Abstract
Single blastomere removal from cleavage-stage embryos, a common procedure used in conjunction with preimplantation genetic diagnosis (PGD), may affect reproductive outcomes. We hypothesized that negative pregnancy outcomes associated with PGD may be due to impairment of placental signaling pathways. The goal of this study was to determine the molecular mechanisms through which placental signaling is deregulated by blastomere removal. Four-cell stage murine embryos produced by in vitro fertilization were subjected to removal of a single blastomere (biopsied) or to the same manipulations without the blastomere removal (controls). Placental tissues from term (18.5 day) pregnancies obtained after embryo transfer were tested for levels of nitrosative species, interleukin 6, signal transducers and activators of transcription (STAT) 1 and 3, suppressors of cytokine signaling (SOCS) 1, 2 and 3 and matrix metalloproteinases (MMP) 1, 2, 3 and 9. Significant increases in nitrosative stress (P < 0.05), phosphorylative activation of STAT1 (P < 0.05) but not STAT3, lower levels of the inhibitors SOCS2 (P < 0.01) and SOCS3 (P < 0.001) and activation of MMP9 (P < 0.001) were observed in placentas derived from biopsied embryos, compared with controls. Such effects could contribute to greater levels of premature membrane rupture, incorrect parturition, preterm birth and intrauterine growth restriction associated with PGD. This work has determined signaling mechanisms that may be responsible for blastomere removal effects on placental function, with the potential to become targets for improving obstetric and neonatal outcomes in assisted reproduction.
Keywords: assisted reproduction technology, in vitro fertilization, suppressors of cytokine signaling
Introduction
The use of assisted reproductive technologies (ART) for conception has nearly doubled in the last decade as more couples face infertility (National Center for Chronic Disease Prevention and Health Promotion, 2011). Following IVF or ICSI, embryos may undergo preimplantation genetic diagnosis (PGD). For the PGD, one or two blastomeres are removed from the cleavage-stage embryo to screen for genetic abnormalities. However, biopsy at the blastocyst stage can also be employed which allows the selection of the best embryo in vitro, and avoids mosaicism at the 8-cell stage (Capalbo et al., 2013). Although cleavage-stage biopsy is no longer common in the clinic, there have been thousands of children conceived this way, and these studies present an avenue to explain any outcomes observed in this population. It is not clear whether this widely used and successful method of embryo analysis contributes to congenital abnormalities or syndromes associated with ART and/or in conjunction with the underlying causes of infertility (Goossens et al., 2012). However, recent studies of the effects of PGD and the outcomes of the procedure indicate that it may increase several negative pregnancy syndromes (Grace and Sinclair, 2009, Liebaers et al., 2010).
One reproductive tissue that blastomere removal procedures may affect is the developing placenta. The placenta plays a pivotal role in pregnancy by functioning as a semi-permeable barrier through which nutrients, gases and wastes are transferred between the maternal and fetal systems (Pelkonen et al., 2006). It also has vital metabolic and endocrine roles to aid in the growth, development and protection of the fetus (Syme et al., 2004; Porterfield and White, 2007). Placental development is tightly regulated. During placentation, fetal-derived cells invade the endometrium of the uterus for implantation and this is mediated by several processes (Agarwal et al., 2005). Cytokines, such as interleukin 6 (IL-6), are secreted by the uterine cells and later by the placenta, to aid efficient blastocyst implantation, placental growth and development, and to maintain pregnancy (Guzeloglu-Kayisli et al., 2009). Oxidative and nitrosative species also support blastocyst implantation by causing decidualization of the endometrium (Agarwal et al., 2005). In addition, secretion of proteolytic matrix metalloproteinase (MMP) enzymes is critical for the degradation and remodeling of extracellular matrices that allow for blastocyst invasion during placentation. Later in pregnancy, these MMPs play important roles during parturition (Cohen et al., 2006). Both IL-6 and nitrosative stress can regulate the secretion and activation of MMPs in the placenta, in part by mitigating negative regulation of MMPs through suppressors of cytokine signaling (SOCS) pathways (Meisser et al., 1999; Capobianco et al., 2012).
Some negative outcomes that have been associated with ART may be due to abnormal placental characteristics (Zhang et al., 2011; Haavaldsen et al., 2012). Increased incidences of pre-eclampsia, placental abruption, placenta previa, premature rupture of membranes and differential expression of genes and proteins have been observed in ART-derived human pregnancies (Källén et al., 2005; Romundstad et al., 2006; Zhang et al., 2008; Collier et al., 2009; Haavaldsen et al., 2012). Previous studies from our laboratories have shown dysregulation of placental UDP-glucuronosyl transferase genes, impaired placental steroid metabolism and transport, and greater placental oxidative stress and inflammation in the mouse model of IVF and ICSI (Collier et al., 2009; Raunig et al., 2011a, b; Collier et al., 2012). These studies considered maternal, placental and fetal outcomes from mouse pregnancies achieved by normal mating when compared with those derived after IVF versus ICSI. More recently, we have demonstrated that blastomere removal from cleavage-stage mouse embryos specifically leads to abnormal steroidogenesis and impaired steroid clearance in placental and fetal tissues (Sugawara et al., 2012). We speculated that the mechanisms of these impaired placental functions may involve nitrosative and oxidative stress including specific signaling pathways known to regulate placental functions. To determine the veracity of this, we used exactly the same tissues as in the 2012 study. This allowed us to test specifically for the effects of blastomere removal in a tightly controlled manner. Although ultimately it would be interesting to compare pregnancies achieved after PGD with natural conception, this would require a complex design accommodating for various aspects associated with PGD (ovarian stimulation, fertilization in vitro, embryo culture, manipulation and transfer) and as such was not pursued at this point.
We have shown that blastomere removal increases nitrosative stress, activates signal transducers and activators of transcription (STAT) signaling concurrent with the reduction in negative feedback by suppressors of cytokine signaling (SOCS) and dysregulates MMP enzymes by increasing active (cleaved) MMP9 and latent MMP2 and MMP3 proteins. Although there are very few reports on specific pregnancy outcomes attributed to PGD and/or embryo biopsy, specific adverse effects including impaired implantation of human embryos after cleavage-stage (but not blastocyst) biopsy have been reported (Scott et al., 2013). Moreover, after PGD it is more common for invasive prenatal screening (such as chorionic villous sampling) to be used to confirm the preimplantation results, which puts the mother and child at increased risk (Audibert et al., 2009). Hence, more research is needed to confirm the safety and effectiveness of this type of screening for pregnancy and birth outcomes.
Materials and Methods
Unless otherwise specified, reagents and chemicals were obtained from Sigma Chemical Company (St Louis, MO, USA). Equine chorionic gonadotrophin (eCG) and hCG were bought from Calbiochem (Spring Valley, CA, USA).
Animals
Mice B6D2F1 (C57BL/6 × DBA/2) and Swiss Webster were obtained from the National Cancer Institute (Raleigh, NC, USA) at 6–8 weeks of age. Oocytes and sperm were collected from B6D2F1 mice and used for IVF to generate embryos that at the 4-cell stage underwent either blastomere biopsy or sham biopsy (puncturing of the zona pellucida with no blastomere removal) as previously described (Sugawara et al. 2012). Swiss Webster mice were used as surrogate dams and were mated with vasectomized males to induce pseudopregnancy before embryo transfer.
Mice were fed ad libitum with a standard diet and maintained in a temperature and light-controlled room (22 °C, 14 h light/10 h dark), in accordance with the guidelines presented in National Research Council's (NRC) ‘Guide for Care and Use of Laboratory Animals’ published by the Institute for Laboratory Animal Research of the National Academy of Science, Bethesda, MD, 2011. The protocol for animal handling and treatment procedures was reviewed and approved by the Animal Care and Use Committee at the University of Hawaii.
