Abstract
Purpose of Review
This review summarizes recently described actions of 1,25-dihydroxyvitamin D beyond its function in calcium homeostasis and bone metabolism.
Recent Findings
1,25-dihydroxyvitamin D stimulates the innate immune system, facilitating the clearance of infections such as tuberculosis. Hypovitaminosis D has been associated with several autoimmune disorders, various malignancies, and cardiovascular risk factors in a number of recent epidemiologic reports. Based on these observational reports, vitamin D and its analogues are being evaluated for the prevention and treatment of a variety of conditions, with early findings showing mixed results.
Summary
The broad tissue distribution of the 25-hydroxyvitamin D 1α-hydroxylase enzyme and the vitamin D receptor establish a role for 1,25-dihydroxyvitamin D in the pathophysiology of various disease states and provide new therapeutic targets for vitamin D and its analogues.
Keywords: Vitamin D, Vitamin D Receptor, 25-hydroxyvitamin D 1α-hydroxylase
INTRODUCTION
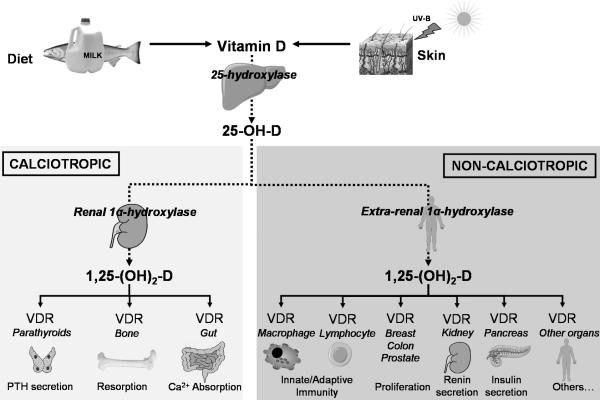
The major sources of vitamin D in humans are cutaneous synthesis, diet, and supplements. The initial step in vitamin D metabolism involves its hepatic hydroxylation, generating 25-hydroxyvitamin D (25-OH-D). 25-OH-D has a half-life in circulation of approximately 2 weeks, making its serum concentration the best biomarker of vitamin D status [1]. 25-OH-D is further hydroxylated by the mitochondrial 25-hydroxyvitamin D-1α-hydroxylase (CYP27B1) enzyme to form 1,25 dihydroxyvitamin D (1,25-(OH)2-D), the physiologically active form of vitamin D. 1,25-(OH)2-D signals via the vitamin D receptor (VDR), a nuclear receptor found in most organs (Table 1). The calciotropic actions of 1,25-(OH)2-D include enhancement of intestinal calcium and phosphorus absorption, suppression of parathyroid hormone secretion, and stimulation of bone resorption via VDR in intestinal, parathyroid, and bone cells respectively (Figure 1). The wide tissue distribution of the VDR led to the recognition of the non-calciotropic actions of 1,25-(OH)2-D (Figure 1), with roles beyond the physiological regulation of calcium transport and bone mineralization. This review summarizes recent studies that have shed light on these non-calciotropic actions.
Table 1.
Cellular and Tissue Distribution of Vitamin D Receptor
| Endocrine | Parathyroid gland, Thyroid, Pituitary, Adrenal |
| CNS | Brain neurons |
| Respiratory | Alveolar cells |
| Cardiovascular | Cardiac muscle |
| Gastrointestinal | Esophagus, Stomach, Hepatocytes, Small and Large Intestine |
| Renal / GU | Kidney, Urethra, Prostate |
| Reproductive | Testis, Ovary, Uterus, Placenta |
| Musculoskeletal | Osteoblasts, Osteocytes, Chondrocytes, Fibroblasts, Striated muscle |
| Epidermis | Skin, Hair follicle, Breast |
| Immune | Thymus, T-cells, B-cells, Macrophages |
Figure 1. Vitamin D Metabolism and Actions.
The calciotropic functions of 1,25-(OH)2-D include the physiological regulation of calcium transport and bone mineralization. The synthesis of circulating 1,25-(OH)2-D which mediates these calciotropic actions is tightly regulated. Non-calciotropic actions involve the activation of vitamin D receptors (VDRs) by locally produced 1,25-(OH)2-D in a number of tissues in a paracrine and autocrine fashion.
IMMUNOMODULATORY EFFECTS OF VITAMIN D
A major non-calciotropic function of 1,25-(OH)2-D is in the regulation of the immune system. Recent studies have unraveled some of the underlying mechanisms, suggesting a potential therapeutic role of vitamin D, its metabolites and its analogues for infectious and auto-immune disorders in humans.
Vitamin D and infectious disorders
Patients with rickets have impaired macrophage phagocytic function [2], which could be reversed by 1,25-(OH)2-D repletion [3]. In mice, targeted ablation of the 25-hydroxyvitamin D 1α-hydroxylase enzyme resulted in enlarged lymph nodes and a reduction in CD4- and CD8- positive peripheral T lymphocytes [4]. These findings suggest that vitamin D plays an essential role in the ability of immune system to fight infections.
Vitamin D and Tuberculosis
A potential role for vitamin D in the control of tuberculosis was first indirectly described in 1849 by Williams who observed that administration of cod liver oil (a major source of vitamin D) to patients with pulmonary tuberculosis “was more beneficial ... than any agent, medicinal, dietetic, or regiminal, that has yet been employed” [5]. Recent studies have shed new light on the mechanism of the anti-microbial action of 1,25-(OH)2-D, a mechanism that involves recognition of Mycobacterium Tuberculosis by toll-like receptors.
