Abstract
Preeclampsia is characterized as new onset maternal hypertension and proteinuria after 20 weeks gestation. Studies suggest that endothelin (ET-1) is a regulator of vascular function in preeclampsia and plays a major role in mediating chronic reduction in uterine perfusion pressure (RUPP)-induced hypertension. We recently demonstrated a role for the autoimmune cytokine interleukin 17 (IL-17) in causing placental oxidative stress and hypertension during pregnancy. In this current study, we investigated the role of ET-1 as a potential mechanism by which TH17 cells and IL-17 mediate hypertension in preeclampsia. While IL-17 infusion into normal pregnant rats increased blood pressure in a dose-responsive manner (98+/-2 mmHg in NP (n=20) to 105+/-3 mmHg in IL-17 (50pg/day, n=20) to 120+/-4 mmHg in IL-17 (100pg/day, n=10) to 123+/-3 mmHg in IL-17 (150 pg/day, n=7), it decreased local endothelin in placentas (NP (n=10) 7.5±0.3; IL-17 (100 pg/day, n=5) 6.4±0.2; IL-17 (150 pg/day, n=12) 4.5+1.5) and renal cortices (NP (n=8) 7.9 + 0.4; IL-17 (100 pg/day, n=6) 7.1±0.4; IL-17 (150 pg/day, n=4) 1.6 +0.7 during pregnancy. In addition, increasing IL-17 directly reduced secretion of ET-1 by human umbilical venous endothelial cells (HUVECs). HUVEC ET-1 secretion decreased from that seen in serum free media 42.7±7.7 pg/ml to 36.2 ± 5.9 pg/ml at 10 pg IL-17 to 31.3 ± 5.1 pg/ml at 10 μg IL-17. Our observations suggest that IL-17 negatively regulates the ET-1 pathway in local tissues and cultured endothelial cells and that the ET-1 pathway is not a mechanism by which IL-17 causes hypertension during pregnancy.
Introduction
Preeclampsia is a hypertensive disorder of pregnancy affecting 5-8% of US pregnancies that is characterized by new onset maternal hypertension and proteinuria after 20 weeks gestation [1-3] Hallmark characteristics of preeclampsia are new onset hypertension with proteinuria, immune activation, increased plasma endothelin (ET-1), and oxidative stress during pregnancy. The hypothesized initiating event leading to preeclampsia is inadequate invasion of trophoblasts into the uterine spiral arteries, leading to reduced uteroplacental perfusion, thereby contributing to the development of placental ischemia, which results in widespread dysfunction of the maternal vascular endothelium [4,5].
Endothelin is a regulator of vascular function in preeclampsia [6]. Studies measuring plasma levels of ET-1 in normal pregnant woman and women with preeclampsia have found that preeclamptic women have 2 to 3-fold higher ET-1 plasma concentrations in the later stages of gestation [7,8]. Increases in the production of ET-1 and activation of the endothelin type A (ETA) receptor may be involved in the pathophysiology of hypertension during preeclampsia. Previous studies have examined a role for ET-1 in mediating hypertension in a placental ischemic rat model and suggest that ET-1 plays a major role in mediating chronic reduction in uterine perfusion pressure (RUPP)-induced hypertension [6,9,10].
An imbalance between two CD4+ T-helper cell populations, CD4+ T-regulatory (TReg) and CD4+ T-helper 17 (TH17) cells, is proposed to be involved in the pathophysiology of preeclampsia [11]. We have previously shown that in the RUPP rat model, placental ischemia stimulates this CD4+ T helper cell imbalance which contributes to excess pro-inflammatory cytokine production, a shift in angiogenic factors, and leads to hypertension during pregnancy [12]. T-helper 17 cells are a subclass of CD4+ cells, characterized by their secretion of the cytokine IL-17, that has also been implicated in a number of autoimmune disorders, including psoriasis, multiple sclerosis, rheumatoid arthritis, irritable bowel syndrome (IBS), and systemic lupus erythematosus (SLE) [13-16]. Autoimmune activities during preeclampsia, such as the production of autoantibodies to the angiotensin II type I receptor (AT1-AA) and increased circulating IL-17, indicate similarities between preeclampsia and autoimmune diseases [17,18]. We have shown that adoptive transfer of RUPP CD4+ T-cells into normal pregnant rats causes hypertension via activation of the ET-1 pathway [12-19]. Furthermore we have shown that infusion of AT1-AA or the pro-inflammatory cytokine TNF alpha (TNFα) into NP rats stimulates ET-1 as a mechanism of hypertension during pregnancy [20,21]. We recently demonstrated a role for the autoimmune cytokine IL-17 in causing placental oxidative stress and hypertension during pregnancy. By infusing IL-17 into normal pregnant rats, we found TH17 cells to be elevated, along with urinary isoprostanes, placental oxidative stress, AT1-AA, and blood pressure compared to the normal pregnant control rat [22] thus suggesting that TH17 cells and IL-17 play a role in mediating preeclampsia.
