Abstract
Objective
To investigate a role of Vitamin D in the pathogenesis of preeclampsia (PE), and to discern any potential benefits of Vitamin D supplementation on hypertension in the RUPP rat model of PE.
Study design
Blood and placentas from normal pregnancies (NP) and PE were collected following elective cesarean delivery without evidence of infection. Circulating Vitamin D was extracted by HPLC and measured via mass spectrometry. Media for placenta explants was supplemented with Vitamin D and exposed to hypoxic (1% O2) or normoxic (6% O2) conditions for 24 hours. ELISAs were performed on media and normalized to total protein to determine cytokine secretion. RUPP rats were supplemented with vitamin D by oral gavage, and blood pressure (MAP) and pup weights were measured in NP and RUPP rats with or without Vitamin D supplementation. Flow cytometry was used to evaluate CD4+ Tcells in control RUPP rats and RUPP rats treated with Vitamin D.
Results
Inflammatory cytokine secretion was higher (p<0.05) while the anti-inflammatory cytokine, IL-10, was significantly lower in the media of PE placentas compared to NP (p=0.005). Vitamin D supplementation decreased hypoxia stimulated pro-inflammatory cytokine secretion (p=0.003) in the media of PE placentas. Vitamin D decreased MAP and circulating CD4+ T cells in the RUPP rat model of PE (p<0.05).
Conclusion
Vitamin D supplementation may be useful in the treatment or prevention of hypertensive disorders in pregnancy.
Keywords: Vitamin D, Hypertension, Preeclampsia, Cytokines
Introduction
Vitamin D levels have been shown to be two fold higher during the third trimester of normal pregnancy than in non-pregnant or postpartum women [1].Vitamin D may be obtained from the diet, but it can also be synthesized after exposure to UV light. 7-dehydrocholesterol is converted to cholecalciferol within the skin after exposure to sunlight. It is then hydroxylated to calcidiol (25(OH) D) in the liver and hydroxylated again in the kidneys to form calcitriol (1,25-dihydroxycholecalciferol). Calcidiol is the easiest form of Vitamin D to measure in serum and calcitriol is the active form of the vitamin. Classical actions of Vitamin D have been described in bone and kidney; however, Vitamin D also has important effects on the immune system, pancreas, cardiovascular system and brain [2]. Importantly, Vitamin D blunts adaptive immune responses while increasing innate immunity. Therefore, Vitamin D analogs may be useful in the prevention and treatment of human autoimmune or hypertensive disorders [3].
Preeclampsia (PE) is a hypertensive disorder of pregnancy usually diagnosed by new onset maternal hypertension and proteinuria after 20 weeks gestation; it is most frequently diagnosed in the latter trimester (>28 weeks)of pregnancy [4-5]. The disorder affects 2-7% of pregnancies in the United States and is a major of cause of maternal morbidity and mortality, intrauterine growth restriction, prenatal death, and preterm birth [6-7]. Although the exact mechanism is unclear, PE is postulated to be caused, in part, by uteroplacental ischemia, which leads to increased antiangiogenic factors, like sFlt-1 and soluble endoglin, as well as T helper 1 (Th1) cells and cytokines, such as tumor necrosis factor-α, interleukin-6 (IL-6), interleukin-8, and interleukin-17 (IL-17) [8]. There also is evidence that a decrease in T helper 2 (Th2) cells and cytokines occurs in the setting of PE [9].
PE occurs more frequently in the winter months, when days are shorter [10]. The lack of sunlight may result in a reduction in the production of sunlight-dependent Vitamin D. A subsequent Vitamin D deficiency (less than 32 ng/mL) has been linked with the development of chronic immune diseases and possibly PE [11-12]. Although a link between Vitamin D deficiency, chronic immune activation and PE is suggested, studies have demonstrated no significant difference in circulating Vitamin D between normal pregnancies and those affected by PE [13] Furthermore, no studies to date have evaluated the effect of Vitamin D supplementation on placental ischemia induced hypertension or the effect it may have of placental cytokine secretion.
