Abstract
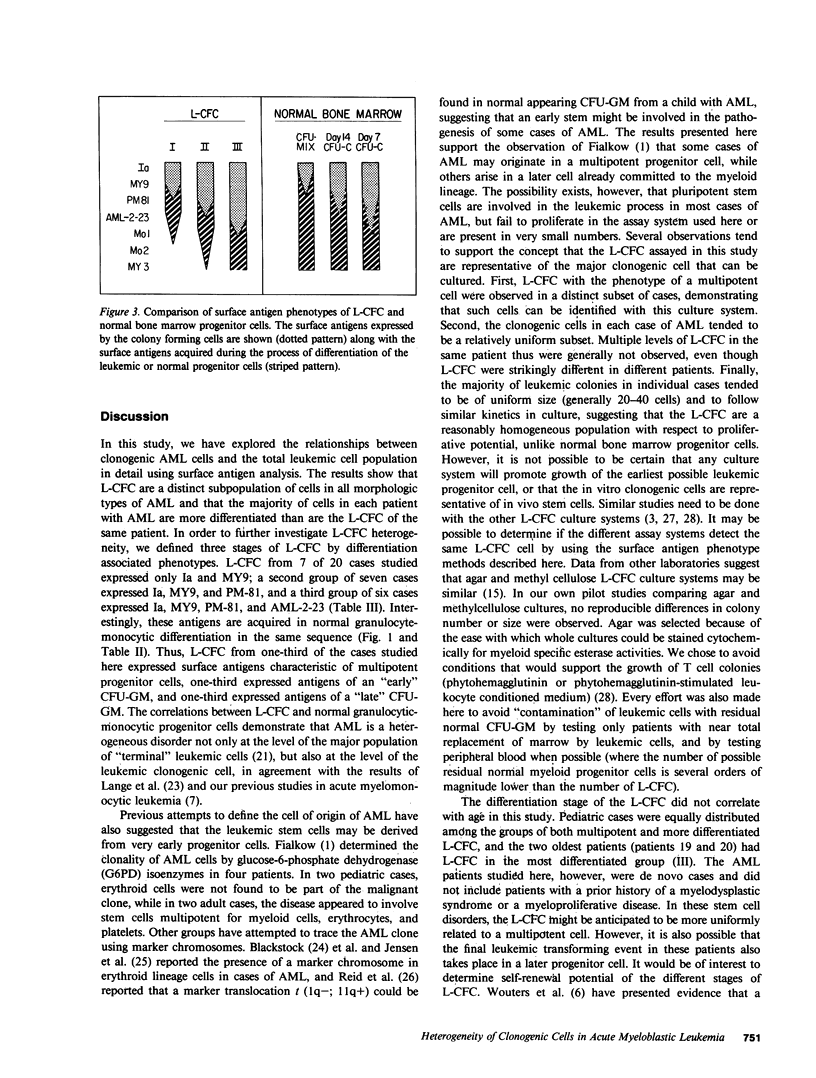
The expression of differentiation-associated surface antigens by the clonogenic leukemic cells from 20 patients with acute myeloblastic leukemia (AML) was studied with a panel of seven cytotoxic monoclonal antibodies (anti-Ia, -MY9, -PM-81, -AML-2-23, -Mol, -Mo2, and -MY3). The surface antigen phenotypes of the clonogenic cells were compared with the phenotypes of the whole leukemic cell population, and with the phenotypes of normal hematopoietic progenitor cells. In each case the clonogenic leukemic cells were found within a distinct subpopulation that was less "differentiated" than the total cell population. Clonogenic leukemic cells from different patients could be divided into three phenotype groups. In the first group (7 of 20 cases), the clonogenic cells expressed surface antigens characteristic of the normal multipotent colony-forming cell (Ia, MY9). These cases tended to have "undifferentiated" (FAB M1) morphology, and the total cell population generally lacked expression of "late" monocyte antigens such as MY3 and Mo2. A second group (seven cases) of clonogenic cells expressed surface antigens characteristic of an "early" (day 14) colony-forming unit granulocyte-monocyte (CFU-GM), and a third group (six cases) was characteristic of a "late" (day 7) CFU-GM. The cases in these latter two groups tended to have myelomonocytic (FAB M4) morphology and to express monocyte surface antigens. These results suggest that the clonogenic cells are a distinct subpopulation in all cases of AML, and may be derived from normal hematopoietic progenitor cells at multiple points in the differentiation pathway. The results further support the possibility that selected monoclonal antibodies have the potential to purge leukemic clonogenic cells from bone marrow in some AML patients without eliminating critical normal progenitor cells.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andreotti P. E., Apgar J. R., Cresswell P. HLA-A2 as a target for cell-mediated lympholysis: evidence from immunoselected HLA-A2 negative mutant cell lines. Hum Immunol. 1980 Jul;1(1):77–86. doi: 10.1016/0198-8859(80)90011-7. [DOI] [PubMed] [Google Scholar]
- Ball E. D., Bernier G. M., Cornwell G. G., 3rd, McIntyre O. R., O'Donnell J. F., Fanger M. W. Monoclonal antibodies to myeloid differentiation antigens: in vivo studies of three patients with acute myelogenous leukemia. Blood. 1983 Dec;62(6):1203–1210. [PubMed] [Google Scholar]
- Ball E. D., Graziano R. F., Fanger M. W. A unique antigen expressed on myeloid cells and acute leukemia blast cells defined by a monoclonal antibody. J Immunol. 1983 Jun;130(6):2937–2941. [PubMed] [Google Scholar]
- Ball E. D., Graziano R. F., Shen L., Fanger M. W. Monoclonal antibodies to novel myeloid antigens reveal human neutrophil heterogeneity. Proc Natl Acad Sci U S A. 1982 Sep;79(17):5374–5378. doi: 10.1073/pnas.79.17.5374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett J. M., Catovsky D., Daniel M. T., Flandrin G., Galton D. A., Gralnick H. R., Sultan C. Proposals for the classification of the acute leukaemias. French-American-British (FAB) co-operative group. Br J Haematol. 1976 Aug;33(4):451–458. doi: 10.1111/j.1365-2141.1976.tb03563.x. [DOI] [PubMed] [Google Scholar]
