Abstract
Immunotherapy for cancer continues to gain both momentum and legitimacy as a rational mode of therapy and a vital treatment component in the emerging era of personalized medicine. Gliomas, and their most malignant form, glioblastoma, remain as a particularly devastating solid tumor whose standard treatment options proffer only modest efficacy and target specificity. Immunotherapy would seem a well-suited choice to address such deficiencies given both the modest inherent immunogenicity of gliomas and the strong desire for treatment specificity within the confines of the toxicity-averse normal brain. This review highlights the caveats and challenges to immunotherapy for primary brain tumors, as well as reviews modalities that have been currently employed or are undergoing active investigation. Tumor immunosuppressive counter measures, peculiarities of CNS immune access, and opportunities for rational treatment design are discussed.
Introduction
In 2010, the FDA approved two immunotherapies, sipuleucel-T (PROVENGE, Dendreon Corp.) (1) and ipilimumab (Yervoy, Bristol-Meyers Squibb) for the treatment of metastatic hormone-refractory prostate cancer and metastatic melanoma, respectively, ushering in a new era for cancer immunotherapy. The state of such approaches for primary brain tumors (most frequently glioblastoma (GBM)) remains, by comparison, in its adolescence, sustaining the “growing pains” specific to the immunologic peculiarities of GBM and the central nervous system (CNS). This review will highlight the current context, clinical applications, and challenges to successful immunotherapy for primary brain tumors, focusing on GBM.
Context: The (Fading) Question of Immune Privilege
In light of historical notions regarding CNS immune privilege, relying on a collection of seemingly “brain-banished” immune cells to deliver a strategic anti-tumor “smart bomb” would appear ill-advised. Such notions draw their origins from the studies of Medawar in the 1940s, in which allogeneic skin grafts transplanted onto the brains of experimental animals escaped rejection (2). Subsequent CNS studies highlighted vague nascent antigen presentation, low HLA-expression, blood-brain barrier (BBB)-imposed restrictions for immune access, and absent lymphatic participation, all conjuring the singular perception of the brain as an immunologic void.
As early as the 1980’s, revised views of the CNS as more “immunologically distinct” were increasingly advanced (3). Nascent CNS mechanisms for antigen uptake/transport, T-cell priming, and immune access are increasingly apparent and remain areas of interest for study. It is now accepted that intracerebral antigens move through CSF in the subarachnoid space, along the olfactory nerve, and across the cribiform plate to the nasal mucosa, where they subsequently drain into cervical lymph nodes (CLN) (4, 5). The CLN may be a requisite initiator to adaptive CNS immune responses, possessing unclear interplay with several brain-resident glial cells that have the capacity to mediate their own mode of HLA-restricted antigen presentation (6).
Regardless, T-cells (and other immune effectors) must be granted access to the CNS in order to mediate these primed responses. Restrictions for such access are imposed by the blood-brain barrier (BBB), which is designed to restrict the promiscuous transport of proteins and other molecules from the circulation to the parenchyma, and which also limits immune cell transit. The BBB likely does not represent the unpassable seal to immune cell trafficking initially purported, however (7). This is particularly true in instances of its disruption, often the case in the setting of GBM (8, 9). Even when it remains undamaged, circulating immune cells are capable of penetrating an intact BBB to perform routine immune surveillance functions (10, 11).
While the molecular events underlying immune trafficking to the CNS are still emerging (12), several studies have reported on the chemokines and adhesion molecules that may be critical (13), some proposing a “CNS homing” phenotype that may be influenced by T-cell expression of the α4β1 integrin (14). Ultimately, the identity and phenotype of immune cells penetrating CNS tumors, the means by which they are not infrequently foiled, and the possibilities for enhancing their homing capacities and anti-tumor functionality represent important are as of investigation.
Clinical Applications: Immunotherapeutic Approaches to GBM
Employed immunotherapeutic modalities for GBM now encompass a wide variety of approaches (Table 1, Fig. 1), the major categories of which are discussed below.
Table 1.
Immunotherapeutic approaches to GBM
| CLASS | SUBTYPE | TARGET TYPE | COMMENTS | ACTIVE TRIALS (EXAMPLES) |
|---|---|---|---|---|
| Antibody | Biological Modifier | Surface molecules/receptors (i.e., HER2, EGFR, tenascin). Can also be used to target other cells as with anti-CD25 and Tregs | Direct activity (i.e., receptor blockade or Fc-mediated cytotoxicity). Local administration more common, unless targeting suppressive immune cells. | NCT01475006, NCT00600054 |
| Antibody/Ligand | Toxin Delivery | Surface molecules/receptors (commonly IL-13R, IL-4R, and transferrin receptor) | Targets toxins to tumor. Common toxins have been altered diphtheria and pseudomonas toxins linked to IL-13 or IL-4 | NCT00880061 |
| Antibody/Ligand | Radionucleotide Delivery | Surface molecules/receptors (commonly EGFR and tenascin) | Targets radionucleotide to tumor, commonly 131I | NCT00003478, NCT00002753 (both completed) |
| ALT | Lymphocyte, Immune effector | Whole tumor antigen, TAA(s), CMV | Difficult production, survival/persistence in vivo can be enhanced with concomitant vaccination or prior myeloconditioning | NCT0114427, NCT01801852 |
| ALT | CARs | Whole tumor antigen, TAA(s), CMV | Chimeric antigen receptor links otherwise non-specific T-cells to tumor surface antigens. Still requires autologous lymphocyte harvests. | NCT01454596, NCT01109095, NCT0073061 NCT01082926 |
| Vaccine | Tumor Cell +/− GM-CSF | Whole tumor antigens | Difficult production. Newer forms employ GM-CSF secreting bystander lines (i.e., K-562) | NCT00694330 |
| Vaccine | Dendritic Cell | Whole tumor antigens, TAA(s), CMV | Multiple methods for production, loading, maturing and delivering, all relatively laborious and none standardized. Relies on nascent T-cells for effect unless combined with ALT. | Numerous active. Examples: NCT01808820, NCT00045968 |
| Vaccine | Antigenic / Peptide(s) | TAA(s) (to date commonly EGFRvIII, IL13Ra2, survivin, EphA2, and WT-1) | Rely on nascent DC and T-cells to effect function. Scalable, “off the shelf production.” HLA-restricted use. | NCT02149225, NCT01920191, NCT02078648, NCT01480479 |
| Vaccine | HSPPC | Unidentified tumor peptides | HSP shuttle peptides to MHC I, enhancing presentation. Antigens remain unidentified. | NCT02122822, NCT01814813 |
| Vaccine | DNA/Viral | TAA(s), cytokine delivery, CMV | Virus used to coat DNA for gene delivery into and expression by APC. | Preclinical to date. |
| Oncolytic Virus | HSV, Adenovirus, Polio | Direct tumor cell lysis / immune recruitment | Avails of predilection for tumor. Some effect immune-mediated. | NCT02031965, NCT00931931, NCT0219716 |
| Immune Checkpoint Blockade | Anti-CTLA-4 | Non-specific T-cell activation, Treg inhibition | Perpetuates T-cell activation. FDA approved for metastatic melanoma. | NCT02017717 |
| Immune Checkpoint Blockade | Anti-PD-1 / PD-L1 | Non-specific T-cell activation, Treg inhibition | Perpetuates T-cell activation. May be better tolerated than anti-CTLA-4. | NCT02017717 |
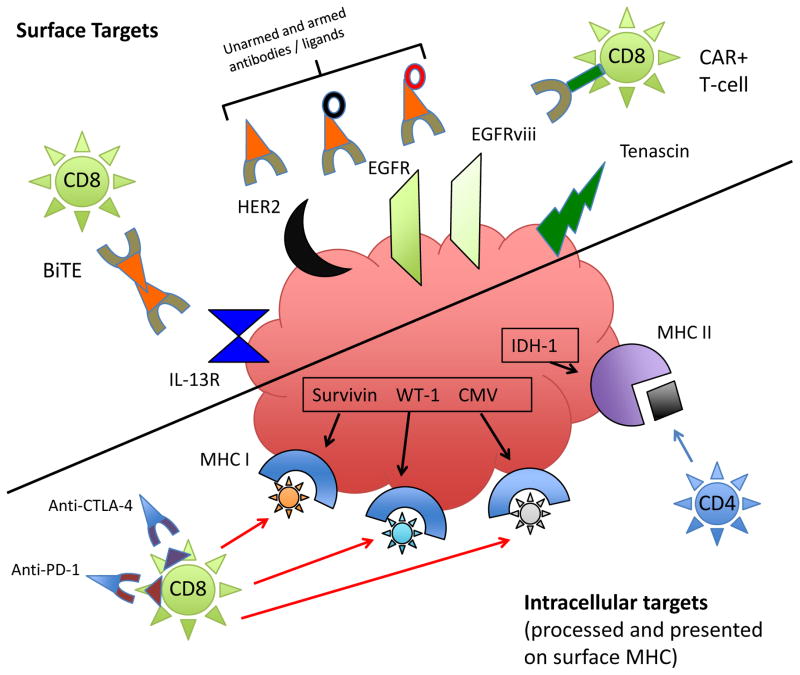
Figure 1.
