Abstract
Starvation in laboratory rodents results in significant alterations in thyroid hormone economy characterized by decreased circulating levels of thyroxine (T4) and 3,5,3'-triiodothyronine (T3) and a decline in serum thyrotropin (TSH) concentration. To investigate this apparent paradox, we have compared in fasted and hypothyroid animals the intracellular parameters mediating thyroid hormone action in the anterior pituitary gland. In vitro saturation analysis combined with quantitation of nuclear T3 content by radioimmunoassay allowed for characterization of pituitary nuclear T3 receptors and estimation of the endogenous fractional receptor occupancy. In rats, thyroidectomized 4 wk earlier, the 10-fold increase in serum TSH levels and decline in peripheral thyroid hormone concentrations were accompanied by a 61% decrease in pituitary nuclear T3 content and a marked decline in fractional T3 receptor occupancy as compared with control animals. In euthyroid animals subjected to short-term starvation (72 h), serum T3, T4, and TSH levels declined by 52, 43, and 48%, respectively. Despite these marked decreases in circulating thyroid hormone levels, pituitary nuclear T3 content in fasted rats declined by only 15% (P less than 0.05) relative to control levels. This modest decline in nuclear T3 content, combined with a 23% decrease in total T3 receptor number, resulted in an estimated fractional receptor occupancy in fasted animals which was equal to or greater than that noted in controls. The effects of fasting and hypothyroidism on the pituitary were further investigated by quantifying low Michaelis constant (Km) T4 5'-deiodinase activity in the crude cytosol fraction of pituitary homogenates. In thyroidectomized animals, maximum velocity was increased ninefold, whereas fasting resulted in a 37% decrease (P less than 0.025) in this parameter compared with controls. Km values were similar in all experimental groups (4.7 +/- 0.6 nM). These results demonstrate that, despite significant reductions in circulating thyroid hormone concentrations and pituitary T4 5'-deiodinase activity, nuclear T3 levels are maintained at relatively normal levels in the pituitary of the fasted animal and fractional T3 receptor occupancy may actually increase. These findings are in marked contrast to those noted in thyroidectomized animals and suggest that the suppression of TSH secretion accompanying starvation in the rat is mediated, at least in part, by local pituitary mechanisms that serve to maintain and possibly enhance nuclear T3 receptor occupancy.
