Abstract
Lysinuric protein intolerance (LPI) is a rare autosomal recessive inborn error of metabolism caused by mutations in SLC7A7, which encodes a component of the dibasic amino acid transporter found in intestinal and renal tubular cells. Patients typically present with vomiting, diarrhea, irritability, failure to thrive, and symptomatic hyperammonemia after protein-rich meals. Long-term complications may include pulmonary alveolar proteinosis, renal disease, and osteoporosis. We present a 5-year-old male who was followed in our skeletal dysplasia clinic for 3 years for multiple fractures, idiopathic osteoporosis, and short stature in the absence of typical features of LPI. Whole exome sequencing performed to determine the etiology of the osteoporosis and speech delay identified a nonsense mutation in SLC7A7. Chromosome microarray analysis identified a deletion involving the second allele of the same gene, and biochemical analysis supported the diagnosis of LPI. Our patient's atypical presentation underscores the importance of maintaining a high index of suspicion for LPI in patients with unexplained fractures and idiopathic osteoporosis, even in the absence of clinical symptoms of hyperammonemia after protein rich meals or other systemic features of classical LPI. This case further demonstrates the utility of whole exome sequencing in diagnosis of unusual presentations of rare disorders for which early intervention may modify the clinical course.
Abbreviations: CMA, chromosomal microarray analysis; LH3, lysyl hydroxylase 3; LPI, lysinuric protein intolerance; WES, whole exome sequencing
Keywords: Lysinuric protein intolerance, Osteoporosis, Bone fractures, SLC7A7
1. Introduction
Lysinuric protein intolerance (LPI, OMIM # 222700) is an autosomal recessive inborn error of metabolism associated with mutations in SLC7A7, the gene encoding solute carrier family 7 (amino acid transporter light chain), member 7 [1], [2]. This protein binds to solute carrier family 3 (amino acid transporter heavy chain), member 2, to form the heterodimeric y + L cationic amino acid transporter which transports arginine, lysine, and ornithine at the basolateral membrane of intestinal and renal tubular cells. In LPI, decreased transporter activity results in low plasma lysine, ornithine, and arginine. It has been proposed that patients manifest with clinical symptoms of urea cycle disorders because of deficiencies of urea cycle intermediates within the cellular compartment.
Classically, patients with LPI develop vomiting and diarrhea after introduction of protein-rich foods. Other presenting features may include failure to thrive, protein avoidance, and neurologic symptoms of hyperammonemia such as lethargy, abnormal behavior, and hypotonia after protein-rich meals. Rare presentations include systemic lupus erythematosus [3], [4], [5] or interstitial lung disease [6]. Long-term complications of LPI may include pulmonary alveolar proteinosis, dyslipidemia, hematologic abnormalities, macrophage activation syndrome, renal disease, and osteoporosis. Treatment involves dietary protein restriction and citrulline supplementation to replete urea cycle intermediates. Oral lysine supplementation and nitrogen-scavenging agents have also been used.
In the present report, we describe a 5-year-old male with short stature and speech delay who was followed for idiopathic osteoporosis and fragility fractures. Whole exome sequencing (WES) with chromosomal microarray analysis (CMA) revealed a diagnosis of LPI, demonstrating that idiopathic osteoporosis should raise suspicion for LPI.
2. Case report
2.1. Clinical description
The patient was born to non-consanguineous parents after an unremarkable 37-week gestation. His birth weight was 3.5 kg (25–50th centile), and length was 50.8 cm (50–75th centile). At 1.5 months of age, he exhibited irritability when weaned from breast milk to infant milk-based formula. These symptoms resolved with transition to another milk-based formula.
The patient had an otherwise uneventful course until 14 months of age, when he developed an intermittent limp and had periods in which he reverted from walking to crawling for a single day. At 22 months of age, he sustained a non-displaced right supracondylar fracture following a witnessed fall. At 24 months of age, he fractured his left distal humeral diaphysis also after a fall.
