Abstract
Background
Mean arterial pressure (MAP) below the lower limit of cerebral autoregulation during cardiopulmonary bypass (CPB) is associated with complications after cardiac surgery. However, simply raising empiric MAP targets during CPB might result in MAP above the upper limit of autoregulation (ULA), causing cerebral hyperperfusion in some patients and predisposing them to cerebral dysfunction after surgery. We hypothesized that MAP above an ULA during CPB is associated with postoperative delirium.
Methods
Autoregulation during CPB was monitored continuously in 491 patients with the cerebral oximetry index (COx) in this prospective observational study. COx represents Pearson's correlation coefficient between low-frequency changes in regional cerebral oxygen saturation (measured with near-infrared spectroscopy) and MAP. Delirium was defined throughout the postoperative hospitalization based on clinical detection with prospectively defined methods.
Results
Delirium was observed in 45 (9.2%) patients. Mechanical ventilation for >48 h [odds ratio (OR), 3.94; 95% confidence interval (CI), 1.72–9.03], preoperative antidepressant use (OR, 3.0; 95% CI, 1.29–6.96), prior stroke (OR, 2.79; 95% CI, 1.12–6.96), congestive heart failure (OR, 2.68; 95% CI, 1.28–5.62), the product of the magnitude and duration of MAP above an ULA (mm Hg h; OR, 1.09; 95% CI, 1.03–1.15), and age (per year of age; OR, 1.01; 95% CI, 1.01–1.07) were independently associated with postoperative delirium.
Conclusions
Excursions of MAP above the upper limit of cerebral autoregulation during CPB are associated with risk for delirium. Optimizing MAP during CPB to remain within the cerebral autoregulation range might reduce risk of delirium.
Clinical trial registration
clinicaltrials.gov NCT00769691 and NCT00981474.
Keywords: cardiac surgery, cardiopulmonary bypass, cerebral autoregulation, delirium
Editor's key points.
Cerebral hyperperfusion attributable to arterial pressure above an upper limit of autoregulation could contribute to delirium after cardiopulmonary bypass (CPB).
Cerebral autoregulation was measured using cerebral oximetry, and postoperative delirium was prospectively assessed in cardiac surgery patients.
Mean arterial pressure above the upper limit of cerebral autoregulation during CPB was associated with increased risk of delirium.
Mean arterial pressure (MAP) targets during cardiopulmonary bypass (CPB) are arbitrarily chosen based mostly on historical practices.1,2 These practices are grounded largely on older data showing that cerebral blood flow (CBF) autoregulation remains functional during CPB when alpha-stat pH management is used.2–4 Consequently, MAP as low as 50 mm Hg is viewed as well-tolerated because CBF is perceived not to be compromised. More recently, our group has found that the lower limit of CBF autoregulation in patients monitored continuously during CPB ranges between 40 and 90 mm Hg and that the actual limit is difficult to predict based on preoperative arterial pressure and patient medical history.5–7 We have found that higher magnitudes and longer durations of MAP below the lower limit of autoregulation are associated with a higher risk of complications.8,9
One approach to address potential organ hypoperfusion during CPB might be to simply raise current MAP targets to a higher level.10 However, empirically raising MAP targets during CPB might result in arterial pressure that is above the upper limit of autoregulation (ULA) in some patients. In such cases, CBF would increase proportionately with MAP and could cause cerebral hyperperfusion that may lead to microvascular changes, alterations in the blood–brain barrier, cerebral oedema, and perhaps a more subtle presentation of cerebral dysfunction.11–13 Delirium is one manifestation of cerebral dysfunction that has been associated with subsequent adverse events.14,15 In nonsurgical settings, cerebral hyperperfusion is the proposed mechanism for delirium in patients with hypertensive emergencies.16 However, little information is available on whether MAP above an ULA during CPB adversely affects patient outcomes. In this study, we hypothesized that the magnitude and duration of MAP above the upper limit of CBF autoregulation during CPB is associated with postoperative delirium.
Methods
From April 2008 to January 2013, patients undergoing cardiac surgery with CPB were enrolled in prospective studies of CBF autoregulation monitoring as described.5–9,17 The current study represents a retrospective analysis of these data.
All procedures received the approval of the Institutional Review Board (Committee 1) of The Johns Hopkins Medical Institutions (protocol number NA_00027003), and all patients provided written informed consent.
Perioperative care
Patients received routine institutional care, including continuous, direct, radial artery arterial pressure monitoring. Midazolam, fentanyl, isoflurane, and pancuronium or vecuronium were used for anaesthesia and muscle relaxation. Non-pulsatile flow between 2.0 and 2.4 litre min−1 m−2 was used for CPB. Isoflurane concentration was maintained between 0.5 and 1.0% through the gas inflow of the oxygenator. Alpha-stat pH management was used, and oxygenation and normocarbia were ensured by continuous in-line arterial blood gas monitoring that was calibrated hourly. Arterial pressure targets were chosen empirically based on institutional standard of care. Phenylephrine was administered to treat low arterial pressure based on institutional standards.