Full text
PDF

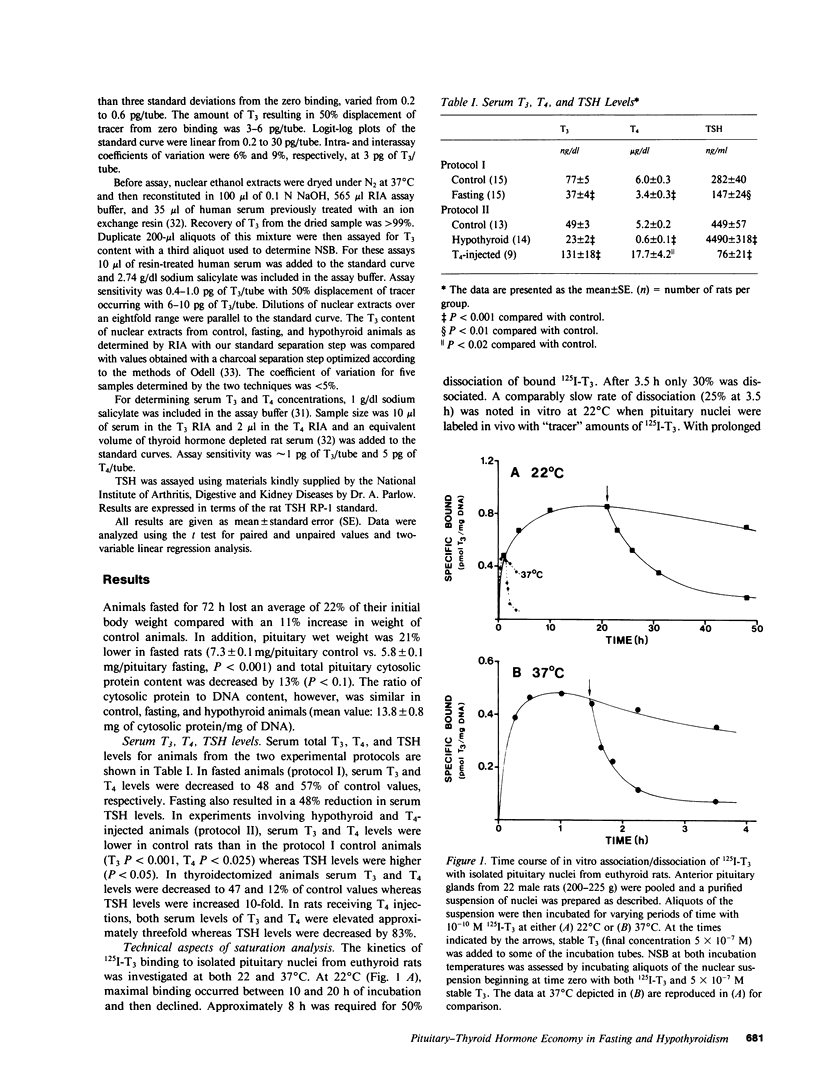
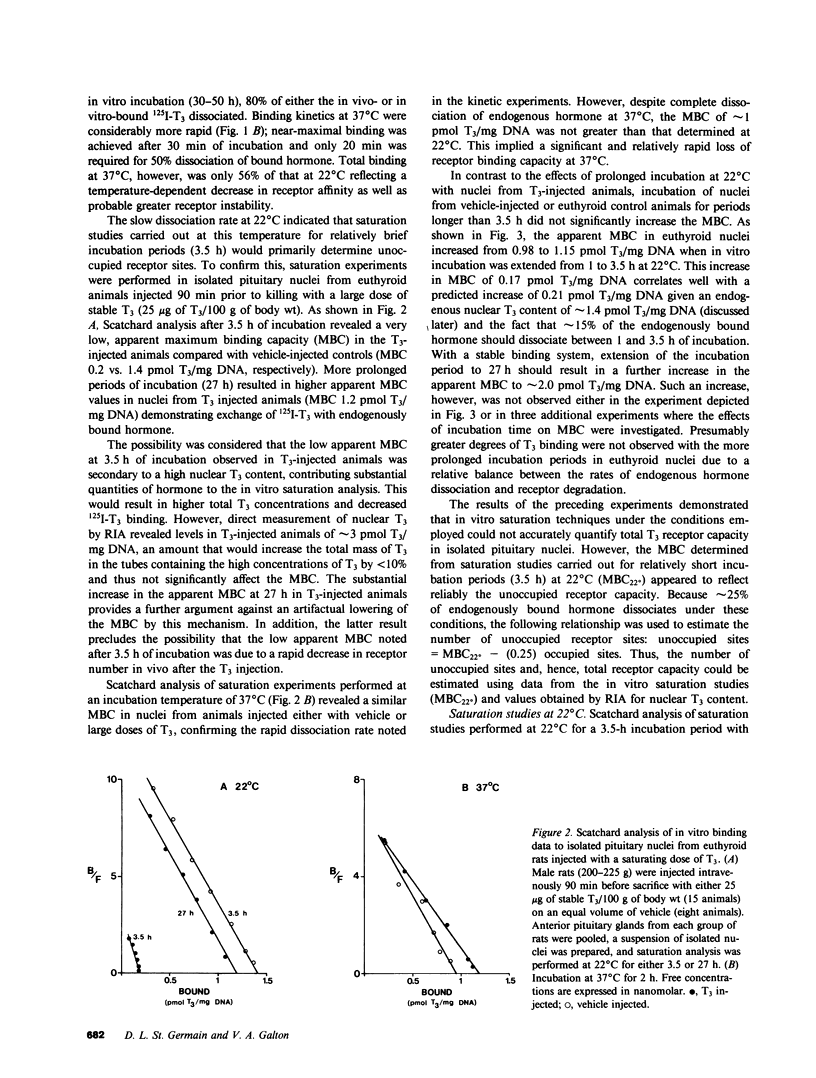
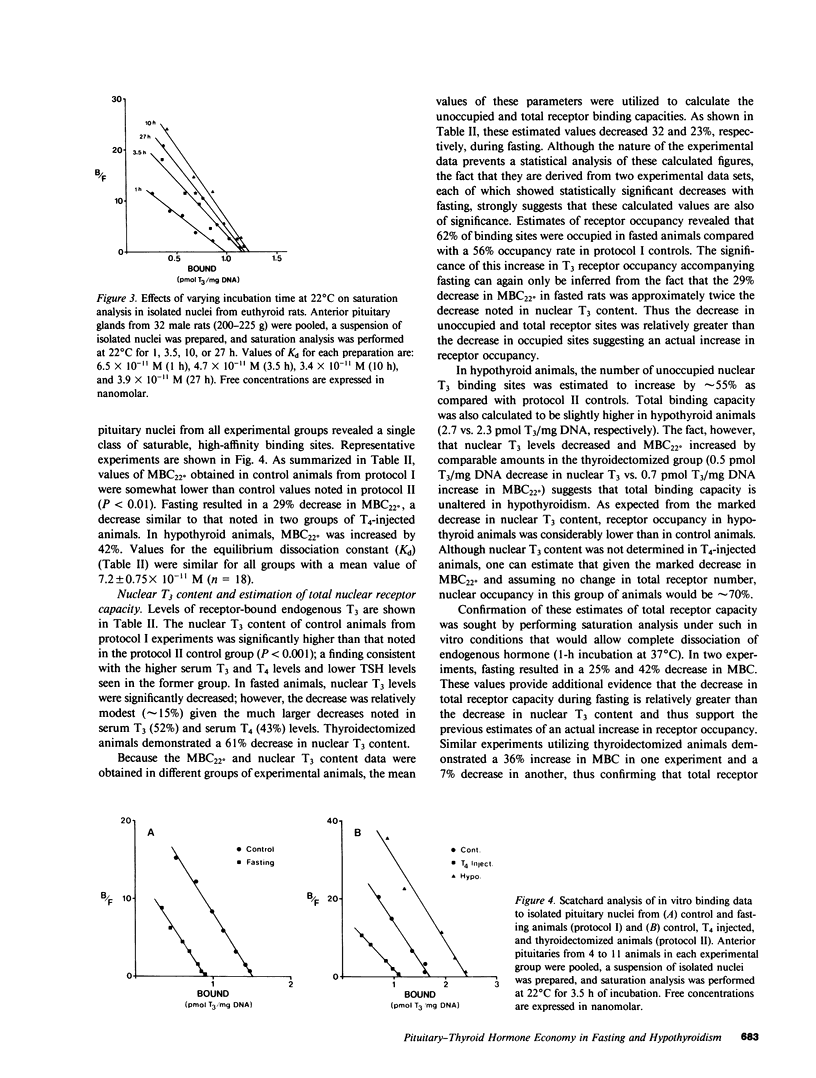
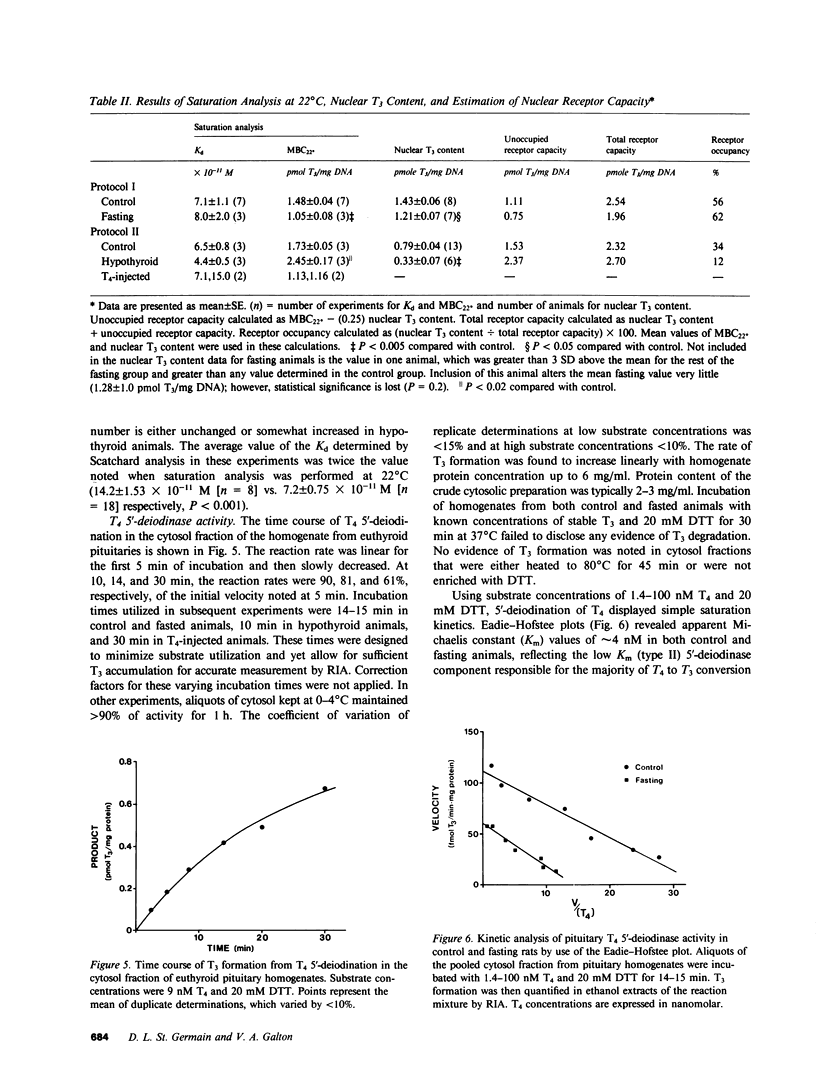



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Azizi F. Effect of dietary composition on fasting-induced changes in serum thyroid hormones and thyrotropin. Metabolism. 1978 Aug;27(8):935–942. doi: 10.1016/0026-0495(78)90137-3. [DOI] [PubMed] [Google Scholar]
- BURTON K. A study of the conditions and mechanism of the diphenylamine reaction for the colorimetric estimation of deoxyribonucleic acid. Biochem J. 1956 Feb;62(2):315–323. doi: 10.1042/bj0620315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balsam A., Sexton F., Ingbar S. H. Effects of dietary manipulation on the in vitro generation of 3,5,3'-triiodothyronine from thyroxine in rat liver preparations. Life Sci. 1981 Apr 13;28(15-16):1727–1736. doi: 10.1016/0024-3205(81)90344-1. [DOI] [PubMed] [Google Scholar]
- Bavli S. Z. Fasting, but not glucagon administration, decreases anterior pituitary nuclear thyroid hormone receptors in rats. Metabolism. 1980 Jul;29(7):636–642. doi: 10.1016/0026-0495(80)90108-0. [DOI] [PubMed] [Google Scholar]
- Beumont P. J., George G. C., Pimstone B. L., Vinik A. I. Body weight and the pituitary response to hypothalamic releasing hormones in patients with anorexia nervosa. J Clin Endocrinol Metab. 1976 Sep;43(3):487–496. doi: 10.1210/jcem-43-3-487. [DOI] [PubMed] [Google Scholar]
- Burman K. D., Smallridge R. C., Burge J. R., Carlson D., Wartofsky L. Ipodate restores the fasting-induced decrement in thyrotropin secretion. J Clin Endocrinol Metab. 1983 Sep;57(3):597–602. doi: 10.1210/jcem-57-3-597. [DOI] [PubMed] [Google Scholar]
- Burman K. D., Wartofsky L., Dinterman R. E., Kesler P., Wannemacher R. W., Jr The effect of T3 and reverse T3 administration on muscle protein catabolism during fasting as measured by 3-methylhistidine excretion. Metabolism. 1979 Aug;28(8):805–813. doi: 10.1016/0026-0495(79)90206-3. [DOI] [PubMed] [Google Scholar]
