Abstract
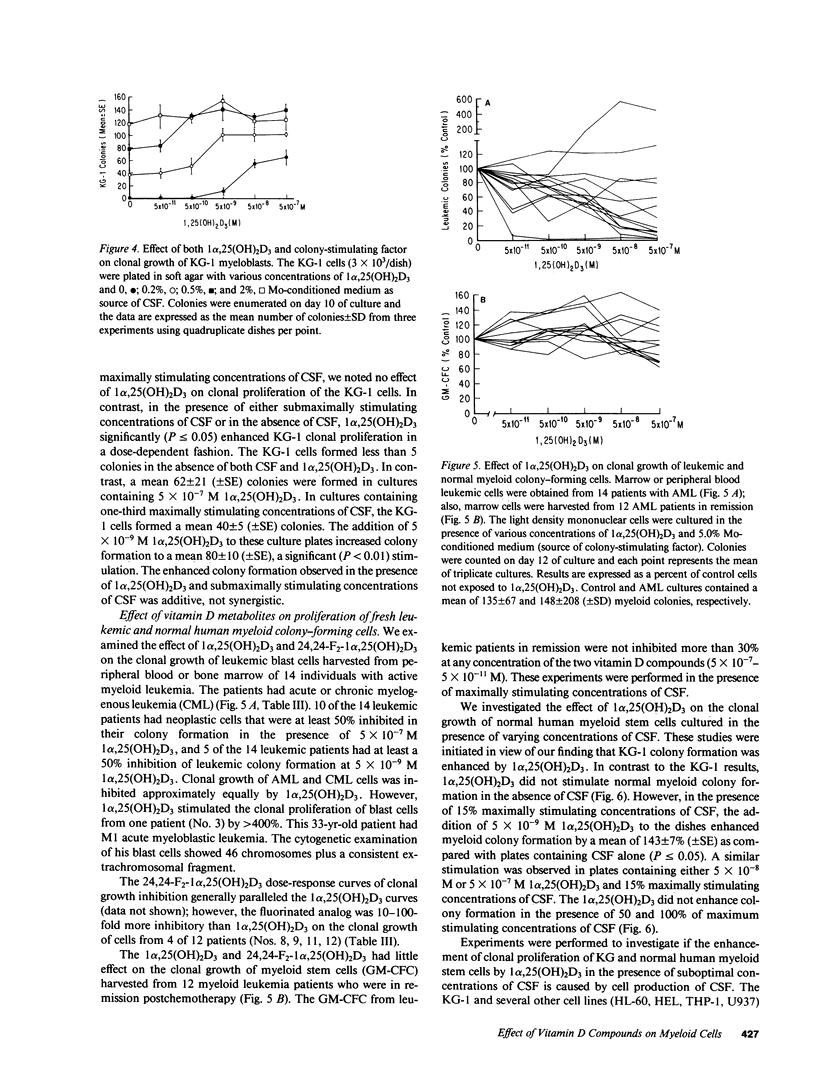
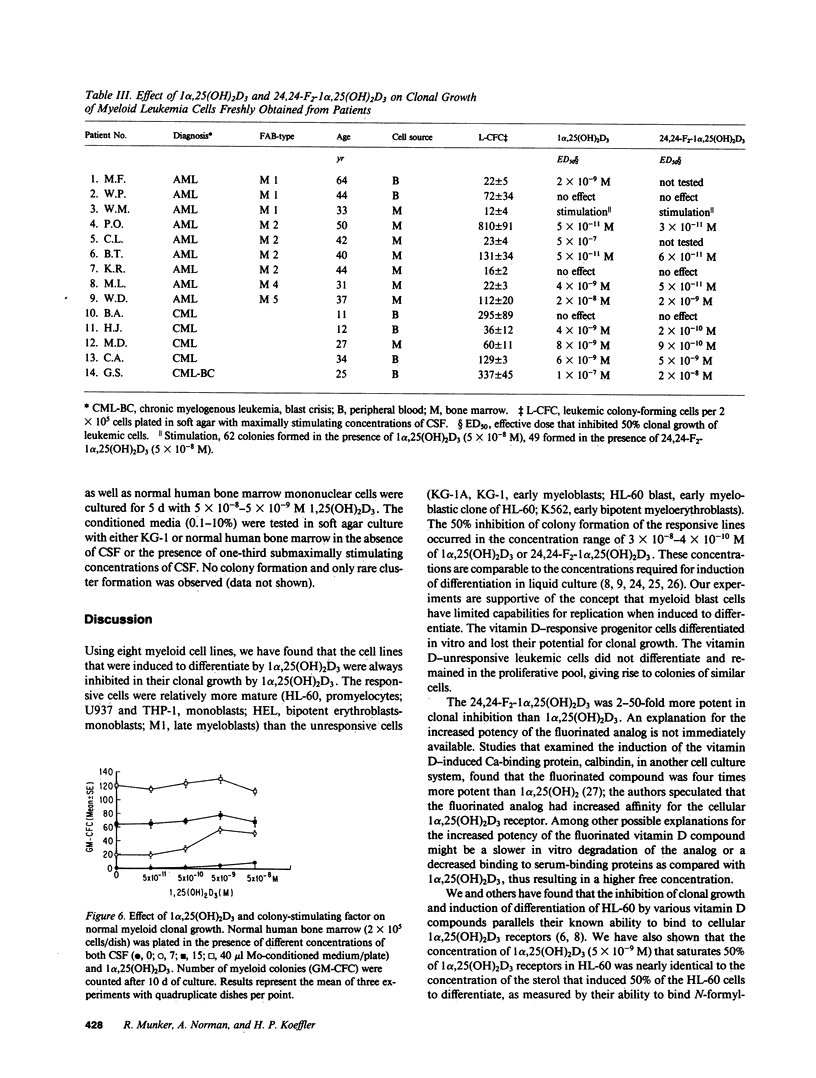
We examined the effect of 1 alpha,25-dihydroxyvitamin D3 (1 alpha,25(OH)2D3) and a variety of vitamin D analogs on proliferation and differentiation of normal and leukemic myeloid clonogenic cells. Only cells from myeloid leukemic lines that contained relatively mature cells (HL-60, U937, THP, HEL, M1) were induced to differentiate and were inhibited in their clonal growth by exposure to 1 alpha,25(OH)2D3 (50% inhibition, 3 X 10(-8)-8 X 10(-10) M). A fluorinated analog of vitamin D was 5-10-fold more potent than 1 alpha,25(OH)2D3. Cells from a human myeloblast line (KG-1) and normal human granulocyte-monocyte stem cells (GM-CFC), both of which depend on colony-stimulating factor (CSF) for clonal growth, were stimulated in their clonal proliferation by 1 alpha,25(OH)2D3 in the presence of suboptimal concentrations of CSF. Leukemic cells from 10 of 14 patients with myeloid leukemia, but not normal GM-CFC from 12 patients in remission, were markedly inhibited in their clonal proliferation by 1 alpha,25(OH)2D3. Our results suggest that 1 alpha,25(OH)2D3 may be a cofactor in hematopoiesis and that vitamin D analogs may have a differential effect on normal versus leukemic growth.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abe E., Miyaura C., Sakagami H., Takeda M., Konno K., Yamazaki T., Yoshiki S., Suda T. Differentiation of mouse myeloid leukemia cells induced by 1 alpha,25-dihydroxyvitamin D3. Proc Natl Acad Sci U S A. 1981 Aug;78(8):4990–4994. doi: 10.1073/pnas.78.8.4990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins S. J., Gallo R. C., Gallagher R. E. Continuous growth and differentiation of human myeloid leukaemic cells in suspension culture. Nature. 1977 Nov 24;270(5635):347–349. doi: 10.1038/270347a0. [DOI] [PubMed] [Google Scholar]
- Corradino R. A., DeLuca H. F., Tanaka Y., Ikekawa N., Kobayashi Y. A fluorinated vitamin D3 analog with biopotency greater than 1 alpha, 25-dihydroxy vitamin D3. Biochem Biophys Res Commun. 1980 Oct 31;96(4):1800–1803. doi: 10.1016/0006-291x(80)91383-2. [DOI] [PubMed] [Google Scholar]