Targeting typical GBM antigens. Immunotherapy takes a variety of forms that may ultimately target glioma surface antigens or antigens expressed in the cytoplasm that are processed and presented in the context of MHC class I. In this figure, a sampling of surface (left) and intracellular (right) glioma antigens that have been commonly targeted are represented, along with pertinent immunotherapeutic effectors. Some of the primary modalities targeting surface antigens/receptors are antibodies/ligands (unarmed or armed with toxins (black ring) or radionucleotides (red ring)); BiTEs, which recruit T-cells to the tumor cell surface; and CAR+ T-cells, which provide surface-antigen specificity to otherwise non-reactive T-cells. For intracellular antigens presented in the context of MHC I, T-cells are the primary effectors. These may be adoptively transferred (ALT), or activated by DC, antigenic, HSPPC, or DNA/viral vaccines. Their activity may also be non-specifically perpetuated by immune checkpoint blockade with antibodies to CTLA-4 and PD-1, for instance, which can also inhibit Treg-mediated T-cell suppression. Recent work to build a vaccine against a mutated IDH-1 has revealed mostly class II epitopes for mutation-spanning peptides, which may provide a target to CD4 T-cells in the context of low levels of glioma class II MHC, or may stimulate a CD4 helper response via APC-mediated class II MHC presentation to CD4 T-cells.
Surface-directed passive immunotherapies (antibodies and targeted toxins)
Antibody and targeted toxin therapies remain some of the oldest investigated immunotherapies for brain tumors (reviewed in (15)). The ultimate goal is specific binding of a molecule or receptor on the tumor surface, with the deployed agent serving in one of a number of defined capacities: as biologic response modifiers (i.e., EGFR blockade) (16) or as delivery vehicles for tumoricidal toxins (i.e., diphtheria, pseudomonas) (17, 18) or radionucleotides (131I) (19). Many clinical trials have been conducted over the years, most of these being Phase I/II studies. Classically, surface targets have included EGFR, tenascin, transferrin receptor, and the IL-13 and IL-4 receptors. The non-permissiveness for large protein passage across the BBB often limits treatment delivery to intrathecal routes or directly into resection cavities, but some recent Phase II successes are reported employing systemic antibody delivery to pediatric patients with diffuse intrapontineglioma (where delivery into a resection cavity is precluded) (16).
This treatment mode is further limited by the passivity of the instigated immunity, with the duration of immune response tethered to the half-life of the agent delivered. Persistent treatment effects can develop, but likely depend on the recruitment of subsequent T-cell immunity. Some contemporary antibody therapies then aim to solicit and direct T-cells not otherwise specific for tumor by employing bi-specificity for a tumor target and the T-cell receptor (bispecific T-cell engagers (BiTEs)). These remain in preclinical testing (20).
Adoptive lymphocyte transfer (ALT)
Multiple strategies have looked to precipitate T-cell activation with the most “simple” being direct enlistment of T-cells via adoptive lymphocyte transfer (ALT). Here, autologous T-cells are harvested, trained/expanded/activated ex vivo against tumor, and transferred back to patients either alone or in conjunction with other so-handled immune cells, such as dendritic cells. In its earlier renditions, ALT included the transfer of a variety of immune populations, not just T-cells. These have included peripheral blood mononuclear cells (PBMC) (21); lymphokine/mitogen-activated killer cells (LAK) (22); tumor-infiltrating lymphocytes (TIL) (23); and cytotoxic T-lymphocytes (CTL) (24, 25), administered either systemically (preclinical data supports tumor trafficking (26)) or into the tumor cavity. Targets have varied, and newer renditions have combined ALT with active vaccination (27) and/or prior myelosuppressive regimens (28) (NCT00693095), in efforts to promote survival and functional expansion of the transferred cells in vivo (active trials: NCT0114427, NCT01801852).
Beyond ensuring cell survival, an additional “rate-limiting step” for ALT therapy has been the generation of large numbers of functional tumor-specific T-cells ex vivo. One solution has been the genetic modification of T-cells to express a chimeric antigen receptor (CAR), which specifically binds to tumor antigens in an MHC-unrestricted fashion (29, 30). CARs are fusion genes comprised of a single-chain variable fragment (scFv) antibody or other extracellular domain recognizing the TAA of interest, linked to intracellular signaling modules that mediate T-cell activation upon ligation of the CAR’s extracellular domain. Upon gene transfer of the CAR into T-cells (using viral vectors or electroporation (31)), the transduced T-cell acquires specificity for the targeted TAA, while retaining its endogenous TCR. As a result of this construct, use is limited to cell surface targets, such as IL-13R, EGFRvIII, and HER2 (Phase I/II trials are ongoing or recently completed: NCT01454596, NCT01109095, NCT00730613, NCT01082926).
Vaccines
Much of the immunotherapeutic work in GBM to date has been vaccine-based. Tumor vaccines encompass a broad range of approaches, including cell-based; antigenic; DNA; and viral-derived strategies. Most are intended as therapeutic modalities, initiated after tumor detection. The most prominent exceptions are cervical and hepatocellular carcinomas, where the identification of human papilloma virus and hepatitis B etiologies, respectively (32, 33) confers the ability to vaccinate prophylactically against a cancer. The majority of cancers do not have an identified microbial precipitant, and the ability to vaccinate against a viral target is not similarly afforded. In the case of GBM, the detection of tumor-borne cytomegalovirus (CMV) antigens has sparked debate regarding whether CMV might be etiologic or simply re-expressed / reactivated in an immunosuppressive local environment (34, 35).
Early tumor vaccines comprised “killed or inactivated” tumor cells, eventually genetically engineered to elaborate a variety of immune-stimulating cytokines, most famously, granulocyte-macrophage colony-stimulating factor (GM-CSF) (36). Versions of GM-CSF secreting tumor cell vaccines have been employed for GBM (37, 38), often revealing technical difficulties (38). Current generations are accompanied by an allogeneic tumor cell line (K-562) secreting GM-CSF. These have completed Phase I testing, and results await publication (NCT00694330).
More commonly, vaccine-based therapies for GBM have employed dendritic cells (DC) (39–50), most of which have demonstrated some level of efficacy in phase I/II studies. Definitive phase III evidence for efficacy remains lacking, however, and production is labor-intensive and expensive, with nearly all generating DC from peripheral blood monocytes with the aid of GM-CSF and IL-4. DC have been loaded/pulsed with synthetic versions of glioma-associated antigens/peptides (41, 51, 52); whole tumor cell lysates (40, 43, 45–48); or electroporated/pulsed/transfected with tumor cell or even tumor stem cell RNA (49, 50). After loading, DC are often matured with a cocktail (often some combination of TNF–α, IL-1β, IL-6, PGE2), or more recently with poly I:C, a dsRNA mimic, prior to being delivered, typically intradermally. Presently, there are at least 11 open DC vaccine trials for adult and/or pediatric glioma in the U. S. (NCT0108820, NCT01792505, NCT22010606, NCT01902771, NCT01635283, NCT01204684, NCT01957956, NCT02049489, NCT00626483, NCT00045968, NCT01522820), as well as an additional trial for medulloblastoma/PNET (NCT01326104).
