological study. Brain. 1975 Sep;98(3):341–356. doi: 10.1093/brain/98.3.341. [DOI] [PubMed] [Google Scholar]
- Miller E. D., Jr, Beckman J. J., Woodside J. R., Jr, Althaus M. S., Peach M. J. Blood pressure control during anesthesia: importance of the peripheral sympathetic nervous system and renin. Anesthesiology. 1983 Jan;58(1):32–37. [PubMed] [Google Scholar]
- Murray A., Ewing D. J., Campbell I. W., Neilson J. M., Clarke B. F. RR interval variations in young male diabetics. Br Heart J. 1975 Aug;37(8):882–885. doi: 10.1136/hrt.37.8.882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray R. H., Thompson L. J., Bowers J. A., Albright C. D. Hemodynamic effects of graded hypovolemia and vasodepressor syncope induced by lower body negative pressure. Am Heart J. 1968 Dec;76(6):799–811. doi: 10.1016/0002-8703(68)90266-4. [DOI] [PubMed] [Google Scholar]
- Neubauer B., Christensen N. J. Norepinephrine, epinephrine, and dopamine contents of the cardiovascular system in long-term diabetics. Diabetes. 1976 Jan;25(1):6–10. doi: 10.2337/diab.25.1.6. [DOI] [PubMed] [Google Scholar]
- Oberg B., Thorén P. Increased activity in left ventricular receptors during hemorrhage or occlusion of caval veins in the cat. A possible cause of the vaso-vagal reaction. Acta Physiol Scand. 1972 Jun;85(2):164–173. doi: 10.1111/j.1748-1716.1972.tb05247.x. [DOI] [PubMed] [Google Scholar]
- Robertson D., Johnson G. A., Robertson R. M., Nies A. S., Shand D. G., Oates J. A. Comparative assessment of stimuli that release neuronal and adrenomedullary catecholamines in man. Circulation. 1979 Apr;59(4):637–643. doi: 10.1161/01.cir.59.4.637. [DOI] [PubMed] [Google Scholar]
- Sundlöf G., Wallin B. G. Human muscle nerve sympathetic activity at rest. Relationship to blood pressure and age. J Physiol. 1978 Jan;274:621–637. doi: 10.1113/jphysiol.1978.sp012170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallin B. G., Sundlöf G., Eriksson B. M., Dominiak P., Grobecker H., Lindblad L. E. Plasma noradrenaline correlates to sympathetic muscle nerve activity in normotensive man. Acta Physiol Scand. 1981 Jan;111(1):69–73. doi: 10.1111/j.1748-1716.1981.tb06706.x. [DOI] [PubMed] [Google Scholar]
- Wallin B. G., Sundlöf G. Sympathetic outflow to muscles during vasovagal syncope. J Auton Nerv Syst. 1982 Nov;6(3):287–291. doi: 10.1016/0165-1838(82)90001-7. [DOI] [PubMed] [Google Scholar]
- Watkins P. J., Mackay J. D. Cardiac denervation in diabetic neuropathy. Ann Intern Med. 1980 Feb;92(2 Pt 2):304–307. doi: 10.7326/0003-4819-92-2-304. [DOI] [PubMed] [Google Scholar]
- Young R. J., Ewing D. J., Clarke B. F. Nerve function and metabolic control in teenage diabetics. Diabetes. 1983 Feb;32(2):142–147. doi: 10.2337/diab.32.2.142. [DOI] [PubMed] [Google Scholar]
- Ziegler M. G., Lake C. R., Kopin I. J. The sympathetic-nervous-system defect in primary orthostatic hypotension. N Engl J Med. 1977 Feb 10;296(6):293–297. doi: 10.1056/NEJM197702102960601. [DOI] [PubMed] [Google Scholar]