In vitro fertilization and embryo biopsy
Procedures for IVF, embryo culture, biopsy and transfer, have been described in detail by us previously (Sugawara et al., 2012). Medium T6 (Quinn et al., 1982) was used for IVF and HEPES-buffered Chatot, Zimek and Bavister's medium (HEPES-CZB (Chatot et al., 1989, Kimura and Yanagimachi, 1995) was used for gamete handling and embryo micromanipulation. Medium CZB (Chatot et al., 1989) was used for embryo culture. Both CZB and T6 were maintained in an atmosphere of 5% CO2 in air and HEPES-CZB was maintained in air.
Oocytes were collected from females induced to superovulate with injections of 5 iu eCG and 5 iu hCG given 48 h apart. Epididymal sperm were collected by release from cauda epididymides directly into T6 medium and were capacitated for 1.5 h at 37°C in a humidified atmosphere of 5% CO2. The gametes were co-incubated for 4 h. After gamete co-incubation, oocytes were washed with HEPES-CZB, followed by at least one wash with CZB medium. Only morphologically normal oocytes were selected for culture. We have previously compared CZB and KSOM media in culture of embryos derived from hybrid B6D2F1 mice (used in this study) and did not observe significant differences in the proportions of embryos that developed to blastocysts in vitro and live offspring after embryo transfer. In the past, we tested KSOM for its compatibility with assisted reproduction (ICSI, nuclear transfer) and found that it does not work well when supplemented with HEPES and/or depleted in Ca2+/Mg2+, which are the media variations necessary for injections and/or embryo manipulations.
Fertilized eggs (zygotes with two well-developed pronuclei and extruded second polar body) were cultured in 50 µl drops of CZB medium pre-equilibrated overnight with humidified 5% CO2 in air. After ∼48 h of culture, 4-cell embryos were transferred into Ca2+- and Mg2+-free CZB for 10–20 min to disrupt cell adhesion, and were then transferred to microdrops of Ca2+- and Mg2+-free HEPES-CZB on the micromanipulation dish. Blastomere removal was performed using Eppendorf micromanipulators (Micromanipulator TransferMan, Eppendorf, Hamburg, Germany) with a Piezo-electric actuator (PMM Controller, model PMAS-CT150, PrimeTech, Tsukuba, Japan). The zona pellucida was penetrated with a micropipette (20 µm ID) and one blastomere was aspirated. Control (sham-biopsied) embryos from the same IVF cohorts were subjected to exactly the same manipulations and culture conditions as biopsied embryos but the blastomeres were not removed. Biopsied and control embryos were cultured under the same conditions for subsequent 24 h until they developed to morula/early blastocyst stage. The embryos were then transferred into the oviducts (2–8 per oviduct) of Swiss Webster females mated during the previous night with vasectomized Swiss Webster males. Biopsied and control embryos were transferred into separate females. The 4-cell stage embryo biopsy was chosen because it represents best the 6–8-stage embryo biopsy most commonly used in humans. The 4-cell mouse embryos are not yet compacted and are finalizing the process of embryonic genome activation. Performing biopsy during or after compaction is likely to have negative effects on the developing embryos. In humans, compaction does not start until the 16–32-cell stage, and embryonic genome activation takes place at the 4–8-cell stage.
Tissue collection and processing
Placental tissue was collected after caesarian section on Day 18.5 of gestation. Samples were washed briefly in Dulbecco phosphate-buffered saline (D-PBS) before being placed singly into tubes. Tissues were kept on ice for up to 30 min during collection process and subsequently frozen at −80°C until further processing. Upon processing, placentas were thawed, their wet weight was recorded and they were homogenized 1:4 w:v in 0.1 M Tris-HCl buffer containing 5 mM MgCl2 and 2 mM PMSF (pH 7.4) using a hand-held Tissue Tearor rotor-stator for 30 s (Biospec, Bartlesville, OK, USA). Tissue homogenates were then divided in two and one half was centrifuged at 10 000 × g for 20 min at 4°C to obtain S9 fractions, devoid of mitochondria, nuclei and cellular cytoskeleton. Homogenates and S9 fractions were aliquoted and frozen at −80°C until use. Protein concentration was measured with the Bicinchoninic acid method (Smith et al., 1985) and was adjusted to a normalized value prior to use in assays.
Interleukin 6
Levels of IL-6 were detected using a commercially available mouse tissue ELISA (eBioscience, San Diego, CA, USA) as per the manufacturer's instructions. Briefly, standards or placental lysates (100 µl, 1 mg/ml) were assayed in duplicate and accepted if variation is ≤15%. ELISA was accepted if the standard curve fitted the four-parameter logistic regression (Hill equation) with an R2 ≥ 0.98.
Total nitrosative species (nitrates and nitrites)
Total nitrosative species were detected in placental tissue lysates using a nitrate/nitrite colorimetric assay as per the manufacturer's instructions. Both nitrate and nitrite species were assayed (Cayman Chemical Company, Ann Arbor, MI, USA). Levels of nitrosative species were transformed to µmol/mg protein using a standard curve generated with nitrate and nitrite standards provided in the kit.
Activated (phosphorylated) STAT1 and STAT3
Levels of phosphorylated STAT1 and STAT3 were detected with commercial ELISA (Cell Signaling Technology, Danvers, MA, USA and eBioscience, San Diego, CA, USA respectively). Placental lysates (1 mg/ml) were added to wells of a 96-well plate and the assays carried out as per the manufacturer's instructions.
Analysis of MMP 1, 2, 3, 9 and SOCS1, 2, 3 proteins by western blot
Western blotting was performed as previously described by us (Collier et al., 2012). Briefly, murine placental lysates (5 µg) and positive human liver controls (2 µg, Xenotech, KS, USA) were resolved on denaturing 10% SDS-polyacrylamide gels, transferred to PVDF membrane with semi-dry transfer (Bio-Rad Laboratories, Hercules, CA, USA) and blocked in phosphate-buffered saline with Tween 20 (PBS-T) with 5% nonfat milk powder (MMP antibodies) or 2% fish skin gelatin (SOCS antibodies) overnight. Membranes were washed and incubated with primary antibody with PBS-T and 5% nonfat milk powder or 2% fish skin gelatin for 2 h at room temperature. Antibody dilutions were as follows: MMP1, 1:1000 (Cedarlane Labs, Burlington, NC, USA); MMP2, 1:500 (Abnova, Taipei, Taiwan); MMP3, 1:400 and MMP9, 1:500 (Abcam, Cambridge, MA, USA); SOCS1, 2, 3, 1:100 (Santa Cruz Biotechnology, Santa Cruz, CA, USA). Following primary antibody incubation, membranes were washed and incubated with a secondary biotinylated antibody (1:5000, PBS-T with 2% normal donkey serum, 5% nonfat milk powder or 2% fish skin gelatin) for 1 h at room temperature. Membranes were washed again before final incubation with streptavidin-biotinylated horseradish peroxidase (1:3000, PBS-T) for 1 h, and visualized using enhanced chemiluminescence (ECL) detection (Perkin Elmer, Waltham, MA, USA). Gels were stained with Coomassie blue to verify even protein loading. Band intensity analyses were performed with the ImageJ software (http://rsbweb.nih.gov/ij/) using a pooled human liver band as a reference control for each membrane. The same sample representing a pool of 200 human livers was used on all membranes in a separate lane, and normalization was to levels of expression in that band. This increases sensitivity and reduces background noise caused by normalizing to a different antibody/protein. For each antibody replicate, four mini-gels were run in parallel tanks, off the same power-pack, on the same day, with the same buffer. Transfer to membranes was performed in the same semi-dry transfer cell (which can accommodate four mini-transfers simultaneously) then all four membranes incubated simultaneously with antibodies and ECL developer, for the same amounts of time. For development, membranes were placed on the same 8 × 10 inch film, inside the same development cassette. Despite this, some variation due to technical (rather than biological) differences is expected. Each antibody was optimized so that length of ECL incubation and length of exposure time to film was consistent for all membranes and all samples (although they differed between antibodies). Finally, the area and density of the bands was measured on film images scanned into TIFF image files (Canoscan 900F, MKII, Canon, Inc., Melville NY, USA). A single box was generated directly on the TIFF image within the Image J program. This pixel box was moved across the gel manually, which controls for band selection bias. The background of the individual film was subtracted and the Area:Density calculated as the density of pixels in the box compared with the constant box area, less the average background. The Area:Density relationships within boxes ranged ∼4-fold. These raw Area:Densities were then normalized to the liver control protein by dividing the Area:Density of individual samples by that of the liver control. By normalizing to Area:Density of the liver protein band, the variability observed for individual western bands is diminished to ∼2-fold. Moreover, because three membranes were analyzed for each protein, but only the average of the three Area:Densities was used for analysis, the variability across the set of experiments was lower in the graphs (as can be observed for the error bars), than for individual membranes. Data for individual embryos are presented in Supplementary data, Table S1.