In contrast to cell-based vaccines, “antigenic” vaccines involve the delivery of a protein or peptide antigen itself, often in conjunction with an immune-stimulating adjuvant. This is, in effect, an attempt at in vivo pulsing of nascent DC. Advantages include scalable, “off-the-shelf” production, but HLA-restrictions and reliance upon potentially dysfunctional nascent immune cells impose limitations. Currently identified, glioma-associated antigens (GAAs) include IL13Ra2, HER2, gp100, TRP2, EphA2, survivin, WT1, SOX2, SOX11, MAGE-A1, MAGE-A3, AIM2, SART1, and CMV proteins. Additionally, EGFRvIII and the IDH-1 mutant (R132H) represent truly tumor-specific targets within a subset of tumors, with the latter proffering a newly revealed vaccine target containing mostly class II MHC epitopes (53). A phase I study is set to begin recruiting (NCT02193347).
To date, peptide vaccine trials in glioma have targeted WT-1 (54, 55) and EGFRvIII (41), with ongoing trials targeting collections of GAA, including IL13Ra2, survivin, EphA2, and WT-1 (NCT02149225, NCT01920191, NCT02078648). A study targeting the same antigens in pediatric glioma continues to show tremendous promise and awaits publication (NCT01130077). One of the few phase III immunotherapy trials for gliomais an active study (NCT01480479) targeting EGFRvIII. “CDX110-04” is an international, multicenter, double-blind clinical trial of rindopepimut (EGFRvIII peptide vaccine, Celldex) in which approximately 700 patients with newly diagnosed, resected, EGFRvIII positive GBM, upon completion of standard chemoradiation, are randomized to receive either rindopepimut/GM-CSF or control (keyhole limpet hemocyanin), in combination with standard adjuvant temozolomide.
A unique tumor cell-derived approach administers essentially multiple non-identified peptides in the form of heat shock protein-peptide complexes (HSPPC). HSP are stress-induced proteins that chaperone intracellular peptides from the proteasome to the endoplasmic reticulum, mediating transfer to MHC I. One such HSPPC employing the tumor-isolated HSP glycoprotein-96 (gp-96) (HSPPC-96, VItespen, formerly Oncophage), has served as a vaccination platform in phase III trials for metastatic melanoma and renal cell carcinoma with no survival benefit observed (56, 57). A Phase I study published for glioma in 2012 demonstrated safety as well as antigen-specific peripheral immune responses in 11/12 treated patients (58). Two further early phase clinical trials are ongoing (NCT02122822, NCT01814813, NCT00293423).
There are a variety of viral-based anti-cancer approaches being explored today for GBM, ranging from immune-targeting antigen-delivery systems (59–61) to tumor-targeting suicide gene delivery vectors (62) to directly oncolytic viruses (63–65). The latter two strategies classically employ viruses with specific tissue predilections, with the neural preferences for herpes and polioviruses creating roles in glioma (reviewed in (66)). Viruses have also served as the antigenic target of interest, and as discussed above, studies have uncovered the selective re-expression of latent CMV proteins within glioma cells (34, 35), proffering a potent immunologic target. Multiple clinical trials targeting CMV are currently open (NCT00626483, NCT01109095, NCT00693095).
Immune checkpoint blockade
The physiologic provisions for routine immunologic shutdown are termed “immune checkpoints” and are furnished by molecules on activated T-cells, signaling via which precipitates inactivation (CTLA-4) or even apoptosis (PD-1). Conversely, blockade or antagonism of these same molecules and their intracellular signaling pathways can potentiate T-cell responses, and even render them insensitive to tumor-mediated inhibition (67).
CTLA-4 blockade increases the availability of CD28 co-stimulation, thereby amplifying/perpetuating T-cell activation and either directly or indirectly inhibiting Treg activity, as Tregs similarly express CTLA-4 at high levels (68). Resultant T-cell activation is global and antigen non-specific, creating a response that is potent, but not inherently “directed.” Promising phase III results led to FDA approval of anti-CTLA-4 (ipilimumab, Bristol Myers Squibb) for patients with metastatic melanoma in 2010 (69). Although preclinical studies have proven extremely promising (70–72), multi-center clinical trials in GBM are only now being initiated (NCT02017717). Clinical experience with CNS disease to date has been solely in patients harboring small intracranial melanoma metastases (73), experience which proved safe, yielding no instances of CNS autoimmunity.
Programmed death-1 (PD-1, CD279) is a member of the CD28 family expressed on activated T cells, B cells, dendritic cells, and macrophages (67, 74). PD-1 engages two ligands, PD-L1 (B7-H1) and PD-L2 (B7-DC), both members of the B7 family. PD-L1 is expressed on a variety of immune and non-hematopoietic cells, while PD-L2 is restricted to myeloid cells. The PD-1 pathway functions to down-modulate inflammatory responses under physiological conditions and may be exploited by cancers en route to immunologic escape. PD-1 is also highly expressed on Tregs, and signaling enhances their suppressive function upon ligand engagement. The molecule is detected on a large proportion of TILs, and PD-1 ligands (especially PD-L1) are up-regulated on the surface of numerous tumor types, including GBM (67), a phenomenon linked to inferior clinical outcomes in a variety of cancers (75–77).
Clinical trials with anti-PD-1 (MDX-1106) and anti-PD-L1 (MDX-1105) monoclonal antibodies have been conducted in patients with various solid tumors with promising response rates (78). In some contrast to the results with anti-CTLA-4, anti-PD1 mAbs appear to be better tolerated, although potentially lethal pneumonitis has been observed (67). Clinical trials of anti-PD-1 in GBM are set to begin (NCT02017717) and will employ a combination arm with ipilimumab, given an expectation for synergy (72).
Challenges: Designing, Effecting, and Monitoring Our Success
There is no step along the advance from planning to implementing to assessing immunotherapeutic deployment that does not pose a defined set of challenges to be acknowledged and met. Beginning with trial design, the relative infrequency of GBM limits the obtainable power for single institution studies, which have dominated the landscape as phase I and II studies to date. Large phase III studies become similarly challenging to construct, and a search of clinicaltrials.gov reveals just three active phase III trials that can be classified as immune-based therapies (NCT00045968, NCT01759810, NCT01480479), all of which have required willing industry sponsors (Northwest Biotherapeutics, NeuroVita Clinic, Celldex). Additionally, some trials target newly diagnosed patients, while others enlist patients with recurrence, the latter of whom have almost invariably undergone a variety of previous regimens, many with potential immunologic consequences. Even “newly diagnosed” patients will have typically seen dexamethasone, an established lymphocyte modulator and immunosuppressant. Therefore, trial design must standardize across such influences, as well as strive for multi-institutional recruitment.
Once implemented, immunotherapies face a unique set of contextual difficulties posed specifically by GBM and the severity of its immunologic influence. GBMs are now increasingly recognized as among the most immunosuppressive of solid tumors. Cellular immunity is particularly damaged, with T-cell deficits proving both profound and widespread (79). A thorough review of glioma’s capacities for soliciting immune-compromise is beyond the scope of this account, although exists recently in the literature (3). A brief introduction is offered here, however.
Therapies aimed at stimulating T-cell immunity depend on some abundance of T-cells, yet T-cell lymphopenia is one of the oldest documented immune shortfalls for patients with GBM, harkening back to the studies of Brooks and Roszman in the 1970s (80). Often, lymphopenia has been attributed to the effects of treatment with chemotherapy (temozolomide) and dexamethasone, and while these undoubtedly contribute, increasing evidence is that they merely exacerbate a lymphopenia (particularly CD4) that is already present in a substantial number of treatment-naïve patients (81). Investigations into the source of such lymphopenia are currently underway and yielding interesting results regarding compartmental T-cell re-distributions.