For these reasons, in this current study, we investigated a role for ET-1 as a mechanism by which TH17 cells and IL-17 mediate hypertension in preeclampsia. We tested the hypothesis that the autoimmune cytokine IL-17 mediates hypertension during pregnancy by activating the endothelin-1 system. To test this hypothesis, in vitro experiments were conducted to measure secretion of endothelin by endothelial cells in response to increasing doses of IL-17. Furthermore, in vivo studies were performed to assess kidney and placental expression of preproendothelin in response to increasing doses of IL-17 infusion.
Materials and Methods
In vivo experimental protocols
Pregnant Sprague-Dawley rats purchased from Harlan Sprague Dawley Inc (Indianapolis, IN) were used in the study. Animals were housed in a temperature-controlled room (23 °C) with a 12:12-hour light/dark cycle. All experimental procedures executed in this study were in accordance with the National Institute of Health guidelines for use and care of animals. All protocols were approved by the Institutional Animal Care and Use Committee (IACUC) at the University of Mississippi Medical Center.
An established dose response of IL-17 infusion on blood pressure
Recombinant mouse IL-17, at three consecutive doses (50,100, 150 pg/day) (RnD Systems, Minneapolis, MN), was infused intraperitoneally from day 14 to day 19 of gestation via mini-osmotic pumps (model 2002, Alzet Scientific Corporation) into normal pregnant (NP) rats. Carotid catheters were inserted on day 18 of gestation and blood pressure and blood and tissue were collected on day 19 of gestation.
An established dose response of IL-17 infusion on and kidney and placental ET-1 mRNA Level in pregnant rats
The cortex and medulla of the kidneys from the two higher doses that significantly increased blood pressure were separated immediately after harvesting and quickly frozen in liquid nitrogen and stored at −80°C. Placentas were weighed and quickly frozen in liquid nitrogen and stored at −80°C. Total RNA was extracted using the Totally RNA kit supplied by Ambion after the tissues were crushed in liquid nitrogen with a mortar and pestle. The isolation procedure was then performed as outlined in the instructions provided by the manufacturer. Real-time PCR was utilized, as previously described, to determine tissue preproendothelin-1 levels [21-23]. Briefly, cDNA was synthesized from 1 μg of RNA with Bio-Rad Iscript cDNA reverse transcriptase and real-time PCR was performed using the Bio-Rad Sybre Green Supermix and iCycler. The following primer sequences, provided by Life technologies, were used for PPET as previously described: forward 1, ctaggtctaagcgatccttg, and reverse 1, tctttgtctgcttggc [4,5,20]. Levels of mRNA were calculated using the mathematical formula for 2−ΔΔCt (2avg. Ct gene of interest − avg Ct beta actin) recommended by Applied Biosystems (Applied Biosystems User Bulletin, No. 2, 1997).
In vitro Experimental protocols
Endothelial cell culture
Human umbilical venous endothelial cells (HUVECs; ATCC, Manassas, VA), passage 2, were used for all experiments. Culture media was 50%/50% Dulbecco's modified Eagle's medium/Medium 199 (Gibco, Billings, MT) with 10% fetal bovine serum (Hyclone, Logan, UT) and 10 ml antimycotic–antibiotic (Gibco) at 37 °C in a humidified atmosphere of 5% CO2–20% O2–75% N2. Seventy percent confluent monolayers were incubated for 48 h in serum-free media before IL-17 exposure.