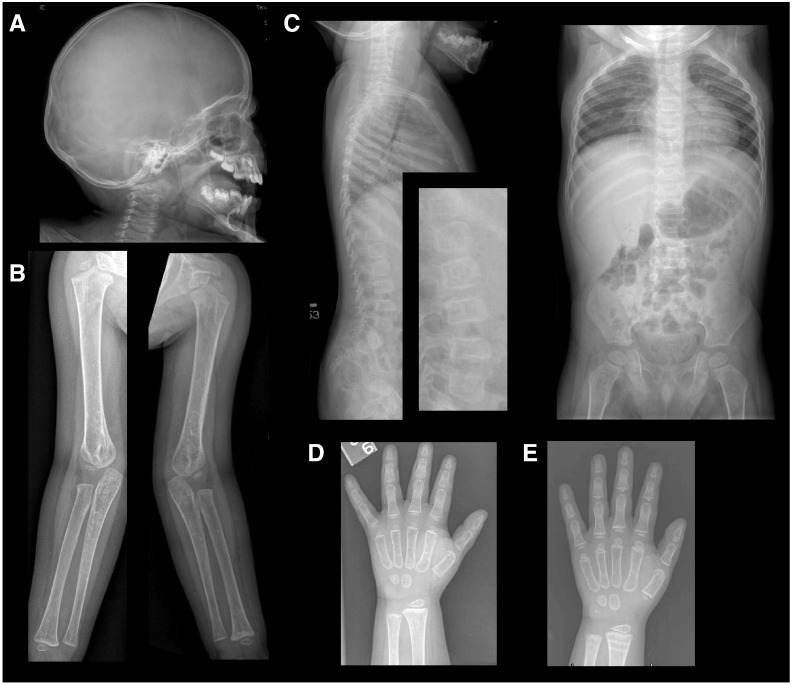
His fragility fractures prompted a skeletal genetic assessment at 26 months of age. Detailed pedigree analysis revealed that he was the only product of the union between his 22-year-old father (Mexican ancestry) and 19-year-old mother (Mexican/Salvadoran ancestry). There was no family history of fragility fractures, dental anomalies, blue sclera, or hearing loss. Physical examination demonstrated a height of 76.1 cm (less than 5th centile), a weight of 10.3 kg (just below 5th centile), and a head circumference of 49 cm (25th–50th centile). Examination was notable for a depressed nasal bridge, prominent cheeks, tented upper lip, mild limping gait, normal skin texture and elasticity, and absence of blue sclera or contractures. Initial investigations revealed mildly elevated phosphorus, normocytic anemia, and elevated alkaline phosphatase (Table 1). A radiographic skeletal survey suggested osteopenia but no wormian bones (Fig. 1). Bone age was consistent with chronological age. The second metacarpal cortical thickness measurement at 3 years (height age of 15 months) was 0.5 mm (mean cortical thickness for 1-year-old males is 1.46 mm with standard deviation of 0.30 mm, and for 2-year-old males is 1.85 mm with standard deviation of 0.39 mm) [7] and, thus, also supported the qualitative assessment of low bone mass on plain radiographs (Fig. 1). An oligonucleotide CMA (Baylor Medical Genetics Laboratory, Version 8.1) did not reveal any causative copy number variation. COL1A1 and COL1A2 sequencing to evaluate for common forms of osteogenesis imperfecta did not identify mutations.
Table 1.
Metabolic bone laboratory values before and after zoledronic acid therapy.
| Laboratory | Initial value | Normal range | After therapy | Normal range |
|---|---|---|---|---|
| Calcium, total | 8.6 mg/dL | 8.6–10.2 mg/dL | 9.0 mg/dL | 8.8–10.1 mg/dL |
| Phosphorus | 5.0 mg/dL | 2.5–4.5 mg/dL | 5.1 mg/dL | 3.1–6.3 mg/dL |
| Alkaline phosphatase | 293 U/L | 40–115 U/L | 319 U/L | 134–346 U/L |
| Vitamin D, 25-hydroxy | 43 ng/mL | 30–100 ng/mL | 30 ng/mL | ≥ 20 ng/mL |
| Vitamin D, 1,25-dihydroxy | 173 pg/mL | 31–87 pg/mL | ||
| PTH, intact | 78 pg/mL | NE | 46 pg/mL | NE |
| Osteocalcin | 31 ng/mL | 9–38 ng/mL | 56 ng/mL | 39–121 ng/mL |
| Collagen N-telopeptide | < 10 nm BCE/L | NE | ||
| Collagen C-telopeptide | 422 pg/mL | 574–1849 pg/mL |
NE—not established; PTH—parathyroid hormone.
Fig. 1.
Skeletal radiographs. (A) Skull films show no evidence of wormian bones. (B, C) Upper extremity and spine films suggest osteopenia. (D) Left-hand radiograph revealed second metacarpal cortical thickness measuring 0.5 mm prior to zoledronic acid. (E) Left-hand radiograph one year later showed second metacarpal cortical thickness of 1 mm.
Over the course of the next 14 months, he had 3 additional fractures (distal right femoral metaphysis, distal right radial metaphysis, and left femoral diaphysis), and bisphosphonate therapy (zoledronic acid) was initiated. Two additional fractures (right tibia/fibula, right proximal humerus) occurred within the first year of zoledronic acid therapy, with no additional fractures thereafter. The second metacarpal cortical thickness measured 1 year after initiating therapy (height age of 2 years) was 1 mm (mean cortical thickness for 2-year-old males is 1.85 mm with standard deviation of 0.39 mm and for 4-year-old males is 2.48 mm with a standard deviation of 0.37 mm) [7]. His short stature persisted with normal growth velocity, and speech delay became apparent between 2 and 3 years of age, prompting further diagnostic evaluation with clinical whole exome sequencing (WES analysis, Baylor Medical Genetics Laboratories) [8].
