Abstract
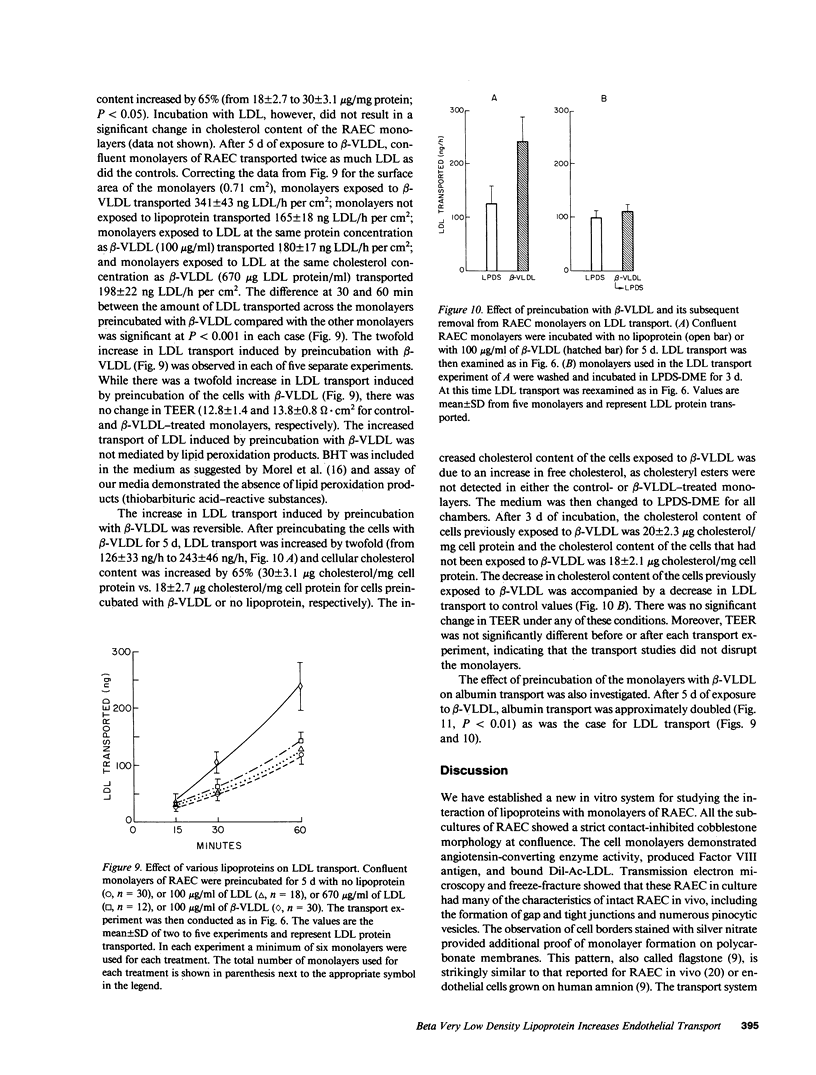
Rabbit aortic endothelial cells (RAEC) were grown on micropore filters in a new device. This system allowed in situ measurement of transendothelial electrical resistance (TEER). The monolayers demonstrated a TEER of 14 +/- 1 omega X cm2 at confluence. No difference was seen in the transport of low density lipoproteins (LDL) across endothelial cell monolayers obtained from normal or Watanabe heritable hyperlipidemic rabbits, indicating that the LDL receptor was not involved in the LDL transport. TEER was inversely correlated with 22Na transport (r2 = 0.93, P = less than 0.001) but not with 125I-LDL transport. The amount of LDL transported at 15 degrees C or across glutaraldehyde-fixed monolayers was half that of the controls at 37 degrees C. Preincubation of the monolayers with rabbit beta-migrating very low density lipoproteins (beta-VLDL) increased cholesterol content by 65%, and the transport of albumin and LDL doubled without a change in TEER. Removal of beta-VLDL from the culture medium resulted in the return of cellular cholesterol content and LDL transport to control values. We conclude that preincubation of RAEC with beta-VLDL resulted in an increased permeability to LDL and albumin, and that beta-VLDL may promote increased transendothelial transport of macromolecules in cholesterol-fed rabbits.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baker D. P., Van Lenten B. J., Fogelman A. M., Edwards P. A., Kean C., Berliner J. A. LDL, scavenger, and beta-VLDL receptors on aortic endothelial cells. Arteriosclerosis. 1984 May-Jun;4(3):248–255. doi: 10.1161/01.atv.4.3.248. [DOI] [PubMed] [Google Scholar]
- Bilheimer D. W., Eisenberg S., Levy R. I. The metabolism of very low density lipoprotein proteins. I. Preliminary in vitro and in vivo observations. Biochim Biophys Acta. 1972 Feb 21;260(2):212–221. doi: 10.1016/0005-2760(72)90034-3. [DOI] [PubMed] [Google Scholar]
- Buonassisi V., Venter J. C. Hormone and neurotransmitter receptors in an established vascular endothelial cell line. Proc Natl Acad Sci U S A. 1976 May;73(5):1612–1616. doi: 10.1073/pnas.73.5.1612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chobanian A. V., Menzoian J. O., Shipman J., Heath K., Haudenschild C. C. Effects of endothelial denudation and cholesterol feeding on in vivo transport of albumin, glucose, and water across rabbit carotid artery. Circ Res. 1983 Dec;53(6):805–814. doi: 10.1161/01.res.53.6.805. [DOI] [PubMed] [Google Scholar]
- Crone C., Christensen O. Electrical resistance of a capillary endothelium. J Gen Physiol. 1981 Apr;77(4):349–371. doi: 10.1085/jgp.77.4.349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fielding P. E., Fielding C. J., Havel R. J., Kane J. P., Tun P. Cholesterol net transport, esterification, and transfer in human hyperlipidemic plasma. J Clin Invest. 1983 Mar;71(3):449–460. doi: 10.1172/JCI110789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fogelman A. M., Haberland M. E., Seager J., Hokom M., Edwards P. A. Factors regulating the activities of the low density lipoprotein receptor and the scavenger receptor on human monocyte-macrophages. J Lipid Res. 1981 Sep;22(7):1131–1141. [PubMed] [Google Scholar]
- Frank J. S., Rich T. L., Beydler S., Kreman M. Calcium depletion in rabbit myocardium. Ultrastructure of the sarcolemma and correlation with the calcium paradox. Circ Res. 1982 Aug;51(2):117–130. doi: 10.1161/01.res.51.2.117. [DOI] [PubMed] [Google Scholar]