Near-infrared spectroscopy-based autoregulation monitoring
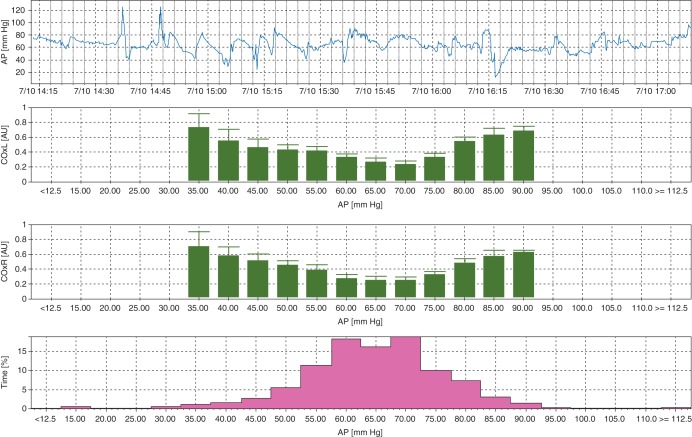
Two sensor pads connected to a near-infrared spectroscopy (NIRS) monitor (INVOS™, Covidien Somanetics™, Boulder, CO, USA) were placed on the patient's forehead before induction of anaesthesia. Our signal processing and analysis has been described elsewhere.5–9,17 Arterial pressure signals from the operating room haemodynamic monitor were digitalized by a data acquisition module (DT9800, Data Translation, Inc., Marlboro, MA, USA); these data, along with digital NIRS-derived regional cerebral oxygen saturation (rScO2) signals, were transferred to a laptop computer using the ICM+ software (University of Cambridge, Cambridge, UK). The signals were then filtered as non-overlapping 10 s mean values that were time-integrated. This method, which is equivalent to having a moving average filter with a 10 s time window and resampling at 0.1 Hz, eliminates high-frequency components caused by respiration and pulse waveforms. Additional high-pass filtering was applied with a DC cutoff set at 0.003 Hz to remove slow drifts associated with haemodilution at initiation of CPB. The monitor calculates a continuous, moving Pearson's correlation coefficient between MAP and rScO2 using a sliding 300 s window updated every 10 s, rendering the variable cerebral oximetry index (COx). COx provides a reliable surrogate of CBF for autoregulation monitoring.6,18 When CBF is within the autoregulatory range, COx approaches zero or is negative. However, when MAP is below or above the limits of autoregulation, COx increases towards 1. Average COx data were placed in 5 mm Hg MAP bins. We define the limit of autoregulation as that MAP when COx increases from <0.3 to >0.3 with low or high MAP (Fig. 1).
Fig 1.
The representative graph of autoregulation monitoring during CPB. The COx represents the correlation coefficient between low-frequency regional cerebral oxygen saturation and MAP. When arterial pressure is above or below the autoregulation threshold, COx approaches 1, but when autoregulation is functional, COx is near zero. In this example, the lower limit of autoregulation based on the MAP at which COx ≥0.3 is ∼55 mm Hg, and an ULA is at a MAP of 75 mm Hg. AP, arterial pressure.
Patient outcomes
Each patient's medical history, medications, and intraoperative and postoperative course were recorded in a study-specific database. The record included complications affecting major organs, as defined by the Society of Thoracic Surgeons National Cardiac Surgery Database (www.sts.org), and acute kidney injury. Baseline serum creatinine was defined as the last value collected before surgery, as measured in the Clinical Chemistry Laboratory of Johns Hopkins Hospital (Roche Diagnostics, Indianapolis, IN, USA). The estimated glomerular filtration rate (eGFR) was calculated using the simplified Modification of Diet in Renal Disease formula.19 Acute kidney injury was diagnosed based on the RIFLE criteria: (i) Risk, defined as an increase in plasma creatinine ×1.5 or decrease in eGFR by >25% from baseline; (ii) Injury, defined as an increase in plasma creatinine ×2 or a decrease in eGFR by >50% from baseline; and (iii) Failure, defined as an increase in plasma creatinine ×3, plasma creatinine ≥350 µmol litre−1, an acute increase in plasma creatinine of ≥44 µmol litre−1 from baseline, or new renal replacement therapy.20 Patients meeting any of the RIFLE criteria were considered to have acute kidney injury. Operative death was defined as death that occurred during the hospitalization in which the operation was performed, even if it was after 30 days, or death that occurred after discharge from the hospital but within 30 days of the procedure.
Assessment for delirium
After patients underwent cardiac surgery, clinical nurses (with clinical training to care for cardiac surgery patients) identified possible neurologic problems (stroke, delirium, confusion, agitation, change in mental status, seizure, coma, or slowness to awaken after surgery) on a daily basis and communicated this information to a charge nurse or nurse practitioner as previously described.6 Research personnel queried the charge nurse (for patients in the intensive care unit) and reviewed daily patient progress sheets (prepared by nurse practitioners for patients on the floor) for evidence of any new neuropsychiatric symptoms. Delirium was defined as the presence of any of the following observations by clinical staff: delirium, confusion, agitation, or change in mental status. We excluded patients with evidence of new focal deficits such as stroke, seizure, or coma.
Data analysis
The lower limit of autoregulation was defined as that decrement of MAP at which COx increased from <0.3 to >0.3.18,21 An ULA was defined as that incremental increase in MAP at which COx increased from <0.3 to >0.3 (Fig. 1). For patients who had COx >0.3 at all MAP during CPB, the optimal MAP was defined as the MAP associated with the lowest COx. In these situations, MAPs that were less than or greater than optimal MAP were considered to be below or above the limit of autoregulation, respectively. Excursions outside the limits of autoregulation were calculated as the sum of the product of the magnitude and duration by which MAP was either below the lower or above the upper autoregulation threshold (mm Hg) per hour of CPB (mm Hg h), which is equivalent to area under the curve (∑ Δ arterial pressure from the limit of autoregulation at each MAP×time on CPB in h at each MAP).
Patients were categorized based on whether they developed delirium after surgery. We analysed continuous data that were normally distributed by Student's t-test and continuous data that were not normally distributed by Mann–Whitney analysis. The analysis of normal distribution was performed by the Kolmogorov–Smirnov test. Dichotomous data were compared with the Fisher exact test. We used backwards step-wise multivariate logistic regression by including those univariate variables that differed between patients with and without postoperative delirium, with a P-value of <0.1.