Those T-cells that do remain in the circulation are hampered by anergy (82, 83), IL-2 system dysfunction (84), TH2-biased responsiveness (85), decreased NKG2D expression (86), and inhibition by disproportionate representations of suppressive regulatory T-cells (Tregs) (81), all products of uniquely potent GBM systemic influences and extrinsic mechanisms for immune-escape. T-cells that do manage activation and tumor-trafficking find themselves faced with equally impressive local and intrinsic means of tumor evasion, including more Tregs (87), IDO expression (88), down regulated MHC and B7 family proteins (89, 90), increased PD-L1 (91), PTEN loss (92), STAT3 expression/activation (93), TGF-β and IL-10 production (94), MICA/B secretion (95, 96), and HLA-E expression (97), all of which serve to sidestep or directly undermine those immune cells present (Fig. 2). Our own sampling of TILs in glioma specimens yields phenotypes rich in CD95, PD-1, PD-L1, CTLA-4, LAG3, and Tim3, strongly indicating immune exhaustion, defined by poor effector function, sustained expression of inhibitory receptors, and an altered transcriptional state (98). We can therefore no longer be satisfied with simply “delivering” immune cells to target, but must better know the fate of those cells and arrive at standardized biomarker and radiographic surrogates/goals for realized immunity across studies. The question is no longer just one of privilege.
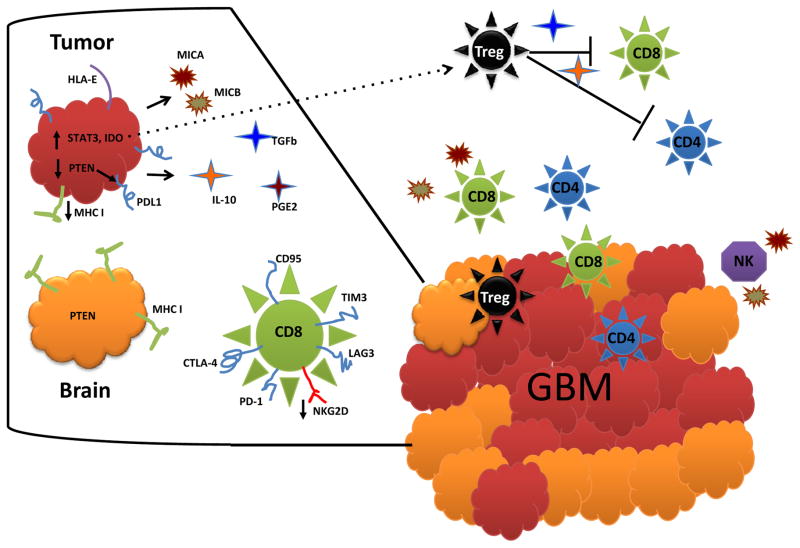
Figure 2.
GBM immuno-evasive and –suppressive mechanisms. GBM employs a variety of mechanisms, both cell intrinsic and extrinsic, meant to sidestep or even directly counter host immune responses. GBM is pictured here as red cells amongst orange normal brain (glial cells). Inset on the left represents magnification of tumor, normal glia, and a CD8 T-cell with typical exhausted phenotype. GBM cell-intrinsic mechanisms (visible on inset) include IDO expression (leading to recruitment of tumor-associated Tregs (black dotted arrow)) (88), down regulated MHC and B7 family proteins (89, 90), increased PD-L1 (91), PTEN loss (which can precipitate PD-L1 expression) (92), STAT3 expression/activation (pleotropic immunosuppression) (93), TGF-β and IL-10 production (causing counterproductive TH2 shifts and elaborating Tregs) (94), MICA/B secretion (inhibiting both T- and NK-cell activity) (95, 96), and HLA-E expression (inhibiting NK cells) (97). Cell-extrinsic mechanisms comprise effects on surrounding and systemic immune cells, and include lymhopenia and depressed cellular immunity. Patient T-cells are hampered by anergy (82, 83), IL-2 system dysfunction (84), TH2-biased responsiveness (85), decreased NKG2D expression (86), and inhibition by disproportionate representations of Tregs (81).
Conclusions and Future Directions
Over the last three decades, tumor immunotherapy has forged forward with substantial strides, constituting a now legitimate and expanding mode of cancer therapy. Successful deployment against GBM, however, requires increasing attention to the “immunologic idiosyncrasies” of gliomas and their microenvironment. We must acknowledge, understand, and counter the limitations imposed by relying on often impaired host cellular immunity to mediate our therapies in an immunologically “distinct” compartment. Such striving for immunologic potency, however, must be balanced by vigilance for autoimmune toxicities, particularly when choosing whole antigen approaches, as the brain is decidedly less tolerant of collateral inflammation than the prostate or skin. Conversely, these concerns must be weighed against fears for tumor immune escape when just a single or small number of antigens are targeted (99).
Immunotherapy is now poised to be a more ubiquitous component to the ever-emerging collage that will be personalized medicine. It will be the responsibility of immunotherapists, then, to determine its optimal place in the broadening context of complementary (or even co-canceling) therapies and tumor genetic backgrounds. GBM, as with cancer more generally, is now recognized as a constellation of genetically distinct diseases. The Cancer Genome Atlas (TCGA) project’s division of GBM into proneural, neural, classical, and mesenchymal classifications highlights tumor phylogenies whose genetic makeup, patient characteristics, prognoses, and responses to traditional therapies all vary definitively (100). The immunophenotypes and efficacy of various immune-based therapies amidst the tumor classes remains almost entirely uncharacterized, however. Such characterization will be an important step to developing personalized treatment combinations predicated on pathological diagnosis and the genomic technologies highlighted in there view by Gajjar and colleagues (101) in this CCR Focus section, and therefore, represents a vital future direction for GBM immunotherapy.
Likewise, the revealing of GBM subclasses may hold some relevance for understanding the differences between responders and non-responders in immunotherapy trials, as well as between patients possessing normal versus defective cellular immunity (often strongly dichotomous). Practically speaking, this means that immunotherapy trials should begin to incorporate GBM subclass and baseline immunophenotype into patient selection and grouping. Pre-treatment factors such as lymphocyte count, steroid exposure, Treg fraction, and T-cell phenotype and responsiveness (as well as a variety of not yet determined immune-markers) are likely to be just as important as (and possibly related to) proneural versus mesenchymal subtype in determining treatment responses and should constitute, at the very least, subgroup analyses in trials. Despite the challenges this will pose, it is the contextual understanding afforded that will permit us to move from simply proof of concept to a realizable goal of therapeutic efficacy.
Acknowledgments
Grant Support
This work was supported by the NIH under award numbers R01-NS085412-01, R01-CA177476-02, and R25-NS065731-05 (to J.H. Sampson); CA1208113 (to A.B. Heimberger); and P50 CA127001 (to A.B. Heimberger, through her institution).
Footnotes
Disclosure of Potential Conflicts of Interest
J.H. Sampson is a consultant/advisory board member for CellDex Therapeutics, and reports receiving a commercial research grant and licensing fees from CellDex Therapeutics for intellectual property related to the EGFRvIII peptide vaccine (CDX-110). A.B. Heimberger is a consultant/advisory board member for Bristol-Myers Squibb; holds patents on WP1066 and the immune modulatory miRNA portfolios; reports receiving a research grant from Merck and licensing fees from CellDex Therapeutics for intellectual property related to the EGFRvIII peptide vaccine (CDX-110). No potential conflicts of interest were disclosed by the other author.