Thiobarbituric acid reactive substances assay
The levels of thiobarbituric acid reactive substances (TBARSs) were quantified using a modification of the method described by Ohkawa et al. (1979). Briefly, placental lysates (50 µl, 1 mg/ml), 0.375% (w/v) thiobarbituric acid solution (1450 µl) and 2% (w/v) butylated hydroxytoluene (14 µl) were added to a microcentrifuge tube and vortexed for 10 s. Samples were heated in a 100°C water bath for 15 min. Following incubation, samples were centrifuged at 13 000g for 5 min at room temperature. Sample supernatant (200 µl) was transferred to an optically clear 96-well microtiter plate in triplicate and the absorbance read at λ = 532 nm. Results were transformed to nmol/mg protein of malondialdehyde equivalents using a standard curve generated with 1,1,3,3-tetraethoxypropane.
Statistical analyses
Statistical analyses were performed using Prism 5.0 with α = 0.05 (GraphPad Prism, San Diego, CA, USA). Biopsied and control groups were compared using Student's t-test and differences between groups were considered significant when α < 0.05. Additionally, the significance of differences birth outcomes between groups was tested with a χ2 test. No correction was applied for multiple comparisons.
Results
Production of fetuses
The data shown in this paper are novel, but are derived from a cohort of pregnancies produced and described earlier (Sugawara et al., 2012). Out of 248 4-cell embryos obtained after IVF, 103 were subjected to single blastomere removal and 88 to sham biopsy (Table I). No differences between two groups were noted in the progression of preimplantation development. Biopsied and control embryos were transferred to pseudo-pregnant females (1–2 per group per experiment). There were no statistically significant differences between the number of fetuses (mean ± SD, 3.4 ± 1.5 versus 4.0. ± 2.7) and embryos that implanted (mean ± SD, 8.7 ± 2.3. versus 10.3 ± 5.9) per pregnant female, and post-implantation development measured by proportion of live fetuses and embryos that implanted was also similar in both groups. For the experiments here we used four matched dam pairs (biopsied and control) that provided the following number of placentas: biopsied (n = 16) and control (n = 15).
Table I.
Production of fetuses after single blastomere biopsy and sham biopsy.
| No. of oocytes inseminated (no experiments) | No. of 2-cell embryos (%)a | No. of 4-cell embryos (%)b | No. of 4-cell embryos biopsied | No. of 4-cell embryos sham biopsied (control) | No. of embryos survived biopsy (%)c | No. of embryos developed to M/EB (%)d | No. of M/EB transferred (No of females) | No. of fetuses at Day 18 (%)e | No. of resorption sites at Day 18 (%)e | No. of implants at Day 18 (%)e |
|---|---|---|---|---|---|---|---|---|---|---|
| 264 (4) | 251 (95) | 248 (98.8) | 103 | n/a | 102 (99) | 102 (100) | 97 (7) | 24 (25) | 37 (38) | 61 (63) |
| n/a | 88 | 85 (97) | 85 (100) | 70 (6) | 16 (23) | 25 (36) | 41 (59) |
M/EB, morula/early blastocyst stage. Two females from control group failed to achieve pregnancy, most likely due to no pseudopregnancy. The proportion of fetuses remained similar between biopsied and control groups regardless whether or not two non-pregnant females were included in data analysis but more resorption sites per female were noted in the control group when they were excluded. These data were shown as Supplementary data, Table SI in Sugawara et al. (2012).
aPercentage calculated from oocytes inseminated.
bPercentage calculated from 2-cell embryos.
cPercentage calculated from 4-cell embryos biopsied.
dPercentage calculated from survived biopsied or sham-biopsied 4-cell embryos.
ePercentage calculated from (control) M/EB transferred.
Interleukin 6
The cytokine IL-6 is critical for many placental functions. The IL-6 secretion is inhibited by SOCS1 prevention of STAT5 signaling, and in turn, IL-6 can also activate MMPs and STAT3 causing tissue remodeling. Blastomere removal did not cause an increase in placental IL-6 levels when compared with placentas from the control group. However, the levels of IL-6 measured here for biopsy group were significantly higher than those previously reported in placentas from pregnancies derived after normal mating and ART in the same strain of mice (30-fold higher, P < 0.001 and 10-fold, P < 0.001, respectively (Raunig et al., 2011b). Measurements of IL-6 for the current and previous study were performed using a commercial ELISA from the same company and lot number.
Phosphorylated STAT1 and STAT3
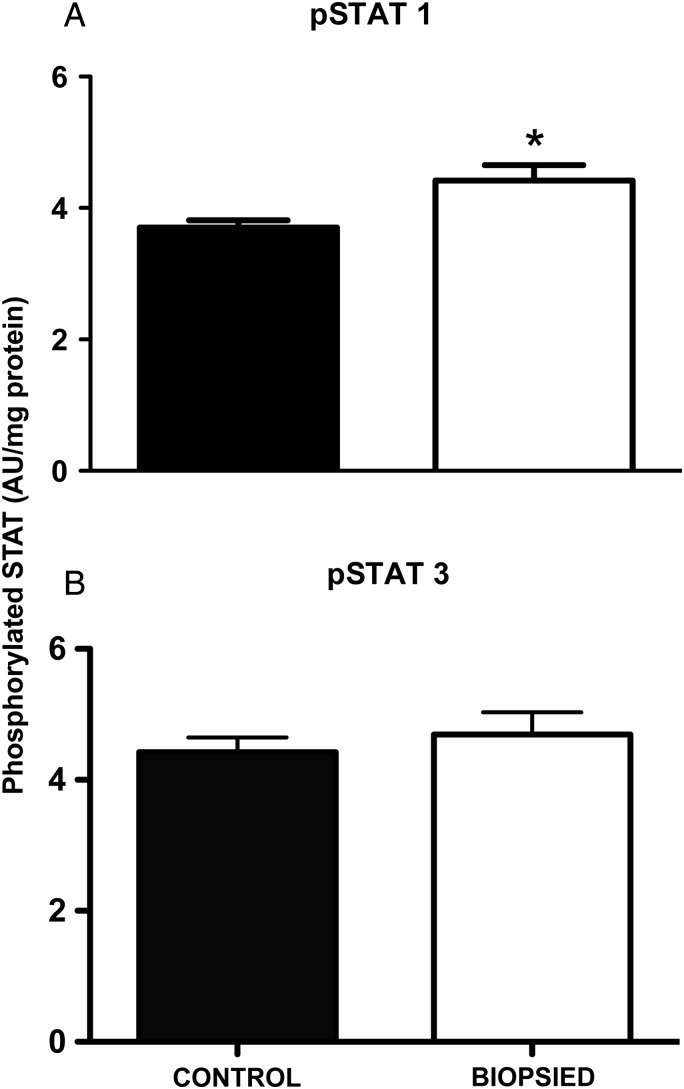
The STAT proteins are activated by phosphorylation, whereupon they translocate to the nucleus and act as transcriptional activators of a plethora of other proteins. Placentas derived from biopsied embryos had significantly higher (P < 0.05) phospho-STAT1 compared with the control group (Fig. 1A). However, there was no statistically significant difference detected in phospho-STAT3 levels between the two groups (Fig. 1B).