Results
Autoregulation data were available from 491 patients. We were able to identify an upper limit of cerebral autoregulation in 303 (62%) patients. The MAP at ULA was [mean (sd)] 90(12) mm Hg [95% confidence interval (CI), 88–91 mm Hg]. Medical characteristics did not differ between patients with and those without a detectable ULA, except that the former were more likely to be male and less likely to have chronic obstructive pulmonary disease. Delirium was observed in 45 (9.2%) patients. Seventy-one per cent of delirium episodes occurred by postoperative Day 2 and 90% occurred by Day 3. The frequency of delirium was four-fold higher in patients whose MAP exceeded an ULA than in patients whose MAP did not exceed the upper limit (12.9 vs 3.2%, P<0.001).
The characteristics for the entire cohort and for patients with and without postoperative delirium are given in Table 1. Patients with delirium were older and were more likely to have a history of congestive heart failure (CHF), prior stroke, and preoperative antidepressant drug use than were those without delirium. Patients who developed delirium also had a longer mean duration of CPB than did those without delirium and longer postoperative hospital length of stay. Furthermore, patients with delirium had higher frequencies of major morbidity and major mortality, perioperative stroke, and mechanical ventilation for >48 h (Table 2).
Table 1.
Subject characteristics. *The P-value refers to a comparison between patients with and without postoperative delirium.†Mean (sd) (95% CI);‡median, inter-quartile range. Aortic, aortic surgery; AVR, aortic valve replacement; CABG, coronary artery bypass graft; CPB, cardiopulmonary bypass; CKD, chronic kidney disease; GFR, glomerular filtration rate; LVAD, left ventricular assisting device placement; MVR, mitral valve replacement or repair; rScO2, regional cerebral oxygen saturation measured with near-infrared spectroscopy; SARI, serotonin antagonist and reuptake inhibitor; SNRI, serotonin norepinephrine reuptake inhibitor; SSRI, selective serotonin reuptake inhibitor; Statins, HMG-CoA reductase inhibitors; TIA, transient ischaemic attack
| Characteristics | Entire population (n=491) | Delirium (n=45) | No delirium (n=446) | P-value* |
|---|---|---|---|---|
| Age (yr)† | 66.2 (11.3) (65.2–67.2) | 69.6 (9.9) (66.6–72.6) | 65.8 (11. 4) (64.8–67.0) | 0.035 |
| Male (%) | 359 (73.1%) | 37 (82.2%) | 322 (72.2%) | 0.162 |
| COBD (%) | 54 (11.0%) | 8 (17.8%) | 46 (10.3%) | 0.134 |
| Diabetes (%) | 191 (38.9%) | 20 (44.4%) | 171 (38.3%) | 0.427 |
| Coronary artery disease (%) | 241 (49.1%) | 25 (55.6%) | 216 (48.4%) | 0.435 |
| CHF (%) | 84 (17.1%) | 14 (31.1%) | 70 (15.7%) | 0.020 |
| Prior myocardial infarction (%) | 123 (25.1%) | 7 (15.6%) | 116 (26.0%) | 0.149 |
| Hypertension (%) | 377 (76.8%) | 39 (86.7%) | 338 (75.8%) | 0.137 |
| Preoperative systolic arterial pressure (mm Hg)† | 136 (21) (134–138) | 136 (21) (134–138) | 134 (19) (128–140) | 0.573 |
| Preoperative mean arterial pressure (mm Hg)† | 93 (14) (92–94) | 93 (14) (92–94) | 93 (14) (92–94) | 0.967 |
| Baseline estimated GFR (ml × min−1 × 1.73 m−2) | 76.0 (25.9) | 70.3 (27.0) | 76.5 (25.8) | 0.128 |
| CKD with dialysis (%) | 12 (2.4%) | 2 (4.4%) | 10 (2.2%) | 0.302 |
| Angiotensin-converting enzyme inhibitors-I (%) | 170 (34.6%) | 19 (42.2%) | 151 (33.9%) | 0.324 |
| Statins (%) | 308 (62.7%) | 27 (60.0%) | 281 (63.0%) | 0.747 |
| Aspirin (%) | 342 (69.7%) | 29 (64.4%) | 313 (70.2%) | 0.496 |
| Beta blocker (%) | 292 (59.5%) | 29 (64.4%) | 263 (59.0%) | 0.527 |
| Antidepressant drug (%) | 46 (9.4%) | 10 (22.2%) | 36 (8.0%) | 0.005 |
| SSRI (%) | 33 (6.7%) | 8 (17.8%) | 25 (5.6%) | 0.006 |
| SNRI (%) | 6 (1.2%) | 1 (2.2%) | 5 (1.1%) | 0.440 |
| SARI (%) | 1 (0.2%) | 0 (0.0%) | 1 (0.2%) | >0.999 |
| Tricyclic/tetracyclic antidepressant (%) | 4 (0.8%) | 1 (2.2%) | 3 (0.7%) | 0.320 |