Effect of IL-17 on endothelin-1 secretion by HUVECs
To determine the direct effects of IL-17 on ET synthesis, we examined ET-1 secretion of endothelial cells exposed to IL-17 in culture. The dose-dependent response to IL-17 on ET-1 production was examined in HUVECs using 10 pg, 10 ng, and 10 μg IL-17 added per ml of serum-free culture media. Following an 8-h experimental incubation, cell culture supernatant was removed and used for ET-1 measurements.
Determination of ET-1
One milliliter of cell culture media was extracted from the culture well before cell harvesting and used to determine concentrations of secreted ET-1. Levels were determined by enzyme-linked immunosorbent assay, specifically with the Parameter human ET, ET-1, from R&D Systems (Minneapolis, MN). Assays were performed following instructions outlined in the manual provided with the kit from R&D Systems.
Statistical analysis
All of the data are expressed as mean ± SEM. Comparisons of control with each experimental group was analyzed by analysis of variance with Student's T test as post hoc analysis. A value of P < .05 was considered statistically significant.
Results
Increasing IL-17 increases blood pressure during pregnancy
IL-17 at three consecutive doses (50,100, 150 pg/day) increased blood pressure from 98+/-2 mmHg in NP (n=20) to 105+/-3 mmHg (n=20) in IL-17 (50pg/day) to 120+/-4 mmHg (n=10) in IL-17 (100pg/day) to 123+/-3 mmHg (n=7) in IL-17 (150 pg/day) (Figure 1).
Figure 1. Increasing IL-17 increases blood pressure during pregnancy.
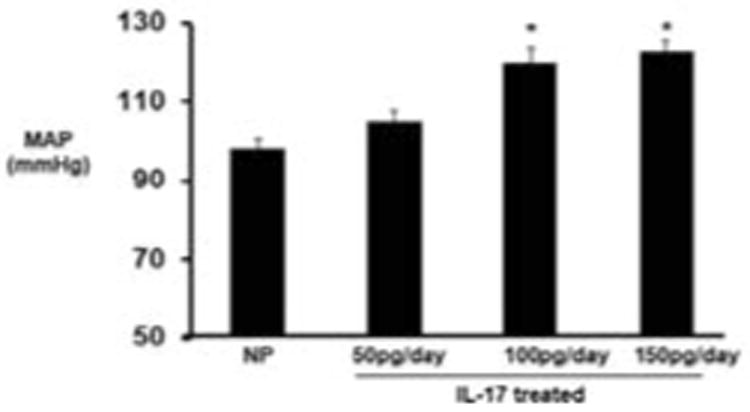
Blood pressure was increased at three consecutive doses of increasing IL-17. NP- 98 mmHg; 50 pg/day IL-17-105 mmHg, 100 pg/day IL-17-120 mmHg, and 150 pg/day IL-17-123 mmHg.
IL-17 decreases kidney and placental preproendothelin mRNA levels
Surprisingly, although IL-17 increases blood pressure in the 100 pg/day and 150 pg/day infusion, ET-1 was not activated. In fact ET-1 significantly decreased with increasing doses of IL-17, independent of blood pressure response. Real-time PCR was used to measure preproendothelin in the placenta and the renal cortex. In (Figure 2), preproendothelin decreased in IL-17 infused NP rats. Preproendothelin levels were lower in the placentas of IL-17 (100 pg/day) at 6.4±0.2 (n=5) and IL-17 (150 pg/day) at 4.5+1.5 (n=12, p < 0.05), as compared to NP rat placentas 7.5+ 0.3(n=10). Similarly there was a decrease in preproendothelin in renal cortices of IL-17 infused NP rats (100 pg/day) 7.1±0.4 (n=6) and (150 pg/day) 1.6 ±0.7 (n=4, p < 0.0001) compared to NP rat 7.9 ± 0.4 (n=8).
Figure 2. Preproendothelin in placenta and renal cortices at different IL-17 doses.