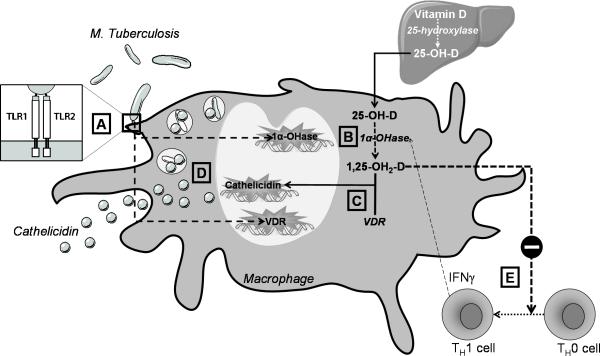
Toll-like receptors (TLRs) are a class of single membrane-spanning receptors that recognize structurally conserved molecules derived from microbes, and activate immune responses. TLR2 in particular contributes to innate immunity against M. tuberculosis [6]. A recent study investigating the underlying mechanisms in humans identified the 1α-hydroxylase and VDR as two genes that are uniquely upregulated in monocytes/macrophages in response to TLR activation by M. tuberculosis [7**]. In the presence of sufficient 25-OH-D, this expression leads to a 1,25-(OH)2-D–dependent induction of the antimicrobial peptide cathelicidin and enhanced killing of intracellular M. tuberculosis (Figure 2). In the same study, sera from African-American subjects with low 25-OH-D concentrations were inefficient in supporting cathelicidin mRNA induction [7**]. This may be the basis for the observation that African-Americans, in whom vitamin D deficiency is more prevalent, have increased susceptibility to tuberculosis [8].
Figure 2. Vitamin D and the Immune Response to Mycobacterium Tuberculosis.
Activation of the Toll-Like Receptor 2/1 (TLR 2/1) heterodimer by Mycobacterium tuberculosis (A) upregulates the expression of 1α-hydroxylase and VDR genes in monocytes and macrophages (B). In the presence of sufficient 25-OH-D, this upregulation leads to a 1,25-(OH)2-D–dependent induction of the antimicrobial peptide cathelicidin (C) and enhanced killing of intracellular M. tuberculosis (D). In a granuloma, surrounding T-helper 1 (TH1) cells produce γ-interferon (INFγ) which further enhances 1α-hydroxylase expression (E). Excess 1,25-(OH)2-D inhibits the differentiation of undifferentiated T-helper cells (TH0) to TH1 cells in a paracrine fashion (E), providing a mechanism to prevent excessive 1,25-(OH)2-D production.
While it appears that 1,25-(OH)2-D plays a cardinal role in antimycobacterial immunity in vitro, most existing studies investigating the effects of vitamin D provision in TB treatment in vivo are methodologically flawed [9]. The only study examining the role of vitamin D added to standard anti-mycobacterial therapy is a randomized placebo-controlled trial in 67 Indonesian patients with pulmonary tuberculosis [10]. Compared with placebo, provision of 10,000 IU vitamin D daily for 6 weeks was associated with a higher rate of sputum conversion and a greater frequency of radiological improvement, with no cases of hypercalcemia observed [10]. In another recent randomized double-blind controlled trial, a single oral dose of 100,000 IU vitamin D enhanced the ability of participants’ whole blood to restrict growth of recombinant reporter mycobacteria in vitro [11]. More prospectively designed and controlled studies are needed to firmly establish whether correcting vitamin D deficiency, a relatively simple and inexpensive measure, reduces the incidence of tuberculosis in at risk populations, and facilitates clearance of the infection in affected individuals.
Other infections
Cathelicidin exhibits a broad spectrum of antimicrobial activity against a number of bacteria, fungi, and viral pathogens [12]. Since induction of cathelicidin expression was recently shown to be vitamin D-dependent [7**], vitamin D could potentially enhance host defense against infections other than tuberculosis, such urinary tract infections [13] and epidemic influenza [14]. Although case control studies have found associations between 25-OH-D deficiency and various infections [15,16], data from randomized trials is extremely limited. Intramuscular 1,25-(OH)2-D co-administration with influenza vaccine in a randomized fashion did not appear to enhance humoral immunity in healthy young volunteers [17]. A trend to less frequent self-reported infections and antibiotic use was observed with vitamin D supplementation in the RECORD trial, a large randomized, placebo-controlled trial of oral vitamin D3 for the secondary prevention of osteoporotic fractures [18]. Additional randomized studies are clearly needed to assess whether vitamin D or its analogues can either prevent or alter the course of an infectious disorder.
Vitamin D and auto-immune disorders
Besides promoting innate immunity, another major effect of 1,25-(OH)2-D on the immune system is the suppression of the adaptive immune system and generation of tolerance and anergy. At the cellular level, dendritic cells exhibit reduced expression of major histocompatibility complex (MHC) class II molecules and adhesion molecules necessary for full T-cell stimulation in the presence of 1,25-(OH)2-D. Furthermore, 1,25-(OH)2-D shifts the balance of helper T-cells from Th1 to Th2 and T-regulatory cells through inhibition of interleukin 12 production. Epidemiologic reports have correlated limited sunlight exposure, reduced dietary vitamin D intake and/or 25-OH-D levels with a number of auto-immune diseases, and studies have shown effectiveness of 1,25-(OH)2-D and its analogs in a variety of animal models of these autoimmune disorders.
Multiple sclerosis
The prevalence of multiple sclerosis (MS) is well known to increase with latitude both north and south of the equator [19]. Although genetic predisposition likely plays a role in this variation [20], the role of sunshine was proposed several decades ago [21], and recent epidemiologic and experimental studies have provided evidence that high levels of vitamin D may decrease the risk of MS. Intake of vitamin D from diet and from supplements was inversely associated with risk of MS in the Nurses’ Health Study and Nurses’ Health Study II [22]. In a nested case-control study among > 7 million US military personnel who had serum samples stored in the Department of Defense Serum Repository, the risk of MS significantly decreased with increasing levels of 25-hydroxyvitamin D [23*]. Studies in experimental allergic encephalomyelitis (EAE), a widely used animal model for human MS disease, also suggest a protective role of 1,25-(OH)2-D in MS, through inhibition of monocyte trafficking to the CNS, and suppression of chemokine synthesis [24]. In a recently published small uncontrolled pilot study, administration of high doses of vitamin D (up to 280,000 IU per week) to MS patients was well-tolerated, with a tendency toward a decrease in the number of gadolinium-enhancing lesions per patient [25]. Randomized controlled studies will address whether vitamin D supplementation can alter the clinical course of MS.