| Aminoketone (%) | 2 (0.4%) | 0 (0.0%) | 2 (0.4%) | >0.999 |
| Current smoker (%) | 68 (13.8%) | 6 (13.3%) | 62 (13.9%) | >0.999 |
| Prior smoker (%) | 206 (42.0%) | 19 (42.2%) | 187 (41.9%) | >0.999 |
| Prior cardiac surgery (%) | 44 (9.0%) | 5 (11.1%) | 39 (8.7%) | 0.583 |
| Prior carotid endarterectomy (%) | 15 (3.1%) | 1 (2.2%) | 14 (3.1%) | >0.999 |
| Prior cerebral vascular accident (%) | 43 (8.8%) | 8 (17.8%) | 35 (7.8%) | 0.046 |
| Prior TIA (%) | 26 (5.3%) | 4 (8.9%) | 22 (4.9%) | 0.284 |
| Peripheral vascular disease (%) | 62 (12.6%) | 7 (15.6%) | 55 (12.3%) | 0.486 |
| Surgical procedure | 0.334 | |||
| CABG (%) | 277 (56.4%) | 20 (44.4%) | 257 (57.6%) | |
| CABG + AVR/MVR (%) | 70 (14.3%) | 9 (20.0%) | 61 (13.7%) | |
| AVR/MVR (%) | 106 (21.6%) | 11 (24.5%) | 95 (21.3%) | |
| Aortic root (%) | 11 (2.2%) | 1 (2.2%) | 10 (2.2%) | |
| LVAD/aortic (%) | 27 (5.5%) | 4 (8.9%) | 23 (5.2%) | |
| Intraoperative transfusion (%) | 244 (49.6%) | 23 (51.1%) | 221 (49.6%) | 0.872 |
| CPB duration (min)† | 112.7 (49.1) (108.4–117.1) | 125.7 (57.4) (108.0–143.0) | 111.4 (48.0) (106.9–115.9) | 0.063 |
| Cross clamp (min)† | 67.9 (32.3) (65.0–70.8) | 72.5 (33.3) (62.5–82.5) | 67.5 (32.2) (64.5–70.5) | 0.322 |
| Average rScO2 during CPB | ||||
| Left (%) | 54 (9.1) (53.0–54.8) | 52 (9.5) (48.6–54.8) | 54 (9.0) (53.2–55.0) | 0.125 |
| Right (%) | 55 (9.8) (53.6–55.5) | 52 (10.1) (48.8–55.4) | 55 (9.8) (53.8–55.8) | 0.105 |
| Product of magnitude × duration of MAP < lower limit of autoregulation (mm Hg h)‡ | 3.448 (1.154–9.696) | 3.542 (1.154–8.258) | 3.433 (1.157–9.871) | 0.849 |
| Product of magnitude × duration of MAP >ULA (mm Hg h)‡ | 0.422 (0–2.588) | 1.693 (0.450–5.720) | 0.315 (0–2.114) | <0.001 |
Table 2.
Postoperative outcomes. *Percentage calculated out of patients who were not on dialysis preoperatively. AKI, acute kidney injury as defined by the RIFLE criteria; IABP, intra-aortic balloon pumping; low cardiac output syndrome, inotrope use >24 h or new requirement for intra-aortic balloon pump insertion. Patients meeting any of the RIFLE criteria were considered to have AKI
| Outcomes | Entire population (n=491) | Delirium (n=45) | No delirium (n=446) | P-value |
|---|---|---|---|---|
| Re-exploration for bleeding (%) | 19 (3.8%) | 1 (2.2%) | 18 (4.0%) | >0.999 |
| Postoperative transfusion (%) | 260 (53.0%) | 29 (64.4%) | 231 (51.8%) | 0.118 |
| AKI within 7 days* (%) | 183 (38.4%) | 17 (39.5%) | 166 (38.3%) | 0.871 |
| Mortality (%) | 24 (4.9%) | 5 (11.1%) | 19 (4.3%) | 0.058 |
| Low cardiac output syndrome (%) | 61 (12.4%) | 9 (20.0%) | 52 (11.7%) | 0.150 |
| Stroke (%) | 21 (4.3%) | 6 (13.3%) | 15 (3.4%) | <0.001 |
| Sepsis (%) | 12 (2.4%) | 3 (6.7%) | 9 (2.0%) | 0.880 |
| Inotropic drug >24 h (%) | 55 (11.2%) | 9 (20.0%) | 46 (10.3%) | 0.077 |
| Mechanical ventilation >48 h (%) | 39 (7.9%) | 11 (24.4%) | 28 (6.3%) | <0.001 |
| New IABP insertion (%) | 23 (4.7%) | 3 (6.7%) | 20 (4.5%) | 0.458 |
| New dialysis (%) | 10 (2.0%) | 2 (4.4%) | 8 (1.8%) | 0.231 |
| 30-day mortality (%) | 24 (4.9%) | 5 (11.1%) | 19 (4.3%) | 0.058 |
| Hospital stay in days (median) | 7 | 10 | 7 | <0.001 |
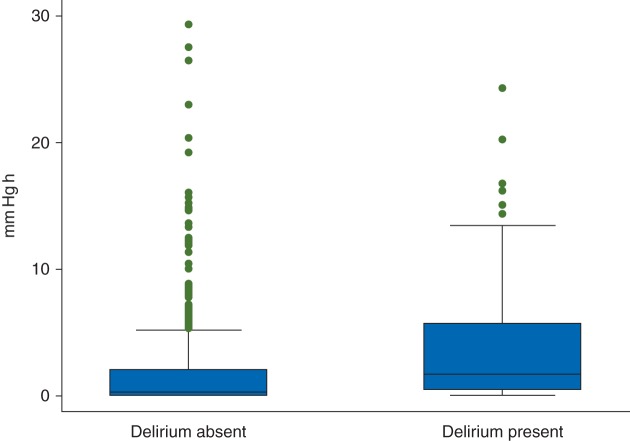
The sum of the product of the magnitude and duration of MAP above an ULA (mm Hg h) was higher for patients who had postoperative delirium than for those without delirium (Fig. 2). However, the sum of the product of the magnitude and duration of MAP below the lower limit of autoregulation did not differ between the two groups. Notably, when we considered crude MAP cutoffs irrespective of autoregulation limits, we found no significant difference between groups in the sum of the product of the magnitude and duration of MAP >80, >85, >90, >95, >100, or >105 mm Hg (Table 3). That is, the relationship between MAP and delirium was present only when MAP was above an ULA and not for any given MAP alone.