Because PE has been associated with immune activation, [2,14-15] we hypothesize that the anti-inflammatory properties of Vitamin D could blunt the effects of hypertension in response to placental ischemia and placental cytokine secretion. We undertook this study to investigate the role of Vitamin D in the pathogenesis of PE, and to discern if there might be potential benefits of Vitamin D supplementation in the RUPP rat model PE.
Materials and Methods
NP and PE women scheduled for elective, non-infected cesarean delivery at Women and Infants Hospital at the University of Mississippi Medical Center were enrolled in our IRB- approved study for the analysis of Vitamin D in correlation with the prevalence of chronic immune activation.
Determination of circulating 1, 25(OH)2D by Mass Spectrometry
Mass spectrometry was used to determine the circulating concentrations of 1, 25(OH)2D (Vitamin D) in women with PE (n=7) or with normal pregnancies (NP; n=12) who were undergoing cesarean delivery at the Women and Infants Hospital at the University of Mississippi Medical Center. Briefly, whole blood was collected and centrifuged to obtain plasma. 10μL of 0.2ng/μL 25-OH vitamin D3 was added to 200μL of plasma to serve as an internal control, followed by the addition of 500μL of acetonitrile. After centrifugation at 10,000g for 15min., the organic phase was transferred to recovery vials and evaporated to dryness under a stream of nitrogen gas. The residue was re-dissolved in HPLC grade water and column (Waters Corp.) purified using ethyl acetate and methanol.
Vitamin D supplementation in placental explants
Placentas were collected from pregnant women with PE and NP (n=3 each) undergoing cesarean delivery. Uniform segments of placental tissue weighing approximately 0.5mg were plated in matrigel-coated (BD Pharmingen) 6-well plates containing media, DMEM + 5% Penicillin-Streptomycin) (Invitrogen) with or without Vitamin D at concentrations of 100μM D2 (Ergocalciferol Oral Solution USP units 0.2mg, County Line Pharmaceuticals, LLC Brookfield, WI)and 100μM D3 (Enfamil D-Vi-Sol Vitamin D supplement Drops 400 IU). Tissue cultures were incubated under hypoxic (1% O2) or normoxic (6% O2) conditions for 24 hours. 1mL of cell culture media was saved for inflammatory cytokine analysis.
Determination of inflammatory cytokine secretion
Enzyme-linked immunosorbent assays (ELISAs) were performed on cell culture supernatant to determine placental explant cytokine secretion. IL-6, sFlt-1, IL-17 and Interleukin-10 (IL-10) concentrations were determined using 100μL of collected media in commercially available ELISA kits from RnD Systems. All assays were carried out in accordance with manufacturer's directions. Total protein was measured in the cell culture media, using a BCA protein quantitation kit from Pierce. The minimal detectable dose (MDD) for the IL-6 ELISA was 0.7pg/mL with an intra-assay/inter-assay precision of 3.1% and 2.7% CV respectively. The MDD for the sFlt-1 ELISA was 3.5pg/mL with an intra-assay/inter-assay precision of CV 3.2% and 7.4%, respectively. The MDD for the IL-17 ELISA was 15 pg/mL with an intra-assay/inter-assay precision of 5.5% and 8.4% CV, respectively. The MDD for the IL-10 ELISA was 3.9 pg/mLwith an intra-assay/inter-assay precision of 4.6% and 6.6% CV, respectively.
Effect of Vitamin D supplementation on blood pressure and chronic elevations in immune cells in the RUPP rat model of PE
All animal studies were performed with 250g timed-pregnant Sprague Dawley rats (Harlan, Indianapolis, IN). Animals were housed in a temperature controlled room with a 12:12 light:dark cycle. All experimental procedures in this study were in accordance with the National Institutes of Health guidelines for use and care of animals and were approved by the Institutional Animal Care and Use Committee at the University of Mississippi Medical Center.