2.2. Molecular and biochemical evaluation
A clinical WES demonstrated an apparently homozygous nonsense mutation in SLC7A7 (Table 2). Sanger sequencing showed that the proband's mother was heterozygous for this mutation but did not demonstrate a mutation in the father. This finding raised the possibility of LPI with an undetected deletion within SLC7A7 on the second allele. A later version CMA with higher exon coverage (Baylor Medical Genetics Laboratories, version 9.1.1) demonstrated a heterozygous 3 Kb deletion involving exons 6 through 11 in SLC7A7 that was confirmed to be present in the father. Deletions of this region of SLC7A7 have been previously described in patients with LPI [9]. Hence, this patient's diagnosis of LPI is due to compound heterozygosity for a nonsense mutation and a multi-exon deletion occurring in trans in the SLC7A7 gene. Lysinuria with low plasma levels of ornithine, arginine, and lysine confirmed the diagnosis of LPI. Interestingly, despite persistent lysinuria, the levels of urine arginine and ornithine were normal and have remained normal on repeat analyses (Table 3). Post-prandial plasma ammonia and urine orotic acid were elevated. Serum lactate dehydrogenase and ferritin were also elevated.
Table 2.
Whole exome sequence results.
| Disease | Inheritance | Gene | Nucleotide | Amino acid | Zygosity | Novel |
|---|---|---|---|---|---|---|
| Class: Damaging | ||||||
| Lysinuric protein intolerance [MIM:222700] | AR | SLC7A7 | c.1122C>A | p.C374X | Hom | Yes |
| Class: VUS | ||||||
| Lysyl hydroxylase 3 deficiency [MIM:612394] | AR | PLOD3 | c.1030G>A | p.A344T | Het | No |
| Wolcott-Rallison syndrome [MIM:226980] | AR | EIF2AK3 | c.2286G>T | p.Q762H | Het | Yes |
AR—autosomal recessive; Het—heterozygous; Hom—homozygous; VUS—variant of unknown clinical significance.
Table 3.
Biochemical evaluation.
| Laboratory | Initial result | With therapy | Discontinued therapya | Normal range |
|---|---|---|---|---|
| Urine protein | 1 + | Negative | Negative | |
| Serum LDH | 2067 U/L | 470–900 U/L | ||
| Ferritin | 248 ng/mL | 10–60 ng/mL | ||
| Pre-prandial ammonia | 38 μmol/L | 25 μmol/L | 22–48 μmol/L | |
| Post-prandial | ||||
| Ammonia | 78 μmol/L | 25 μmol/L | 173 μmol/L | 22–48 μmol/L |
| Urine orotic acid | 206.9 mmol/mol Cr | 0.3–2.8 mmol/mol Cr | ||
| Acylcarnitine profile | Normal | |||
| Plasma amino acids | ||||
| Lysine | 19 μmol/L | 35 μmol/L | 41–225 μmol/L | |
| Arginine | 7 μmol/L | 3 μmol/L | 18–127 μmol/L | |
| Ornithine | 5 μmol/L | 3 μmol/L | 5–100 μmol/L | |
| Glutamine | 1227 μmol/L | 1271 μmol/L | 266–746 μmol/L | |
| Glycine | 270 μmol/L | 384 μmol/L | 92–346 μmol/L | |
| Alanine | 392 μmol/L | 1499 μmol/L | 103–528 μmol/L | |
| Urine amino acids | ||||
| Lysine | 3874 μmol/g Cr | 1931 μmol/g Cr | 8–557 μmol/g Cr | |
| Arginine | 59 μmol/g Cr | 27 μmol/g Cr | 0–117 μmol/g Cr | |
| Ornithine | 9 μmol/g Cr | 10 μmol/g Cr | 0–170 μmol/g Cr | |
Cr—creatinine; LDH—lactate dehydrogenase.
Patient discontinued therapy.
A dietary history revealed that he was self-restricted to between 1.2 and 1.8 g/kg of protein per day without post-prandial vomiting or lethargy. This dietary protein restriction was continued, and citrulline supplementation was initiated. Laboratory evaluation six weeks after introduction of citrulline demonstrated normal pre- and post-prandial ammonia. However, after citrulline was briefly discontinued by the family, his ammonia rose to 173 μmol/L, requiring hospitalization for management. The ammonia returned to the normal range after citrulline was restarted.
3. Discussion
3.1. Osteoporosis as a presenting feature of LPI
Multiple fractures and low bone density are unusual presenting features of LPI. To our knowledge, osteoporosis has been reported as the presenting feature in only one other patient [10]. Unlike our patient who presented with multiple fractures, this previously reported 4-year-old female had a history of only a single fracture [10]. However, radiographs were consistent with osteopenia, and bone biopsy showed reduced bone volumes [10]. Like our patient, she did not have typical neurologic symptoms of hyperammonemia after meals [10]. Despite this, she had vomiting and diarrhea after introduction of table food, and physical examination was notable for hepatomegaly, a characteristic finding in LPI [10].