Fig 2.
Box and whiskers plots comparing the duration and magnitude of arterial pressure above an ULA (mm Hg h) during CPB for patients who did and did not develop postoperative delirium (P < 0.01). The horizontal line in the shaded box represents the median value, and the shaded box represents the inter-quartile range. The error bars below and above the shaded area represent (1.5×) the inter-quartile range; points beyond the error bar are outliers.
Table 3.
The product of the magnitude and duration of MAP above selected cutoffs (mm Hg h). *The cutoffs represent raw MAP irrespective of the upper limits of autoregulation. Data are presented as median (inter-quartile range)
| MAP cutoff* | Delirium (n=45) | No delirium (n=446) | P-value |
|---|---|---|---|
| MAP >80 mm Hg | 8.71 (3.66–15.27) | 5.99 (2.90–11.50) | 0.120 |
| MAP >85 mm Hg | 4.96 (1.45–9.69) | 3.01 (1.18–6.76) | 0.143 |
| MAP >90 mm Hg | 2.23 (0.44–4.80) | 1.36 (0.44–3.39) | 0.196 |
| MAP >95 mm Hg | 0.89 (0.13–2.40) | 0.58 (0.10–1.66) | 0.298 |
| MAP >100 mm Hg | 0.30 (0.05–0.86) | 0.24 (0–0.75) | 0.321 |
| MAP >105 mm Hg | 0.08 (0–0.28) | 0.07 (0–0.31) | 0.634 |
Variables entered into the multivariate logistic regression model included age, prior stroke, duration of CPB, MAP excursions above the upper limit of cerebral autoregulation, MAP excursions below the lower limit of cerebral autoregulation, mechanical ventilation for >48 h after surgery, history of CHF, prior cerebral vascular event, and usage of antidepressant drugs. Factors that had a statistically significant association with risk for delirium after surgery included mechanical ventilation for >48 h after surgery, preoperative antidepressant drug use, prior stroke, CHF, the product of the magnitude and duration of MAP above the upper limit of cerebral autoregulation, and patient age (Table 4). These results suggest that for each unit change in normalized product of the duration and magnitude of MAP above an ULA (mm Hg h), the odds of developing delirium are increased from 3 to 15%.The area under the receiver operator characteristic curve for the logistic model was 0.789 (95% CI, 0.719–0.859). There was no interaction between the sum of the product of the magnitude and duration of MAP above an ULA and other variables, including preoperative systolic arterial pressure, preoperative MAP, and age, so we did not include these interactions in the final model. Finally, we assessed the multivariate model's performance with cross-validation. The cross-validated receiver operator characteristic curve was 0.75 (95% CI, 0.68–0.83).
Table 4.
Variables that had statistically independent association with postoperative delirium based on logistic regression model. MAP, mean arterial pressure; ULA, upper limit of cerebral autoregulation; OR, odds ratio. *For each additional year of age
| Variables | OR | 95% CI | P-value |
|---|---|---|---|
| Mechanical ventilation >48 h | 3.94 | 1.72–9.03 | 0.001 |
| Antidepressant drug | 3.00 | 1.29–6.96 | 0.011 |
| Prior stroke | 2.79 | 1.12–6.96 | 0.027 |
| CHF | 2.68 | 1.28–5.62 | 0.009 |
| Magnitude of MAP above ULA (mm Hg h) | 1.09 | 1.03–1.15 | 0.001 |
| Age* | 1.04 | 1.01–1.072 | 0.030 |
Discussion
We found that the sum of the product of the magnitude and duration of MAP above the upper threshold of cerebral autoregulation during CPB had a statistically significant association with risk for delirium. Other factors associated with delirium included mechanical lung ventilation >48 h, preoperative use of antidepressant drugs, prior stroke, history of CHF, and patient age.
Delirium is an acute disorder of awareness and attention that has a fluctuating course associated with additional new cognitive problems, including disorientation and memory problems unexplained by other causes such as pre-existing dementia. It is characterized by inattention, impaired cognition, and altered perception.14 Because postoperative delirium can lead to increased mortality, longer hospital length of stay, long-term cognitive decline, and other complications, researchers have considerable interest in developing strategies to reduce its frequency.14,15 Basing delirium diagnosis on only clinical signs observed by nurses and physicians, as we did in this study, is likely to underestimate the true frequency of delirium compared with careful assessment by a psychiatrist or with a structured instrument.14,22,23 Nonetheless, we have found in this and other studies that delirium identified by our method is associated with other adverse events, including increased morbidity, length of hospitalization, and mortality.24 Thus, measures to reduce the frequency of this form of delirium might provide a strategy to improve patient outcomes.