- Blackstock A. M., Garson O. M. Direct evidence for involvement of erythroid cells in acute myeloblastic leukaemia. Lancet. 1974 Nov 16;2(7890):1178–1179. doi: 10.1016/s0140-6736(74)90815-0. [DOI] [PubMed] [Google Scholar]
- Buick R. N., Minden M. D., McCulloch E. A. Self-renewal in culture of proliferative blast progenitor cells in acute myeloblastic leukemia. Blood. 1979 Jul;54(1):95–104. [PubMed] [Google Scholar]
- Buick R. N., Till J. E., McCulloch E. A. Colony assay for proliferative blast cells circulating in myeloblastic leukaemia. Lancet. 1977 Apr 16;1(8016):862–863. doi: 10.1016/s0140-6736(77)92818-5. [DOI] [PubMed] [Google Scholar]
- Di Persio J. F., Brennan J. K., Lichtman M. A., Speiser B. L. Human cell lines that elaborate colon-stimulating activity for the marrow cells of man and other species. Blood. 1978 Mar;51(3):507–519. [PubMed] [Google Scholar]
- Fauser A. A., Messner H. A. Granuloerythropoietic colonies in human bone marrow, peripheral blood, and cord blood. Blood. 1978 Dec;52(6):1243–1248. [PubMed] [Google Scholar]
- Ferrero D., Pessano S., Pagliardi G. L., Rovera G. Induction of differentiation of human myeloid leukemias: surface changes probed with monoclonal antibodies. Blood. 1983 Jan;61(1):171–179. [PubMed] [Google Scholar]
- Fialkow P. J., Singer J. W., Adamson J. W., Vaidya K., Dow L. W., Ochs J., Moohr J. W. Acute nonlymphocytic leukemia: heterogeneity of stem cell origin. Blood. 1981 Jun;57(6):1068–1073. [PubMed] [Google Scholar]
- Gallagher R., Collins S., Trujillo J., McCredie K., Ahearn M., Tsai S., Metzgar R., Aulakh G., Ting R., Ruscetti F. Characterization of the continuous, differentiating myeloid cell line (HL-60) from a patient with acute promyelocytic leukemia. Blood. 1979 Sep;54(3):713–733. [PubMed] [Google Scholar]
- Griffin J. D., Larcom P., Schlossman S. F. Use of surface markers to identify a subset of acute myelomonocytic leukemia cells with progenitor cell properties. Blood. 1983 Dec;62(6):1300–1303. [PubMed] [Google Scholar]
- Griffin J. D., Linch D., Sabbath K., Larcom P., Schlossman S. F. A monoclonal antibody reactive with normal and leukemic human myeloid progenitor cells. Leuk Res. 1984;8(4):521–534. doi: 10.1016/0145-2126(84)90001-8. [DOI] [PubMed] [Google Scholar]
- Griffin J. D., Mayer R. J., Weinstein H. J., Rosenthal D. S., Coral F. S., Beveridge R. P., Schlossman S. F. Surface marker analysis of acute myeloblastic leukemia: identification of differentiation-associated phenotypes. Blood. 1983 Sep;62(3):557–563. [PubMed] [Google Scholar]
- Griffin J. D., Ritz J., Beveridge R. P., Lipton J. M., Daley J. F., Schlossman S. F. Expression of MY7 antigen on myeloid precursor cells. Int J Cell Cloning. 1983 Apr;1(1):33–48. doi: 10.1002/stem.5530010106. [DOI] [PubMed] [Google Scholar]
- Griffin J. D., Ritz J., Nadler L. M., Schlossman S. F. Expression of myeloid differentiation antigens on normal and malignant myeloid cells. J Clin Invest. 1981 Oct;68(4):932–941. doi: 10.1172/JCI110348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen M. K., Killmann S. A. Chromosome studies in acute leukaemia. Evidence for chromosomal abnormalities common to erythroblasts and leukaemic white cells. Acta Med Scand. 1967 Jan;181(1):47–53. doi: 10.1111/j.0954-6820.1967.tb07225.x. [DOI] [PubMed] [Google Scholar]