Figure 1.
Activation of placental STAT signaling is increased by blastomere removal for STAT-1 (A) but not STAT-3 (B). STAT-1 and STAT-3 were measured by ELISA with spectrophotometric detection in absorbance units (AU) at 405 nm. Bars are mean ± SEM of n = 15 control and n = 16 biopsied placentas. *P < 0.05, t-test.
SOCS proteins
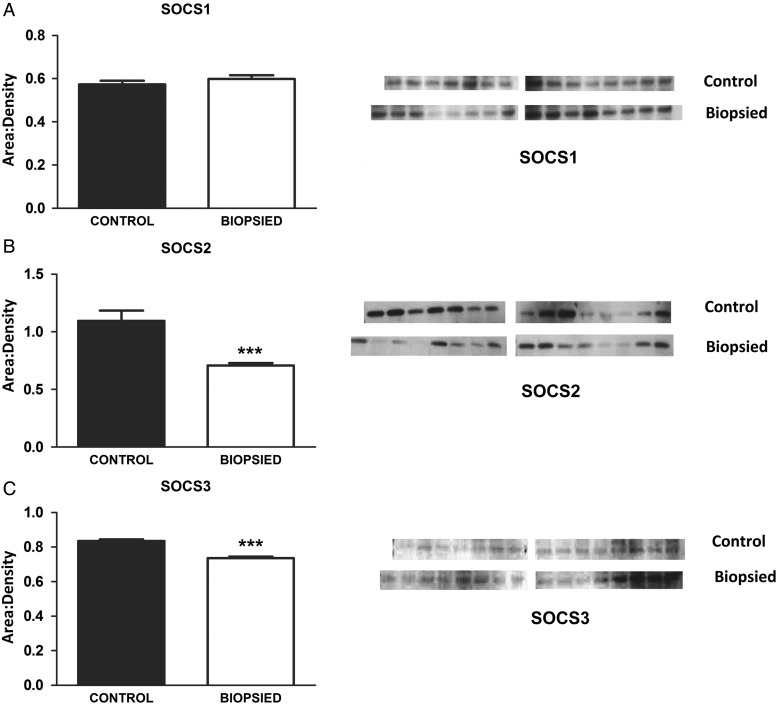
The SOCS proteins are negative feedback inhibitors of cytokines as well as other transcriptional regulators including the STAT pathways. The levels of SOCS1 proteins did not differ between placentas from biopsied and control groups. However, the placentas from biopsied embryos had significantly lower levels of SOCS2 (P < 0.001, Fig. 2B) and SOCS3 (P < 0.001, Fig. 2C).
Figure 2.
The SOCS proteins, which negatively feedback on STAT and other cytokines, are lower in placentas from biopsied embryos. While SOCS1 proteins were unchanged (A), SOCS 2 and 3 were significantly lower in placentas from biopsied embryos (B and C respectively). The images presented are single membranes, representative of the blots obtained. Each protein was blotted three times and the area:densities normalized to blot background as well as to an internal liver control on each membrane (as detailed in materials and methods). The arithmetic mean of the three replicates used to generate graphs, hence the error bars are smaller that visually would appear on the blots. Bars are mean ± SEM of n = 15 control and n = 16 biopsied placentas. ***P < 0.001, t-test.
Nitrosative species
Nitrosative species are reactive with nitric oxide synthase enzymes to cause changes in vascular tone and function, as well as have downstream effects on lipid peroxidation and other oxidative stress pathways. While nitrite species were undetectable, nitrate levels in placentas from biopsy-derived embryos were higher (P < 0.05) than those measured in placentas from control embryos.
Latent and activated MMP proteins
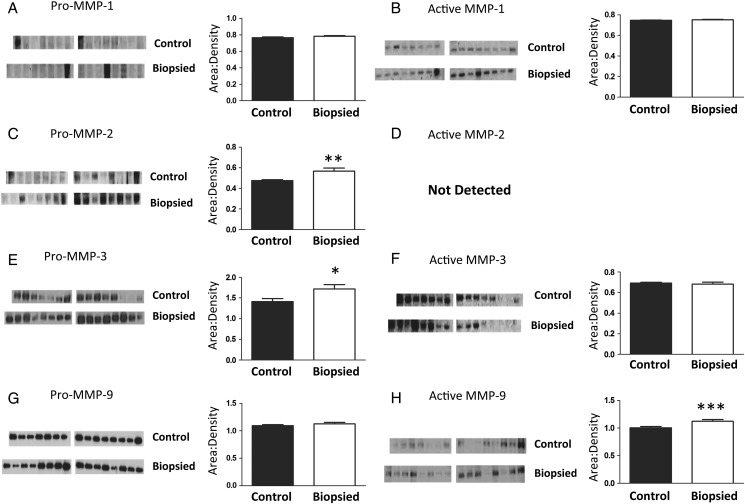
Placentas from biopsied and control groups had similar levels of latent (53 kDa) and active (25 kDa) MMP1 (Fig. 3A and 3B, respectively). Pro-MMP2 was significantly higher in placentas from the biopsied group (72 kDa, P < 0.01, Fig. 3C), but the active form was not detected in either groups (Fig. 3D). Pro-MMP3 (54 kDa) was significantly higher in placentas derived from biopsied embryos than controls (P < 0.05, Fig. 3E), but there were no differences in the active form of MMP3 (44 kDa, Fig. 3F). Finally, pro-MMP9 (102 kDa, Fig. 3G) was not affected by blastomere removal, but activated MMP9 (82 kDa) was significantly higher (P < 0.001) in placentas from the biopsy group compared with control (Fig. 3G). In sum, blastomere removal is associated with significantly greater levels of placental pro-MMP2 and MMP-3 as well as significant activation of MMP9.
Figure 3.
Greater levels of pro-MMP 2 and 3 enzymes and activated MMP-9 are observed in placentas from biopsied when compared with placentas from control embryos. Although MMP1 was unchanged (A and B), levels of latent MMP 2 and 3 (C and E) and activated MMP9 (H) increased. The images presented are single membranes, representative of the blots obtained. Each protein was blotted three times and the area:densities normalized to blot background as well as to an internal liver control on each membrane (as detailed in Materials and Methods). The arithmetic mean of the three replicates used to generate graphs, hence the error bars are smaller that visually would appear on the blots. Bars are mean ± SEM of n = 15 control and n = 16 biopsied placentas. *P < 0.05, **P < 0.01, ***P < 0.001, t-test. ND, not detected.
TBARS assay
The thiobarbituric acid reactive species are a surrogate measure of lipid peroxidation, which can be one effect of activation of STATs and MMPs and/or increases in nitrosative and oxidative species. Levels of thiobarbituric acid reactive species were significantly higher (P < 0.01) in biopsied placentas compared with control, indicating that the downstream effects of these signaling pathways are resulting in lipid peroxidation in biopsied embryos where blastomeres are removed, but not in controls where the zona was pricked but no cellular material removed.