The role of arterial pressure aberrations in the aetiology of postoperative delirium is not clear. The small number of studies that have addressed this issue have focused on hypotension, produced conflicting findings, varied in the timing of arterial pressure recordings (e.g. every 5 min in some studies), focused on non-cardiac surgery, had small number of patients, and were associated with other limitations.25,26 Our work showing wide variability in the lower limit of autoregulation suggests that defining hypotension with arbitrary arterial pressure cutoffs is an imprecise method for determining the relationship between arterial pressure and delirium. By using a more precise method of determining autoregulation thresholds, we found that MAP below the lower limit of autoregulation was not associated with delirium but that MAP above ULA during CPB was associated with the risk for delirium. Notably, we found no relationship between MAP and delirium when MAP was analysed based on simple MAP cutoffs from 80 to 105 mm Hg irrespective of the upper autoregulation limit. This finding suggests that it is not MAP alone that increases risk for delirium, but rather MAP above the vasomotor constraints of autoregulation. It is possible that MAP above the upper threshold of autoregulation might lead to an increase in CBF that exceeds cerebral metabolic demand, resulting in excessive cerebral microembolic load, endothelial damage, and compromise to the blood–brain barrier. These aberrations expose patients to neuroinflammation, which has been suggested to contribute to delirium susceptibility.14 A similar mechanism has been proposed to explain acute delirium associated with hypertensive emergencies.16 An interesting finding of our study was that the mean preoperative MAP [93(14)] was similar to the MAP at an ULA measured during general anaesthesia and CPB [90(12) mm Hg]. Arterial pressure obtained immediately before surgery when the patient is under stress likely does not reflect true baseline arterial pressure. It is further likely that the conditions associated with CPB, including general anaesthesia, haemodilution, a non-pulsatile circulation, and systemic inflammation, could have affected the actual ULA observed and/or its relationship with subsequent risk for delirium.
Several investigations have noted an association between pre-existing depression and risk for delirium after cardiac surgery.27,28 The mechanistic link for this relationship is not known, but Kazmierski and colleagues28 have proposed that activation of the hypothalamic-pituitary axis leading to high cortisol levels might be the cause. We found that anti-depressant usage before surgery increased risk for delirium nearly four-fold. Because imbalance of central nervous system neurotransmission is believed to be one possible mechanism of delirium, our findings suggest that preoperative use of antidepressants and/or failure to restart this therapy early after surgery might contribute to risk for delirium.14 However, the use of anti-depressant medication might simply identify patients with other comorbidities that increase the risk for adverse outcomes. In a retrospective study of data collected from 375 US hospitals, patients who were undergoing major non-cardiac surgery and taking selective serotonin reuptake inhibitors were more likely to have obesity, chronic pulmonary disease, and hypothyroidism.29 Even after statistically adjusting for these and other factors, the use of selective serotonin reuptake inhibitors was associated with a higher risk for in-hospital mortality, bleeding, and hospital readmission within 30 days of the initial surgery.29
Other factors found to have a statistically significant association with delirium were consistent with those observed in other studies, including prior stroke and age.14,22 The presence of CHF might indicate individuals with elevations in baseline cytokine levels that might be exacerbated by the heightened inflammatory responses to surgical trauma and CPB. This increase in cytokines might contribute to neuroinflammation and susceptibility to delirium.14,30,31 Prolonged mechanical ventilation might signify patients who are more critically ill, and such patients might receive sedation with benzodiazepines, a drug class linked to postoperative delirium.32
To our knowledge, this is the largest series to date of patients who have had cerebral autoregulation measured continuously during CPB. However, our study is associated with several limitations. The lack of a reference standard patient interview by a psychiatrist or clinically trained researcher in the assessment for delirium is an important limitation. Relying on bedside nurse identification has been reported to have low sensitivity and high specificity for diagnosis of delirium, but it might under-recognize hypoactive forms of delirium.33 Further, the more complex medical conditions in patients who developed delirium could conceivably have resulted in the need for more intensive nursing care. If nurses observed such patients more closely than they observed the less complex patients, they might have been more likely to identify delirium in them, producing bias in the data. Regardless, our results could be biased in favour of patients with hyperactive forms of delirium. Nonetheless, assessments for delirium throughout the day by nurses allowed for continuous observation and detection of waxing and waning symptoms. Moreover, monitoring patients during every day of their hospitalization may have provided better delirium detection than a one-time assessment. Although our current delirium detection method might have missed some patients with subclinical delirium, the identified group had significantly worse outcomes and adverse events than non-delirious patients, including increased morbidity, length of hospitalization, and mortality.24 However, our results must be considered in the context of the relatively small number of cases of delirium that we observed. As a result, our multivariate model, though significant, might have been ‘over-fitted’, which could impact the external validity of our findings.
Vasoconstriction of scalp blood vessels after administration of drugs such as phenylephrine could confound the accuracy of rScO2 measurements.34,35 Scalp blood flow, however, is not autoregulated. Since COx measures the relationship between low-frequency changes in rScO2 and mean arterial pressure, the administration of vasoconstrictors properties should have minimal effects on COx.
In previous laboratory and clinical investigations,6,18 we have validated the use of COx for autoregulation monitoring and shown that a COx >0.3 has the best sensitivity and specificity for identifying the limits of autoregulation.21 Nonetheless, any COx cutoff value is somewhat arbitrary compared with the more precise method of monitoring CBF while raising or lowering arterial pressure by pharmacological or mechanical means. However, the ethics of such an approach would be questionable because cardiac patients have a high frequency of cerebral vascular disease and risk factors for neurological complications. In clinical practice, the optimal MAP can be chosen based on the pressure associated with the most preserved autoregulation rather than on a priori cutoffs.36 Because our results are based on observational data, however, they must be viewed only as an association. They do not necessarily support the prediction that pharmacologically maintaining MAP within the autoregulation range will reduce the frequency of delirium.
Our current findings, together with our previous results showing a relationship between a MAP below the lower limit of autoregulation and adverse patient outcomes, suggest that both ‘low’ and ‘high’ MAP during CPB can have adverse effects.5–9 These findings challenge the existing standard of care whereby MAP targets are chosen empirically during CPB. Optimizing MAP during CPB to remain within the cerebral autoregulation range might provide a strategy for modifying the risk for adverse patient outcomes, including postoperative delirium.