Systemic Lupus Erythematosus
The threefold greater incidence of systemic lupus erythematosus (SLE) in African-Americans and increased morbidity and mortality compared to Caucasians [26] has been attributed to lower serum 25-OH-D concentrations [27]. In support of this hypothesis, 25-OH-D levels were significantly lower in recently diagnosed SLE patients compared to controls, and severe deficiency was associated with the presence of renal disease and photosensitivity [28]. In a different cohort of lupus patients, severe 25-OH-D deficiency was associated with higher disease activity measures consistent with a poorer general health status, but lower levels of antibodies to double-stranded DNA (dsDNA) than in patients with normal serum 25-OH-D [29]. In a murine model of lupus, the MRL/lpr mouse, treatment with 22-oxa-1,25-dihydroxyvitamin D3, an analog of 1,25-(OH)2-D significantly improved longevity and reduced proteinuria [30]. On the other hand, vitamin D intake from food and supplements was not associated with risk of SLE in two large cohorts, the Nurses’ Health Study and Nurses’ Health Study II (186,389 women) [31]. Thus, the role of vitamin D deficiency in the pathogenesis of SLE and whether vitamin D supplementation can improve the course of the disease is still not entirely proven.
Type 1 diabetes
Type 1 diabetes mellitus (T1DM) is another auto-immune disorder that may be modulated by vitamin D [32]. In non-obese diabetic (NOD) mice, a mouse model of human T1DM, vitamin D deficiency increases the incidence of diabetes [33], and oral administration of 1,25-(OH)2-D prevents disease onset [34]. On the other hand, NOD mice lacking the VDR had the same course of disease as NOD mice with functional VDR, despite having worse immune abnormalities [35].
Low serum 25-OH-D levels have been reported in patients with T1DM from several countries [36,37]. While use of cod liver oil [38] or vitamin D supplementation [39,40] in infancy were associated with a decreased risk of T1DM in older uncontrolled studies, no association was found between an intermediate dose of vitamin D supplementation during infancy and development of diabetes-related autoantibodies at the age of 1 and 2.5 years [41]. An ongoing randomized controlled trial is currently recruiting individuals at risk for T1DM in Manitoba, Canada to address this issue [42].
ANTI-PROLIFERATIVE PROPERTIES OF VITAMIN D
A major non-calciotriopic action of 1,25-(OH)2-D is its modulation of benign and malignant hyperproliferative conditions. This property was first clinically applied in the treatment of psoriasis, a recurrent inflammatory skin disorder, characterized by keratinocyte hyperproliferation and abnormal differentiation. Calcipotriol (calcipotriene or Dovonex®), a synthetic 1,25-(OH)2-D analog chemically engineered to be metabolized quickly in systemic circulation and hence a less calcemic compound than 1,25-(OH)2-D, has become first-line therapy for plaque psoriasis, either as monotherapy or in combination.
Vitamin D and Cancer Epidemiology
Recent epidemiologic studies have described inverse associations between biomarkers of sunlight exposure, dairy products and/or dietary vitamin D and the risk of various malignancies including colon cancer [43], prostate cancer [44] breast cancer [45], and pancreatic cancer [46]. In Caucasians, solar UV-B exposure was inversely correlated with incidence and mortality for ten cancers: bladder, colon, Hodgkin lymphoma, myeloma, other biliary, prostate, rectum, stomach, uterus, and vulva [47]. Most recently, in data from the National Health and Nutrition Examination Study (NHANES) III, serum 25-OH-D levels was not associated with total cancer mortality, although in the same study, individuals with higher 25-OH-D levels had significantly lower risk of colon cancer mortality [48*]. A variety of experimental animal models and in vitro studies have shown that 1,25-(OH)2-D exerts anti-neoplastic effects by regulating cellular proliferation, differentiation, apoptosis, and angiogenesis.
Vitamin D and Cancer Prevention and Treatment
While the association between hypovitaminosis D and the incidence of various malignancies is relatively well-established, the role of vitamin D supplementation in the prevention and treatment of cancer is still not entirely clear. In a 4-year study of 1179 community-dwelling postmenopausal women randomly assigned to calcium supplementation alone, calcium and vitamin D supplementation, or placebo, cancer incidence was significantly lower in the Ca+D women than in the placebo control subjects (P< 0.03), and serum 25-OHD level was a significant independent predictor of cancer risk [49*]. The results of this study are limited because of the small number of actual malignancies observed, but also suggest that calcium itself may play a big role beyond that of vitamin D. The largest randomized placebo-controlled study of vitamin D and the risk of colon cancer is the Women's Health Initiative, in which over 36,000 women were randomized to receive 400 IU vitamin D along with 1,000 mg elemental calcium or placebo daily [50*]. The incidence of invasive colorectal cancer after 7 years did not differ significantly between women assigned to calcium/vitamin D supplementation and those assigned to placebo. It is plausible that the dose of vitamin D used was too small to show an effect, and poor compliance and vitamin D use by placebo treated patients may have masked a potential protective effect. In men with advanced, androgen-insensitive prostate cancer treatment the use of DN-101, a 1,25-(OH)2-D analog, along with docetaxel was associated with improved survival, although DN-101 did not produce a statistically significant improvement in the PSA response rate, the primary end point of the study [51]. A follow-up phase III clinical trial was halted early due to a higher death rate among DN-101-treated patients, although the cause of the excess mortality has not been reported.
The anti-neoplastic properties of 1,25-(OH)2-D include regulating apoptosis and angiogenesis, and promoting cellular proliferation of normal and cancerous cells while inducing their terminal differentiation [52]. The major side effect of vitamin D and calcitriol that limits their expanded use and clinical development as anti-neoplastic agents is hypercalcemia. Consequently, vitamin D analogs with less hypercalcemic liability have been / are being developed as preventative and therapeutic agents [53**], and certain compounds are currently in various stages of clinical investigation [54].
VITAMIN D AND CARDIOVASCULAR DISEASE / RISK FACTORS
In small case-control studies, 25-OH-D deficiency is associated with a greater incidence of myocardial infarction [55,56]. In the Framingham Offspring Study, 25-OH-D deficiency was associated with incident cardiovascular disease, particularly in hypertensive individuals [57*]. This link may be in part related to the association of lower 25-OH-D levels with a number of traditional cardiovascular risk factors including obesity, hypertension, hyperglycemia and hypertriglyceridemia [58*], the metabolic syndrome [59] as well as other confounders such as lower advanced age, socioeconomic class, and decreased physical activity.