Discussion
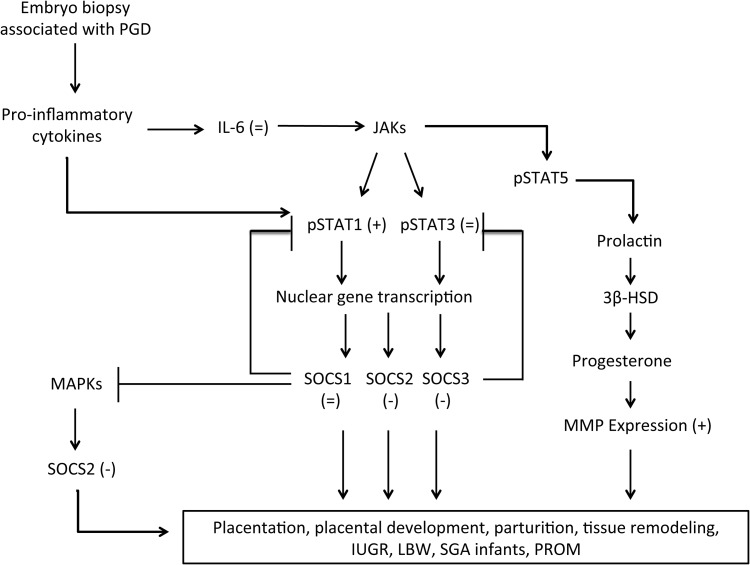
The aim of this study was to determine whether single blastomere removal, a procedure commonly used for diagnosing birth defects before embryo transfer, affects placental functions, even at late stages of pregnancy. Empirically, if effects of blastomere removal on the placenta can be observed at term (as we have done here), this demonstrates effects of the procedure that endure throughout pregnancy. As we have previously demonstrated abnormalities at the tissue and protein levels from ART, with and without PGD (Collier et al., 2009; Raunig et al. 2011a, b; Sugawara et al., 2012), we wished to determine the molecular signaling mechanisms affected by the PGD procedure which may cause dysfunction in placental tissues. The integrity and function of critical signaling pathways in the placental repertoire, namely: nitrosative stress, IL-6, STAT, SOCS and MMP activation were assessed. These pathways of placental function and parturition are inter-related and contain many points of cross-talk and multiple common outcomes and a schematic diagram (Fig. 4) is provided to aid discussion. These are also signaling pathways commonly implicated in pregnancy syndromes such as pre-eclampsia, spontaneous fetal loss and premature rupture of the membranes (Goossens et al., 2009). To our knowledge, this the first study aiming to determine the molecular mechanisms behind the effects of blastomere removal on placental signaling and function at term.
Figure 4.
Schematic diagram showing the inter-related inflammatory SOCS, STAT and MMP signaling pathways. Arrows indicate stimulation and capped lines indicate feed-back inhibition. Bracketed figures in each part of the cascade indicate that blastomere removal caused increases (+), decreases (−) or no change (=), when compared with controls for which the zona pellucida was pierced but blastomere was not removed.
Our previous work examining pregnancies obtained as a result of normal mating, IVF and ICSI demonstrated that both ART procedures were associated with placental inflammation through increases in IL-6 (Raunig et al., 2011b). In this study, we determined that blastomere removal in conjunction with IVF did not increase placental IL-6 levels when compared with placentas from embryos that had undergone a sham-biopsy procedure. This is supported by our data showing no changes in SOCS1 protein levels between placentas from biopsied and control embryos. The SOCS1 protein is an upstream inhibitor of IL-6 secretion through the STAT5 pathway. It also prevents other inflammatory cytokine production by inhibiting the mitogen-activated protein kinase (MAPK) pathway (Yoshimura et al., 2007). Therefore, blastomere biopsy does not increase inflammatory signaling through SOCS1, STAT5, IL-6 and MAPK pathways. However, the levels of IL-6 observed in this study were ∼30 times higher than those reported by us earlier in placentas from normally mated mice and ∼10 times higher than in placentas from mice generated by IVF but not subjected to any further manipulations (Raunig et al., 2011b). This suggests that the mode of conception (in vitro versus in vivo) as well as prolonged embryo culture and zona pellucida piercing, but not the blastomere removal, lead to induction of placental IL-6. While IL-6 is one activator of STAT3 and MMP pathways, it is not the sole agent and other mechanisms, such as greater oxidative stress (previously demonstrated to occur in murine IVF placentas (Raunig et al., 2011b) and increased nitrosative stress (as shown here), can also independently activate these pathways.
The STAT signaling factors were also activated by the removal of a blastomere during the embryo biopsy procedure, as observed by increased levels of phosphorylated STAT1 and also significantly reduced expression of feedback inhibitors SOCS2 and SOCS3 in placentas derived from biopsied embryos. That STAT1, and not STAT3, was significantly activated is not surprising since STAT3 is thought to be primarily activated by IL-6 (Ivashkiv, 2000), which did not differ between biopsy and control groups. Because SOCS3 is the primary inhibitor of STAT3 (Yoshimura et al., 2007) it was somewhat contradictory to see that although SOCS3 was down-regulated in placentas from the biopsy group, we did not detect increased STAT3 due to loss of negative feedback. The explanation for this is likely that IL-6 signaling is a stronger mediator of STAT3 activation than SOCS3 feedback inhibition, although differences in the relative sensitivity between ELISA and the western blotting method may also have impacted the results. The SOCS3 protein also prevents the activation of STAT1 (Yoshimura et al., 2007); therefore, lower levels of SOCS3 observed here do correlate with higher levels of activated STAT1. In contrast, STAT1 is more strongly regulated by oxidative stress, which we have demonstrated is increased in murine IVF placentas (Raunig et al, 2011b). Additionally, activation of STAT1 also activates inducible nitric oxide synthase and so the greater levels of nitrosative stress observed may both contribute to and/or be a consequence of activated STAT1 signaling. Both activation of STAT1 and greater nitrosative signaling are known to have downstream effects activating MMP signaling and cause defects in intercellular communication (Yoshimura et al., 2007). Finally, greater STAT1 ought to have caused greater SOCS1, but this did not occur. We speculate that this is because the sensitivity of the ELISA for STAT1 was higher than the sensitivity of western blot for SOCS1.
Down-regulation of SOCS3, as observed here, has been previously associated with pre-eclampsia in human pregnancies (Fitzgerald et al., 2009), while SOCS3 null mice exhibited embryonic lethality and placental defects (Alexander, 2002). The SOCS proteins tend to be induced by cytokines, hence they act as classical negative feedback inhibitors of cytokine signaling (Yoshimura et al., 2007). However, SOCS proteins are also induced by other molecules including lipopolysaccharide, isoproterenol, statin drugs and molecules that up-regulate cyclic AMP (Yoshimura et al., 2007). Of particular relevance for this study, SOCS1 expression in immature fetal thymocytes is developmentally regulated due to the fact that cytokine signaling is necessarily depressed in the fetus (Yoshimura et al., 2007). Consequently, changes in SOCS proteins have important consequences for the regulation of the immune system in utero and for fetal development.
Metalloproteinases are important mediators of intercellular communication, primarily through their ability to break down extracellular matrices. In the placenta they are particularly involved in placentation, membrane rupture and cleavage from the uterine wall during parturition. These proteins exist as either latent (pro-protein) or activated (cleaved) forms, the latter having catalytic activity. The increases in levels of latent MMP2 and 3 and activated MMP9 proteins in placentas from biopsied embryos may be due to the individual or combined effects of greater STAT1 signaling and higher levels of nitrosative stress. Both MMP9 and MMP3 (the latter to a lesser extent) increase in the placenta toward the end of pregnancy in order to execute their roles in degrading the extracellular matrix and causing membrane rupture during parturition (Hulboy et al., 1997). Higher levels of MMP9 in the amniotic fluid have been associated with women who experienced preterm premature rupture of membranes, leading to premature labor (Athayde et al., 1998). Because we terminated pregnancies by caesarian section at Day 18.5, we cannot assess whether premature labor would result from increased MMP activities in our mouse model. Nevertheless, our data in the context of published human studies imply that increased incidence of premature labor in ART cases involving PGD may be due to blastomere removal increasing MMP activation.
Latent MMP proteins can also become activated by chemical modification of the thiol group in the pro-domain (Parks et al., 2004). Inflammatory responses, such as through IL-6, reactive oxygen or nitrogen species can all modify the pro-domain thiol residue In fact, Capobianco et al. (2012) demonstrated that increased levels of nitric oxide species and formation of peroxynitrites can lead to over-activation of MMP2 and 9 in human term placentas; these results both support and are supported by our data. Clearly, MMP signaling at both a functional level and downstream signaling pathways is altered by blastomere removal from embryos.