- Cahill G. F., Jr Role of T3 in fasted man. Life Sci. 1981 Apr 13;28(15-16):1721–1726. doi: 10.1016/0024-3205(81)90343-x. [DOI] [PubMed] [Google Scholar]
- Campbell G. A., Kurcz M., Marshall S., Meites J. Effects of starvation in rats on serum levels of follicle stimulating hormone, luteinizing hormone, thyrotropin, growth hormone and prolactin; response to LH-releasing hormone and thyrotropin-releasing hormone. Endocrinology. 1977 Feb;100(2):580–587. doi: 10.1210/endo-100-2-580. [DOI] [PubMed] [Google Scholar]
- Carr F. E., Seelig S., Mariash C. N., Schwartz H. L., Oppenheimer J. H. Starvation and hypothyroidism exert an overlapping influence on rat hepatic messenger RNA activity profiles. J Clin Invest. 1983 Jul;72(1):154–163. doi: 10.1172/JCI110953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter W. J., Shakir K. M., Hodges S., Faas F. H., Wynn J. O. Effect of thyroid hormone on metabolic adaptation to fasting. Metabolism. 1975 Oct;24(10):1177–1183. doi: 10.1016/0026-0495(75)90154-7. [DOI] [PubMed] [Google Scholar]
- Cheng S. Y. Characterization of binding and uptake of 3,3',5-triido-L-thyronine in cultured mouse fibroblasts. Endocrinology. 1983 May;112(5):1754–1762. doi: 10.1210/endo-112-5-1754. [DOI] [PubMed] [Google Scholar]
- Cheron R. G., Kaplan M. M., Larsen P. R. Physiological and pharmacological influences on thyroxine to 3,5,3'-triiodothyronine conversion and nuclear 3,5,3'-triiodothyronine binding in rat anterior pituitary. J Clin Invest. 1979 Nov;64(5):1402–1414. doi: 10.1172/JCI109598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chopra I. J. Alterations in monodeiodination of iodothyronines in the fasting rat: effects of reduced nonprotein sulfhydryl groups and hypothyroidism. Metabolism. 1980 Feb;29(2):161–167. doi: 10.1016/0026-0495(80)90141-9. [DOI] [PubMed] [Google Scholar]
- Chopra I. J., Carlson H. E., Solomon D. H. Comparison of inhibitory effects of 3,5,3'-triiodothyronine (T3), thyroxine (T4), 3,3,',5'-triiodothyronine (rT3), and 3,3'-diiodothyronine (T2) on thyrotropin-releasing hormone-induced release of thyrotropin in the rat in vitro. Endocrinology. 1978 Aug;103(2):393–402. doi: 10.1210/endo-103-2-393. [DOI] [PubMed] [Google Scholar]
- Coulombe P., Ruel J., Faure R., Dussault J. H. Pituitary nuclear triiodothyronine receptors during development in the rat. Am J Physiol. 1983 Jul;245(1):E81–E86. doi: 10.1152/ajpendo.1983.245.1.E81. [DOI] [PubMed] [Google Scholar]