Although not typically a presenting feature, osteoporosis and osteopenia are observed in many patients with LPI. A recent case series reported 6 Turkish patients with LPI (age range: 11 to 36 years) who had bone mineral density Z scores ranging from − 2.1 to − 5.8 [11]. Likewise, in a cohort of 9 Italian patients, 2 patients had osteoporosis [12]. In a study of 29 Finnish patients (age range: 3.7 to 47.9 years), 13 had radiographic signs of osteoporosis [13]. Fifty-seven fractures were reported in 20 of these patients with the number of fractures per patient ranging from 0 to 7 [13], [14]. Most fractures occurred before 15 years of age, and as in our patient, the fractures in childhood were associated with minor trauma [13]. Interestingly, fractures occurred as frequently in patients with osteoporosis as in patients without radiologic evidence of osteoporosis [13], [14].
3.2. Treatment of osteoporosis in LPI
The efficacy of recommended therapy for improving the osteoporosis in LPI remains unclear. Although citrulline supplementation resulted in radiographic improvement in bone density in one patient [10], a 2-year study of 19 patients (age range: 1.9 to 32.7 years) who were treated with citrulline with or without lysine supplementation demonstrated no improvement in osteoporosis [15]. Bone density was not assessed in a trial of oral lysine supplementation [16]. There is a single report of the use of alendronate in an 11-year-old female with LPI, with improvement in bone density after one year of therapy [17]. Zoledronic acid was started in our patient prior to his diagnosis of LPI, and within the first year of therapy, decreased fracture frequency was noted.
3.3. Pathophysiology of osteoporosis in LPI
The mechanism underlying osteoporosis and fractures in LPI is unknown. Low vitamin D and calcium, features not seen in our patient, have been attributed to protein self-restriction [18]. However, rickets-like changes on radiographs are rare in LPI patients [13] and were not seen in our patient. The presence of elevated hydroxyproline in serum and urine of LPI patients suggests increased collagen degradation [14]. Furthermore, the finding of decreased collagen synthesis in patient fibroblasts suggests that the process of collagen turnover may be affected [14]. Decreased levels of circulating cationic amino acids may also contribute to this potential defect in collagen turnover [14]. Alternatively, increased nitric oxide may contribute to many of the phenotypes associated with LPI, as patients have elevated plasma nitrate levels, and increased nitrite production has been noted in fibroblasts from patients [19]. Whether this increased nitric oxide synthesis plays a role in bone homeostasis remains unclear. Of note, exogenous nitrate supplementation has been shown to be beneficial for osteoporosis [20].
The atypical presentation of our patient raised the possibility that one or more variants in other genes may be modifying the phenotype. We reviewed all exome sequence variants listed in the original clinical and expanded clinical reports for any association with fragility fractures or reduced bone mineral density (Table 1, Supplementary Tables 1 and 2). Our patient carries a heterozygous missense mutation in PLOD3, a gene that encodes lysyl hydroxylase 3 (LH3). LH3 is involved in the hydroxylation of lysyl residues and O-glycosylation of hydroxylysyl residues in several types of collagen [21], and it is plausible that a defect in lysine modification might enhance the bone fragility phenotype caused by defective lysine transport. Compound heterozygous mutations in PLOD3 have been described in one family with several congenital malformations, including skeletal anomalies and scoliosis, as well as osteoporosis with fragility fractures [22], but to our knowledge, there is no known association between PLOD3 heterozygous variants and low bone mineral density. Further review of the literature for loci associated with reduction in bone mineral density did not reveal any additional polymorphic variants that might contribute to our patient's predisposition to bone fragility [23], [24], [25], [26], [27], [28], [29], [30], [31].
3.4. Clinical utility of WES
Another unusual feature of our patient's presentation is the isolated lysinuria with normal urine ornithine and arginine levels. These urine amino acid results suggest that the diagnosis of LPI might be missed on urine biochemistry alone, and plasma amino acid analysis should be used to complement urine studies in cases of suspected LPI. Two prior case reports describe patients who initially presented with normal urine levels of dibasic amino acids [10], [32]. These reports, in conjunction with the finding of isolated lysinuria in our patient, underscore both the value of DNA-based diagnosis such as WES, and the importance of maintaining a high index of suspicion for LPI, even in the face of non-diagnostic biochemical analysis. This case also supports the inclusion of SLC7A7 as a bona fide low bone mass-causing gene in targeted analyses in the context of sequencing panels [33].
4. Conclusions
LPI should be considered in the differential diagnosis for osteoporosis and multiple fractures in the setting of short stature, particularly when other features of osteogenesis imperfecta (e.g., blue sclera, wormian bones) are absent. As in our patient, these skeletal features may be the only presenting signs of this rare disorder. Furthermore, this case demonstrates the clinical utility of next-generation WES to diagnose patients with idiopathic severe osteoporosis and, in this case, facilitate initiation of appropriate surveillance studies and treatment.
Acknowledgments
J.E.P. was supported by the Medical Genetics Research Fellowship ProgramNIH/NIGMS NIH T32 GM07526. L.C.B. was supported by the Genzyme/ACMG Foundation for Genetic and Genomic Medicine Medical Genetics Training Award in Clinical Biochemical Genetics and the Medical Genetics Research Fellowship Program NIH/NIGMS NIH T32 GM07526. This work was supported by the Baylor College of Medicine Intellectual and Developmental Disabilities Research Center (HD024064) from the Eunice Kennedy Shriver National Institute of Child Health & Human Development (NICHD P01 HD070394, B.H.L.).