Additionally, since blastomere removal was associated with greater levels of nitrosative stress, the direct effects of active nitrogen species on the placental vasculature (independent of STAT, SOCS or MMP signaling pathways) should be considered. The villous placenta expresses nitric oxide synthase enzymes involved in placental vasculogenesis and angiogenesis, and these are also critical for dynamic regulation of endothelial tone and function throughout pregnancy (Vatish et al., 2006). Nitric oxide synthases have been extensively studied, and alterations of normal nitrosative stress are associated with several negative pregnancy outcomes directly through effects on placental vascular tone, including pre-eclampsia, intrauterine growth restriction, gestational diabetes mellitus and placental cell death (Coughlan et al., 2004; Vatish et al., 2006; Al-Gubory et al., 2010). Therefore, although the STAT, SOCS and MMP signaling factors represent more complex and intricate mechanisms of placental function, the classical effects of nitrous species on the placental vasculature are an extremely logical explanation for negative obstetric effects caused by blastomere removal.
One question that arises is how alike are mouse and human placentas? The gestational period in humans is 40 weeks, significantly longer than 20 days of gestation in mice. Placentation in mice takes around half of gestation, while in humans the placenta is fully functional after one-third of the gestational period (12 weeks) (Malassiné et al., 2003). In both mice and humans, the site of chemical and gas exchange is the trophoblast. In humans, trophoblasts form a single cell layer separating fetal and maternal circulations (Gude et al., 2004), but in mice the analogous region comprise syncytiotrophoblasts, chorionic trophoblasts, fetal blood vessels and stroma forming the decidual, labyrinth and syncytiotrophoblast zones (Malassiné et al., 2003; Niemand et al., 2003). In spite of these structural differences, considerable similarities exist between mouse and human placentas and several genes necessary for mouse placental development are expressed in analogous manner in humans (Rossant and Cross, 2001). Moreover, the signaling pathways we have examined here are conserved across most mammalian species (Mann et al., 2004; Rivera et al., 2008) and there are several examples from the immunology, recurrent spontaneous miscarriage and pre-eclamptic literature demonstrating essentially the same mechanisms in mice as in humans (reviewed Clark, 2014).
Another aspect of this study of interest is how manipulations at such an early stage of conception can cause effects that are noticeable at term. It has been shown in bovine models that negative reproductive outcomes after somatic cell transfer stem from altered placentation at very early stages (Chavatte-Palmer et al., 2012). Moreover, in humans it is known that pregnancy complications such as recurrent spontaneous miscarriage and pre-eclampsia can be caused by incomplete early blastocyst invasion, which manifests later (Al-Khan et al., 2011). While our recent publications have documented functional placental changes (Collier et al., 2009; Raunig et al., 2011a, b; Collier et al., 2012), this study begins to determine the mechanisms behind the observed findings. With respect to prolonged embryo culture, other groups have demonstrated placenta effects in the mouse model, where prolonged embryo culture changes placental and fetal development (Delle Piane et al., 2012) including nutrient transport functions (Bloise et al., 2012). In our model, because culture conditions were the same for both groups, we contend that piercing the zona pellucida itself is bringing about changes over-and-above those caused by embryo culture. Moreover, while this is the first evidence for perturbation of these specific pathways (SOCS, Jak/STAT, MMPs) and the reasons for these changes are speculative, other studies have indicated that at least some placental changes caused by preimplantation manipulation are through aberrant changes in imprinted genes, including epigenetic methylation patterns (Mann et al., 2004; Rivera et al., 2008; deWaal et al., 2014). It will now be important to investigate how early in gestation this placental dysfunction appears, how it develops and what are the associated molecular changes.
Although the differences between biopsied and control groups described herein are not orders of magnitude in difference, we believe they are still biologically significant. Small but significant changes to placental cellular signaling molecules (including MMPs, Garcia-Lopez et al., 2007), receptors (Bebbere et al., 2013) and DNA methylation (Katari et al., 2009) in both humans and other species, have proven in the past to have strong biological effects. Indeed, with respect to MMPs, single nucleotide polymorphisms that change their activity <2-fold have been linked with premature membrane rupture (Fujimoto et al., 2002)
Based on these findings, we propose that perturbation of the SOCS, STAT and MMP signaling pathways caused by blastomere removal performed as part of the PGD may be a contributing factor for obstetric complications including premature membrane rupture, premature labor and dysfunctional labor, that have been associated with ART pregnancies (Goossens et al., 2009). Additionally, inflammatory mediators such as increased nitric oxide may contribute to altering these pathways and/or can have direct effects of their own. While assisted reproductive techniques (including PGD) have helped many couples conceive, it is of great importance to understand how and why negative pregnancy outcomes can occur. Our results demonstrate dysregulation in placental signaling at term; further investigations at earlier time points may be useful to identify the precise timing of this dysregulation in placenta to determine whether processes such as invasion and placentation, pregnancy maintenance, fetal growth and parturition are affected. The study presented can contribute to improving and refining ART by identifying optimal and non-optimal technologies at the preimplantation stage. By extending such studies in a longitudinal manner, we may begin to unravel the mechanisms of obstetric and neonatal outcomes. If the best practices in ART can be identified, any increased risks of the procedures can be minimized in future and promote successful pregnancy, good obstetric and neonatal outcomes.
Supplementary data
Supplementary data are available at http://molehr.oxfordjournals.org/ online.
Authors' roles
B.S. provided intellectual contributions, performed bench work, analyzed data and wrote the manuscript; A.S. performed PGD experiments, animal husbandry and edited the manuscript; M.A.W. provided intellectual contributions, performed animal husbandry, edited the manuscript and provided funding for these studies. A.C.C. provided intellectual impetus, analyzed the data, wrote and edited the manuscript and provided funding for the studies.
Funding
This material is based on work supported by NIGMS 8P20GM103457-5 (Project 4) to ACC, NIGMS 8P20GM103457-5 (Project 2) and NIH HD072380 and Hawaii Community Foundation to MAW.
Conflict of interest
The authors have no conflicts of interest to declare.