- Furie M. B., Cramer E. B., Naprstek B. L., Silverstein S. C. Cultured endothelial cell monolayers that restrict the transendothelial passage of macromolecules and electrical current. J Cell Biol. 1984 Mar;98(3):1033–1041. doi: 10.1083/jcb.98.3.1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goetzl E. J., Austen K. F. A neutrophil-immobilizing factor derived from human leukocytes. I. Generation and partial characterization. J Exp Med. 1972 Dec 1;136(6):1564–1580. doi: 10.1084/jem.136.6.1564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein J. L., Kita T., Brown M. S. Defective lipoprotein receptors and atherosclerosis. Lessons from an animal counterpart of familial hypercholesterolemia. N Engl J Med. 1983 Aug 4;309(5):288–296. doi: 10.1056/NEJM198308043090507. [DOI] [PubMed] [Google Scholar]
- Haraldsson B., Johansson B. R. Changes in transcapillary exchange induced by perfusion fixation with glutaraldehyde, followed by measurements of capillary filtration coefficient, diffusion capacity and albumin clearance. Acta Physiol Scand. 1985 May;124(1):99–106. doi: 10.1111/j.1748-1716.1985.tb07637.x. [DOI] [PubMed] [Google Scholar]
- Hennig B., Shasby D. M., Fulton A. B., Spector A. A. Exposure to free fatty acid increases the transfer of albumin across cultured endothelial monolayers. Arteriosclerosis. 1984 Sep-Oct;4(5):489–497. doi: 10.1161/01.atv.4.5.489. [DOI] [PubMed] [Google Scholar]
- King G. L., Johnson S. M. Receptor-mediated transport of insulin across endothelial cells. Science. 1985 Mar 29;227(4694):1583–1586. doi: 10.1126/science.3883490. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Loskutoff D. J., Edgington T. E. Synthesis of a fibrinolytic activator and inhibitor by endothelial cells. Proc Natl Acad Sci U S A. 1977 Sep;74(9):3903–3907. doi: 10.1073/pnas.74.9.3903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morel D. W., DiCorleto P. E., Chisolm G. M. Endothelial and smooth muscle cells alter low density lipoprotein in vitro by free radical oxidation. Arteriosclerosis. 1984 Jul-Aug;4(4):357–364. doi: 10.1161/01.atv.4.4.357. [DOI] [PubMed] [Google Scholar]
- POOLE J. C., SANDERS A. G., FLOREY H. W. The regeneration of aortic endothelium. J Pathol Bacteriol. 1958 Jan;75(1):133–143. doi: 10.1002/path.1700750116. [DOI] [PubMed] [Google Scholar]
- Renkin E. M., Curry F. E. Endothelial permeability: pathways and modulations. Ann N Y Acad Sci. 1982;401:248–259. doi: 10.1111/j.1749-6632.1982.tb25724.x. [DOI] [PubMed] [Google Scholar]
- Schwartz S. M. Selection and characterization of bovine aortic endothelial cells. In Vitro. 1978 Dec;14(12):966–980. doi: 10.1007/BF02616210. [DOI] [PubMed] [Google Scholar]
- Simionescu M., Simionescu N., Palade G. E. Segmental differentiations of cell junctions in the vascular endothelium. Arteries and veins. J Cell Biol. 1976 Mar;68(3):705–723. doi: 10.1083/jcb.68.3.705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simionescu N., Simionescu M., Palade G. E. Recent studies on vascular endothelium. Ann N Y Acad Sci. 1976;275:64–75. doi: 10.1111/j.1749-6632.1976.tb43338.x. [DOI] [PubMed] [Google Scholar]
- Territo M., Berliner J. A., Fogelman A. M. Effect of monocyte migration on low density lipoprotein transport across aortic endothelial cell monolayers. J Clin Invest. 1984 Dec;74(6):2279–2284. doi: 10.1172/JCI111655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Lenten B. J., Fogelman A. M., Hokom M. M., Benson L., Haberland M. E., Edwards P. A. Regulation of the uptake and degradation of beta-very low density lipoprotein in human monocyte macrophages. J Biol Chem. 1983 Apr 25;258(8):5151–5157. [PubMed] [Google Scholar]
- Wiklund O., Carew T. E., Steinberg D. Role of the low density lipoprotein receptor in penetration of low density lipoprotein into rabbit aortic wall. Arteriosclerosis. 1985 Mar-Apr;5(2):135–141. doi: 10.1161/01.atv.5.2.135. [DOI] [PubMed] [Google Scholar]