On gestational day (GD) 14,under isoflurane anesthesia normal pregnant rats underwent a reduction in uterine perfusion pressure (RUPP) with the application of a constrictive silver clip (0.203mm) to the aorta superior to the iliac bifurcation while ovarian collateral circulation to the uterus was reduced with restrictive clips (0.100mm) to the bilateral uterine arcades at the ovarian end [16-18]. Vitamin D2 100μL/mL as well as Vitamin D3 100μL/mL was administered to RUPP rats daily via oral gavage on GDs 14 through 18. On GD18 indwelling carotid catheters were inserted into all groups for blood pressure measurements. On GD19 mean arterial pressure, (MAP) was monitored with a pressure transducer (Cobe III Transducer CDX Sema) and continuously recorded for 45 minutes following a one-hour stabilization period. Immediately after blood pressure collection, maternal tissues were harvested and whole blood was collected. Four groups of pregnant rats were examined: Normal pregnant (NP, n=5), Reduced uterine perfusion pressure (RUPP, n=6), RUPP+Vitamin D2 @100μL/mL (RUPP+100D2; n=5), RUPP+Vitamin D3 @100μL/mL (RUPP+100D3; n=7).
Determination of CD4+ T lymphocytes
Flow cytometry was used to determine the effect of Vitamin D on CD4+ T helper cells in RUPP rats. 1mL of whole blood was prepared for lymphocyte isolation by dilution with 3mLs of RPMI 1640 (Invitrogen) and layered over a Ficoll-Hypaque gradient (Lymphoprep®, Accurate Chemical Corp) according to the manufacturer's directions and centrifuged at 1600RPM for 25min. The suspended layer was collected and diluted with 10mLs of RPMI 1640 and centrifuged at 1600RPM for 10 minutes. The supernatant was discarded and the cells were blocked with 250μL mouse and goat blocking buffer (0.5% mouse serum + 0.5% goat serum+ FACS buffer) for 15 minutes. After incubation, 1mL of FACS Wash (RPMI 1640 + 1% heat-inactivated fetal bovine serum + 5% 0.5M ethylenediaminetetraacetic acid) was added to the lymphocytes and centrifuged at 1600RPM for 10 minutes. The supernatant was discarded and the lymphocytes were reconstituted with FACS buffer (RPMI 1640 + 1% heat-inactivated fetal bovine serum + 5% 0.5M ethylenediaminetetraacetic acid + 0.5% Tween20).
Equal numbers of lymphocytes (1 × 106) were incubated for 30min. at 4°C with antibodies against mouse CD4. After washing, cells were labeled with secondary antibodies fluorescein isothyiocyanate (FITC; Southern biotech) for 30min. at 4°C. Cells were washed and suspended in 500μL of FACS fixative (50% FACS Buffer + 50% of 10% buffered formalin). As a negative control, for each individual rat, cells were incubated with anti-FITC alone. Cells were analyzed for single staining on a Gallios flow cytometer (Beckman Coulter). The percent of positive staining cells above the negative control was collected for each individual rat and mean values for each group was calculated.
Statistical analysis
All data are expressed as mean + standard error mean. Differences between multiple groups were analyzed via one-way analysis of variance (ANOVA) and post-hoc analyses were obtained through Bonferoni. Values of P < 0.05 were considered significant.
Results
Circulating Vitamin D is not significantly different between NP and PE patients
To determine if PE is associated with a decrease in circulating Vitamin D, we examined Vitamin D levels via mass spectrometry. As seen in previous studies by Powe et al, [13] there were no significant differences in circulating Vitamin D levels in patients with PE (n=7) compared to NP (n=12) Vitamin D levels decreased from 6.063±1.243 ng/200μL in the NP group to 5.266±0.75 in the PE group (p=0.65).