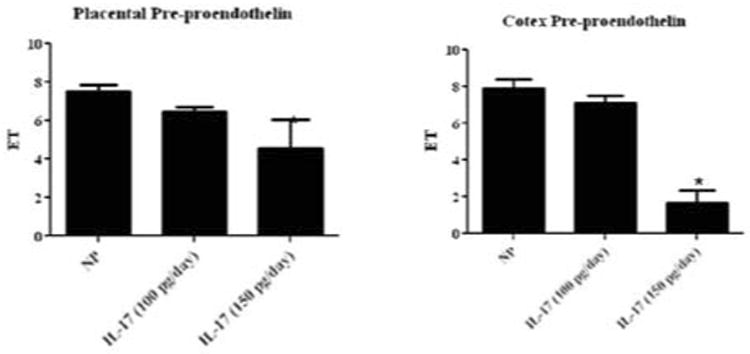
Preproendothelin was not altered in placenta of NP rats infused with IL-17 (4.5 ± 1.5) compared to NP rats (7.5 ± 0.3). Renal cortex levels of preproendothelin of NP rats infused with IL-17 were not significantly different from those in NP rats (1.6 +0.7 vs 7.9 + 0.4).
IL-17 does not alter HUVEC secretion of endothelin
Human umbilical venous endothelial cells were cultured in serum free media or increasing doses of IL-17. Endothelin secretion by HUVECs was not increased at any dose of IL-17 administered in culture (36.2 ± 5.9 at10 pg, 43.9 ± 4.5 at 10 ng, 31.3 ± 5.1 at 10 μg) compared to culture in serum free media only (42.7 ±7.7, Figure 3).
Figure 3. IL-17 does not alter ET-1 secretion by HUVECs.
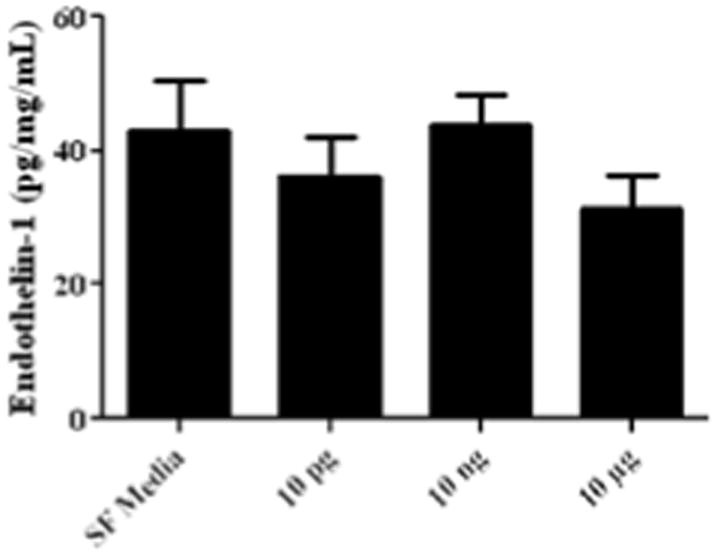
HUVECs were cultured in serum-free media with various doses of IL-17 (10 pg, 10 ng, and 10 μg) and ET-1 secretion was measured using a commercial ELISA kit. Endothelin secretion by HUVECs was not increased at any dose of IL-17 administered in culture (10 pg- 36.2 ± 5.9, 10 ng- 43.9 ± 4.5, 10 μg- 31.3 ± 5.1) compared to culture in serum free media only (42.7 ±7.7).
Discussion
The role of endothelin (ET-1) in regulation of vascular function is well established. With hypertension as a hallmark characteristic of preeclampsia, much attention has been given to the role of ET-1 as a key factor in the pathology of preeclampsia [24,25]. A number of studies have shown a role for ET-1 in the development of hypertension and increased oxidative stress in preeclampsia [24-26]. The anti-angiogenic protein, sFLT-1, the AT1-AA, and pro-inflammatory cytokines have all been shown to induce hypertension in preeclampsia through production of ET-1 [24-27]. Recent studies demonstrate a role for the proinflammatory autoimmune cytokine IL-17 in the pathophysiology of preeclampsia [11,22-28]. However, the mechanism by which IL-17 causes pathology in preeclampsia is yet unknown. We therefore tested the hypothesis that IL-17 mediates pathology in preeclampsia through ET-1 production.