Footnotes
Supplementary data to this article can be found online at http://dx.doi.org/10.1016/j.ymgmr.2014.03.004.
Contributor Information
Jennifer E. Posey, Email: jp042803@bcm.edu.
Lindsay C. Burrage, Email: burrage@bcm.edu.
Marcus J. Miller, Email: marcus.miller@bcm.edu.
Pengfei Liu, Email: pengfeil@bcm.edu.
Matthew T. Hardison, Email: mhardison@ncpmr.com.
Sarah H. Elsea, Email: sarah.elsea@bcm.edua.
Qin Sun, Email: qsun@bcm.edu.
Yaping Yang, Email: yapingy@bcm.edu.
Alecia S. Willis, Email: willa36@labcorp.com.
Alan E. Schlesinger, Email: aeschles@texaschildrenshospital.org.
Carlos A. Bacino, Email: cbacino@bcm.edu.
Brendan H. Lee, Email: blee@bcm.edu.
Appendix A. Supplementary data
Supplementary Tables
References
- 1.Borsani G., Bassi M.T., Sperandeo M.P., De Grandi A., Buoninconti A., Riboni M., Manzoni M., Incerti B., Pepe A., Andria G., Ballabio A., Sebastio G. SLC7A7, encoding a putative permease-related protein, is mutated in patients with lysinuric protein intolerance. Nat. Genet. 1999;21:297–301. doi: 10.1038/6815. [DOI] [PubMed] [Google Scholar]
- 2.Torrents D., Mykkanen J., Pineda M., Feliubadalo L., Estevez R., de Cid R., Sanjurjo P., Zorzano A., Nunes V., Huoponen K., Reinikainen A., Simell O., Savontaus M.L., Aula P., Palacin M. Identification of SLC7A7, encoding y+LAT-1, as the lysinuric protein intolerance gene. Nat. Genet. 1999;21:293–296. doi: 10.1038/6809. [DOI] [PubMed] [Google Scholar]
- 3.Kamoda T., Nagai Y., Shigeta M., Kobayashi C., Sekijima T., Shibasaki M., Nakamura N. Lysinuric protein intolerance and systemic lupus erythematosus. Eur. J. Pediatr. 1998;157:130–131. doi: 10.1007/s004310050784. [DOI] [PubMed] [Google Scholar]
- 4.Parsons H., Snyder F., Bowen T., Klassen J., Pinto A. Immune complex disease consistent with systemic lupus erythematosus in a patient with lysinuric protein intolerance. J. Inherit. Metab. Dis. 1996;19:627–634. doi: 10.1007/BF01799838. [DOI] [PubMed] [Google Scholar]
- 5.Aoki M., Fukao T., Fujita Y., Watanabe M., Teramoto T., Kato Y., Suzuki Y., Kondo N. Lysinuric protein intolerance in siblings: complication of systemic lupus erythematosus in the elder sister. Eur. J. Pediatr. 2001;160:522–523. doi: 10.1007/pl00008455. [DOI] [PubMed] [Google Scholar]
- 6.Kerem E., Elpelg O.N., Shalev R.S., Rosenman E., Bar Ziv Y., Branski D. Lysinuric protein intolerance with chronic interstitial lung disease and pulmonary cholesterol granulomas at onset. J. Pediatr. 1993;123:275–278. doi: 10.1016/s0022-3476(05)81703-2. [DOI] [PubMed] [Google Scholar]
- 7.Garn S.M., Poznanski A.K., Nagy J.M. Bone measurement in the differential diagnosis of osteopenia and osteoporosis. Radiology. 1971;100:509–518. doi: 10.1148/100.3.509. [DOI] [PubMed] [Google Scholar]
- 8.Bainbridge M.N., Hu H., Muzny D.M., Musante L., Lupski J.R., Graham B.H., Chen W., Gripp K.W., Jenny K., Wienker T.F., Yang Y., Sutton V.R., Gibbs R.A., Ropers H.H. De novo truncating mutations in ASXL3 are associated with a novel clinical phenotype with similarities to Bohring–Opitz syndrome. Genome Med. 2013;5:11. doi: 10.1186/gm415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Font-Llitjos M., Rodriguez-Santiago B., Espino M., Sillue R., Manas S., Gomez L., Perez-Jurado L.A., Palacin M., Nunes V. Novel SLC7A7 large rearrangements in lysinuric protein intolerance patients involving the same AluY repeat. Eur. J. Hum. Genet. 2009;17:71–79. doi: 10.1038/ejhg.2008.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Carpenter T.O., Levy H.L., Holtrop M.E., Shih V.E., Anast C.S. Lysinuric protein intolerance presenting as childhood osteoporosis. Clinical and skeletal response to citrulline therapy. N. Engl. J. Med. 1985;312:290–294. doi: 10.1056/NEJM198501313120506. [DOI] [PubMed] [Google Scholar]