Vitamin D supplementation blunts hypoxia induced inflammatory cytokine secretion from PE placentas
To determine if Vitamin D supplementation could reduce inflammation associated with hypoxia and preeclampsia, we cultured placental explants with 100μM D2 or 100μM D3. After 24hrs of tissue culture, hypoxia significantly increased IL-6 secretion from 38.41±22.1 to 114.3±19.61 pg/mg/mL (n=3; p=0.05; Figure 2A) in PE placentas. Addition of Vitamin D3 to media of PE tissue explants blunted IL-6 secretion compared to placental explants without Vitamin D supplementation (p=0.13; Figure 1A).
Figure 2. Vitamin D supplementation decreases mean arterial pressure (MAP) and immune cells in an animal model of Preeclampsia.

Reduction of uterine perfusion pressure (RUPP) significantly increased MAP compared to NP rats when measured on gestational day 19. Administration of Vitamin D on gestational days 14-18 to RUPP rats decreased RUPP induced MAP (A) and RUPP induced increases in circulating CD4+ T lymphocytes (B) *denotes P<0.05, **P<0.005 compared to the indicated groups.
Figure 1. Vitamin D supplementation decreases placental inflammatory response to hypoxia.
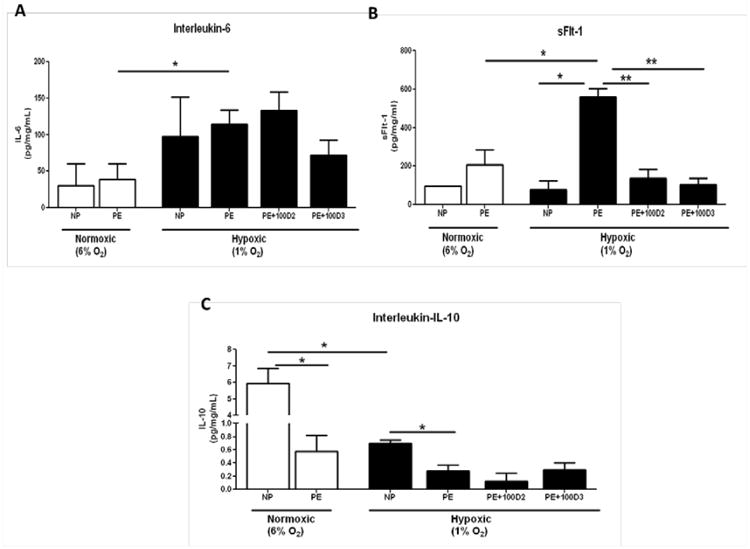
Hypoxia significantly increased IL-6 secretion from placental explants collected from women with preeclampsia (A). Hypoxia increases sFlt-1 secretion from preeclamptic placental explants compared to normoxic preeclamptic explants and hypoxic explants from women with normal pregnancies. Vitamin D supplementation significantly decreased hypoxia induced sFlt-1 secretion in preeclamptic placental explants. (B) IL-10 secretion was significantly decreased in preeclamptic placental explants compared to normal pregnancy explants under both hypoxic and normoxic conditions. (C) * denotes P <0.05, ** P<0.005 compared to the indicated groups.
Hypoxia significantly increased placental secretion of sFlt-1 from 207.1±78.98 to 559.3±43.06 pg/mg/mL (p=0.04; (Figure 1B) from PE placentas. Vitamin D supplementation significantly decreased sFlt-1 secretion in response to hypoxia from PE placentas with both Vitamin D2 (135+47.93pg/mg/mL; p=0.008) and Vitamin D3 (103.4+32.8pg/mg/mL; p=0.003; Figure 1B) when compared to PE tissue explants without Vitamin D. Hypoxia did not significantly increase sFlt-1 secretion from NP placentas compared to normoxic NP placentas (p=0.71; Figure 1B), however PE placentas secreted significantly more sFlt-1 in response to hypoxia compared to NP placentas exposed to hypoxia (p=0.01; Figure 1B).