Preproendothelin mRNA expression was assessed, via realtime PCR, in placental and renal tissues of both NP rats and NP rats infused with IL-17 with a significant decrease in ET-1 mRNA expression only at the highest dose of IL-17 (150 pg/day). These data suggest that the IL-17 does not mediate pathology in preeclampsia using the ET-1 pathway, as observed with other pro-inflammatory cytokines. In vitro experiments in which HUVECs were cultured in serum-free media supplemented with IL-17 also did not show as increase in ET-1 secretion, further validating our observations that IL-17 may potentially down regulate the ET-1 pathway in endothelial cells. It may be that in the whole animal other forces such as TNFα and AT1-AA must overcome the negative regulation of IL-17 on ET-1 to stimulate activation of this pathway during preeclampsia or in response to placental ischemia during pregnancy. However, we did not further investigate any transcriptional or translational modifications that may have occurred in ET-1 processing in vivo or in vitro. Although the experimental groups were small, our data show that both local PPET and ET-1 secretion from endothelial cells were significantly decreased; convincingly indicating a negative regulation of ET -1 in response to increasing doses of IL-17.
Data from previous studies in our laboratory demonstrate a role for IL-17 in mediating preeclampsia pathology; however, this study has shown the ET-1 pathway is not the mechanism by which IL-17 causes progression of preeclampsia. The physiological role of IL-17, when secreted by TH17 cells, is to recruit host defense molecules, such as neutrophils, to sites of infection. IL-17 stimulates neutrophils and macrophages to produce reactive oxygen species (ROS) as a defense mechanism against microbial infection. However, ROS is a non-specific defense mechanism of the innate immune system, and can result in damage to normal host tissue as well. Reactive oxygen species is increased in preeclampsia, and we have previously published data which show that infusion of IL-17 into normal pregnant rats increases placental and whole body oxidative stress as well as hypertension in pregnancy [22].
It is possible that IL-17 regulates pathology in preeclampsia in ways similar to its pathological regulation in other autoimmune diseases. For example, Doreau, et al. determined that IL-17 regulates survival and proliferation of B-cells and their differentiation into antibody-secreting cells [29]. Therefore, IL-17 may regulate pathology in preeclampsia by regulating B-cell proliferation and differentiation which could be directly involved in AT1-AA production. IL-17 has also been shown to recruit T-cells and cause adherence of these cells to endothelial cells [15-30]. Moreover, IL-17 is believed to be directly involved in development of glomerulonephritis in systemic lupus erythematosus, suggesting that IL-17 may cause pathology in preeclampsia by acting directly upon the kidney [15-31]. While data from our and other studies identify IL-17 as a viable target for treatment of preeclampsia as well as other autoimmune disorders, future studies into the mechanism of action by which IL-17 causes pathology will be important to understand the molecular pathways that are involved in the development and progression of preeclampsia [22-32]. Moreover, elucidation of new pathways involved in the pathology of preeclampsia may provide new therapeutic targets for the treatment and possible cure that could preserve the pregnancy and result in more favorable outcome for mother and fetus.
Acknowledgments
This work was supported by NIH grants HL78147 and HL51971 and HD067541 and HL105324. RD is supported by the German Research Foundation (DFG 631/7-1).