A number of studies have described lower serum 25-OH-D levels among obese individuals, typically associated with elevation in serum PTH, and similar serum 1,25-(OH)2-D when compared with non-obese controls [60-62]. The association between obesity and hypovitaminosis D may be in part due to reduced vitamin D bioavailability from sequestration in adipose tissue, as obese individuals exhibit a blunted rise in serum vitamin D in response to sun light and to orally supplemented vitamin D [63]. While reduced sun exposure related to a more sedentary lifestyle is another postulated mechanism linking obesity to hypovitaminosis D, this theory was refuted in a recent study [64]. Among morbidly obese individuals, 25-OH-D deficiency was more prevalent in those with the metabolic syndrome [60], suggesting that factors besides adiposity may be important. Relationships between hypovitaminosis D and insulin resistance and inflammation should be further explored in this observed clinical association.
The relationship between vitamin D and blood pressure was first described over two decades ago [65], and 1,25-(OH)2-D was shown to negatively regulate the renin-angiotensin-aldosterone system (RAAS) in studies in VDR knock-out mice [66]. Recent reports on the relationship between vitamin D and the risk of hypertension from three large independent prospective cohorts, the Nurses’ Health Study I and II, and the Health Professionals’ Follow-up Study have yielded conflicting results: While intake of vitamin D was not associated with the risk of developing hypertension [67], lower serum 25-OH-D levels conferred a greater risk of incident hypertension [68].
Hypovitaminosis D has also been linked to greater risk for type 2 diabetes and worse glycemic control in a number of case control studies [69*]. In NHANES III, lower serum 25-OH-D levels was associated with a greater degree of insulin resistance as measured by HOMA (Homeostasis Model for Assessment of insulin resistance), and a higher prevalence of diabetes mellitus among Caucasians and Hispanics [70]. Potential mechanisms underlying this association include the influence of vitamin D on insulin secretion and peripheral actions. Interventional studies examining the role of vitamin D administration on insulin resistance and the risk of type 2 diabetes in humans are not entirely conclusive [69*]. Short-term interventional studies with a number of vitamin D metabolites did not show changes in fasting serum glucose or insulin [71,72]. On the other hand, compared with placebo, combined vitamin D and calcium supplementation for 3 years was associated with a smaller rise in fasting serum glucose in subjects with impaired fasting glucose [73].
It remains unclear whether the association of 25-OH-D deficiency with increased cardiovascular disease can be entirely explained by traditional cardiovascular risk factors or if additional mechanism(s) are involved. In fact, 1,25-(OH)2-D may have additional effects via modulation of pro- and anti-inflammatory cytokines linked to cardiovascular disease such as TNF-α and IL-10 [74], and through direct activation of the VDR expressed in cardiac myocytes and vascular smooth muscle cells [75,76]. On the other hand, a potentially detrimental action of 1,25-(OH)2-D on the cardiovascular system is its promotion of vascular calcification [77].
IMPLICATIONS OF THE NON-CALCITRIOPIC ACTIONS OF VITAMIN D IN PATIENTS WITH CHRONIC KIDNEY DISEASE
The non-calciotropic effects of vitamin D are modulated by the presence of extra-renal 1α-hydroxylase enzyme which metabolizes 25-OH-D to 1,25-(OH)2-D locally in a number of tissues. This highlights the importance of adequate serum 25-OH-D levels as a substrate for these effects. The serum concentration of 25-OH-D estimated as optimal for both calciotropic (PTH suppression, intestinal calcium absorption, fracture prevention) and non-calciotropic outcomes (colorectal cancer, muscle strength) is >30 ng/ml (75 nmol/L) [78], although this level has been disputed by some [79]. The vast majority of patients with renal insufficiency are 25-OH-D deficient by this criteria [80], and repletion of 25-OH-D stores may potentially ameliorate the immune and cardiovascular function in these patients via non-calciotropic mechanisms. This is highlighted by a recent nested case-control study sample of 1,000 hemodialysis patients in whom severe 25-OH-D deficiency was associated with increased mortality within 90 days of hemodialysis initiation [80]. Thus, until data from prospective controlled studies become available, ergocalciferol administration to patients with stage 3 and 4 chronic kidney disease with serum 25-OH-D < 30 ng/ml should be strongly considered, especially since such a treatment also has a favorable effect on serum PTH [81].
Treatment of patients on hemodialysis with 1,25-(OH)2-D or its analogs for PTH suppression has been associated with improved survival in several large cohort retrospective uncontrolled studies [80,82-84]. However, a recent meta-analysis of published randomized controlled studies did not substantiate this survival advantage [85*]. It is unclear whether this disparity in the findings is due to the small number of subjects reviewed in the meta-analysis or because of the nonrandom assignment of therapy in the observational studies (resulting in differences in baseline characteristics of patients, with sicker individuals not receiving vitamin D analogs). Large prospective randomized trials are needed to settle this debate. Even when such studies become available, it will be difficult to identify the isolated effects of vitamin D on mortality from effects of changes in serum calcium, phosphorus and PTH which occur during supplementation with 1,25-(OH)2-D and its analogs.
CONCLUSION
The wide tissue distribution of the 1α-hydroxylase enzyme and the VDR establish a role for vitamin D beyond its function in calcium homeostasis and bone metabolism. Accumulating evidence suggests that adequate vitamin D levels are required for prevention of malignancy and optimal function of the immune and cardiovascular systems. While vitamin D and its analogs have a potential therapeutic role for a range of medical conditions, clinical studies to date have not conclusively demonstrated efficacy.
ACKNOWLEDGEMENT
The author is supported by the National Institutes of Health (K23RR21710).