- Douer D., Koeffler H. P. Retinoic acid enhances colony-stimulating factor-induced clonal growth of normal human myeloid progenitor cells in vitro. Exp Cell Res. 1982 Mar;138(1):193–198. doi: 10.1016/0014-4827(82)90105-7. [DOI] [PubMed] [Google Scholar]
- Douer D., Koeffler H. P. Retinoic acid. Inhibition of the clonal growth of human myeloid leukemia cells. J Clin Invest. 1982 Feb;69(2):277–283. doi: 10.1172/JCI110450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freake H. C., Iwasaki J., McCarthy D. M. Specific uptake of 1,25-dihydroxycholecalciferol by human chronic myeloid leukemia cells. Cancer Res. 1984 Aug;44(8):3627–3631. [PubMed] [Google Scholar]
- Golde D. W., Quan S. G., Cline M. J. Human T lymphocyte cell line producing colony-stimulating activity. Blood. 1978 Nov;52(5):1068–1072. [PubMed] [Google Scholar]
- Honma Y., Hozumi M., Abe E., Konno K., Fukushima M., Hata S., Nishii Y., DeLuca H. F., Suda T. 1 alpha,25-Dihydroxyvitamin D3 and 1 alpha-hydroxyvitamin D3 prolong survival time of mice inoculated with myeloid leukemia cells. Proc Natl Acad Sci U S A. 1983 Jan;80(1):201–204. doi: 10.1073/pnas.80.1.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichikawa Y. Differentiation of a cell line of myeloid leukemia. J Cell Physiol. 1969 Dec;74(3):223–234. doi: 10.1002/jcp.1040740303. [DOI] [PubMed] [Google Scholar]
- Koeffler H. P., Amatruda T., Ikekawa N., Kobayashi Y., DeLuca H. F. Induction of macrophage differentiation of human normal and leukemic myeloid stem cells by 1,25-dihydroxyvitamin D3 and its fluorinated analogues. Cancer Res. 1984 Dec;44(12 Pt 1):5624–5628. [PubMed] [Google Scholar]
- Koeffler H. P., Bar-Eli M., Territo M. C. Phorbol ester effect on differentiation of human myeloid leukemia cell lines blocked at different stages of maturation. Cancer Res. 1981 Mar;41(3):919–926. [PubMed] [Google Scholar]
- Koeffler H. P., Billing R., Lusis A. J., Sparkes R., Golde D. W. An undifferentiated variant derived from the human acute myelogenous leukemia cell line (KG-1). Blood. 1980 Aug;56(2):265–273. [PubMed] [Google Scholar]
- Koeffler H. P., Golde D. W. Acute myelogenous leukemia: a human cell line responsive to colony-stimulating activity. Science. 1978 Jun 9;200(4346):1153–1154. doi: 10.1126/science.306682. [DOI] [PubMed] [Google Scholar]
