Abstract
Background.
Intoxication with ethylene glycol happen all around the world and without rapid recognition and early treatment, mortality from this is high.
Methods.
In our study, we retrospectively analysed six cases of ethylene glycol intoxication in our department. We measured ethylene glycol or glycolate levels, lactate levels and calculated the osmolal and anion gap.
Results.
Data from six patients admitted to the nephrology department between 1999 and 2011 with ethylene glycol poisoning are reported. All patients were men. The mean pH on admission was 7.15 ± 0.20 and the anion and osmolal gap were elevated in five of six patients. Four patients had an acute kidney injury and one patient had an acute-on-chronic kidney injury. All patients survived and after being discharged, two patients required chronic intermittent haemodialysis. Interestingly, at the time of admission, all patients had elevated lactate levels but there was no linear regression between toxic levels and lactate levels and no linear correlation was found between initial lactate levels and anion gap and osmolal gap.
Conclusions.
The initial diagnosis of ethylene glycol poisoning is difficult and poisoning with ethylene glycol is rare but life threatening and needs rapid recognition and early treatment. Therefore, intoxication with ethylene glycol should not be misdiagnosed as lactic acidosis in patients with metabolic acidosis and elevated lactate levels.
Keywords: acute kidney injury, ethylene glycol poisoning, lactate levels
Introduction
Hundreds of fatal intoxication cases with methanol and glycol occur around the world annually as small quantities of these alcohols can produce significant toxicity. Furthermore, these agents are widely available in household and commercial products (automotive anti-freeze and de-icing solutions, solvents, cleaners, windshield wiper fluid, fuels and other industrial products). Ethylene glycol has a sweet taste, is colourless and poisoning is more frequent than methanol intoxication. Poisoning induced by alcohols requires rapid recognition and early treatment as they can cause neurological changes, acute kidney injury and death.
The mortality rate of ethylene glycol intoxication ranges between 1 and 22% depending on the amount of alcohol ingestion and the time span between alcohol ingestion and initiation of therapy [1–3].
Because of the widespread availability of toxic alcohols, ingestion should be suspected in patients with a typical medical history consistent with ingestion, metabolic acidosis and a widened anion and osmolal gap in the serum chemistry.
The study objective was to analyse retrospectively the course and outcome of six cases of ethylene glycol intoxication. Additionally, the role of lactate levels in patients with ethylene glycol poisoning was investigated.
Materials and methods
Data from six patients admitted to the emergency department between 1999 and 2011 because of ethylene glycol poisoning are reported. The initial diagnosis based on a detailed clinical history in combination with a detailed laboratory work-up with sufficient evidence of ethylene glycol intake in combination with the presence of metabolic acidosis with elevation of the osmolal or anion gaps. Anion gap was calculated using the standard formula (Na+ − Cl− − HCO3 −) and corrected using the Figge equation: {4.4 – [observed serum albumin (g/dL) × 2.5] + AG}. Osmolal gap was calculated by substracting the osmolality measured (osmometer) from the osmolality calculated with the standard formula. Calculated serum osmolality (mOsm/L)= (2 × Na+)+ Urea (mmol/L) + glucose (mmol/L) + 1.25 × ethanol (mmol/L). Serum levels of ethylene glycol were measured by gas chromatography and routine procedures were used to test all other parameters (arterial blood gas, electrolytes, osmolality, urine analysis, etc.).
Due to the metabolic acidosis, all patients were treated with sodium bicarbonate infusions and received volume expansion with saline to promote diuresis. When necessary, haemodialysis (HD) was performed via a temporary dialysis catheter.
Statistical analysis
Data are reported as mean ± SD unless otherwise specified. Linear regression was calculated using the coefficient of determination R 2 with .