Importantly, IL-10, an anti-inflammatory cytokine, was significantly decreased in PE placental explants (0.57+0.24pg/mg/mL) cultured at normoxic conditions compared to NP placental explants 5.95±0.89 pg/mg/mL (p=0.005). Similar results were seen when IL-10 secretion from PE explants (0.27+0.09pg/mg/mL) was compared to IL-10 secretion from NP placental explants (0.69+0.04pg/mg/mL; p=0.05; Figure 1C) under hypoxic conditions. Vitamin D supplementation did not significantly increase IL-10 secretion in PE placentas exposed to hypoxia (p=0.61; Figure 1C).
Vitamin D supplementation decreases mean arterial pressure (MAP) in RUPP rat model of preeclampsia
As we have previously reported, [16,18-19]. MAP was significantly increased in RUPP rats (123+3.5mmHg) compared to NP rats (108.8+4.3mmHg; p=0.03; Figure 2A). Administration of VitaminD2 did not significantly decrease MAP (117.6+2mmHg; p=0.24) compared to RUPPs, however MAP did significantly decrease in response to administration of VitaminD3 (111±3.24mmHg; 0.04; Figure 2A). Vitamin D supplementation did not prevent the placental ischemia induced decrease in pup weight when compared to RUPP.
Vitamin D supplementation decreases CD4+ T Cells in RUPP rat model of preeclampsia
We have previously reported that placental ischemia in the RUPP rat is associated with increased circulating CD4+ T cells similar to what is seen in women with PE [18-19]. To determine if Vitamin D supplementation could prevent the RUPP mediated increase in CD4+ T cells, we measured circulating CD4+ T cells in rats treated with Vitamin D. As previously reported, RUPP significantly increased circulating CD4+ T cells compared to NP rats (p=0.009; Figure 2B). Vitamin D3 administration significantly decreased circulating CD4+ T cells (p=0.01; Figure 2B) as did administration of Vitamin D2 (0.08; Figure 2B).
Comments
Vitamin D has several known functions within the immune system including down regulation of Th1 cytokines as well as induction of the Th2 pathway. Avoiding an imbalance between the two is crucial to normal pregnancy [20]. In the adaptive immune system, 1,25(OH)2D inhibits IgG production, proliferation, and differentiation of B lymphocytes, proliferation of T lymphocytes, and proliferation of T helper 1 (Th1) cells and the cytokines they produce [15,21-23]. Previous studies have demonstrated that low vitamin D levels impair normal Th1 and Th2 cytokine balance, resulting in a higher Th1 cytokine expression. This adversely affects immunological tolerance of embryo implantation, suggesting that Vitamin D deficiency may also be associated with the higher Th1 expression seen in preeclampsia [20]. The present study sought to determine if there is, in fact, a link between Vitamin D deficiency and preeclampsia, and to determine if Vitamin D supplementation would alter Th1 and Th2 cytokine secretion in the placentas removed from patients with preeclampsia.
IL-6 and sFlt-1 secretion were increased in the media of PE placentas compared to NP, and was further heightened by hypoxia in PE placental explants compared to NP placental explants. Importantly, secretion of the anti-inflammatory cytokine IL-10 was significantly decreased from preeclamptic placentas compared to healthy NP placentas (Figure 1). Additionally, Vitamin D supplementation decreased inflammatory cytokine secretion from PE placentas compared to NP placentas.