References
- 1.Kantoff PW, Higano CS, Shore ND, Berger ER, Small EJ, Penson DF, et al. Sipuleucel-T immunotherapy for castration-resistant prostate cancer. N Engl J Med. 2010;363:411–22. doi: 10.1056/NEJMoa1001294. [DOI] [PubMed] [Google Scholar]
- 2.Medawar PB. Immunity to homologous grafted skin; the fate of skin homografts transplanted to the brain, to subcutaneous tissue, and to the anterior chamber of the eye. Br J Exp Pathol. 1948;29:58–69. [PMC free article] [PubMed] [Google Scholar]
- 3.Dunn GP, Fecci PE, Curry WT. Cancer immunoediting in malignant glioma. Neurosurgery. 2012;71(2):201–23. doi: 10.1227/NEU.0b013e31824f840d. [DOI] [PubMed] [Google Scholar]
- 4.Cserr HF, Knopf PM. Cervical lymphatics, the blood-brain barrier and the immunoreactivity of the brain: a new view. Immunol Today. 1992;13:507–12. doi: 10.1016/0167-5699(92)90027-5. [DOI] [PubMed] [Google Scholar]
- 5.Cserr HF, Harling-Berg CJ, Knopf PM. Drainage of brain extracellular fluid into blood and deep cervical lymph and its immunological significance. Brain Pathol. 1992;2:269–76. doi: 10.1111/j.1750-3639.1992.tb00703.x. [DOI] [PubMed] [Google Scholar]
- 6.Aloisi F, Ria F, Adorini L. Regulation of T-cell responses by CNS antigen-presenting cells: different roles for microglia and astrocytes. Immunol Today. 2000;21:141–7. doi: 10.1016/s0167-5699(99)01512-1. [DOI] [PubMed] [Google Scholar]
- 7.Bechmann I, Galea I, Perry VH. What is the blood-brain barrier (not)? Trends Immunol. 2007;28:5–11. doi: 10.1016/j.it.2006.11.007. [DOI] [PubMed] [Google Scholar]
- 8.Long DM. Capillary ultrastructure and the blood-brain barrier in human malignant brain tumors. J Neurosurg. 1970;32:127–44. doi: 10.3171/jns.1970.32.2.0127. [DOI] [PubMed] [Google Scholar]
- 9.Vajkoczy P, Menger MD. Vascular microenvironment in gliomas. J Neurooncol. 2000;50:99–108. doi: 10.1023/a:1006474832189. [DOI] [PubMed] [Google Scholar]
- 10.Hickey WF, Hsu BL, Kimura H. T-lymphocyte entry into the central nervous system. J Neurosci Res. 1991;28:254–60. doi: 10.1002/jnr.490280213. [DOI] [PubMed] [Google Scholar]
- 11.Ransohoff RM, Kivisakk P, Kidd G. Three or more routes for leukocyte migration into the central nervous system. Nat Rev Immunol. 2003;3:569–81. doi: 10.1038/nri1130. [DOI] [PubMed] [Google Scholar]
- 12.Engelhardt B. Molecular mechanisms involved in T cell migration across the blood-brain barrier. J Neural Transm. 2006;113:477–85. doi: 10.1007/s00702-005-0409-y. [DOI] [PubMed] [Google Scholar]
- 13.Calzascia T, Di Berardino-Besson W, Wilmotte R, Masson F, de Tribolet N, Dietrich PY, et al. Cutting edge: cross-presentation as a mechanism for efficient recruitment of tumor-specific CTL to the brain. J Immunol. 2003;171:2187–91. doi: 10.4049/jimmunol.171.5.2187. [DOI] [PubMed] [Google Scholar]
- 14.Masson F, Calzascia T, Di Berardino-Besson W, de Tribolet N, Dietrich PY, Walker PR. Brain microenvironment promotes the final functional maturation of tumor-specific effector CD8+ T cells. J Immunol. 2007;179:845–53. doi: 10.4049/jimmunol.179.2.845. [DOI] [PubMed] [Google Scholar]
- 15.Fecci PE, Sampson JH. Clinical immunotherapy for brain tumors. Neuroimaging Clin N Am. 2002;12:641–64. doi: 10.1016/s1052-5149(02)00027-8. [DOI] [PubMed] [Google Scholar]
- 16.Bartels U, Wolff J, Gore L, Dunkel I, Gilheeney S, Allen J, et al. Phase 2 study of safety and efficacy of nimotuzumab in pediatric patients with progressive diffuse intrinsic pontine glioma. Neuro Oncol. 2014 May 20; doi: 10.1093/neuonc/nou091. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Laske DW, Youle RJ, Oldfield EH. Tumor regression with regional distribution of the targeted toxin TF-CRM107 in patients with malignant brain tumors. Nat Med. 1997;3:1362–8. doi: 10.1038/nm1297-1362. [DOI] [PubMed] [Google Scholar]
- 18.Kunwar S, Prados MD, Chang SM, Berger MS, Lang FF, Piepmeier JM, et al. Direct intracerebral delivery of cintredekin besudotox (IL13-PE38QQR) in recurrent malignant glioma: a report by the Cintredekin Besudotox Intraparenchymal Study Group. J Clin Oncol. 2007;25:837–44. doi: 10.1200/JCO.2006.08.1117. [DOI] [PubMed] [Google Scholar]
- 19.Reardon DA, Akabani G, Coleman RE, Friedman AH, Friedman HS, Herndon JE, 2nd, et al. Phase II trial of murine (131) I-labeled antitenascin monoclonal antibody 81C6 administered into surgically created resection cavities of patients with newly diagnosed malignant gliomas. J Clin Oncol. 2002;20:1389–97. doi: 10.1200/JCO.2002.20.5.1389. [DOI] [PubMed] [Google Scholar]
- 20.Choi BD, Kuan CT, Cai M, Archer GE, Mitchell DA, Gedeon PC, et al. Systemic administration of a bispecific antibody targeting EGFRvIII successfully treats intracerebral glioma. Proc Natl Acad Sci U S A. 2013;110:270–5. doi: 10.1073/pnas.1219817110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Steinbok P, Thomas JP, Grossman L, Dolman CL. Intratumoral autologous mononuclear cells in the treatment of recurrent glioblastoma multiforme. A phase 1 (toxicity) study. J Neurooncol. 1984;2:147–51. doi: 10.1007/BF00177901. [DOI] [PubMed] [Google Scholar]
- 22.Hayes RL, Koslow M, Hiesiger EM, Hymes KB, Hochster HS, Moore EJ, et al. Improved long term survival after intracavitary interleukin-2 and lymphokine-activated killer cells for adults with recurrent malignant glioma. Cancer. 1995;76:840–52. doi: 10.1002/1097-0142(19950901)76:5<840::aid-cncr2820760519>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 23.Quattrocchi KB, Miller CH, Cush S, Bernard SA, Dull ST, Smith M, et al. Pilot study of local autologous tumor infiltrating lymphocytes for the treatment of recurrent malignant gliomas. J Neurooncol. 1999;45:141–57. doi: 10.1023/a:1006293606710. [DOI] [PubMed] [Google Scholar]
- 24.Tsurushima H, Liu SQ, Tuboi K, Matsumura A, Yoshii Y, Nose T, et al. Reduction of end-stage malignant glioma by injection with autologous cytotoxic T lymphocytes. Jpn J Cancer Res. 1999;90:536–45. doi: 10.1111/j.1349-7006.1999.tb00781.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kitahara T, Watanabe O, Yamaura A, Makino H, Watanabe T, Suzuki G, et al. Establishment of interleukin 2 dependent cytotoxic T lymphocyte cell line specific for autologous brain tumor and its intracranial administration for therapy of the tumor. J Neurooncol. 1987;4:329–36. doi: 10.1007/BF00195603. [DOI] [PubMed] [Google Scholar]
- 26.Prins RM, Shu CJ, Radu CG, Vo DD, Khan-Farooqi H, Soto H, et al. Anti-tumor activity and trafficking of self, tumor-specific T cells against tumors located in the brain. Cancer Immunol Immunother. 2008;57:1279–89. doi: 10.1007/s00262-008-0461-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Holladay FP, Heitz-Turner T, Bayer WL, Wood GW. Autologous tumor cell vaccination combined with adoptive cellular immunotherapy in patients with grade III/IV astrocytoma. J Neurooncol. 1996;27:179–89. doi: 10.1007/BF00177482. [DOI] [PubMed] [Google Scholar]