Results
Between 1999 und 2011, six patients with ethylene glycol intoxication were admitted to our emergency department. All patients were men and the mean age was 59.3 ± 20.8 (range 22–84) years. Five of six patients were somnolent at the time of admission to the emergency department, therefore it was not possible to obtain these patients' medical histories. Two of the six patients (29%) required mechanical ventilation, four patients had an acute kidney injury (57%) and one patient had an acute-on-chronic kidney injury. Only one patient (16.7%) presented urinary oxalate crystals at time of admission and all patients had ethanol levels in the normal range. All patients with acute kidney injury received HD (Table 1). Mean pH on admission was 7.15 ± 0.20 (range 6.83–7.41), mean initial anion gap was 27.8 ± 12.4 mmol/L (range 7–43.5) and mean osmolal gap was 33.3 ± 5.99 mOsm/L (range 22–40). Mean base deficit on admission was 18.6 ± 10.9 (range –27.7 to 0.9) (Table 2). Five of six patients had an acidosis in the blood gas analysis on admission. One patient (Case 4) had no acidosis because the intoxication was only 30 min before blood gas analysis in the emergency department. This patient had severe learning difficulties and was referred to the emergency room by ambulance with his parents. The parents reported that he had ingested several mouthfuls of windshield wiper fluid only 30 min ago. He was only treated with intravenous ethanol. Under this therapy, he developed no acidosis and was discharged from the intensive care unit 1 day later. All other patients received HD for initial therapy, four of six patients received intravenous ethanol. No patient died, two patients required dialysis on a regular basis, three times a week after being discharged from hospital. Case 2 was admitted with an acute kidney injury and required regular HD sessions for a total of 14 days until recovery of kidney function, while Case 6 still requires chronic intermittent HD (CIHD) and an implantation of a tunnelled dialysis catheter was done for long-term access for dialysis.
Table 1.
Clinical data of patients intoxicated with ethylene glycola
| Case | Age | Toxic levels (mg/dL) | Treatment | Mechanical ventilation | Prognosis |
| 1 | 84 | 50 | ET + HD | No | Alive, CIHD |
| 2 | 61 | ND | ET + HD | No | Alive, no HD |
| 3 | 62 | 450 | HD | No | Alive, no HD |
| 4 | 22 | 840 | ET | No | Alive, no HD |
| 5 | 56 | 370 | HD | Yes | Alive, no HD |
| 6 | 71 | 550 | ET + HD | Yes | Alive, CIHD |
Toxic levels include ethylene glycol levels or glycolate levels (Case 1); ET, ethanol; ND, not determined.
Table 2.
Laboratory test results on admission in intoxicated patients
| Case | Na+ (mmol/L) | K+ (mmol/L) | Cl− (mmol/L) | Anion gap (mmol/L) | Osmolal gap (mOsm/L | pH | PCO2 (mmHg) | Base deficit (mmol/L) | Creatinine (mg/dL) | Urea (mg/dL) | Lactate (mmol/L) | Urinary oxalate crystals present |
| 1 | 140 | 8.5 | 100 | 43.5 | 35 | 6.826 | 32.5 | 27.7 | 18 | 419 | 3774.4 | No |
| 2 | 141 | 5.6 | 101 | 31 | 22 | 7.141 | 106 | −24.5 | 2.1 | 28 | 252.2 | Yes |
| 3 | 139 | 3.9 | 104 | 21 | 40 | 7.288 | 28 | −12 | 1.4 | 54 | 486.4 | No |
| 4 | 139 | 3.3 | 107 | 7 | 34 | 7.415 | 39.6 | 0.9 | 0.9 | 18 | 162.1 | No |
| 5 | 134 | 3.7 | 93 | 32 | 34 | 7.094 | 18.7 | −24.1 | 1.4 | 55 | 495.4 | No |
| 6 | 137 | 5.1 | 98 | 32 | 35 | 7.163 | 11.7 | −24.1 | 1.8 | 37 | 333.3 | No |
Interestingly, all patients had elevated lactate levels [mean 917.3 mmol/L ± 1405.7 (range 162–3774)] on admission, but there was no linear regression between toxic levels and lactate levels [R2 (coefficient of determination) = 0.27]. No linear correlation was found between initial lactate levels and anion gap (R 2 = 0.10), no linear correlation was found between initial lactate levels and osmolal gap (R 2 = 0.31) and no correlation was found between toxic levels and anion or osmolal gap (R 2 = 0.12, R 2 = 0.68).