- Koeffler H. P., Hirji K., Itri L. 1,25-Dihydroxyvitamin D3: in vivo and in vitro effects on human preleukemic and leukemic cells. Cancer Treat Rep. 1985 Dec;69(12):1399–1407. [PubMed] [Google Scholar]
- Koeffler H. P., Reichel H., Bishop J. E., Norman A. W. gamma-Interferon stimulates production of 1,25-dihydroxyvitamin D3 by normal human macrophages. Biochem Biophys Res Commun. 1985 Mar 15;127(2):596–603. doi: 10.1016/s0006-291x(85)80202-3. [DOI] [PubMed] [Google Scholar]
- Kuribayashi T., Tanaka H., Abe E., Suda T. Functional defect of variant clones of a human myeloid leukemia cell line (HL-60) resistant to 1 alpha,25-dihydroxyvitamin D3. Endocrinology. 1983 Dec;113(6):1992–1998. doi: 10.1210/endo-113-6-1992. [DOI] [PubMed] [Google Scholar]
- Lozzio C. B., Lozzio B. B. Human chronic myelogenous leukemia cell-line with positive Philadelphia chromosome. Blood. 1975 Mar;45(3):321–334. [PubMed] [Google Scholar]
- Major P. P., Griffin J. D., Minden M., Kufe D. W. A blast subclone of the HL-60 human promyelocytic cell line. Leuk Res. 1981;5(4-5):429–430. doi: 10.1016/0145-2126(81)90018-7. [DOI] [PubMed] [Google Scholar]
- Mangelsdorf D. J., Koeffler H. P., Donaldson C. A., Pike J. W., Haussler M. R. 1,25-Dihydroxyvitamin D3-induced differentiation in a human promyelocytic leukemia cell line (HL-60): receptor-mediated maturation to macrophage-like cells. J Cell Biol. 1984 Feb;98(2):391–398. doi: 10.1083/jcb.98.2.391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin P., Papayannopoulou T. HEL cells: a new human erythroleukemia cell line with spontaneous and induced globin expression. Science. 1982 Jun 11;216(4551):1233–1235. doi: 10.1126/science.6177045. [DOI] [PubMed] [Google Scholar]
- Matsui T., Nakao Y., Kobayashi N., Kishihara M., Ishizuka S., Watanabe S., Fujita T. Phenotypic differentiation-linked growth inhibition in human leukemia cells by active vitamin D3 analogues. Int J Cancer. 1984 Feb 15;33(2):193–202. doi: 10.1002/ijc.2910330207. [DOI] [PubMed] [Google Scholar]
- McCarthy D., Hibbin J., San Miguel J. F., Freake H., Rodrigues B., Andrews C., Pinching A., Catovsky D., Goldman J. M. The effect of vitamin D3 metabolites on normal and leukemic bone marrow cells in vitro. Int J Cell Cloning. 1984 Jul;2(4):227–242. doi: 10.1002/stem.5530020403. [DOI] [PubMed] [Google Scholar]