The RUPP rat model of placental ischemia is a well established model of PE that has increased blood pressure, renal dysfunction and importantly an altered immune system similar to that seen in women with PE [16,24-28]. To determine if Vitamin D supplementation could potentially decrease blood pressure and immune cells in the RUPP model of preeclampsia, we administered the two most common forms of Vitamin D to pregnant RUPP rats. Vitamin D2 supplementation decreased hypertension in response to placental ischemia during pregnancy in the RUPP rat model. However, Vitamin D3 normalized mean arterial pressure in RUPP rats to the level of NP rats (Figure 2A). A recent meta-analysis found that decreased circulating Vitamin D is associated with an increased risk of hypertension in healthy populations [29]. Although circulating Vitamin D is not significantly less in PE compared to NP, the current study highlights the need to further determine the plausibility of Vitamin D supplementation in not only healthy individuals but also for those at risk for PE. Vitamin D supplementation could decrease pro-inflammatory events that plague preeclamptic women and have been shown to further stimulate anti-angiogenic cascade. Therefore, Vitamin D supplementation could benefit these women greatly by interacting with multiple pathways.
During placental ischemia Th1 lymphocytes are increased and contribute to inflammatory cytokine secretion [30-32]. We recently reported that in the RUPP rat model of placental ischemia, there is an increase in CD4+ T helper cells which increases arterial pressure and circulating levels of IL-6, IL-17 and sFlt-1[18]. In the current study we determined that Vitamin D supplementation in RUPP rats significantly decreased circulating CD4+ T cells. It is important to note that both forms of Vitamin D used for supplementation in the current study decreased circulating CD4+ T cells and blood pressure in RUPP rats, thereby suggesting that one potential mechanism whereby Vitamin D may work to decrease mean arterial pressure is through a reduction in inflammatory cytokine secreting CD4+ T cells. These findings suggest that Vitamin D supplementation may be useful in the treatment or prevention of hypertensive disease in human pregnancy.
Acknowledgments
Sources of Financial Support: DEPT OF OB/GYN: Marie Darby, Kedra Wallace, Janae Moseley, Judith Heath, James N. Martin Jr, Christine Purser, Rodney Baker, Michelle Owens, Babbette LaMarca. Pregnancy Complicated by Chronic Hypertension: A State of Vitamin D Deficiency and Immune Activation. Poster presented at Society for Maternal-Fetal Medicine 33rd Annual Meeting; 2013 Feb 11-16; San Francisco, CA.
References
- 1.Shin JS, Choi MY, Longtine MS, Nelson DM. Vitamin D effects on pregnancy and the placenta. Placenta. 2010;31:1027–1034. doi: 10.1016/j.placenta.2010.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bikle D. Nonclassic actions of vitamin D. J Clin Endocrinol Metab. 2009;94:26–34. doi: 10.1210/jc.2008-1454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Adorini L. Intervention in autoimmunity: the potential of vitamin D receptor agonists. Cell Immunol. 2005;233:115–124. doi: 10.1016/j.cellimm.2005.04.013. [DOI] [PubMed] [Google Scholar]
- 4.Working group report on high blood pressure in pregnancy. National Instititutes of Health; Washington, DC: 2000. [Google Scholar]
- 5.ACOG Committee on Obstetric Practice. ACOG practice bulletin. Diagnosis and management of preeclampsia and eclampsia. Number 33, January 2002. American College of Obstetricians and Gynecologists. Int J Gynaecol Obstet. 2002;77:67–75. [PubMed] [Google Scholar]