- 28.Sanchez-Perez LA, Choi BD, Archer GE, Cui X, Flores C, Johnson LA, et al. Myeloablative temozolomide enhances CD8(+) T-cell responses to vaccine and is required for efficacy against brain tumors in mice. PloS One. 2013;8:e59082. doi: 10.1371/journal.pone.0059082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gross G, Waks T, Eshhar Z. Expression of immunoglobulin-T-cell receptor chimeric molecules as functional receptors with antibody-type specificity. Proc Natl Acad Sci U S A. 1989;86:10024–8. doi: 10.1073/pnas.86.24.10024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gross G, Eshhar Z. Endowing T cells with antibody specificity using chimeric T cell receptors. FASEB J. 1992;6:3370–8. doi: 10.1096/fasebj.6.15.1464371. [DOI] [PubMed] [Google Scholar]
- 31.June CH, Blazar BR, Riley JL. Engineering lymphocyte subsets: tools, trials and tribulations. Nat Revi Immunol. 2009;9:704–16. doi: 10.1038/nri2635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Walboomers JM, Jacobs MV, Manos MM, Bosch FX, Kummer JA, Shah KV, et al. Human papillomavirus is a necessary cause of invasive cervical cancer worldwide. J Pathol. 1999;189:12–9. doi: 10.1002/(SICI)1096-9896(199909)189:1<12::AID-PATH431>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 33.Benvegnu L, Fattovich G, Noventa F, Tremolada F, Chemello L, Cecchetto A, et al. Concurrent hepatitis B and C virus infection and risk of hepatocellular carcinoma in cirrhosis. A prospective study. Cancer. 1994;74:2442–8. doi: 10.1002/1097-0142(19941101)74:9<2442::aid-cncr2820740909>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 34.Cobbs CS, Harkins L, Samanta M, Gillespie GY, Bharara S, King PH, et al. Human cytomegalovirus infection and expression in human malignant glioma. Cancer Res. 2002;62:3347–50. [PubMed] [Google Scholar]
- 35.Mitchell DA, Xie W, Schmittling R, Learn C, Friedman A, McLendon RE, et al. Sensitive detection of human cytomegalovirus in tumors and peripheral blood of patients diagnosed with glioblastoma. Neuro Oncol. 2008;10:10–8. doi: 10.1215/15228517-2007-035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dranoff G, Jaffee E, Lazenby A, Golumbek P, Levitsky H, Brose K, et al. Vaccination with irradiated tumor cells engineered to secrete murine granulocyte-macrophage colony-stimulating factor stimulates potent, specific, and long-lasting anti-tumor immunity. Proc Natl Acad Sci U S A. 1993;90:3539–43. doi: 10.1073/pnas.90.8.3539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Clavreul A, Piard N, Tanguy JY, Gamelin E, Rousselet MC, Leynia P, et al. Autologous tumor cell vaccination plus infusion of GM-CSF by a programmable pump in the treatment of recurrent malignant gliomas. J Clin Neurosci. 2010;17:842–8. doi: 10.1016/j.jocn.2009.11.017. [DOI] [PubMed] [Google Scholar]
- 38.Parney IF, Chang LJ, Farr-Jones MA, Hao C, Smylie M, Petruk KC. Technical hurdles in a pilot clinical trial of combined B7-2 and GM-CSF immunogene therapy for glioblastomas and melanomas. J Neurooncol. 2006;78:71–80. doi: 10.1007/s11060-005-9058-0. [DOI] [PubMed] [Google Scholar]
- 39.Okada H, Kalinski P, Ueda R, Hoji A, Kohanbash G, Donegan TE, et al. Induction of CD8+ T-cell responses against novel glioma-associated antigen peptides and clinical activity by vaccinations with {alpha}-type 1 polarized dendritic cells and polyinosinic-polycytidylic acid stabilized by lysine and carboxymethyl cellulose in patients with recurrent malignant glioma. J Clin Oncol. 2011;29:330–6. doi: 10.1200/JCO.2010.30.7744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Prins RM, Soto H, Konkankit V, Odesa SK, Eskin A, Yong WH, et al. Gene expression profile correlates with T-cell infiltration and relative survival in glioblastoma patients vaccinated with dendritic cell immunotherapy. Clin Cancer Res. 2011;17:1603–15. doi: 10.1158/1078-0432.CCR-10-2563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sampson JH, Archer GE, Mitchell DA, Heimberger AB, Herndon JE, 2nd, Lally-Goss D, et al. An epidermal growth factor receptor variant III-targeted vaccine is safe and immunogenic in patients with glioblastoma multiforme. Mol Cancer Ther. 2009;8:2773–9. doi: 10.1158/1535-7163.MCT-09-0124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wheeler CJ, Black KL, Liu G, Mazer M, Zhang XX, Pepkowitz S, et al. Vaccination elicits correlated immune and clinical responses in glioblastoma multiforme patients. Cancer Res. 2008;68:5955–64. doi: 10.1158/0008-5472.CAN-07-5973. [DOI] [PubMed] [Google Scholar]
- 43.Yu JS, Liu G, Ying H, Yong WH, Black KL, Wheeler CJ. Vaccination with tumor lysate-pulsed dendritic cells elicits antigen-specific, cytotoxic T-cells in patients with malignant glioma. Cancer Res. 2004;64:4973–9. doi: 10.1158/0008-5472.CAN-03-3505. [DOI] [PubMed] [Google Scholar]
- 44.Yu JS, Wheeler CJ, Zeltzer PM, Ying H, Finger DN, Lee PK, et al. Vaccination of malignant glioma patients with peptide-pulsed dendritic cells elicits systemic cytotoxicity and intracranial T-cell infiltration. Cancer Res. 2001;61:842–7. [PubMed] [Google Scholar]
- 45.Ardon H, Van Gool SW, Verschuere T, Maes W, Fieuws S, Sciot R, et al. Integration of autologous dendritic cell-based immunotherapy in the standard of care treatment for patients with newly diagnosed glioblastoma: results of the HGG-2006 phase I/II trial. Cancer Immunol Immunother. 2012;61:2033–44. doi: 10.1007/s00262-012-1261-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.De Vleeschouwer S, Fieuws S, Rutkowski S, Van Calenbergh F, Van Loon J, Goffin J, et al. Postoperative adjuvant dendritic cell-based immunotherapy in patients with relapsed glioblastoma multiforme. Clin Cancer Res. 2008;14:3098–104. doi: 10.1158/1078-0432.CCR-07-4875. [DOI] [PubMed] [Google Scholar]
- 47.Yamanaka R, Abe T, Yajima N, Tsuchiya N, Homma J, Kobayashi T, et al. Vaccination of recurrent glioma patients with tumour lysate-pulsed dendritic cells elicits immune responses: results of a clinical phase I/II trial. Br J Cancer. 2003;89:1172–9. doi: 10.1038/sj.bjc.6601268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yamanaka R, Homma J, Yajima N, Tsuchiya N, Sano M, Kobayashi T, et al. Clinical evaluation of dendritic cell vaccination for patients with recurrent glioma: results of a clinical phase I/II trial. Clin Cancer Res. 2005;11:4160–7. doi: 10.1158/1078-0432.CCR-05-0120. [DOI] [PubMed] [Google Scholar]