References
- 1.Noris M, Perico N, Remuzzi G. Mechanisms of disease: Pre-eclampsia. Nat Clin Pract Nephrol. 2005;1:98–114. doi: 10.1038/ncpneph0035. [DOI] [PubMed] [Google Scholar]
- 2.George EM, Granger JP. Recent insights into the pathophysiology of preeclampsia. Expert Rev Obstet Gynecol. 2010;5:557–566. doi: 10.1586/eog.10.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Roberts JM, Lain KY. Recent Insights into the pathogenesis of pre-eclampsia. Placenta. 2002;23:359–372. doi: 10.1053/plac.2002.0819. [DOI] [PubMed] [Google Scholar]
- 4.LaMarca BD, Alexander BT, Gilbert JS, Ryan MJ, Sedeek M, Murphy SR, et al. Pathophysiology of hypertension in response to placental ischemia during pregnancy: a central role for endothelin? Gend Med. 2008;5(Suppl A):S133–138. doi: 10.1016/j.genm.2008.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.LaMarca BB, Cockrell K, Sullivan E, Bennett W, Granger JP. Role of endothelin in mediating tumor necrosis factor-induced hypertension in pregnant rats. Hypertension. 2005;46:82–86. doi: 10.1161/01.HYP.0000169152.59854.36. [DOI] [PubMed] [Google Scholar]
- 6.Granger JP, Abram S, Stec D, Chandler D, LaMarca B. Endothelin, the kidney, and hypertension. Curr Hypertens Rep. 2006;8:298–303. doi: 10.1007/s11906-006-0068-x. [DOI] [PubMed] [Google Scholar]
- 7.Granger JP, Alexander BT, Llinas MT, Bennett WA, Khalil RA. Pathophysiology of hypertension during preeclampsia linking placental ischemia with endothelial dysfunction. Hypertension. 2001;38:718–722. doi: 10.1161/01.hyp.38.3.718. [DOI] [PubMed] [Google Scholar]
- 8.Khalil RA, Granger JP. Vascular mechanisms of increased arterial pressure in preeclampsia: lessons from animal models. Am J Physiol Regul Integr Comp Physiol. 2002;283:R29–45. doi: 10.1152/ajpregu.00762.2001. [DOI] [PubMed] [Google Scholar]
- 9.Granger JP, LaMarca BB, Cockrell K, Sedeek M, Balzi C, Chandler D, et al. Reduced uterine perfusion pressure (RUPP) model for studying cardiovascular-renal dysfunction in response to placental ischemia. Methods Mol Med. 2006;122:383–392. doi: 10.1385/1-59259-989-3:381. [DOI] [PubMed] [Google Scholar]
- 10.Alexander BT, Rinewalt AN, Cockrell KL, Massey MB, Bennett WA, Granger JP, et al. Endothelin type a receptor blockade attenuates the hypertension in response to chronic reductions in uterine perfusion pressure. Hypertension. 2001;37:485–489. doi: 10.1161/01.hyp.37.2.485. [DOI] [PubMed] [Google Scholar]
- 11.Santner-Nanan B, Peek MJ, Khanam R, Richarts L, Zhu E, Fazekas de St Groth B, et al. Systemic increase in the ratio between Foxp3+ and IL-17-producing CD4+ T cells in healthy pregnancy but not in preeclampsia. J Immunol. 2009;183:7023–7030. doi: 10.4049/jimmunol.0901154. [DOI] [PubMed] [Google Scholar]
- 12.Wallace K, Richards S, Dhillon P, Weimer A, Edholm ES, Bengten E, et al. CD4+ T-helper cells stimulated in response to placental ischemia mediate hypertension during pregnancy. Hypertension. 2011;57:949–955. doi: 10.1161/HYPERTENSIONAHA.110.168344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mai J, Wang H, Yang XF. Th 17 cells interplay with Foxp3+ Tregs in regulation of inflammation and autoimmunity. Front Biosci (Landmark Ed) 2010;15:986–1006. doi: 10.2741/3657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wilke CM, Bishop K, Fox D, Zou W. Deciphering the role of Th17 cells in human disease. Trends Immunol. 2011;32:603–611. doi: 10.1016/j.it.2011.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yang J, Chu Y, Yang X, Gao D, Zhu L, Yang X, et al. Th17 and natural Treg cell population dynamics in systemic lupus erythematosus. Arthritis Rheum. 2009;60:1472–1483. doi: 10.1002/art.24499. [DOI] [PubMed] [Google Scholar]
- 16.Yap DY, Lai KN. The role of cytokines in the pathogenesis of systemic lupus erythematosus - from bench to bedside. Nephrology (Carlton) 2013;18:243–255. doi: 10.1111/nep.12047. [DOI] [PubMed] [Google Scholar]
- 17.Granger JP, Alexander BT, Bennett WA, Khalil RA. Pathophysiology of pregnancy-induced hypertension. Am J Hypertens. 2001;14:178S–185S. doi: 10.1016/s0895-7061(01)02086-6. [DOI] [PubMed] [Google Scholar]