- Moore M. A., Gabrilove J., Sheridan A. P. Myeloid leukemic cell differentiation induced by human postendotoxin serum and vitamin analogues. Haematol Blood Transfus. 1983;28:327–337. doi: 10.1007/978-3-642-68761-7_63. [DOI] [PubMed] [Google Scholar]
- Norman A. W., Roth J., Orci L. The vitamin D endocrine system: steroid metabolism, hormone receptors, and biological response (calcium binding proteins). Endocr Rev. 1982 Fall;3(4):331–366. doi: 10.1210/edrv-3-4-331. [DOI] [PubMed] [Google Scholar]
- Olsson I., Gullberg U., Ivhed I., Nilsson K. Induction of differentiation of the human histiocytic lymphoma cell line U-937 by 1 alpha,25-dihydroxycholecalciferol. Cancer Res. 1983 Dec;43(12 Pt 1):5862–5867. [PubMed] [Google Scholar]
- Provvedini D. M., Tsoukas C. D., Deftos L. J., Manolagas S. C. 1,25-dihydroxyvitamin D3 receptors in human leukocytes. Science. 1983 Sep 16;221(4616):1181–1183. doi: 10.1126/science.6310748. [DOI] [PubMed] [Google Scholar]
- Reitsma P. H., Rothberg P. G., Astrin S. M., Trial J., Bar-Shavit Z., Hall A., Teitelbaum S. L., Kahn A. J. Regulation of myc gene expression in HL-60 leukaemia cells by a vitamin D metabolite. Nature. 1983 Dec 1;306(5942):492–494. doi: 10.1038/306492a0. [DOI] [PubMed] [Google Scholar]
- Rigby W. F., Shen L., Ball E. D., Guyre P. M., Fanger M. W. Differentiation of a human monocytic cell line by 1,25-dihydroxyvitamin D3 (calcitriol): a morphologic, phenotypic, and functional analysis. Blood. 1984 Nov;64(5):1110–1115. [PubMed] [Google Scholar]
- Sundström C., Nilsson K. Establishment and characterization of a human histiocytic lymphoma cell line (U-937). Int J Cancer. 1976 May 15;17(5):565–577. doi: 10.1002/ijc.2910170504. [DOI] [PubMed] [Google Scholar]
- Tanaka H., Abe E., Miyaura C., Kuribayashi T., Konno K., Nishii Y., Suda T. 1 alpha,25-Dihydroxycholecalciferol and a human myeloid leukaemia cell line (HL-60). Biochem J. 1982 Jun 15;204(3):713–719. doi: 10.1042/bj2040713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuchiya S., Yamabe M., Yamaguchi Y., Kobayashi Y., Konno T., Tada K. Establishment and characterization of a human acute monocytic leukemia cell line (THP-1). Int J Cancer. 1980 Aug;26(2):171–176. doi: 10.1002/ijc.2910260208. [DOI] [PubMed] [Google Scholar]