REFERENCES
- 1.Holick MF. The use and interpretation of assays for vitamin D and its metabolites. J Nutr. 1990;120(Suppl 11):1464–1469. doi: 10.1093/jn/120.suppl_11.1464. [DOI] [PubMed] [Google Scholar]
- 2.Stroder J, Kasal P. Evaluation of phagocytosis in rickets. Acta Paediatr Scand. 1970;59:288–292. doi: 10.1111/j.1651-2227.1970.tb09005.x. [DOI] [PubMed] [Google Scholar]
- 3.Bar-Shavit Z, et al. 1,25-dihydroxyvitamin D3 and the regulation of macrophage function. Calcif Tissue Int. 1981;33:673–676. doi: 10.1007/BF02409507. [DOI] [PubMed] [Google Scholar]
- 4.Panda DK, et al. Targeted ablation of the 25-hydroxyvitamin D 1alpha-hydroxylase enzyme: evidence for skeletal, reproductive, and immune dysfunction. Proc Natl Acad Sci U S A. 2001;98:7498–7503. doi: 10.1073/pnas.131029498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Williams CJB. Cod liver oil in phthisis. London J Med. 1849;1:1–18. [PMC free article] [PubMed] [Google Scholar]
- 6.Stenger S, Modlin RL. Control of Mycobacterium tuberculosis through mammalian Toll-like receptors. Curr Opin Immunol. 2002;14:452–457. doi: 10.1016/s0952-7915(02)00355-2. [DOI] [PubMed] [Google Scholar]
- 7**.Liu PT, et al. Toll-like receptor triggering of a vitamin D-mediated human antimicrobial response. Science. 2006;311:1770–1773. doi: 10.1126/science.1123933. [Vitamin D was shown to mediate the activation of toll-like receptors by M. Tuberculosis lipoproteins in macrophages and the subsequent mycobacterial killing by cathelicidin, an antimicrobial peptide in this landmark publication.] [DOI] [PubMed] [Google Scholar]
- 8.Stead WW, et al. Racial differences in susceptibility to infection by Mycobacterium tuberculosis. N Engl J Med. 1990;322:422–427. doi: 10.1056/NEJM199002153220702. [DOI] [PubMed] [Google Scholar]
- 9.Martineau AR, et al. Vitamin D in the treatment of pulmonary tuberculosis. J Steroid Biochem Mol Biol. 2007;103:793–798. doi: 10.1016/j.jsbmb.2006.12.052. [DOI] [PubMed] [Google Scholar]
- 10.Nursyam EW, Amin Z, Rumende CM. The effect of vitamin D as supplementary treatment in patients with moderately advanced pulmonary tuberculous lesion. Acta Med Indones. 2006;38:3–5. [PubMed] [Google Scholar]
- 11.Martineau AR, et al. A single dose of vitamin D enhances immunity to mycobacteria. Am J Respir Crit Care Med. 2007;176:208–213. doi: 10.1164/rccm.200701-007OC. [DOI] [PubMed] [Google Scholar]
- 12.Durr UH, Sudheendra US, Ramamoorthy A. LL-37, the only human member of the cathelicidin family of antimicrobial peptides. Biochim Biophys Acta. 2006;1758:1408–1425. doi: 10.1016/j.bbamem.2006.03.030. [DOI] [PubMed] [Google Scholar]
- 13.Zasloff M. Antimicrobial peptides, innate immunity, and the normally sterile urinary tract. J Am Soc Nephrol. 2007;18:2810–2816. doi: 10.1681/ASN.2007050611. [DOI] [PubMed] [Google Scholar]
- 14.Cannell JJ, et al. Epidemic influenza and vitamin D. Epidemiol Infect. 2006;134:1129–1140. doi: 10.1017/S0950268806007175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Muhe L, et al. Case-control study of the role of nutritional rickets in the risk of developing pneumonia in Ethiopian children. Lancet. 1997;349:1801–1804. doi: 10.1016/S0140-6736(96)12098-5. [DOI] [PubMed] [Google Scholar]
- 16.Wayse V, et al. Association of subclinical vitamin D deficiency with severe acute lower respiratory infection in Indian children under 5 y. Eur J Clin Nutr. 2004;58:563–567. doi: 10.1038/sj.ejcn.1601845. [DOI] [PubMed] [Google Scholar]
- 17.Kriesel JD, Spruance J. Calcitriol (1,25-dihydroxy-vitamin D3) coadministered with influenza vaccine does not enhance humoral immunity in human volunteers. Vaccine. 1999;17:1883–1888. doi: 10.1016/s0264-410x(98)00476-9. [DOI] [PubMed] [Google Scholar]
- 18.Avenell A, et al. Vitamin D supplementation to prevent infections: a sub-study of a randomised placebo-controlled trial in older people (RECORD trial, ISRCTN 51647438). Age Ageing. 2007;36:574–577. doi: 10.1093/ageing/afm091. [DOI] [PubMed] [Google Scholar]
- 19.Kurtzke JF. Geography in multiple sclerosis. J. Neurol. 1977;215:1–26. doi: 10.1007/BF00312546. [DOI] [PubMed] [Google Scholar]
- 20.Compston A, Sawcer S. Genetic analysis of multiple sclerosis. Curr Neurol Neurosci Rep. 2002;2:259–266. doi: 10.1007/s11910-002-0085-3. [DOI] [PubMed] [Google Scholar]
- 21.Acheson ED, Bachrach CA, Wright FM. Some comments on the relationship of the distribution of multiple sclerosis to latitude, solar radiation, and other variables. Acta Psychiatr Scand Suppl. 1960;35:132–147. doi: 10.1111/j.1600-0447.1960.tb08674.x. [DOI] [PubMed] [Google Scholar]
- 22.Munger KL, et al. Vitamin D intake and incidence of multiple sclerosis. Neurology. 2004;62:60–65. doi: 10.1212/01.wnl.0000101723.79681.38. [DOI] [PubMed] [Google Scholar]
- 23*.Munger KL, et al. Serum 25-hydroxyvitamin D levels and risk of multiple sclerosis. JAMA. 2006;296:2832–2838. doi: 10.1001/jama.296.23.2832. [Case-control study based on a depository of serum samples showing a greater risk for multiple sclerosis in vitamin D deficient Caucasian individuals.] [DOI] [PubMed] [Google Scholar]