- 11.Guzel-Ozanturk A., Ozgul R.K., Unal O., Hismi B., Aydin H.I., Sivri S., Tokatli A., Coskun T., Aksoz E., Dursun A. Molecular and clinical evaluation of Turkish patients with lysinuric protein intolerance. Gene. 2013;521:293–295. doi: 10.1016/j.gene.2013.03.033. [DOI] [PubMed] [Google Scholar]
- 12.Parenti G., Sebastio G., Strisciuglio P., Incerti B., Pecoraro C., Terracciano L., Andria G. Lysinuric protein intolerance characterized by bone marrow abnormalities and severe clinical course. J. Pediatr. 1995;126:246–251. doi: 10.1016/s0022-3476(95)70552-x. [DOI] [PubMed] [Google Scholar]
- 13.Svedstrom E., Parto K., Marttinen M., Virtama P., Simell O. Skeletal manifestations of lysinuric protein intolerance. A follow-up study of 29 patients. Skelet. Radiol. 1993;22:11–16. doi: 10.1007/BF00191519. [DOI] [PubMed] [Google Scholar]
- 14.Parto K., Penttinen R., Paronen I., Pelliniemi L., Simell O. Osteoporosis in lysinuric protein intolerance. J. Inherit. Metab. Dis. 1993;16:441–450. doi: 10.1007/BF00710296. [DOI] [PubMed] [Google Scholar]
- 15.Rajantie J., Simell O., Rapola J., Perheentupa J. Lysinuric protein intolerance: a two-year trial of dietary supplementation therapy with citrulline and lysine. J. Pediatr. 1980;97:927–932. doi: 10.1016/s0022-3476(80)80422-7. [DOI] [PubMed] [Google Scholar]
- 16.Tanner L.M., Nanto-Salonen K., Niinikoski H., Huoponen K., Simell O. Long-term oral lysine supplementation in lysinuric protein intolerance. Metabolism. 2007;56:185–189. doi: 10.1016/j.metabol.2006.09.011. [DOI] [PubMed] [Google Scholar]
- 17.Gomez L., Garcia-Cazorla A., Gutierrez A., Artuch R., Varea V., Martin J., Pinillos S., Vilaseca M.A. Treatment of severe osteoporosis with alendronate in a patient with lysinuric protein intolerance. J. Inherit. Metab. Dis. 2006;29:687. doi: 10.1007/s10545-006-0236-9. [DOI] [PubMed] [Google Scholar]
- 18.Tanner L.M., Nanto-Salonen K., Venetoklis J., Kotilainen S., Niinikoski H., Huoponen K., Simell O. Nutrient intake in lysinuric protein intolerance. J. Inherit. Metab. Dis. 2007;30:716–721. doi: 10.1007/s10545-007-0558-2. [DOI] [PubMed] [Google Scholar]
- 19.Mannucci L., Emma F., Markert M., Bachmann C., Boulat O., Carrozzo R., Rizzoni G., Dionisi-Vici C. Increased NO production in lysinuric protein intolerance. J. Inherit. Metab. Dis. 2005;28:123–129. doi: 10.1007/s10545-005-5954-x. [DOI] [PubMed] [Google Scholar]
- 20.Jamal S.A., Cummings S.R., Hawker G.A. Isosorbide mononitrate increases bone formation and decreases bone resorption in postmenopausal women: a randomized trial. J. Bone Miner. Res. 2004;19:1512–1517. doi: 10.1359/JBMR.040716. [DOI] [PubMed] [Google Scholar]
- 21.Sricholpech M., Perdivara I., Nagaoka H., Yokoyama M., Tomer K.B., Yamauchi M. Lysyl hydroxylase 3 glucosylates galactosylhydroxylysine residues in type I collagen in osteoblast culture. J. Biol. Chem. 2011;286:8846–8856. doi: 10.1074/jbc.M110.178509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Salo A.M., Cox H., Farndon P., Moss C., Grindulis H., Risteli M., Robins S.P., Myllyla R. A connective tissue disorder caused by mutations of the lysyl hydroxylase 3 gene. Am. J. Hum. Genet. 2008;83:495–503. doi: 10.1016/j.ajhg.2008.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Duncan E.L., Danoy P., Kemp J.P., Leo P.J., McCloskey E., Nicholson G.C., Eastell R., Prince R.L., Eisman J.A., Jones G., Sambrook P.N., Reid I.R., Dennison E.M., Wark J., Richards J.B., Uitterlinden A.G., Spector T.D., Esapa C., Cox R.D., Brown S.D., Thakker R.V., Addison K.A., Bradbury L.A., Center J.R., Cooper C., Cremin C., Estrada K., Felsenberg D., Gluer C.C., Hadler J., Henry M.J., Hofman A., Kotowicz M.A., Makovey J., Nguyen S.C., Nguyen T.V., Pasco J.A., Pryce K., Reid D.M., Rivadeneira F., Roux C., Stefansson K., Styrkarsdottir U., Thorleifsson G., Tichawangana R., Evans D.M., Brown M.A. Genome-wide association study using extreme truncate selection identifies novel genes affecting bone mineral density and fracture risk. PLoS Genet. 