- DOWD J. E., RIGGS D. S. A COMPARISON OF ESTIMATES OF MICHAELIS-MENTEN KINETIC CONSTANTS FROM VARIOUS LINEAR TRANSFORMATIONS. J Biol Chem. 1965 Feb;240:863–869. [PubMed] [Google Scholar]
- DeGroot L. J., Coleoni A. H., Rue P. A., Seo H., Martino E., Refetoff S. Reduced nuclear triiodothyronine receptors in starvation-induced hypothyroidism. Biochem Biophys Res Commun. 1977 Nov 7;79(1):173–178. doi: 10.1016/0006-291x(77)90076-6. [DOI] [PubMed] [Google Scholar]
- Degroot L. J., Torresani J., Carrayon P., Tirard A. Factors influencing triiodothyronine binding properties of liver nuclear receptors. Acta Endocrinol (Copenh) 1976 Oct;83(2):293–304. doi: 10.1530/acta.0.0830293. [DOI] [PubMed] [Google Scholar]
- Eberhardt N. L., Valcana T., Timiras P. S. Triiodothyronine nuclear receptors: an in vitro comparison of the binding of triiodothyronine to nuclei of adult rat liver, cerebral hemisphere, and anterior pituitary. Endocrinology. 1978 Feb;102(2):556–561. doi: 10.1210/endo-102-2-556. [DOI] [PubMed] [Google Scholar]
- Eckel J., Rao G. S., Rao M. L., Breuer H. Uptake of L-tri-iodothyronine by isolated rat liver cells. A process partially inhibited by metabolic inhibitors; attempts to distinguish between uptake and binding to intracellular proteins. Biochem J. 1979 Aug 15;182(2):473–491. doi: 10.1042/bj1820473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galton V. A. Binding of thyroid hormones in vivo by hepatic nuclei of Rana catesbeiana tadpoles. Endocrinology. 1980 Mar;106(3):859–866. doi: 10.1210/endo-106-3-859. [DOI] [PubMed] [Google Scholar]
- Gardner D. F., Kaplan M. M., Stanley C. A., Utiger R. D. Effect of tri-iodothyronine replacement on the metabolic and pituitary responses to starvation. N Engl J Med. 1979 Mar 15;300(11):579–584. doi: 10.1056/NEJM197903153001102. [DOI] [PubMed] [Google Scholar]
- Gavin L. A., Bui F., McMahon F., Cavalieri R. R. Sequential deiodination of thyroxine to 3,3'-diiodothyronine via 3,5,3'-triiodothyronine and 3,3',5'-triiodothyronine in rat liver homogenate. The effects of fasting versus glucose feeding. J Biol Chem. 1980 Jan 10;255(1):49–54. [PubMed] [Google Scholar]