Discussion
The initial diagnosis of ethylene glycol poisoning is difficult and is probably an often underdiagnosed cause of severe metabolic acidosis especially in the emergency department. Due to the mental state of some patients at the time of admission, gaining information on a patient's medical history is not always possible. The toxicity of ethylene glycol can be divided into three phases: early toxicity, 30 min–12 h, a central nervous system depressant phase can be noticed with stupor, nausea, vomiting and seizures. Later, 12–24 h after intoxication, a cardiorespiratory phase appears with onset of tachypnoea (compensate for systemic acidosis), hypotension or congestive heart failure. Twenty-four hours to 72 h after ingestion, the patients suffer from flank pain and oxalate crystalluria, often followed by the onset of acute oliguric kidney injury with the necessity of renal replacement therapy. It is important to know that these ‘phases’ could be present together. Patients with ethylene glycol intoxication are in a life-threatening situation and rapid early recognition and treatment are crucial as, if left untreated, morbidity and mortality are high [1–3]. Measurement of the serum levels of ethylene glycol or the degradation products is helpful, but not in case of emergency because analysis takes several days. Mostly, the patients present with a metabolic acidosis (86% at time of presentation) with an elevated anion gap or widened osmolal gap on admission [4–6]. The differential diagnosis of a metabolic acidosis with an elevated anion gap is difficult. They can be divided in an increasing of acid production (lactic acidosis, ketoacidosis or ingestion of methanol, ethylene glycol or aspirin) and a decreased renal acid excretion (chronic kidney disease) (Table 3). In cases with lactic acidosis, diabetic ketoacidosis or alcoholic ketoacidosis, the plasma osmolal gap is elevated as well.
Table 3.
Causes of metabolic acidosis with increased anion gap
| Increased acid production |
| Lactic acidosis |
| Type A: associated with clinical evidence of poor tissue perfusion or oxygenation of blood |
| Type B: associated with no clinical evidence of poor tissue perfusion or oxygenation of blood |
| Type D: associated with several causes of the short bowel syndrome with metabolization and absorption of D-lactic acid into the systemic circulation |
| Ingestions |
| Methanol |
| Ethylene glycol |
| Toluene (if early) |
| Aspirin |
| Diethylene glycol |
| Propylene glycol |
| Ketoacidosis |
| Diabetes mellitus |
| Alcohol-associated |
| Starvation |
| Pyroglutamic acid (5-oxoproline) |
| Decreased renal acid excretion |
| Chronic kidney disease |
A mild elevation of serum anion gap can occur in metabolic alkalosis, which may contribute to a rise in albumin concentration due to extracellular volume depletion or an increase in the number of negative charges per albumin molecule, since the pH is further away from the isoelectric point for albumin of ∼5.4 [7]. Large quantities of ethanol can raise the osmolal gap more than it would be expected based on the molecular weight [8].
In our case series, five of six patients (83%) required HD. HD is the most effective method to remove both toxic acid metabolites and parent alcohols rapidly [4]. In the emergency department, a nephrologist should be consulted immediately if toxic alcohol ingestion is suspected and the patient has evidence of end-organ dysfunction or acidaemia. Treatment with HD is recommended in the setting of known ethylene glycol ingestion if either there is a high anion gap metabolic acidosis, regardless of drug level, or there is evidence of end-organ damage (e.g. renal failure, visual changes). Additionally, HD is suggested in patients with a suspected toxic ethylene glycol ingestion who present with a severe but otherwise unexplained anion gap metabolic acidosis and a wide plasma osmolal gap. HD is often unnecessary in patients with a normal or near normal pH and whether the serum creatinine remains normal [4].
The American Academy of Clinical Toxicology recommends fomepizole in patients with ethylene poisoning in early stages to inhibit the alcohol dehydrogenase [4]. The alcohol dehydrogenase is the initial step in the metabolic pathway and metabolizes ethylene glycol to glycoaldehyde. In Europe, fomepizole is not a routine treatment. Intravenous ethanol and HD are the most important therapies in patients with ethylene poisoning. Maintaining of appropriate ethanol levels is difficult in everyday practice, therefore, frequent testing and infusion adjustments are mandatory. An intravenous loading dose of 800 mg/kg ethanol in a 10% vol/vol solution will raise serum ethanol concentrations by ∼100 mg/dL. A maintenance infusion of 80–160 mg/kg/h, or more during HD, should be titrated according to serial ethanol concentrations.
Data regarding a superiority of fomepizole versus ethanol and/or HD are lacking and the drug is very expensive. The ethylene glycol metabolism is summarized in Figure 1.
Fig. 1.