- 18.Lamarca B. The role of immune activation in contributing to vascular dysfunction and the pathophysiology of hypertension during preeclampsia. Minerva Ginecol. 2010;62:105–120. [PMC free article] [PubMed] [Google Scholar]
- 19.Wallace K, Novotny S, Heath J, Moseley J, Martin JN, Jr, Owens MY, et al. Hypertension in response to CD4(+) T cells from reduced uterine perfusion pregnant rats is associated with activation of the endothelin-1 system. Am J Physiol Regul Integr Comp Physiol. 2012;303:R144–149. doi: 10.1152/ajpregu.00049.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.LaMarca B, Wallace K, Herse F, Wallukat G, Martin JN, Jr, Weimer A, et al. Hypertension in response to placental ischemia during pregnancy: role of B lymphocytes. Hypertension. 2011;57:865–871. doi: 10.1161/HYPERTENSIONAHA.110.167569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.LaMarca BB, Bennett WA, Alexander BT, Cockrell K, Granger JP. Hypertension produced by reductions in uterine perfusion in the pregnant rat: role of tumor necrosis factor-alpha. Hypertension. 2005;46:1022–1025. doi: 10.1161/01.HYP.0000175476.26719.36. [DOI] [PubMed] [Google Scholar]
- 22.Dhillion P, Wallace K, Herse F, Scott J, Wallukat G, Heath J, et al. IL-17-mediated oxidative stress is an important stimulator of AT1-AA and hypertension during pregnancy. Am J Physiol Regul Integr Comp Physiol. 2012;303:353–358. doi: 10.1152/ajpregu.00051.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.LaMarca BD, Gilbert J, Granger JP. Recent progress toward the understanding of the pathophysiology of hypertension during preeclampsia. Hypertension. 2008;51:982–988. doi: 10.1161/HYPERTENSIONAHA.107.108837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.George EM, Granger JP. Endothelin: key mediator of hypertension in preeclampsia. Am J Hypertens. 2011;24:964–969. doi: 10.1038/ajh.2011.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jain A. Endothelin-1: a key pathological factor in pre-eclampsia? Reprod Biomed Online. 2012;25:443–449. doi: 10.1016/j.rbmo.2012.07.014. [DOI] [PubMed] [Google Scholar]
- 26.Tam Tam KB, George E, Cockrell K, Arany M, Speed J, Martin JN, Jr, et al. Endothelin type A receptor antagonist attenuates placental ischemia-induced hypertension and uterine vascular resistance. Am J Obstet Gynecol. 2011;204:330. doi: 10.1016/j.ajog.2011.01.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gilbert JS, Ryan MJ, LaMarca BB, Sedeek M, Murphy SR, Granger JP, et al. Pathophysiology of hypertension during preeclampsia: linking placental ischemia with endothelial dysfunction. Am J Physiol Heart Circ Physiol. 2008;294:541–550. doi: 10.1152/ajpheart.01113.2007. [DOI] [PubMed] [Google Scholar]
- 28.Darmochwal-Kolarz D, Kludka-Sternik M, Tabarkiewicz J, Kolarz B, Rolinski J, Leszczynska-Gorzelak B, et al. The predominance of Th17 lymphocytes and decreased number and function of Treg cells in preeclampsia. J Reprod Immunol. 2012;93:75–81. doi: 10.1016/j.jri.2012.01.006. [DOI] [PubMed] [Google Scholar]
- 29.vDoreau A, Belot A, Bastid J, Riche B, Trescol-Biemont MC, Ranchin B, et al. Interleukin 17 acts in synergy with B cell-activating factor to influence B cell biology and the pathophysiology of systemic lupus erythematosus. Nat Immunol. 2009;10:778–785. doi: 10.1038/ni.1741. [DOI] [PubMed] [Google Scholar]
- 30.Kebir H, Kreymborg K, Ifergan I, Dodelet-Devillers A, Cayrol R, Bernard M, et al. Human TH17 lymphocytes promote blood-brain barrier disruption and central nervous system inflammation. Nat Med. 2007;13:1173–1175. doi: 10.1038/nm1651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pisitkun P, Ha HL, Wang H, Claudio E, Tivy CC, Zhou H, et al. Interleukin-17 cytokines are critical in development of fatal lupus glomerulonephritis. Immunity. 2012;37:1104–1115. doi: 10.1016/j.immuni.2012.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Clark DN, Markham JL, Sloan CS, Poole BD. Cytokine inhibition as a strategy for treating systemic lupus erythematosus. Clin Immunol. 2013;148:335–343. doi: 10.1016/j.clim.2012.11.001. [DOI] [PubMed] [Google Scholar]