- Gavin L. A., Moeller M. Somatostatin inhibits rat hepatic T4-5'-deiodinase. The effect is independent of the associated hypoinsulinemia. J Clin Invest. 1983 Dec;72(6):2020–2030. doi: 10.1172/JCI111167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris A. R., Fang S. L., Azizi F., Lipworth L., Vagenakis A. G., Barverman L. E. Effect of starvation on hypothalamic-pituitary-thyroid function in the rat. Metabolism. 1978 Sep;27(9):1074–1083. doi: 10.1016/0026-0495(78)90153-1. [DOI] [PubMed] [Google Scholar]
- Henson L. C., Heber D. Whole body protein breakdown rates and hormonal adaptation in fasted obese subjects. J Clin Endocrinol Metab. 1983 Aug;57(2):316–319. doi: 10.1210/jcem-57-2-316. [DOI] [PubMed] [Google Scholar]
- Hugues J. N., Reinberg A., Jordan D., Sebaoun J., Modigliani E., Burger A. G. Effects of starvation on circadian variations of plasma TSH in rats. Acta Endocrinol (Copenh) 1982 Nov;101(3):403–407. doi: 10.1530/acta.0.1010403. [DOI] [PubMed] [Google Scholar]
- Kaplan M. M. Thyroxine 5'-monodeiodination in rat anterior pituitary homogenates. Endocrinology. 1980 Feb;106(2):567–576. doi: 10.1210/endo-106-2-567. [DOI] [PubMed] [Google Scholar]
- Larsen P. R., Bavli S. Z., Castonguay M., Jove R. Direct radioimmunoassay of nuclear 3,5,3' triiodothyronine in rat anterior pituitary. J Clin Invest. 1980 Mar;65(3):675–681. doi: 10.1172/JCI109713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsen P. R. Direct immunoassay of triiodothyronine in human serum. J Clin Invest. 1972 Aug;51(8):1939–1949. doi: 10.1172/JCI107000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naito K., Inada M., Mashio Y., Tanaka K., Ishii H., Nishikawa M., Imura H. Modulation of T4 5'-monodeiodination in rat anterior pituitary and liver homogenates by thyroid states and fasting. Endocrinol Jpn. 1981 Dec;28(6):793–798. doi: 10.1507/endocrj1954.28.793. [DOI] [PubMed] [Google Scholar]
- Odell W. D. Use of charcoal to separate antibody complexes from free ligand in radioimmunoassay. Methods Enzymol. 1980;70(A):274–279. doi: 10.1016/s0076-6879(80)70056-3. [DOI] [PubMed] [Google Scholar]
- Oppenheimer J. H., Schwartz H. L. Factors determining the level of activity of 3,5,3'-triiodothyronine-responsive hepatic enzymes in the starved rat. Endocrinology. 1980 Nov;107(5):1460–1468. doi: 10.1210/endo-107-5-1460. [DOI] [PubMed] [Google Scholar]