- 6.Sibai BM, Caritis S, Hauth J National Institute of Child Health and Human Development Maternal-Fetal Medicine Units Network. What we have learned about preeclampsia. Semin Perinatol. 2003;27:239–46. doi: 10.1016/s0146-0005(03)00022-3. [DOI] [PubMed] [Google Scholar]
- 7.Hutcheon JA, Lisonkova S, Joseph KS. Epidemiology of pre-eclampsia and the other hypertensive disorders of pregnancy. Best Pract Res Clin Obstet Gynaecol. 2011;25:391–403. doi: 10.1016/j.bpobgyn.2011.01.006. [DOI] [PubMed] [Google Scholar]
- 8.Visser N, van Rijn BB, Rijkers GT, Franx A, Bruinse HW. Inflammatory changes in preeclampsia: current understanding of the maternal innate and adaptive immune response. Obstet Gynecol Surv. 2007;62:191–201. doi: 10.1097/01.ogx.0000256779.06275.c4. [DOI] [PubMed] [Google Scholar]
- 9.Southcombe J, Redman C, Sargent I. Peripheral blood invariant natural killer T cells throughout pregnancy and in preeclamptic women. J Reprod Immunol. 2010;87:52–59. doi: 10.1016/j.jri.2010.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Haugen M, Brantsaeter AL, Trogstad L, Alexander J, Roth C, Magnus P, et al. Vitamin D supplementation and reduced risk of preeclampsia in nulliparous women. Epidemiology. 2009;20:720–726. doi: 10.1097/EDE.0b013e3181a70f08. [DOI] [PubMed] [Google Scholar]
- 11.Bodnar LM, Catov JM, Simhan HN, Holick MF, Powers RW, Roberts JM. Maternal vitamin D deficiency increases the risk of preeclampsia. J Clin Endocrinol Metab. 2007;92:3517–3522. doi: 10.1210/jc.2007-0718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Halhali A, Tovar AR, Torres N, Bourges H, Garabedian M, Larrea F. Preeclampsia is associated with low circulating levels of insulin-like growth factor I and 1,25-dihydroxyvitamin D in maternal and umbilical cord compartments. J Clin Endocrinol Metab. 2000;85:1828–1833. doi: 10.1210/jcem.85.5.6528. [DOI] [PubMed] [Google Scholar]
- 13.Powe CE, Seely EW, Rana S, Bhan I, Ecker J, Karumanchi SA, et al. First trimester vitamin D, vitamin D binding protein, and subsequent preeclampsia. Hypertension. 2010;56:758–763. doi: 10.1161/HYPERTENSIONAHA.110.158238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Adams JS, Liu PT, Chun R, Modlin RL, Hewison M. Vitamin D in defense of the human immune response. Ann N Y Acad Sci. 2007;1117:94–105. doi: 10.1196/annals.1402.036. [DOI] [PubMed] [Google Scholar]
- 15.Adams JS, Hewison M. Unexpected actions of vitamin D: new perspectives on the regulation of innate and adaptive immunity. Nat Clin Pract Endocrinol Metab. 2008;4:80–90. doi: 10.1038/ncpendmet0716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Granger JP, LaMarca BB, Cockrell K, Sedeek M, Balzi C, Chandler D, et al. Reduced uterine perfusion pressure (RUPP) model for studying cardiovascular-renal dysfunction in response to placental ischemia. Methods Mol Med. 2006;122:383–392. doi: 10.1385/1-59259-989-3:381. [DOI] [PubMed] [Google Scholar]
- 17.LaMarca BD, Ryan MJ, Gilbert JS, Murphy SR, Granger JP. Inflammatory cytokines in the pathophysiology of hypertension during preeclampsia. Curr Hypertens Rep. 2007;9:480–485. doi: 10.1007/s11906-007-0088-1. [DOI] [PubMed] [Google Scholar]
- 18.Wallace K, Richards S, Dhillon P, Weimer A, Edholm ES, Bengten E, et al. CD4+ T-helper cells stimulated in response to placental ischemia mediate hypertension during pregnancy. Hypertension. 2011;57:949–955. doi: 10.1161/HYPERTENSIONAHA.110.168344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.LaMarca B, Wallace K, Herse F, Wallukat G, Martin JN, Jr, Weimer A, et al. Hypertension in response to placental ischemia during pregnancy: role of B lymphocytes. Hypertension. 2011;57:865–871. doi: 10.1161/HYPERTENSIONAHA.110.167569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hyppönen E. Vitamin D for the prevention of preeclampsia? A hypothesis. Nutr Rev. 2005;63:225–232. doi: 10.1301/nr.2005.jul.225-232. [DOI] [PubMed] [Google Scholar]