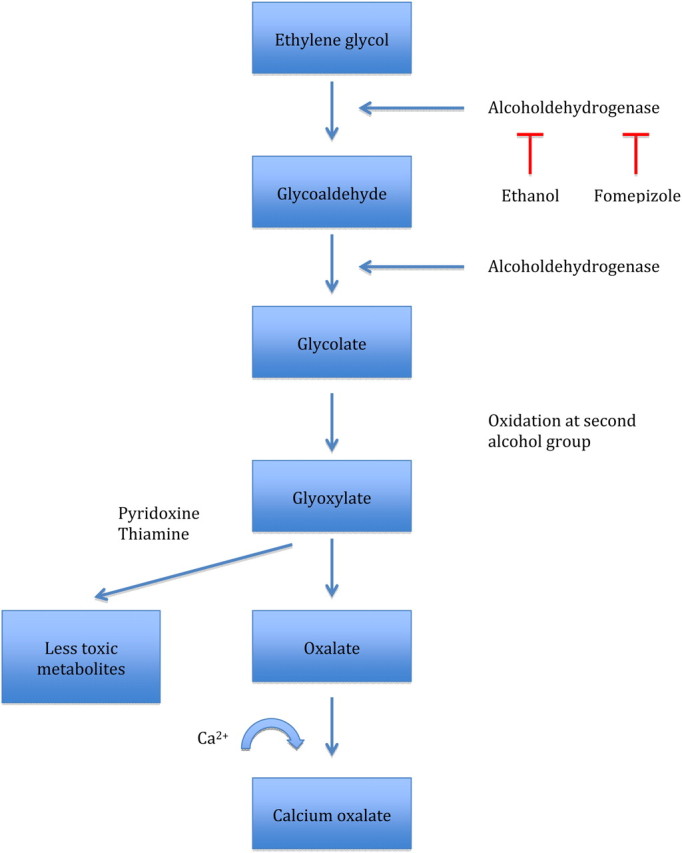
Ethylene glycol metabolism.
The role of lactate is still under discussion. In 1968, Gabow et al. [9] showed that patients with ethylene glycol poisoning often present with elevations in their serum lactate concentration. The question is whether such elevations of lactate levels (usually minor) are substantial or are likely errors of measurement of some laboratory instruments that cannot differentiate between lactate and glycolate, which is structurally similar to lactate and occurs when ethylene glycol is metabolized to glycolate [10–15]. Meng et al. [16] recommend that elevated lactate concentrations on blood gas analysers should be confirmed by a chemistry analyser in the differential diagnosis of ethylene glycol poisoning. Other studies showed that lactic acidosis is more prominent in patients with the highest serum glycolate levels [5, 9]. In our case series, all patients had elevated serum lactate levels, but there was no correlation between glycol levels and lactate levels (R 2 = 0.27). There was no correlation between serum lactate levels and osmolal or anion gap (R 2 = 0.12, R 2 = 0.68) as it is reported between osmolal gap and serum alcohol levels [17].
Traditionally, strong elevated serum lactate levels in a severely acidotic patient suggest diagnoses like tissue hypoxia, ketoacidosis or metformin poisoning. It is important not to exclude poisoning with ethylene glycol as a possible cause of severe acidosis as you can see in our cases. Especially in continental Europe, blood gases are still under-utilized in the management of acutely ill patients in the emergency department, and especially junior trainees should have the ability to interpret the results of a blood gas analysis.
In conclusion, poisoning with ethylene glycol is rare but life threatening and needs rapid recognition and early treatment. Therefore, intoxication with ethylene glycol should not be misdiagnosed as a lactic acidosis in patients with metabolic acidosis and elevated lactate levels.
To our knowledge, our work is the largest case series in the present literature and emphasizes the importance of a structured work-up of metabolic disturbances.
Acknowledgments
Conflict of interest statement. None declared.