- Oppenheimer J. H., Schwartz H. L., Koerner D., Surks M. I. Limited binding capacity sites for L-triiodothyronine in rat liver nuclei. Nuclear-cytoplasmic interrelation, binding constants, and cross-reactivity with L-thyroxine. J Clin Invest. 1974 Mar;53(3):768–777. doi: 10.1172/JCI107615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oppenheimer J. H., Schwartz H. L., Surks M. I. Tissue differences in the concentration of triiodothyronine nuclear binding sites in the rat: liver, kidney, pituitary, heart, brain, spleen, and testis. Endocrinology. 1974 Sep;95(3):897–903. doi: 10.1210/endo-95-3-897. [DOI] [PubMed] [Google Scholar]
- PEARSON J. D., VEALL N., VETTER H. A practical method for plasma albumin turnover studies. Strahlentherapie. 1958;107(SONDERBD):290–297. [PubMed] [Google Scholar]
- Palmblad J., Levi L., Burger A., Melander A., Westgren U., von Schenck H., Skude G. Effects of total energy withdrawal (fasting) on thelevels of growth hormone, thyrotropin, cortisol, adrenaline, noradrenaline, T4, T3, and rT3 in healthy males. Acta Med Scand. 1977 Jan;201(1-2):15–22. doi: 10.1111/j.0954-6820.1977.tb15648.x. [DOI] [PubMed] [Google Scholar]
- Samuels H. H., Stanley F., Casanova J. Depletion of L-3,5,3'-triiodothyronine and L-thyroxine in euthyroid calf serum for use in cell culture studies of the action of thyroid hormone. Endocrinology. 1979 Jul;105(1):80–85. doi: 10.1210/endo-105-1-80. [DOI] [PubMed] [Google Scholar]
- Samuels H. H., Stanley F., Shapiro L. E. Modulation of thyroid hormone nuclear receptor levels by 3,5,3'-triiodo-L-thyronine in GH1 cells. Evidence for two functional components of nuclear-bound receptor and relationship to the induction of growth hormone synthesis. J Biol Chem. 1977 Sep 10;252(17):6052–6060. [PubMed] [Google Scholar]
- Seelig S., Schwartz H. L., Oppenheimer J. H. Limitations in the conventional analysis of the interaction of triiodothyronine with solubilized nuclear receptor sites. Inapparent binding of triiodothyronine to nonspecific binding sites. J Biol Chem. 1981 Mar 10;256(5):2154–2161. [PubMed] [Google Scholar]
- Silva J. E., Dick T. E., Larsen P. R. The contribution of local tissue thyroxine monodeiodination to the nuclear 3,5,3'-triiodothyronine in pituitary, liver, and kidney of euthyroid rats. Endocrinology. 1978 Oct;103(4):1196–1207. doi: 10.1210/endo-103-4-1196. [DOI] [PubMed] [Google Scholar]