- 21.Rigby WF, Stacy T, Fanger MW. Inhibition of T lymphocyte mitogenesis by 1,25-dihydroxyvitamin D3 (calcitriol) J Clin Invest. 1984;74:1451–1455. doi: 10.1172/JCI111557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen S, Sims GP, Chen XX, Gu YY, Chen S, Lipsky PE. Modulatory effects of 1,25-dihydroxyvitamin D3 on human B cell differentiation. J Immunol. 2007;179:1634–1647. doi: 10.4049/jimmunol.179.3.1634. [DOI] [PubMed] [Google Scholar]
- 23.Lemire JM, Adams JS, Sakai R, Jordan SC. 1 alpha,25-dihydroxyvitamin D3 suppresses proliferation and immunoglobulin production by normal human peripheral blood mononuclear cells. J Clin Invest. 1984;74:657–661. doi: 10.1172/JCI111465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Alexander BT, Kassab SE, Miller MT, Abram SR, Reckelhoff JF, Bennett WA, et al. Reduced uterine perfusion pressure during pregnancy in the rat is associated with increases in arterial pressure and changes in renal nitric oxide. Hypertension. 2001;37:1191–1195. doi: 10.1161/01.hyp.37.4.1191. [DOI] [PubMed] [Google Scholar]
- 25.Alexander BT, Rinewalt AN, Cockrell KL, Massey MB, Bennett WA, Granger JP. Endothelin type a receptor blockade attenuates the hypertension in response to chronic reductions in uterine perfusion pressure. Hypertension. 2001;37:485–489. doi: 10.1161/01.hyp.37.2.485. [DOI] [PubMed] [Google Scholar]
- 26.Roberts L, LaMarca BB, Fournier L, Bain J, Cockrell K, Granger JP. Enhanced endothelin synthesis by endothelial cells exposed to sera from pregnant rats with decreased uterine perfusion. Hypertension. 2006;47:615–618. doi: 10.1161/01.HYP.0000197950.42301.dd. [DOI] [PubMed] [Google Scholar]
- 27.Sedeek M, Gilbert JS, LaMarca BB, Sholook M, Chandler DL, Wang Y, et al. Role of reactive oxygen species in hypertension produced by reduced uterine perfusion in pregnant rats. Am J Hypertens. 2008;21:1152–1156. doi: 10.1038/ajh.2008.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wallace K, Novotny S, Heath J, Moseley J, Martin JN, Jr, Owens MY, et al. Hypertension in response to CD4(+) T cells from reduced uterine perfusion pregnant rats is associated with activation of the endothelin-1 system. Am J Physiol Regul Integr Comp Physiol. 2012;303:R144–149. doi: 10.1152/ajpregu.00049.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kunutsor SK, Apekey TA, Steur M. Vitamin D and risk of future hypertension: meta-analysis of 283,537 participants. Eur J Epidemiol. 2013;28:205–221. doi: 10.1007/s10654-013-9790-2. [DOI] [PubMed] [Google Scholar]
- 30.Chen Q, Guo F, Jin HY, Lau S, Stone P, Chamley L. Phagocytosis of apoptotic trophoblastic debris protects endothelial cells against activation. Placenta. 2012;33:548–553. doi: 10.1016/j.placenta.2012.03.007. [DOI] [PubMed] [Google Scholar]
- 31.Schonkeren D, van der Hoorn ML, Khedoe P, Swings G, van Beelen E, Claas F, et al. Differential distribution and phenotype of decidual macrophages in preeclamptic versus control pregnancies. Am J Pathol. 2011;178:709–717. doi: 10.1016/j.ajpath.2010.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nagamatsu T, Schust DJ. The immunomodulatory roles of macrophages at the maternal-fetal interface. Reprod Sci. 2010;17:209–218. doi: 10.1177/1933719109349962. [DOI] [PubMed] [Google Scholar]