2011;7:e1001372. doi: 10.1371/journal.pgen.1001372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Estrada K., Styrkarsdottir U., Evangelou E., Hsu Y.H., Duncan E.L., Ntzani E.E., Oei L., Albagha O.M., Amin N., Kemp J.P., Koller D.L., Li G., Liu C.T., Minster R.L., Moayyeri A., Vandenput L., Willner D., Xiao S.M., Yerges-Armstrong L.M., Zheng H.F., Alonso N., Eriksson J., Kammerer C.M., Kaptoge S.K., Leo P.J., Thorleifsson G., Wilson S.G., Wilson J.F., Aalto V., Alen M., Aragaki A.K., Aspelund T., Center J.R., Dailiana Z., Duggan D.J., Garcia M., Garcia-Giralt N., Giroux S., Hallmans G., Hocking L.J., Husted L.B., Jameson K.A., Khusainova R., Kim G.S., Kooperberg C., Koromila T., Kruk M., Laaksonen M., Lacroix A.Z., Lee S.H., Leung P.C., Lewis J.R., Masi L., Mencej-Bedrac S., Nguyen T.V., Nogues X., Patel M.S., Prezelj J., Rose L.M., Scollen S., Siggeirsdottir K., Smith A.V., Svensson O., Trompet S., Trummer O., van Schoor N.M., Woo J., Zhu K., Balcells S., Brandi M.L., Buckley B.M., Cheng S., Christiansen C., Cooper C., Dedoussis G., Ford I., Frost M., Goltzman D., Gonzalez-Macias J., Kahonen M., Karlsson M., Khusnutdinova E., Koh J.M., Kollia P., Langdahl B.L., Leslie W.D., Lips P., Ljunggren O., Lorenc R.S., Marc J., Mellstrom D., Obermayer-Pietsch B., Olmos J.M., Pettersson-Kymmer U., Reid D.M., Riancho J.A., Ridker P.M., Rousseau F., Slagboom P.E., Tang N.L., Urreizti R., Van Hul W., Viikari J., Zarrabeitia M.T., Aulchenko Y.S., Castano-Betancourt M., Grundberg E., Herrera L., Ingvarsson T., Johannsdottir H., Kwan T., Li R., Luben R., Medina-Gomez C., Palsson S.T., Reppe S., Rotter J.I., Sigurdsson G., van Meurs J.B., Verlaan D., Williams F.M., Wood A.R., Zhou Y., Gautvik K.M., Pastinen T., Raychaudhuri S., Cauley J.A., Chasman D.I., Clark G.R., Cummings S.R., Danoy P., Dennison E.M., Eastell R., Eisman J.A., Gudnason V., Hofman A., Jackson R.D., Jones G., Jukema J.W., Khaw K.T., Lehtimaki T., Liu Y., Lorentzon M., McCloskey E., Mitchell B.D., Nandakumar K., Nicholson G.C., Oostra B.A., Peacock M., Pols H.A., Prince R.L., Raitakari O., Reid I.R., Robbins J., Sambrook P.N., Sham P.C., Shuldiner A.R., Tylavsky F.A., van Duijn C.M., Wareham N.J., Cupples L.A., Econs M.J., Evans D.M., Harris T.B., Kung A.W., Psaty B.M., Reeve J., Spector T.D., Streeten E.A., Zillikens M.C., Thorsteinsdottir U., Ohlsson C., Karasik D., Richards J.B., Brown M.A., Stefansson K., Uitterlinden A.G., Ralston S.H., Ioannidis J.P., Kiel D.P., Rivadeneira F. Genome-wide meta-analysis identifies 56 bone mineral density loci and reveals 14 loci associated with risk of fracture. Nat. Genet. 2012;44:491–501. doi: 10.1038/ng.2249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Styrkarsdottir U., Halldorsson B.V., Gretarsdottir S., Gudbjartsson D.F., Walters G.B., Ingvarsson T., Jonsdottir T., Saemundsdottir J., Snorradottir S., Center J.R., Nguyen T.V., Alexandersen P., Gulcher J.R., Eisman J.A., Christiansen C., Sigurdsson G., Kong A., Thorsteinsdottir U., Stefansson K. New sequence variants associated with bone mineral density. Nat. Genet. 2009;41:15–17. doi: 10.1038/ng.284. [DOI] [PubMed] [Google Scholar]