- Silva J. E., Larsen P. R. Contributions of plasma triiodothyronine and local thyroxine monodeiodination to triiodothyronine to nuclear triiodothyronine receptor saturation in pituitary, liver, and kidney of hypothyroid rats. Further evidence relating saturation of pituitary nuclear triiodothyronine receptors and the acute inhibition of thyroid-stimulating hormone release. J Clin Invest. 1978 May;61(5):1247–1259. doi: 10.1172/JCI109041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva J. E., Larsen P. R. Pituitary nuclear 3,5,3'-triiodothyronine and thyrotropin secretion: an explanation for the effect of thyroxine. Science. 1977 Nov 11;198(4317):617–620. doi: 10.1126/science.199941. [DOI] [PubMed] [Google Scholar]
- Spindler B. J., MacLeod K. M., Ring J., Baxter J. D. Thyroid hormone receptors. Binding characteristics and lack of hormonal dependency for nuclear localization. J Biol Chem. 1975 Jun 10;250(11):4113–4119. [PubMed] [Google Scholar]
- Thrall C. L., Yanagihara T. Alterations of nuclear thyroid hormone receptors in cerebral cortex in vivo. J Neurochem. 1982 Mar;38(3):669–674. doi: 10.1111/j.1471-4159.1982.tb08683.x. [DOI] [PubMed] [Google Scholar]
- Visser T. J., Kaplan M. M., Leonard J. L., Larsen P. R. Evidence for two pathways of iodothyronine 5'-deiodination in rat pituitary that differ in kinetics, propylthiouracil sensitivity, and response to hypothyroidism. J Clin Invest. 1983 Apr;71(4):992–1002. doi: 10.1172/JCI110854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wartofsky L., Burman K. D. Alterations in thyroid function in patients with systemic illness: the "euthyroid sick syndrome". Endocr Rev. 1982 Spring;3(2):164–217. doi: 10.1210/edrv-3-2-164. [DOI] [PubMed] [Google Scholar]
- Yeakley J. M., Balasubramanian K., Harrison R. W. Comparison of glucocorticoid-receptor binding kinetics with predictions from a biomolecular model. J Biol Chem. 1980 May 10;255(9):4182–4188. [PubMed] [Google Scholar]
- van Doorn J., van der Heide D., Roelfsema F. Sources and quantity of 3,5,3'-triiodothyronine in several tissues of the rat. J Clin Invest. 1983 Nov;72(5):1778–1792. doi: 10.1172/JCI111138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Overbeck K., Lemarchand-Béraud T. Modulation of thyroid hormone nuclear receptor levels by L-triiodothyronine (T3) in the rat pituitary. Mol Cell Endocrinol. 1983 Dec;33(2-3):281–292. doi: 10.1016/0303-7207(83)90173-9. [DOI] [PubMed] [Google Scholar]