Supplementary Material
References
- Agarwal A, Gupta S, Sharma R. Role of oxidative stress in female reproduction. Reprod Biol Endocrinol. 2005;3:28. doi: 10.1186/1477-7827-3-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander WS. Suppressors of cytokine signalling (SOCS) in the immune system. Nat Rev Immunol. 2002;2:410–416. doi: 10.1038/nri818. [DOI] [PubMed] [Google Scholar]
- Al-Gubory KH, Fowler PA, Garrel C. The roles of cellular reactive oxygen species, oxidative stress and antioxidants in pregnancy outcomes. Internat J Biochem Cell Biol. 2010;42:1634–1650. doi: 10.1016/j.biocel.2010.06.001. [DOI] [PubMed] [Google Scholar]
- Al-Khan A, Aye IL, Barsoum I, Borbely A, Cebral E, Cerchi G, Clifton VL, Collins S, Cotechini T, Davey A, et al. IFPA Meeting 2010 Workshops Report II: Placental pathology; trophoblast invasion; fetal sex; parasites and the placenta; decidua and embryonic or fetal loss; trophoblast differentiation and syncytialisation. Placenta. 2011;32(Suppl. 2):S90–S99. doi: 10.1016/j.placenta.2010.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Athayde N, Edwin SS, Romero R, Gomez R, Maymon E, Pacora P, Menon R. A role for matrix metalloproteinase-9 in spontaneous rupture of the fetal membranes. AJOG. 1998;179:1248–1253. doi: 10.1016/s0002-9378(98)70141-3. [DOI] [PubMed] [Google Scholar]
- Audibert F, Wilson RD, Allen V, Blight C, Brock JA, Désilets VA, Gagnon A, Johnson JA, Langlois S, Wyatt P. Genetics, committee. preimplantation genetic testing. J Obstet Gynecol Canada. 2009;31:761–775. doi: 10.1016/s1701-2163(16)34284-0. [DOI] [PubMed] [Google Scholar]
- Bebbere D, Bauersachs S, Fürst RW, Reichenbach HD, Reichenbach M, Medugorac I, Ulbrich SE, Wolf E, Ledda S, Hiendleder S. Tissue-specific and minor inter-individual variation in imprinting of IGF2R is a common feature of Bos taurus Concepti and not correlated with fetal weight. PLoS One. 2013;8:e59564. doi: 10.1371/journal.pone.0059564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloise E, Lin W, Liu X, Simbulan R, Kolahi KS, Petraglia F, Maltepe E, Donjacour A, Rinaudo P. Impaired placental nutrient transport in mice generated by in vitro fertilization. Endocrinology. 2012;153:3457–3467. doi: 10.1210/en.2011-1921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capalbo A, Wright G, Elliott T, Ubaldi FM, Rienzi L, Nagy ZP. FISH reanalysis of inner cell mass and trophectoderm samples of previously array-CGH screened blastocysts shows high accuracy of diagnosis and no major diagnostic impact of mosaicism at the blastocyst stage. Hum Reprod. 2013;28:2298–2307. doi: 10.1093/humrep/det245. [DOI] [PubMed] [Google Scholar]
- Capobianco E, White V, Sosa M, Di Marco I, Basualdo MN, Faingold MC, Jawerbaum A. Regulation of matrix metalloproteinases 2 and 9 activities by peroxynitrites in term placentas from type 2 diabetic patients. Reprod Sci. 2012;19:814–822. doi: 10.1177/1933719111434544. [DOI] [PubMed] [Google Scholar]
- Chatot CL, Ziomek CA, Bavister BD, Lewis JL, Torres I. An improved culture medium supports development of random-bred 1-cell mouse embryos in vitro. J Reprod Fertil. 1989;86:679–688. doi: 10.1530/jrf.0.0860679. [DOI] [PubMed] [Google Scholar]
- Chavatte-Palmer P, Camous S, Jammes H, Le Cleac'h N, Guillomot M, Lee RS. Placental perturbations induce the developmental abnormalities often observed in bovine somatic cell nuclear transfer. Placenta. 2012;33:S99–S104. doi: 10.1016/j.placenta.2011.09.012. [DOI] [PubMed] [Google Scholar]
- Clark DA. The use and misuse of animal analog models of human pregnancy disorders. J Reprod Immunol. 2014;101:1–8. doi: 10.1016/j.jri.2014.02.006. [DOI] [PubMed] [Google Scholar]
- Cohen M, Meisser A, Bischof P. Metalloproteinases and human placental invasiveness. Placenta. 2006;27:783–793. doi: 10.1016/j.placenta.2005.08.006. [DOI] [PubMed] [Google Scholar]
- Collier AC, Miyagi SJ, Yamauchi Y, Ward MA. Assisted reproduction technologies impair placental steroid metabolism. J Steroid Biochem Mol Biol. 2009;116:21–28. doi: 10.1016/j.jsbmb.2009.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collier AC, Milam KA, Rougée LRA, Sugawara A, Yamauchi Y, Ward MA. Upregulation of Ugt1a genes in placentas and fetal livers in a murine model of assisted reproduction. Placenta. 2012;33:77–80. doi: 10.1016/j.placenta.2011.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coughlan MT, Vervaart PP, Permezel M, Georgiou HM, Rice GE. Altered placental oxidative stress status in gestational diabetes mellitus. Placenta. 2004;25:78–84. doi: 10.1016/S0143-4004(03)00183-8. [DOI] [PubMed] [Google Scholar]
- Delle Piane L, Lin W, Liu X, Donjacour A, Minasi P, Revelli A, Maltepe E, Rinaudo PF. Effect of the method of conception and embryo transfer procedure on mid-gestation placenta and fetal development in an IVF mouse model. Hum Reprod. 2012;25:2039–2046. doi: 10.1093/humrep/deq165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- deWaal E, Mak W, Calhoun S, Stein P, Ord T, Krapp C, Coutifaris C, Schultz RM, Bartolomei MS. In vitro culture increases the frequency of stochastic epigenetic errors at imprinted genes in placental tissues from mouse concepti produced through assisted reproductive technologies. Biol Reprod. 2014;90:22. doi: 10.1095/biolreprod.113.114785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald JS, Toth B, Jeschke U, Schleussner E, Markert UR. Knocking off the suppressors of cytokine signaling (SOCS): their roles in mammalian pregnancy. J Reprod Immunol. 2009;83:117–123. doi: 10.1016/j.jri.2009.07.010. [DOI] [PubMed] [Google Scholar]
- Fujimoto T, Parry S, Urbanek M, Sammel M, Macones G, Kuivaniemi H, Romero R, Strauss JF., III A single nucleotide polymorphism in the matrix metalloproteinase-1 (MMP-1) promoter influences amnion cell MMP-1 expression and risk for preterm premature rupture of the fetal membranes. J Biol Chem. 2002;277:6296–6302. doi: 10.1074/jbc.M107865200. [DOI] [PubMed] [Google Scholar]
- Garcia-Lopez G, Vadillo-Ortega F, Merchant-Larios H, Maida-Claros R, Osorio M, Soriano-Becerril D, Flores-Herrera H, Beltran-Montoya J, Garfias-Becerra Y, Zaga-Clavellina V. Evidence of in vitro differential secretion of 72 and 92 kDa type IV collagenases after selective exposure to lipopolysaccharide in human fetal membranes. Mol Hum Reprod. 2007;13:409–418. doi: 10.1093/molehr/gam025. [DOI] [PubMed] [Google Scholar]
- Goossens V, Harton G, Moutou C, Traeger-Synodinos J, Van Rij M, Harper JC. ESHRE PGD Consortium data collection IX: cycles from January to December 2006 with pregnancy follow-up to October 2007. Hum Reprod. 2009;24:1786–1810. doi: 10.1093/humrep/dep059. [DOI] [PubMed] [Google Scholar]
- Goossens V, Traeger-Synodinos J, Coonen E, De Rycke M, Moutou C, Pehlivan T, Derks-Smeets IA, Harton G. ESHRE PGD Consortium data collection XI: cycles from January to December 2008 with pregnancy follow-up to October 2009. Hum Reprod. 2012;27:1887–1911. doi: 10.1093/humrep/des106. [DOI] [PubMed] [Google Scholar]
- Grace KS, Sinclair KD. Assisted reproductive technology, epigenetics, and long-term health: a developmental time bomb still ticking. Semin Reprod Med. 2009;27:409–416. doi: 10.1055/s-0029-1237429. [DOI] [PubMed] [Google Scholar]
- Gude NM, Roberts CT, Kalionis B, King RG. Growth and function of the normal human placenta. Thromb Res. 2004;114:397–407. doi: 10.1016/j.thromres.2004.06.038. [DOI] [PubMed] [Google Scholar]