Authors' contributions
D.H., M.O., T.R., C.B., and C.W.H.: study design, patient recruitment, data collection, data analysis, and writing first draft of the paper; H.A., A.G., R.G., K.J.N., and F.S.: data analysis and writing first draft of the paper.
Funding
Funded in part by grant-in-aid number 103363 from the Mid-Atlantic Affiliate of the American Heart Association (C.W.H.); grants R01HL092259 (C.W.H.), R01AG033615 (F.S.), and R01AG029656-01A1 (F.S.) from the National Institutes of Health; Mentored Career Development Award 5KL2RR025006 and R03 AG042331 from the National Institutes of Health (C.B.); and the Jahnigen Career Development Award (C.B.).
Declaration of interest
D.H. has received funding from the Japan Heart Foundation/Bayer Yakuhin Research Grant Abroad; C.W.H. has received research funding from Covidien, Inc. (Boulder, CO, USA), the makers of the near-infrared spectroscopy monitors used in this study.
Acknowledgement
We wish to thank Claire Levine for her editorial assistance in preparation of this manuscript.
References
- 1.Hogue CW, Jr, Palin CA, Arrowsmith JE. Cardiopulmonary bypass management and neurologic outcomes: an evidence-based appraisal of current practices. Anesth Analg. 2006;103:21–37. doi: 10.1213/01.ANE.0000220035.82989.79. [DOI] [PubMed] [Google Scholar]
- 2.Stockard JJ, Bickford RG, Schauble JF. Pressure-dependent cerebral ischemia during cardiopulmonary bypass. Neurology. 1973;23:521–9. doi: 10.1212/wnl.23.5.521. [DOI] [PubMed] [Google Scholar]
- 3.Greeley WJ, Ungerleider RM, Kern FH, Brusino FG, Smith LR, Reves JG. Effects of cardiopulmonary bypass on cerebral blood flow in neonates, infants, and children. Circulation. 1989;80:I209–15. [PubMed] [Google Scholar]
- 4.Taylor KM. The hemodynamics of cardiopulmonary bypass. Sem Thorac Cardiovasc Surg. 1990;2:300–12. [PubMed] [Google Scholar]
- 5.Joshi B, Ono M, Brown C, et al. Predicting the limits of cerebral autoregulation during cardiopulmonary bypass. Anesth Analg. 2012;114:503–10. doi: 10.1213/ANE.0b013e31823d292a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brady K, Joshi B, Zweifel C, et al. Real-time continuous monitoring of cerebral blood flow autoregulation using near-infrared spectroscopy in patients undergoing cardiopulmonary bypass. Stroke. 2010;41:1951–6. doi: 10.1161/STROKEAHA.109.575159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ono M, Joshi B, Brady K, et al. Risks for impaired cerebral autoregulation during cardiopulmonary bypass and postoperative stroke. Br J Anaesth. 2012;109:391–8. doi: 10.1093/bja/aes148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ono M, Arnaoutakis GJ, Fine DM, et al. Blood pressure excursions below the cerebral autoregulation threshold during cardiac surgery are associated with acute kidney injury. Crit Care Med. 2013;41:464–71. doi: 10.1097/CCM.0b013e31826ab3a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ono M, Brady K, Easley RB, et al. Duration and magnitude of blood pressure below cerebral autoregulation threshold during cardiopulmonary bypass is associated with major morbidity and operative mortality. J Thorac Cardiovasc Surg. 2014;147:483–9. doi: 10.1016/j.jtcvs.2013.07.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gold JP, Charlson ME, Williams-Russo P, et al. Improvement of outcomes after coronary artery bypass. A randomized trial comparing intraoperative high versus low mean arterial pressure. J Thorac Cardiovasc Surg. 1995;110:1302–11. doi: 10.1016/S0022-5223(95)70053-6. [DOI] [PubMed] [Google Scholar]
- 11.Henriksen L, Hjelms E, Lindeburgh T. Brain hyperperfusion during cardiac operations. Cerebral blood flow measured in man by intra-arterial injection of xenon 133: evidence suggestive of intraoperative microembolism. J Thorac Cardiovasc Surg. 1983;86:202–8. [PubMed] [Google Scholar]
- 12.Plochl W, Cook DJ. Quantification and distribution of cerebral emboli during cardiopulmonary bypass in the swine: the impact of PaCO2. Anesthesiology. 1999;90:183–90. doi: 10.1097/00000542-199901000-00024. [DOI] [PubMed] [Google Scholar]
- 13.Harris DN, Bailey SM, Smith PL, Taylor KM, Oatridge A, Bydder GM. Brain swelling in first hour after coronary artery bypass surgery. Lancet. 1993;342:586–7. doi: 10.1016/0140-6736(93)91412-f. [DOI] [PubMed] [Google Scholar]
- 14.Inouye SK. Delirium in older persons. N Engl J Med. 2006;354:1157–65. doi: 10.1056/NEJMra052321. [DOI] [PubMed] [Google Scholar]
- 15.Saczynski JS, Marcantonio ER, Quach L, et al. Cognitive trajectories after postoperative delirium. N Engl J Med. 2012;367:30–9. doi: 10.1056/NEJMoa1112923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Aggarwal M, Khan IA. Hypertensive crisis: hypertensive emergencies and urgencies. Cardiol Clin. 2006;24:135–46. doi: 10.1016/j.ccl.2005.09.002. [DOI] [PubMed] [Google Scholar]