- 49.Vik-Mo EO, Nyakas M, Mikkelsen BV, Moe MC, Due-Tonnesen P, Suso EM, et al. Therapeutic vaccination against autologous cancer stem cells with mRNA-transfected dendritic cells in patients with glioblastoma. Cancer Immunol Immunother. 2013;62:1499–509. doi: 10.1007/s00262-013-1453-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Caruso DA, Orme LM, Neale AM, Radcliff FJ, Amor GM, Maixner W, et al. Results of a phase 1 study utilizing monocyte-derived dendritic cells pulsed with tumor RNA in children and young adults with brain cancer. Neuro Oncol. 2004;6:236–46. doi: 10.1215/S1152851703000668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Akiyama Y, Oshita C, Kume A, Iizuka A, Miyata H, Komiyama M, et al. alpha-type-1 polarized dendritic cell-based vaccination in recurrent high-grade glioma: a phase I clinical trial. BMC Cancer. 2012;12:623. doi: 10.1186/1471-2407-12-623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Prins RM, Wang X, Soto H, Young E, Lisiero DN, Fong B, et al. Comparison of glioma-associated antigen peptide-loaded versus autologous tumor lysate-loaded dendritic cell vaccination in malignant glioma patients. J Immunother. 2013;36:152–7. doi: 10.1097/CJI.0b013e3182811ae4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Schumacher T, Bunse L, Pusch S, Sahm F, Wiestler B, Quandt J, et al. A vaccine targeting mutant IDH1 induces antitumour immunity. Nature. 2014;512:324–7. doi: 10.1038/nature13387. [DOI] [PubMed] [Google Scholar]
- 54.Izumoto S, Tsuboi A, Oka Y, Suzuki T, Hashiba T, Kagawa N, et al. Phase II clinical trial of Wilms tumor 1 peptide vaccination for patients with recurrent glioblastoma multiforme. J Neurosurg. 2008;108:963–71. doi: 10.3171/JNS/2008/108/5/0963. [DOI] [PubMed] [Google Scholar]
- 55.Yajima N, Yamanaka R, Mine T, Tsuchiya N, Homma J, Sano M, et al. Immunologic evaluation of personalized peptide vaccination for patients with advanced malignant glioma. Clin Cancer Res. 2005;11:5900–11. doi: 10.1158/1078-0432.CCR-05-0559. [DOI] [PubMed] [Google Scholar]
- 56.Wood C, Srivastava P, Bukowski R, Lacombe L, Gorelov AI, Gorelov S, et al. An adjuvant autologous therapeutic vaccine (HSPPC-96; vitespen) versus observation alone for patients at high risk of recurrence after nephrectomy for renal cell carcinoma: a multicentre, open-label, randomised phase III trial. Lancet. 2008;372:145–54. doi: 10.1016/S0140-6736(08)60697-2. [DOI] [PubMed] [Google Scholar]
- 57.Testori A, Richards J, Whitman E, Mann GB, Lutzky J, Camacho L, et al. Phase III comparison of vitespen, an autologous tumor-derived heat shock protein gp96 peptide complex vaccine, with physician’s choice of treatment for stage IV melanoma: the C-100-21 Study Group. J Clin Oncol. 2008;26:955–62. doi: 10.1200/JCO.2007.11.9941. [DOI] [PubMed] [Google Scholar]
- 58.Crane CA, Han SJ, Ahn B, Oehlke J, Kivett V, Fedoroff A, et al. Individual patient-specific immunity against high-grade glioma after vaccination with autologous tumor derived peptides bound to the 96 KD chaperone protein. Clin Cancer Res. 2013;19:205–14. doi: 10.1158/1078-0432.CCR-11-3358. [DOI] [PubMed] [Google Scholar]
- 59.Cheema TA, Fecci PE, Ning J, Rabkin SD. Immunovirotherapy for the treatment of glioblastoma. Oncoimmunology. 2014;3:e27218. doi: 10.4161/onci.27218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Cheema TA, Wakimoto H, Fecci PE, Ning J, Kuroda T, Jeyaretna DS, et al. Multifaceted oncolytic virus therapy for glioblastoma in an immunocompetent cancer stem cell model. Proc Natl Acad Sci U S A. 2013;110:12006–11. doi: 10.1073/pnas.1307935110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ghulam Muhammad AK, Candolfi M, King GD, Yagiz K, Foulad D, Mineharu Y, et al. Antiglioma immunological memory in response to conditional cytotoxic/immune-stimulatory gene therapy: humoral and cellular immunity lead to tumor regression. Clin Cancer Res. 2009;15:6113–27. doi: 10.1158/1078-0432.CCR-09-1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Chiocca EA, Aguilar LK, Bell SD, Kaur B, Hardcastle J, Cavaliere R, et al. Phase IB study of gene-mediated cytotoxic immunotherapy adjuvant to up-front surgery and intensive timing radiation for malignant glioma. J Clin Oncol. 2011;29:3611–9. doi: 10.1200/JCO.2011.35.5222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gromeier M, Lachmann S, Rosenfeld MR, Gutin PH, Wimmer E. Intergeneric poliovirus recombinants for the treatment of malignant glioma. Proc Natl Acad Sci U S A. 2000;97:6803–8. doi: 10.1073/pnas.97.12.6803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Markert JM, Medlock MD, Rabkin SD, Gillespie GY, Todo T, Hunter WD, et al. Conditionally replicating herpes simplex virus mutant, G207 for the treatment of malignant glioma: results of a phase I trial. Gene Ther. 2000;7:867–74. doi: 10.1038/sj.gt.3301205. [DOI] [PubMed] [Google Scholar]
- 65.Jiang H, Gomez-Manzano C, Lang FF, Alemany R, Fueyo J. Oncolytic adenovirus: preclinical and clinical studies in patients with human malignant gliomas. Curr Gene Ther. 2009;9:422–7. doi: 10.2174/156652309789753356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Fecci PE, Gromeier M, Sampson JH. Viruses in the treatment of brain tumors. Neuroimaging Clin N Am. 2002;12:553–70. doi: 10.1016/s1052-5149(02)00028-x. [DOI] [PubMed] [Google Scholar]
- 67.Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2012;12:252–64. doi: 10.1038/nrc3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Peggs KS, Quezada SA, Chambers CA, Korman AJ, Allison JP. Blockade of CTLA-4 on both effector and regulatory T cell compartments contributes to the antitumor activity of anti-CTLA-4 antibodies. J Exp Med. 2009;206:1717–25. doi: 10.1084/jem.20082492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hodi FS, O’Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363:711–23. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Agarwalla P, Barnard Z, Fecci P, Dranoff G, Curry WT., Jr Sequential immunotherapy by vaccination with GM-CSF-expressing glioma cells and CTLA-4 blockade effectively treats established murine intracranial tumors. J Immunother. 2012;35:385–9. doi: 10.1097/CJI.0b013e3182562d59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Fecci PE, Ochiai H, Mitchell DA, Grossi PM, Sweeney AE, Archer GE, et al. Systemic CTLA-4 blockade ameliorates glioma-induced changes to the CD4+ T cell compartment without affecting regulatory T-cell function. Clin Cancer Res. 2007;13:2158–67. doi: 10.1158/1078-0432.CCR-06-2070. [DOI] [PubMed] [Google Scholar]
- 72.Wainwright DA, Chang AL, Dey M, Balyasnikova IV, Kim C, Tobias AL, et al. Durable therapeutic efficacy utilizing combinatorial blockade against IDO, CTLA-4 and PD-L1 in mice with brain tumors. Clin Cancer Res. 2014 Apr 1; doi: 10.1158/1078-0432.CCR-14-0514. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Margolin K, Ernstoff MS, Hamid O, Lawrence D, McDermott D, Puzanov I, et al. Ipilimumab in patients with melanoma and brain metastases: an open-label, phase 2 trial. Lancet Oncol. 2012;13:459–65. doi: 10.1016/S1470-2045(12)70090-6. [DOI] [PubMed] [Google Scholar]
- 74.Sharpe AH, Wherry EJ, Ahmed R, Freeman GJ. The function of programmed cell death 1 and its ligands in regulating autoimmunity and infection. Nat Immunol. 2007;8:239–45. doi: 10.1038/ni1443. [DOI] [PubMed] [Google Scholar]