- 24.Pedersen LB, et al. 1,25-dihydroxyvitamin D3 reverses experimental autoimmune encephalomyelitis by inhibiting chemokine synthesis and monocyte trafficking. J Neurosci Res. 2007;85:2480–2490. doi: 10.1002/jnr.21382. [DOI] [PubMed] [Google Scholar]
- 25.Kimball SM, et al. Safety of vitamin D3 in adults with multiple sclerosis. Am J Clin Nutr. 2007;86:645–651. doi: 10.1093/ajcn/86.3.645. [DOI] [PubMed] [Google Scholar]
- 26.Alarcon GS, et al. Systemic lupus erythematosus in three ethnic groups: III. A comparison of characteristics early in the natural history of the LUMINA cohort. LUpus in MInority populations: NAture vs. Nurture. Lupus. 1999;8:197–209. doi: 10.1191/096120399678847704. [DOI] [PubMed] [Google Scholar]
- 27.Arnson Y, Amital H, Shoenfeld Y. Vitamin D and autoimmunity: new aetiological and therapeutic considerations. Ann Rheum Dis. 2007;66:1137–1142. doi: 10.1136/ard.2007.069831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kamen DL, et al. Vitamin D deficiency in systemic lupus erythematosus. Autoimmun Rev. 2006;5:114–117. doi: 10.1016/j.autrev.2005.05.009. [DOI] [PubMed] [Google Scholar]
- 29.Thudi A, et al. Vitamin D levels and disease status in Texas patients with systemic lupus erythematosus. Am J Med Sci. 2008;335:99–104. doi: 10.1097/MAJ.0b013e318134eeb6. [DOI] [PubMed] [Google Scholar]
- 30.Abe J, et al. Prevention of immunological disorders in MRL/l mice by a new synthetic analogue of vitamin D3: 22-oxa-1 alpha,25-dihydroxyvitamin D3. J Nutr Sci Vitaminol (Tokyo) 1990;36:21–31. doi: 10.3177/jnsv.36.21. [DOI] [PubMed] [Google Scholar]
- 31.Costenbader KH, et al. Vitamin D intake and risks of systemic lupus erythematosus and rheumatoid arthritis in women. Ann Rheum Dis. 2008 doi: 10.1136/ard.2007.072736. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Harris SS. Vitamin D and type 1 diabetes. Am J Clin Nutr. 2004;79:889–890. doi: 10.1093/ajcn/79.5.889. [DOI] [PubMed] [Google Scholar]
- 33.Giulietti A, et al. Vitamin D deficiency in early life accelerates Type 1 diabetes in non-obese diabetic mice. Diabetologia. 2004;47:451–462. doi: 10.1007/s00125-004-1329-3. [DOI] [PubMed] [Google Scholar]
- 34.Zella JB, McCary LC, DeLuca HF. Oral administration of 1,25-dihydroxyvitamin D3 completely protects NOD mice from insulin-dependent diabetes mellitus. Arch Biochem Biophys. 2003;417:77–80. doi: 10.1016/s0003-9861(03)00338-2. [DOI] [PubMed] [Google Scholar]
- 35.Gysemans C, et al. Unaltered diabetes presentation in NOD mice lacking the vitamin D receptor. Diabetes. 2008;57:269–275. doi: 10.2337/db07-1095. [DOI] [PubMed] [Google Scholar]
- 36.Littorin B, et al. Lower levels of plasma 25-hydroxyvitamin D among young adults at diagnosis of autoimmune type 1 diabetes compared with control subjects: results from the nationwide Diabetes Incidence Study in Sweden (DISS). Diabetologia. 2006;49:2847–2852. doi: 10.1007/s00125-006-0426-x. [DOI] [PubMed] [Google Scholar]
- 37.Pozzilli P, et al. Low levels of 25-hydroxyvitamin D3 and 1,25-dihydroxyvitamin D3 in patients with newly diagnosed type 1 diabetes. Horm Metab Res. 2005;37:680–683. doi: 10.1055/s-2005-870578. [DOI] [PubMed] [Google Scholar]
- 38.Stene LC, Joner G. Use of cod liver oil during the first year of life is associated with lower risk of childhood-onset type 1 diabetes: a large, population-based, case-control study. Am J Clin Nutr. 2003;78:1128–1134. doi: 10.1093/ajcn/78.6.1128. [DOI] [PubMed] [Google Scholar]
- 39.Hypponen E, et al. Intake of vitamin D and risk of type 1 diabetes: a birth-cohort study. Lancet. 2001;358:1500–1503. doi: 10.1016/S0140-6736(01)06580-1. [DOI] [PubMed] [Google Scholar]
- 40.Vitamin D supplement in early childhood and risk for Type I (insulin-dependent) diabetes mellitus. The EURODIAB Substudy 2 Study Group. Diabetologia. 1999;42:51–54. doi: 10.1007/s001250051112. [DOI] [PubMed] [Google Scholar]
- 41.Brekke HK, Ludvigsson J. Vitamin D supplementation and diabetes-related autoimmunity in the ABIS study. Pediatr Diabetes. 2007;8:11–14. doi: 10.1111/j.1399-5448.2006.00223.x. [DOI] [PubMed] [Google Scholar]
- 42.Wicklow BA, Taback SP. Feasibility of a type 1 diabetes primary prevention trial using 2000 IU vitamin D3 in infants from the general population with increased HLA-associated risk. Ann N Y Acad Sci 2006. 1079:310–312. doi: 10.1196/annals.1375.047. [DOI] [PubMed] [Google Scholar]
- 43.Wu K, et al. A nested case control study of plasma 25-hydroxyvitamin D concentrations and risk of colorectal cancer. J Natl Cancer Inst. 2007;99:1120–1129. doi: 10.1093/jnci/djm038. [DOI] [PubMed] [Google Scholar]
- 44.Giovannucci E. Strengths and limitations of current epidemiologic studies: vitamin D as a modifier of colon and prostate cancer risk. Nutr Rev. 2007;65:S77–S79. doi: 10.1111/j.1753-4887.2007.tb00345.x. [DOI] [PubMed] [Google Scholar]
- 45.Knight JA, et al. Vitamin D and reduced risk of breast cancer: a population-based case-control study. Cancer Epidemiol Biomarkers Prev. 2007;16:422–429. doi: 10.1158/1055-9965.EPI-06-0865. [DOI] [PubMed] [Google Scholar]
- 46.Skinner HG, et al. Vitamin D intake and the risk for pancreatic cancer in two cohort studies. Cancer Epidemiol Biomarkers Prev. 2006;15:1688–1695. doi: 10.1158/1055-9965.EPI-06-0206. [DOI] [PubMed] [Google Scholar]