- 17.Joshi B, Brady K, Lee J, et al. Impaired autoregulation of cerebral blood flow during rewarming from hypothermic cardiopulmonary bypass and its potential association with stroke. Anesth Analg. 2010;110:321–8. doi: 10.1213/ANE.0b013e3181c6fd12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brady KM, Lee JK, Kibler KK, et al. Continuous time-domain analysis of cerebrovascular autoregulation using near-infrared spectroscopy. Stroke. 2007;38:2818–25. doi: 10.1161/STROKEAHA.107.485706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Levey AS, Coresh J, Greene T, et al. Expressing the Modification of Diet in Renal Disease Study equation for estimating glomerular filtration rate with standardized serum creatinine values. Clin Chem. 2007;53:766–72. doi: 10.1373/clinchem.2006.077180. [DOI] [PubMed] [Google Scholar]
- 20.Lassnigg A, Schmid ER, Hiesmayr M, et al. Impact of minimal increases in serum creatinine on outcome in patients after cardiothoracic surgery: do we have to revise current definitions of acute renal failure? Crit Care Med. 2008;36:1129–37. doi: 10.1097/CCM.0b013e318169181a. [DOI] [PubMed] [Google Scholar]
- 21.Brady KM, Mytar JO, Kibler KK, et al. Noninvasive autoregulation monitoring with and without intracranial pressure in the naive piglet brain. Anesth Analg. 2010;111:191–5. doi: 10.1213/ANE.0b013e3181e054ba. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sieber FE. Postoperative delirium in the elderly surgical patient. Anesthesiol Clin. 2009;27:451–64. doi: 10.1016/j.anclin.2009.07.009. table of contents. [DOI] [PubMed] [Google Scholar]
- 23.Simon SE, Bergmann MA, Jones RN, Murphy KM, Orav EJ, Marcantonio ER. Reliability of a structured assessment for nonclinicians to detect delirium among new admissions to postacute care. J Am Med Dir Assoc. 2006;7:412–5. doi: 10.1016/j.jamda.2006.02.006. [DOI] [PubMed] [Google Scholar]
- 24.Gottesman RF, Grega MA, Bailey MM, et al. Delirium after coronary artery bypass graft surgery and late mortality. Ann Neurol. 2010;67:338–44. doi: 10.1002/ana.21899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Williams-Russo P, Sharrock NE, Mattis S, et al. Randomized trial of hypotensive epidural anesthesia in older adults. Anesthesiology. 1999;91:926–35. doi: 10.1097/00000542-199910000-00011. [DOI] [PubMed] [Google Scholar]
- 26.Marcantonio ER, Goldman L, Orav EJ, Cook EF, Lee TH. The association of intraoperative factors with the development of postoperative delirium. Am J Med. 1998;105:380–4. doi: 10.1016/s0002-9343(98)00292-7. [DOI] [PubMed] [Google Scholar]
- 27.Rudolph JL, Jones RN, Levkoff SE, et al. Derivation and validation of a preoperative prediction rule for delirium after cardiac surgery. Circulation. 2009;119:229–36. doi: 10.1161/CIRCULATIONAHA.108.795260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kazmierski J, Banys A, Latek J, Bourke J, Jaszewski R. Cortisol levels and neuropsychiatric diagnosis as markers of postoperative delirium: a prospective cohort study. Crit Care. 2013;17:R38. doi: 10.1186/cc12548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Auerbach AD, Vittinghoff E, Maselli J, Pekow PS, Young JQ, Lindenauer PK. Perioperative use of selective serotonin reuptake inhibitors and risks for adverse outcomes of surgery. JAMA Intern Med. 2013;173:1075–81. doi: 10.1001/jamainternmed.2013.714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gullestad L, Ueland T, Vinge LE, Finsen A, Yndestad A, Aukrust P. Inflammatory cytokines in heart failure: mediators and markers. Cardiology. 2012;122:23–35. doi: 10.1159/000338166. [DOI] [PubMed] [Google Scholar]
- 31.Levy JH, Tanaka KA. Inflammatory response to cardiopulmonary bypass. Ann Thorac Surg. 2003;75:S715–20. doi: 10.1016/s0003-4975(02)04701-x. [DOI] [PubMed] [Google Scholar]
- 32.Pandharipande PP, Pun BT, Herr DL, et al. Effect of sedation with dexmedetomidine vs lorazepam on acute brain dysfunction in mechanically ventilated patients: the MENDS randomized controlled trial. JAMA. 2007;298:2644–53. doi: 10.1001/jama.298.22.2644. [DOI] [PubMed] [Google Scholar]
- 33.Inouye SK, Foreman MD, Mion LC, Katz KH, Cooney LM., Jr Nurses’ recognition of delirium and its symptoms: comparison of nurse and researcher ratings. Arch Intern Med. 2001;161:2467–73. doi: 10.1001/archinte.161.20.2467. [DOI] [PubMed] [Google Scholar]
- 34.Meng L, Cannesson M, Alexander BS, et al. Effect of phenylephrine and ephedrine bolus treatment on cerebral oxygenation in anaesthetized patients. Br J Anaesth. 2011;107:209–17. doi: 10.1093/bja/aer150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cho SY, Kim SJ, Jeong CW, et al. Under general anesthesia arginine vasopressin prevents hypotension but impairs cerebral oxygenation during arthroscopic shoulder surgery in the beach chair position. Anesth Analg. 2013;117:1436–43. doi: 10.1213/ANE.0b013e3182a8fa97. [DOI] [PubMed] [Google Scholar]
- 36.Aries MJ, Czosnyka M, Budohoski KP, et al. Continuous determination of optimal cerebral perfusion pressure in traumatic brain injury. Crit Care Med. 2012;40:2456–63. doi: 10.1097/CCM.0b013e3182514eb6. [DOI] [PubMed] [Google Scholar]