- 26.Guo Y., Tan L.J., Lei S.F., Yang T.L., Chen X.D., Zhang F., Chen Y., Pan F., Yan H., Liu X., Tian Q., Zhang Z.X., Zhou Q., Qiu C., Dong S.S., Xu X.H., Guo Y.F., Zhu X.Z., Liu S.L., Wang X.L., Li X., Luo Y., Zhang L.S., Li M., Wang J.T., Wen T., Drees B., Hamilton J., Papasian C.J., Recker R.R., Song X.P., Cheng J., Deng H.W. Genome-wide association study identifies ALDH7A1 as a novel susceptibility gene for osteoporosis. PLoS Genet. 2010;6:e1000806. doi: 10.1371/journal.pgen.1000806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rivadeneira F., Styrkarsdottir U., Estrada K., Halldorsson B.V., Hsu Y.H., Richards J.B., Zillikens M.C., Kavvoura F.K., Amin N., Aulchenko Y.S., Cupples L.A., Deloukas P., Demissie S., Grundberg E., Hofman A., Kong A., Karasik D., van Meurs J.B., Oostra B., Pastinen T., Pols H.A., Sigurdsson G., Soranzo N., Thorleifsson G., Thorsteinsdottir U., Williams F.M., Wilson S.G., Zhou Y., Ralston S.H., van Duijn C.M., Spector T., Kiel D.P., Stefansson K., Ioannidis J.P., Uitterlinden A.G. Twenty bone-mineral-density loci identified by large-scale meta-analysis of genome-wide association studies. Nat. Genet. 2009;41:1199–1206. doi: 10.1038/ng.446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Styrkarsdottir U., Halldorsson B.V., Gretarsdottir S., Gudbjartsson D.F., Walters G.B., Ingvarsson T., Jonsdottir T., Saemundsdottir J., Center J.R., Nguyen T.V., Bagger Y., Gulcher J.R., Eisman J.A., Christiansen C., Sigurdsson G., Kong A., Thorsteinsdottir U., Stefansson K. Multiple genetic loci for bone mineral density and fractures. N. Engl. J. Med. 2008;358:2355–2365. doi: 10.1056/NEJMoa0801197. [DOI] [PubMed] [Google Scholar]
- 29.Xiong D.H., Liu X.G., Guo Y.F., Tan L.J., Wang L., Sha B.Y., Tang Z.H., Pan F., Yang T.L., Chen X.D., Lei S.F., Yerges L.M., Zhu X.Z., Wheeler V.W., Patrick A.L., Bunker C.H., Guo Y., Yan H., Pei Y.F., Zhang Y.P., Levy S., Papasian C.J., Xiao P., Lundberg Y.W., Recker R.R., Liu Y.Z., Liu Y.J., Zmuda J.M., Deng H.W. Genome-wide association and follow-up replication studies identified ADAMTS18 and TGFBR3 as bone mass candidate genes in different ethnic groups. Am. J. Hum. Genet. 2009;84:388–398. doi: 10.1016/j.ajhg.2009.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang L., Choi H.J., Estrada K., Leo P.J., Li J., Pei Y.F., Zhang Y., Lin Y., Shen H., Liu Y.Z., Liu Y., Zhao Y., Zhang J.G., Tian Q., Wang Y.P., Han Y., Ran S., Hai R., Zhu X.Z., Wu S., Yan H., Liu X., Yang T.L., Guo Y., Zhang F., Guo Y.F., Chen Y., Chen X., Tan L., Deng F.Y., Deng H., Rivadeneira F., Duncan E.L., Lee J.Y., Han B.G., Cho N.H., Nicholson G.C., McCloskey E., Eastell R., Prince R.L., Eisman J.A., Jones G., Reid I.R., Sambrook P.N., Dennison E.M., Danoy P., Yerges-Armstrong L.M., Streeten E.A., Hu T., Xiang S., Papasian C.J., Brown M.A., Shin C.S., Uitterlinden A.G., Deng H.W. Multistage genome-wide association meta-analyses identified two new loci for bone mineral density. Hum. Mol. Genet. 2014;23:1923–1933. doi: 10.1093/hmg/ddt575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Richards J.B., Rivadeneira F., Inouye M., Pastinen T.M., Soranzo N., Wilson S.G., Andrew T., Falchi M., Gwilliam R., Ahmadi K.R., Valdes A.M., Arp P., Whittaker P., Verlaan D.J., Jhamai M., Kumanduri V., Moorhouse M., van Meurs J.B., Hofman A., Pols H.A., Hart D., Zhai G., Kato B.S., Mullin B.H., Zhang F., Deloukas P., Uitterlinden A.G., Spector T.D. Bone mineral density, osteoporosis, and osteoporotic fractures: a genome-wide association study. Lancet. 2008;371:1505–1512. doi: 10.1016/S0140-6736(08)60599-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Parini R., Vegni M., Pontiggia M., Melotti D., Corbetta C., Rossi A., Piceni Sereni L. A difficult diagnosis of lysinuric protein intolerance: association with glucose-6-phosphate dehydrogenase deficiency. J. Inherit. Metab. Dis. 1991;14:833–834. doi: 10.1007/BF01799959. [DOI] [PubMed] [Google Scholar]
- 33.Sule G., Campeau P.M., Zhang V.W., Nagamani S.C., Dawson B.C., Grover M., Bacino C.A., Sutton V.R., Brunetti-Pierri N., Lu J.T., Lemire E., Gibbs R.A., Cohn D.H., Cui H., Wong L.J., Lee B.H. Next-generation sequencing for disorders of low and high bone mineral density. Osteoporos. Int. 2013;24:2253–2259. doi: 10.1007/s00198-013-2290-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Tables