- 47.Boscoe FP, Schymura MJ. Solar ultraviolet-B exposure and cancer incidence and mortality in the United States, 1993-2002. BMC Cancer. 2006;6:264. doi: 10.1186/1471-2407-6-264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48*.Freedman DM, et al. Prospective Study of Serum Vitamin D and Cancer Mortality in the United States. J Natl Cancer Inst. 2007;99:1594–1602. doi: 10.1093/jnci/djm204. [In the NHANES III, baseline serum 25-hydroxy vitamin D levels were not associated with total cancer mortality after 6- to 12-year follow up, although an intriguing difference was noted in colon and possibly breast cancer mortality according to baseline vitamin D status.] [DOI] [PubMed] [Google Scholar]
- 49*.Lappe JM, et al. Vitamin D and calcium supplementation reduces cancer risk: results of a randomized trial. Am J Clin Nutr. 2007;85:1586–1591. doi: 10.1093/ajcn/85.6.1586. [The risk of cancer was reduced with calcium and vitamin D supplementation in this randomized study, although the total number of cancer diagnosed was small.] [DOI] [PubMed] [Google Scholar]
- 50*.Wactawski-Wende J, et al. Calcium plus vitamin D supplementation and the risk of colorectal cancer. N Engl J Med. 2006;354:684–696. doi: 10.1056/NEJMoa055222. [Calcium and vitamin D supplementation did not alter the risk of invasive colorectal cancer in postmenopausal women in this large clinical trial, although vitamin D deficiency was associated with a greater risk of cancer in a nested case-control study. The dose of vitamin D used may have been too small to display a preventive effect.] [DOI] [PubMed] [Google Scholar]
- 51.Beer TM, et al. Double-blinded randomized study of high-dose calcitriol plus docetaxel compared with placebo plus docetaxel in androgen-independent prostate cancer: a report from the ASCENT Investigators. J Clin Oncol. 2007;25:669–674. doi: 10.1200/JCO.2006.06.8197. [DOI] [PubMed] [Google Scholar]
- 52.Agoston ES, et al. Vitamin D analogs as anti-carcinogenic agents. Anticancer Agents Med Chem. 2006;6:53–71. doi: 10.2174/187152006784111369. [DOI] [PubMed] [Google Scholar]
- 53**.Ma Y, et al. Identification and characterization of noncalcemic, tissue-selective, nonsecosteroidal vitamin D receptor modulators. J Clin Invest. 2006;116:892–904. doi: 10.1172/JCI25901. [The authors report on new non-steroidal vitamin D receptor modulators with tissue selective activity and less calcemic actions than calcitriol.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Deeb KK, Trump DL, Johnson CS. Vitamin D signalling pathways in cancer: potential for anticancer therapeutics. Nat Rev Cancer. 2007;7:684–700. doi: 10.1038/nrc2196. [DOI] [PubMed] [Google Scholar]
- 55.Scragg R, et al. Myocardial infarction is inversely associated with plasma 25-hydroxyvitamin D3 levels: a community-based study. Int J Epidemiol. 1990;19:559–563. doi: 10.1093/ije/19.3.559. [DOI] [PubMed] [Google Scholar]
- 56.Vik B, et al. Tromso Heart Study: vitamin D metabolism and myocardial infarction. Br Med J. 1979;2:176. doi: 10.1136/bmj.2.6183.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57*.Wang TJ, et al. Vitamin D Deficiency and Risk of Cardiovascular Disease. Circulation Jan. 2008;117:503–11. doi: 10.1161/CIRCULATIONAHA.107.706127. [Vitamin D deficiency was associated with associated with increased cardiovascular risk after controlling for established cardiovascular risk factors in a large, ambulatory cohort followed longitudinally.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Martins D, et al. Prevalence of cardiovascular risk factors and the serum levels of 25-hydroxyvitamin D in the United States: data from the Third National Health and Nutrition Examination Survey. Arch Intern Med. 2007;167:1159–1165. doi: 10.1001/archinte.167.11.1159. [DOI] [PubMed] [Google Scholar]
- 59.Ford ES, et al. Concentrations of serum vitamin D and the metabolic syndrome among U.S. adults. Diabetes Care. 2005;28:1228–1230. doi: 10.2337/diacare.28.5.1228. [DOI] [PubMed] [Google Scholar]
- 60.Botella-Carretero JI. Vitamin D deficiency is associated with the metabolic syndrome in morbid obesity. Clin Nutr. 2007;26:573–80. doi: 10.1016/j.clnu.2007.05.009. [DOI] [PubMed] [Google Scholar]
- 61.Vilarrasa N, et al. Low 25-hydroxyvitamin D concentrations in obese women: their clinical significance and relationship with anthropometric and body composition variables. J Endocrinol Invest. 2007;30:653–8. doi: 10.1007/BF03347445. [DOI] [PubMed] [Google Scholar]
- 62.Reinehr T, et al. Vitamin D status and parathyroid hormone in obese children before and after weight loss. Eur J Endocrinol. 2007;157:225–32. doi: 10.1530/EJE-07-0188. [DOI] [PubMed] [Google Scholar]
- 63.Wortsman J, et al. Decreased bioavailability of vitamin D in obesity. Am J Clin Nutr. 2000;72:690–693. doi: 10.1093/ajcn/72.3.690. [DOI] [PubMed] [Google Scholar]
- 64.Harris SS, Dawson-Hughes B. Reduced sun exposure does not explain the inverse association of 25-hydroxyvitamin D with percent body fat in older adults. J Clin Endocrinol Metab. 2007;92:3155–3157. doi: 10.1210/jc.2007-0722. [DOI] [PubMed] [Google Scholar]