- 75.Thompson RH, Gillett MD, Cheville JC, Lohse CM, Dong H, Webster WS, et al. Costimulatory B7-H1 in renal cell carcinoma patients: Indicator of tumor aggressiveness and potential therapeutic target. Proc Natl Acad Sci U S A. 2004;101:17174–9. doi: 10.1073/pnas.0406351101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hamanishi J, Mandai M, Iwasaki M, Okazaki T, Tanaka Y, Yamaguchi K, et al. Programmed cell death 1 ligand 1 and tumor-infiltrating CD8+ T lymphocytes are prognostic factors of human ovarian cancer. Proc Natl Acad Sci U S A. 2007;104:3360–5. doi: 10.1073/pnas.0611533104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Inman BA, Sebo TJ, Frigola X, Dong H, Bergstralh EJ, Frank I, et al. PD-L1 (B7-H1) expression by urothelial carcinoma of the bladder and BCG-induced granulomata: associations with localized stage progression. Cancer. 2007;109:1499–505. doi: 10.1002/cncr.22588. [DOI] [PubMed] [Google Scholar]
- 78.Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366:2443–54. doi: 10.1056/NEJMoa1200690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Dix AR, Brooks WH, Roszman TL, Morford LA. Immune defects observed in patients with primary malignant brain tumors. J Neuroimmunol. 1999;100:216–32. doi: 10.1016/s0165-5728(99)00203-9. [DOI] [PubMed] [Google Scholar]
- 80.Brooks WH, Roszman TL, Mahaley MS, Woosley RE. Immunobiology of primary intracranial tumours. II. Analysis of lymphocyte subpopulations in patients with primary brain tumours. Clin Exp Immunol. 1977;29:61–6. [PMC free article] [PubMed] [Google Scholar]
- 81.Fecci PE, Mitchell DA, Whitesides JF, Xie W, Friedman AH, Archer GE, et al. Increased regulatory T-cell fraction amidst a diminished CD4 compartment explains cellular immune defects in patients with malignant glioma. Cancer Res. 2006;66:3294–302. doi: 10.1158/0008-5472.CAN-05-3773. [DOI] [PubMed] [Google Scholar]
- 82.Roszman TL, Brooks WH. Immunobiology of primary intracranial tumours. III. Demonstration of a qualitative lymphocyte abnormality in patients with primary brain tumours. Clin Exp Immunol. 1980;39:395–402. [PMC free article] [PubMed] [Google Scholar]
- 83.Morford LA, Elliott LH, Carlson SL, Brooks WH, Roszman TL. T cell receptor-mediated signaling is defective in T cells obtained from patients with primary intracranial tumors. J Immunol. 1997;159:4415–25. [PubMed] [Google Scholar]
- 84.Ashkenazi E, Deutsch M, Tirosh R, Weinreb A, Tsukerman A, Brodie C. A selective impairment of the IL-2 system in lymphocytes of patients with glioblastomas: increased level of soluble IL-2R and reduced protein tyrosine phosphorylation. Neuroimmunomodulation. 1997;4:49–56. doi: 10.1159/000097315. [DOI] [PubMed] [Google Scholar]
- 85.Ausiello CM, Palma C, Maleci A, Spagnoli GC, Amici C, Antonelli G, et al. Cell mediated cytotoxicity and cytokine production in peripheral blood mononuclear cells of glioma patients. Eur J Cancer. 1991;27:646–50. doi: 10.1016/0277-5379(91)90235-6. [DOI] [PubMed] [Google Scholar]
- 86.Crane CA, Han SJ, Barry JJ, Ahn BJ, Lanier LL, Parsa AT. TGF-beta downregulates the activating receptor NKG2D on NK cells and CD8+ T cells in glioma patients. Neuro Oncol. 2010;12:7–13. doi: 10.1093/neuonc/nop009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.El Andaloussi A, Lesniak MS. An increase in CD4+CD25+FOXP3+ regulatory T cells in tumor-infiltrating lymphocytes of human glioblastoma multiforme. Neuro Oncol. 2006;8:234–43. doi: 10.1215/15228517-2006-006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Wainwright DA, Balyasnikova IV, Chang AL, Ahmed AU, Moon KS, Auffinger B, et al. IDO expression in brain tumors increases the recruitment of regulatory T cells and negatively impacts survival. Clin Cancer Res. 2012;18:6110–21. doi: 10.1158/1078-0432.CCR-12-2130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Facoetti A, Nano R, Zelini P, Morbini P, Benericetti E, Ceroni M, et al. Human leukocyte antigen and antigen processing machinery component defects in astrocytic tumors. Clin Cancer Res. 2005;11:8304–11. doi: 10.1158/1078-0432.CCR-04-2588. [DOI] [PubMed] [Google Scholar]
- 90.Anderson RC, Anderson DE, Elder JB, Brown MD, Mandigo CE, Parsa AT, et al. Lack of B7 expression, not human leukocyte antigen expression, facilitates immune evasion by human malignant gliomas. Neurosurgery. 2007;60:1129–36. doi: 10.1227/01.NEU.0000255460.91892.44. [DOI] [PubMed] [Google Scholar]
- 91.Wintterle S, Schreiner B, Mitsdoerffer M, Schneider D, Chen L, Meyermann R, et al. Expression of the B7-related molecule B7-H1 by glioma cells: a potential mechanism of immune paralysis. Cancer Res. 2003;63:7462–7. [PubMed] [Google Scholar]
- 92.Parsa AT, Waldron JS, Panner A, Crane CA, Parney IF, Barry JJ, et al. Loss of tumor suppressor PTEN function increases B7-H1 expression and immunoresistance in glioma. Nat Med. 2007;13:84–8. doi: 10.1038/nm1517. [DOI] [PubMed] [Google Scholar]
- 93.Abou-Ghazal M, Yang DS, Qiao W, Reina-Ortiz C, Wei J, Kong LY, et al. The incidence, correlation with tumor-infiltrating inflammation, and prognosis of phosphorylated STAT3 expression in human gliomas. Clin Cancer Res. 2008;14:8228–35. doi: 10.1158/1078-0432.CCR-08-1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Roussel E, Gingras MC, Grimm EA, Bruner JM, Moser RP. Predominance of a type 2 intratumoural immune response in fresh tumour-infiltrating lymphocytes from human gliomas. Clin Exp Immunol. 1996;105:344–52. doi: 10.1046/j.1365-2249.1996.d01-753.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Friese MA, Platten M, Lutz SZ, Naumann U, Aulwurm S, Bischof F, et al. MICA/NKG2D-mediated immunogene therapy of experimental gliomas. Cancer Res. 2003;63:8996–9006. [PubMed] [Google Scholar]
- 96.Eisele G, Wischhusen J, Mittelbronn M, Meyermann R, Waldhauer I, Steinle A, et al. TGF-beta and metalloproteinases differentially suppress NKG2D ligand surface expression on malignant glioma cells. Brain. 2006;129:2416–25. doi: 10.1093/brain/awl205. [DOI] [PubMed] [Google Scholar]
- 97.Wischhusen J, Friese MA, Mittelbronn M, Meyermann R, Weller M. HLA-E protects glioma cells from NKG2D-mediated immune responses in vitro: implications for immune escape in vivo. J Neuropathol Exp Neurol. 2005;64:523–8. doi: 10.1093/jnen/64.6.523. [DOI] [PubMed] [Google Scholar]
- 98.Wherry EJ. T cell exhaustion. Nat Immunol. 2011;12:492–9. doi: 10.1038/ni.2035. [DOI] [PubMed] [Google Scholar]
- 99.Sampson JH, Heimberger AB, Archer GE, Aldape KD, Friedman AH, Friedman HS, et al. Immunologic escape after prolonged progression-free survival with epidermal growth factor receptor variant III peptide vaccination in patients with newly diagnosed glioblastoma. J Clin Oncol. 2010;28:4722–9. doi: 10.1200/JCO.2010.28.6963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Verhaak RG, Hoadley KA, Purdom E, Wang V, Qi Y, Wilkerson MD, et al. Integrated genomic analysis identifies clinically relevant subtypes of glioblastoma characterized by abnormalities in PDGFRA, IDH1, EGFR, and NF1. Cancer Cell. 2010;17:98–110. doi: 10.1016/j.ccr.2009.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Gajjar A, Pfister SM, Taylor MD, Gilbertson RJ. Molecular insights into pediatric brain tumors have the potential to transform therapy. Clin Cancer Res. 2014;20:xxx–xxx. doi: 10.1158/1078-0432.CCR-14-0833. [DOI] [PMC free article] [PubMed] [Google Scholar]