- Guzeloglu-Kayisli O, Kayisli UA, Taylor HS. The role of growth factors and cytokines during implantation: endocrine and paracrine interactions. Semin Reprod Med. 2009;27:62–79. doi: 10.1055/s-0028-1108011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haavaldsen C, Tanbo T, Eskild A. Placental weight in singleton pregnancies with and without assisted reproductive technology: a population study of 536 567 pregnancies. Hum Reprod. 2012;27:576–582. doi: 10.1093/humrep/der428. [DOI] [PubMed] [Google Scholar]
- Hulboy DL, Rudolph LA, Matrisian LM. Matrix metalloproteinases as mediators of reproductive function. Mol Hum Reprod. 1997;3:27–45. doi: 10.1093/molehr/3.1.27. [DOI] [PubMed] [Google Scholar]
- Ivashkiv LB. Jak-STAT signaling pathways in cells of the immune system. Rev Immunogenet. 2000;2:220–230. [PubMed] [Google Scholar]
- Källén B, Finnström O, Nygren KG, Otterblad Olausson P, Wennerholm U-B. In vitro fertilisation in Sweden: obstetric characteristics, maternal morbidity and mortality. BJOG. 2005;112:1529–1535. doi: 10.1111/j.1471-0528.2005.00745.x. [DOI] [PubMed] [Google Scholar]
- Katari S, Turan N, Bibikova M, Erinle O, Chalian R, Foster M, Gaughan JP, Coutifaris C, Sapienza C. DNA methylation and gene expression differences in children conceived in vitro or in vivo. Mol Hum Genet. 2009;18:3769–3778. doi: 10.1093/hmg/ddp319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura Y, Yanagimachi R. Intracytoplasmic sperm injection in the mouse. Biol Reprod. 1995;52:709–720. doi: 10.1095/biolreprod52.4.709. [DOI] [PubMed] [Google Scholar]
- Liebaers I, Desmyttere S, Verpoest W, De Rycke M, Staessen C, Sermon K, Devroey P, Haentjens P, Bonduelle M. Report on a consecutive series of 581 children born after blastomere biopsy for preimplantation genetic diagnosis. Hum Reprod. 2010;25:275–282. doi: 10.1093/humrep/dep298. [DOI] [PubMed] [Google Scholar]
- Malassiné A, Frendo JL, Evain-Brion D. A comparison of placental development and endocrine functions between the human and mouse model. Hum Reprod Update. 2003;9:531–539. doi: 10.1093/humupd/dmg043. [DOI] [PubMed] [Google Scholar]
- Mann MR, Lee SS, Doherty AS, Verona RI, Nolen LD, Schultz RM, Bartolomei MS. Selective loss of imprinting in the placenta following preimplantation development in culture. Development. 2004;131:3727–3735. doi: 10.1242/dev.01241. [DOI] [PubMed] [Google Scholar]
- Meisser A, Cameo P, Islami D, Campana A, Bischof P. Effects of interleukin-6 (IL-6) on cytotrophoblastic cells. Mol Hum Reprod. 1999;5:1055–1058. doi: 10.1093/molehr/5.11.1055. [DOI] [PubMed] [Google Scholar]
- National Center for Chronic Disease Prevention and Health Promotion Department of Reproductive Health. Assisted reproductive technology: success rates, national summary and fertility clinic reports 2009. 2011. Atlanta, GA, USA: Centers for Disease Control and Prevention.
- Niemand C, Nimmesgern A, Haan S, Fischer P, Schaper F, Rossaint R, Heinrich PC, Müller-Newen G. Activation of STAT3 by IL-6 and IL-10 in primary human macrophages is differentially modulated by suppressor of cytokine signaling 3. J Immunol. 2003;170:3263–3272. doi: 10.4049/jimmunol.170.6.3263. [DOI] [PubMed] [Google Scholar]
- Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem. 1979;95:351–358. doi: 10.1016/0003-2697(79)90738-3. [DOI] [PubMed] [Google Scholar]
- Parks WC, Wilson CL, Lopez-Boado YS. Matrix metalloproteinases as modulators of inflammation and innate immunity. Nat Rev Immunol. 2004;4:617–629. doi: 10.1038/nri1418. [DOI] [PubMed] [Google Scholar]
- Pelkonen O, Vahakangas K, Gupta RC. Placental toxicity of organophosphate and carbamate pesticides. In: Gupta RC, editor. Toxicology of Organophosphate and Carbamate Compounds. Burlington, MA, USA: Academic Press; 2006. [Google Scholar]
- Porterfield SP, White BA. Endocrine Physiology. 3rd edn. Philadelphia, PA, USA: Mosby Elsevier; 2007. [Google Scholar]
- Quinn P, Barros C, Whittingham DG. Preservation of hamster oocytes to assay the fertilizing capacity of human spermatozoa. J Reprod Fertil. 1982;66:161–168. doi: 10.1530/jrf.0.0660161. [DOI] [PubMed] [Google Scholar]
- Raunig JM, Yamauchi Y, Ward MA, Collier AC. Assisted reproduction technologies alter steroid delivery to the mouse fetus during pregnancy. J Steroid Biochem Mol Biol. 2011a;126:26–34. doi: 10.1016/j.jsbmb.2010.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raunig JM, Yamauchi Y, Ward MA, Collier AC. Placental inflammation and oxidative stress in the mouse model of assisted reproduction. Placenta. 2011b;32:852–858. doi: 10.1016/j.placenta.2011.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivera RM, Stein P, Weaver JR, Mager J, Schultz RM, Bartolomei MS. Manipulations of mouse embryos prior to implantation result in aberrant expression of imprinted genes on day 9.5 of development. Hum Mol Genet. 2008;17:1–14. doi: 10.1093/hmg/ddm280. [DOI] [PubMed] [Google Scholar]
- Romundstad LB, Romundstad PR, Sunde A, von Düring V, Skjærven R, Vatten LJ. Increased risk of placenta previa in pregnancies following IVF/ICSI; a comparison of ART and non-ART pregnancies in the same mother. Hum Reprod. 2006;21:2353–2358. doi: 10.1093/humrep/del153. [DOI] [PubMed] [Google Scholar]
- Rossant J, Cross JC. Placental development: lessons from mouse mutants. Nat Rev Genet. 2001;2:538–548. doi: 10.1038/35080570. [DOI] [PubMed] [Google Scholar]
- Scott RT, Jr, Upham KM, Forman EJ, Zhao T, Treff NR. Cleavage-stage biopsy significantly impairs human embryonic implantation potential while blastocyst biopsy does not: a randomized and paired clinical trial. Fertil Steril. 2013;100:624–630. doi: 10.1016/j.fertnstert.2013.04.039. [DOI] [PubMed] [Google Scholar]
- Smith PK, Krohn RI, Hermanson GT, Mallia AK, Gartner FH, Provenzano MD, Fujimoto EK, Goeke NM, Olson BJ, Klenk DC. Measurement of protein using bicinchoninic acid. Anal Biochem. 1985;150:76–85. doi: 10.1016/0003-2697(85)90442-7. [DOI] [PubMed] [Google Scholar]
- Sugawara A, Sato B, Bal E, Collier AC, Ward MA. Blastomere removal from cleavage-stage mouse embryos alters steroid metabolism during pregnancy. Biol Reprod. 2012;87:1–9. doi: 10.1095/biolreprod.111.097444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Syme MR, Paxton JW, Keelan JA. Drug transfer and metabolism by the human placenta. Clin Pharmacokinet. 2004;43:487–514. doi: 10.2165/00003088-200443080-00001. [DOI] [PubMed] [Google Scholar]
- Vatish M, Randeva HS, Grammatopoulos DK. Hormonal regulation of placental nitric oxide and pathogenesis of pre-eclampsia. Trends Mol Med. 2006;12:223–233. doi: 10.1016/j.molmed.2006.03.003. [DOI] [PubMed] [Google Scholar]
- Yoshimura A, Naka T, Kubo M. SOCS proteins, cytokine signalling and immune regulation. Nat Rev Immunol. 2007;7:454–465. doi: 10.1038/nri2093. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Zhang Y-L, Feng C, Wu Y-T, Liu A-X, Sheng J-Z, Cai J, Huang H-F. Comparative proteomic analysis of human placenta derived from assisted reproductive technology. Proteomics. 2008;8:4344–4356. doi: 10.1002/pmic.200800294. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Zhao W, Jiang Y, Zhang R, Wang J, Li C, Zhao H, Gao L, Cui Y, Zhou Z, et al. Ultrastructural study on human placentae from women subjected to assisted reproductive technology treatments. Biol Reprod. 2011;85:635–642. doi: 10.1095/biolreprod.110.090589. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.