- 65.Resnick LM, Muller FB, Laragh JH. Calcium-regulating hormones in essential hypertension. Relation to plasma renin activity and sodium metabolism. Ann Intern Med. 1986;105:649–654. doi: 10.7326/0003-4819-105-5-649. [DOI] [PubMed] [Google Scholar]
- 66.Li YC, et al. 1,25-Dihydroxyvitamin D(3) is a negative endocrine regulator of the renin-angiotensin system. J Clin Invest. 2002;110:229–238. doi: 10.1172/JCI15219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Forman JP, et al. Vitamin D intake and risk of incident hypertension: results from three large prospective cohort studies. Hypertension. 2005;46:676–682. doi: 10.1161/01.HYP.0000182662.82666.37. [DOI] [PubMed] [Google Scholar]
- 68.Forman JP, et al. Plasma 25-hydroxyvitamin D levels and risk of incident hypertension. Hypertension. 2007;49:1063–1069. doi: 10.1161/HYPERTENSIONAHA.107.087288. [DOI] [PubMed] [Google Scholar]
- 69*.Pittas AG, et al. The role of vitamin D and calcium in type 2 diabetes. A systematic review and meta-analysis. J Clin Endocrinol Metab. 2007;92:2017–2029. doi: 10.1210/jc.2007-0298. [Comprehensive review of the literature linking calcium and vitamin D to type 2 diabetes and insulin secretion and action.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Scragg R, Sowers M, Bell C. Serum 25-hydroxyvitamin D, diabetes, and ethnicity in the Third National Health and Nutrition Examination Survey. Diabetes Care. 2004;27:2813–2818. doi: 10.2337/diacare.27.12.2813. [DOI] [PubMed] [Google Scholar]
- 71.Ljunghall S, et al. Treatment with one-alpha-hydroxycholecalciferol in middle-aged men with impaired glucose tolerance--a prospective randomized double-blind study. Acta Med Scand. 1987;222:361–367. doi: 10.1111/j.0954-6820.1987.tb10684.x. [DOI] [PubMed] [Google Scholar]
- 72.Fliser D, et al. No effect of calcitriol on insulin-mediated glucose uptake in healthy subjects. Eur J Clin Invest. 1997;27:629–633. doi: 10.1046/j.1365-2362.1997.1520699.x. [DOI] [PubMed] [Google Scholar]
- 73.Pittas AG, et al. The effects of calcium and vitamin D supplementation on blood glucose and markers of inflammation in nondiabetic adults. Diabetes Care. 2007;30:980–986. doi: 10.2337/dc06-1994. [DOI] [PubMed] [Google Scholar]
- 74.Schleithoff SS, et al. Vitamin D supplementation improves cytokine profiles in patients with congestive heart failure: a double-blind, randomized, placebo-controlled trial. Am J Clin Nutr. 2006;83:754–759. doi: 10.1093/ajcn/83.4.754. [DOI] [PubMed] [Google Scholar]
- 75.Inoue T, Kawashima H. 1,25-Dihydroxyvitamin D3 stimulates 45Ca2+-uptake by cultured vascular smooth muscle cells derived from rat aorta. Biochem Biophys Res Commun. 1988;152:1388–394. doi: 10.1016/s0006-291x(88)80439-x. [DOI] [PubMed] [Google Scholar]
- 76.O'Connell TD, Simpson RU. Immunochemical identification of the 1,25-dihydroxyvitamin D3 receptor protein in human heart. Cell Biol Int. 1996;20:621–624. doi: 10.1006/cbir.1996.0081. [DOI] [PubMed] [Google Scholar]
- 77.Jono S, et al. 1,25-Dihydroxyvitamin D3 increases in vitro vascular calcification by modulating secretion of endogenous parathyroid hormone-related peptide. Circulation. 1998;98:1302–6. doi: 10.1161/01.cir.98.13.1302. [DOI] [PubMed] [Google Scholar]
- 78.Bischoff-Ferrari HA, et al. Estimation of optimal serum concentrations of 25-hydroxyvitamin D for multiple health outcomes. Am J Clin Nutr. 2006;84:18–28. doi: 10.1093/ajcn/84.1.18. [DOI] [PubMed] [Google Scholar]
- 79.Bouillon R, Norman AW, Lips P. Vitamin D deficiency. N Engl J Med. 2007;357:1980–1. doi: 10.1056/NEJMc072359. [DOI] [PubMed] [Google Scholar]
- 80.Wolf M, et al. Vitamin D levels and early mortality among incident hemodialysis patients. Kidney Int. 2007;72:1004–1013. doi: 10.1038/sj.ki.5002451. [DOI] [PubMed] [Google Scholar]
- 81.Al-Aly Z, et al. Changes in serum 25-hydroxyvitamin D and plasma intact PTH levels following treatment with ergocalciferol in patients with CKD. Am J Kidney Dis. 2007;50:59–68. doi: 10.1053/j.ajkd.2007.04.010. [DOI] [PubMed] [Google Scholar]
- 82.Teng M, et al. Activated injectable vitamin D and hemodialysis survival: A historical cohort study. J Am Soc Nephrol. 2005;16:1115–1125. doi: 10.1681/ASN.2004070573. [DOI] [PubMed] [Google Scholar]
- 83.Kalantar-Zadeh K, et al. Survival predictability of time-varying indicators of bone disease in maintenance hemodialysis patients. Kidney Int. 2006;70:771–780. doi: 10.1038/sj.ki.5001514. [DOI] [PubMed] [Google Scholar]
- 84.Melamed ML, et al. Changes in serum calcium, phosphate, and PTH and the risk of death in incident dialysis patients: A longitudinal study. Kidney Int. 2006;70:351–357. doi: 10.1038/sj.ki.5001542. [DOI] [PubMed] [Google Scholar]
- 85*.Palmer SC, et al. Meta-analysis: vitamin D compounds in chronic kidney disease. Ann Intern Med. 2007;147:840–53. doi: 10.7326/0003-4819-147-12-200712180-00004. [This meta-analysis of 76 randomized trials did not find evidence that 1,25-(OH)2-D or vitamin D analogs lowers mortality or reduces the need for parathyroidectomy in CKD patients.] [DOI] [PubMed] [Google Scholar]