References
- 1.Porter WH, Rutter PW, Bush BA, et al. Ethylene glycol toxicity: the role of serum glycolic acid in hemodialysis. J Toxicol Clin Toxicol. 2001;39:607–615. doi: 10.1081/clt-100108493. [DOI] [PubMed] [Google Scholar]
- 2.Karlson-Stiber C, Persson H. Ethylene glycol poisoning: experiences from an epidemic in Sweden. J Toxicol Clin Toxicol. 1992;30:565–574. doi: 10.3109/15563659209017942. [DOI] [PubMed] [Google Scholar]
- 3.Litovitz TL, Klein-Schwartz W, White S, et al. 1999 annual report of the American Association of Poison Control Centers Toxic Exposure Surveillance System. Am J Emerg Med. 2000;18:517–574. doi: 10.1053/ajem.2000.9261. [DOI] [PubMed] [Google Scholar]
- 4.Barceloux DG, Krenzelok EP, Olson K, et al. American Academy of Clinical Toxicology Practice Guidelines on the treatment of ethylene glycol poisoning. Ad Hoc Committee. J Toxicol Clin Toxicol. 1999;37:537–560. doi: 10.1081/clt-100102445. [DOI] [PubMed] [Google Scholar]
- 5.Eder AF, McGrath CM, Dowdy YG, et al. Ethylene glycol poisoning: toxicokinetic and analytical factors affecting laboratory diagnosis. Clin Chem. 1998;44:168–177. [PubMed] [Google Scholar]
- 6.Moriarty RW, McDonald RH., Jr The spectrum of ethylene glycol poisoning. Clin Toxicol. 1974;7:583–596. doi: 10.3109/15563657408988033. [DOI] [PubMed] [Google Scholar]
- 7.Madias NE, Ayus JC, Adrogue HJ. Increased anion gap in metabolic alkalosis: the role of plasma-protein equivalency. N Engl J Med. 1979;300:1421–1423. doi: 10.1056/NEJM197906213002507. [DOI] [PubMed] [Google Scholar]
- 8.Purssell RA, Pudek M, Brubacher J, et al. Derivation and validation of a formula to calculate the contribution of ethanol to the osmolal gap. Ann Emerg Med. 2001;38:653–659. doi: 10.1067/mem.2001.119455. [DOI] [PubMed] [Google Scholar]
- 9.Gabow PA, Clay K, Sullivan JB, et al. Organic acids in ethylene glycol intoxication. Ann Intern Med. 1986;105:16–20. doi: 10.7326/0003-4819-105-1-16. [DOI] [PubMed] [Google Scholar]
- 10.Porter WH, Crellin M, Rutter PW, et al. Interference by glycolic acid in the Beckman synchron method for lactate: a useful clue for unsuspected ethylene glycol intoxication. Clin Chem. 2000;46:874–875. [PubMed] [Google Scholar]
- 11.Shirey T, Sivilotti M. Reaction of lactate electrodes to glycolate. Crit Care Med. 1999;27:2305–2307. doi: 10.1097/00003246-199910000-00050. [DOI] [PubMed] [Google Scholar]
- 12.Eder AF, Dowdy YG, Gardiner JA, et al. Serum lactate and lactate dehydrogenase in high concentrations interfere in enzymatic assay of ethylene glycol. Clin Chem. 1996;42:1489–1491. [PubMed] [Google Scholar]
- 13.Morgan TJ, Clark C, Clague A. Artifactual elevation of measured plasma L-lactate concentration in the presence of glycolate. Crit Care Med. 1999;27:2177–2179. doi: 10.1097/00003246-199910000-00017. [DOI] [PubMed] [Google Scholar]
- 14.Brindley PG, Butler MS, Cembrowski G, et al. Falsely elevated point-of-care lactate measurement after ingestion of ethylene glycol. CMAJ. 2007;176:1097–1099. doi: 10.1503/cmaj.061288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Verelst S, Vermeersch P, Desmet K. Ethylene glycol poisoning presenting with a falsely elevated lactate level. Clin Toxicol (Phila) 2009;47:236–238. doi: 10.1080/15563650802432954. [DOI] [PubMed] [Google Scholar]
- 16.Meng QH, Adeli K, Zello GA, et al. Elevated lactate in ethylene glycol poisoning: true or false? Clin Chim Acta. 2010;411:601–604. doi: 10.1016/j.cca.2010.01.003. [DOI] [PubMed] [Google Scholar]
- 17.Peces C, Gonzalez E, Olivas E, et al. [Effectiveness of pre-emptive hemodialysis with high-flux membranes for the treatment of life-threatening alcohol poisoning] Nefrologia. 2008;28:413–418. [PubMed] [Google Scholar]