- Kubota K., Preisler H. D., Sagawa K., Minowada J. Comparison between agar and methylcellulose cultures of human leukemic cells. Cancer Res. 1981 Aug;41(8):3052–3057. [PubMed] [Google Scholar]
- Lange B., Ferrero D., Pessano S., Palumbo A., Faust J., Meo P., Rovera G. Surface phenotype of clonogenic cells in acute myeloid leukemia defined by monoclonal antibodies. Blood. 1984 Sep;64(3):693–700. [PubMed] [Google Scholar]
- Löwenberg B., Hagemeijer A., Swart K. Karyotypically distinct subpopulations in acute leukemia with specific growth requirements. Blood. 1982 Mar;59(3):641–645. [PubMed] [Google Scholar]
- Löwenberg B., Swart K., Hagemeijer A. PHA-induced colony formation in acute non-lymphocytic and chronic myeloid leukemia. Leuk Res. 1980;4(1):143–149. doi: 10.1016/0145-2126(80)90053-3. [DOI] [PubMed] [Google Scholar]
- Minden M. D., Till J. E., McCulloch E. A. Proliferative state of blast cell progenitors in acute myeloblastic leukemia (AML). Blood. 1978 Sep;52(3):592–600. [PubMed] [Google Scholar]
- Moore M. A., Williams N., Metcalf D. In vitro colony formation by normal and leukemic human hematopoietic cells: characterization of the colony-forming cells. J Natl Cancer Inst. 1973 Mar;50(3):603–623. doi: 10.1093/jnci/50.3.603. [DOI] [PubMed] [Google Scholar]
- Perussia B., Lebman D., Ip S. H., Rovera G., Trinchieri G. Terminal differentiation surface antigens of myelomonocytic cells are expressed in human promyelocytic leukemia cells (HL60) treated with chemical inducers. Blood. 1981 Oct;58(4):836–843. [PubMed] [Google Scholar]
- Phillips P. G., Chikkappa G., Brinson P. S. A triple stain technique to evaluate monocyte, neutrophil, and eosinophil proliferation in soft agar cultures. Exp Hematol. 1983 Jan;11(1):10–17. [PubMed] [Google Scholar]
- Pike B. L., Robinson W. A. Human bone marrow colony growth in agar-gel. J Cell Physiol. 1970 Aug;76(1):77–84. doi: 10.1002/jcp.1040760111. [DOI] [PubMed] [Google Scholar]
- Reid M. M., Tantravahi R., Grier H. E., O'Toole S., Miller B. A., Lipton J. M., Weinstein H. J., Nathan D. G. Detection of leukemia-related karyotypes in granulocyte/macrophage colonies from a patient with acute myelomonocytic leukemia. N Engl J Med. 1983 Jun 2;308(22):1324–1328. doi: 10.1056/NEJM198306023082204. [DOI] [PubMed] [Google Scholar]
- Ritz J., Sallan S. E., Bast R. C., Jr, Lipton J. M., Clavell L. A., Feeney M., Hercend T., Nathan D. G., Schlossman S. F. Autologous bone-marrow transplantation in CALLA-positive acute lymphoblastic leukemia after in-vitro treatment with J5 monoclonal antibody and complement. Lancet. 1982 Jul 10;2(8289):60–63. doi: 10.1016/s0140-6736(82)91686-5. [DOI] [PubMed] [Google Scholar]
- Todd R. F., 3rd, Griffin J. D., Ritz J., Nadler L. M., Abrams T., Schlossman S. F. Expression of normal monocyte-macrophage differentiation antigens on HL60 promyelocytes undergoing differentiation induced by leukocyte-conditioned medium or phorbol diester. Leuk Res. 1981;5(6):491–495. doi: 10.1016/0145-2126(81)90119-3. [DOI] [PubMed] [Google Scholar]
- Todd R. F., 3rd, Nadler L. M., Schlossman S. F. Antigens on human monocytes identified by monoclonal antibodies. J Immunol. 1981 Apr;126(4):1435–1442. [PubMed] [Google Scholar]
- Wouters R., Löwenberg B. On the maturation order of AML cells: a distinction on the basis of self-renewal properties and immunologic phenotypes. Blood. 1984 Mar;63(3):684–689. [PubMed] [Google Scholar]


