Abstract
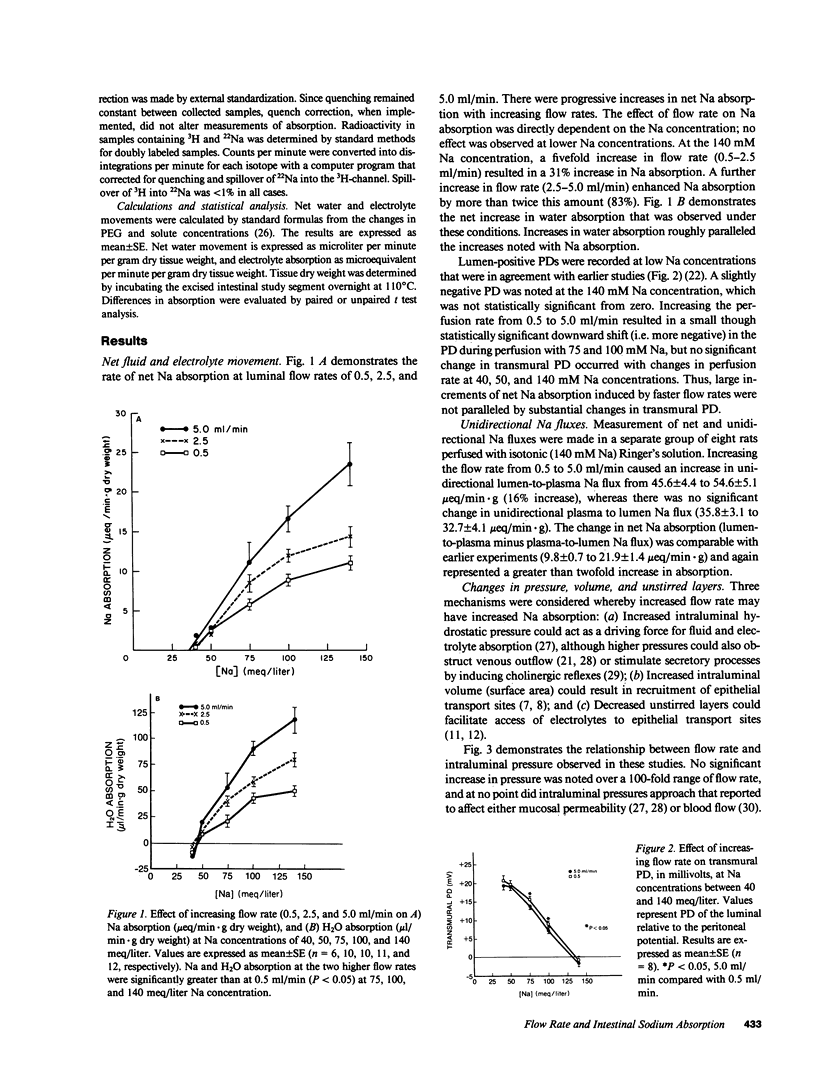
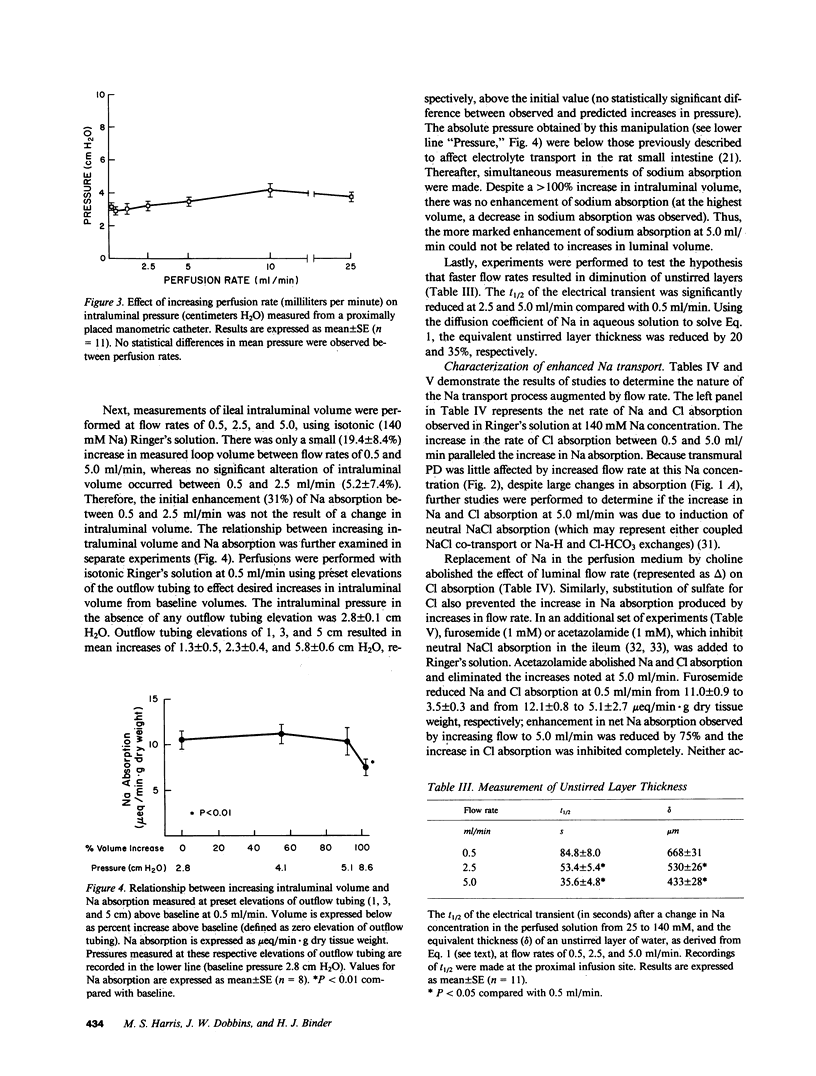
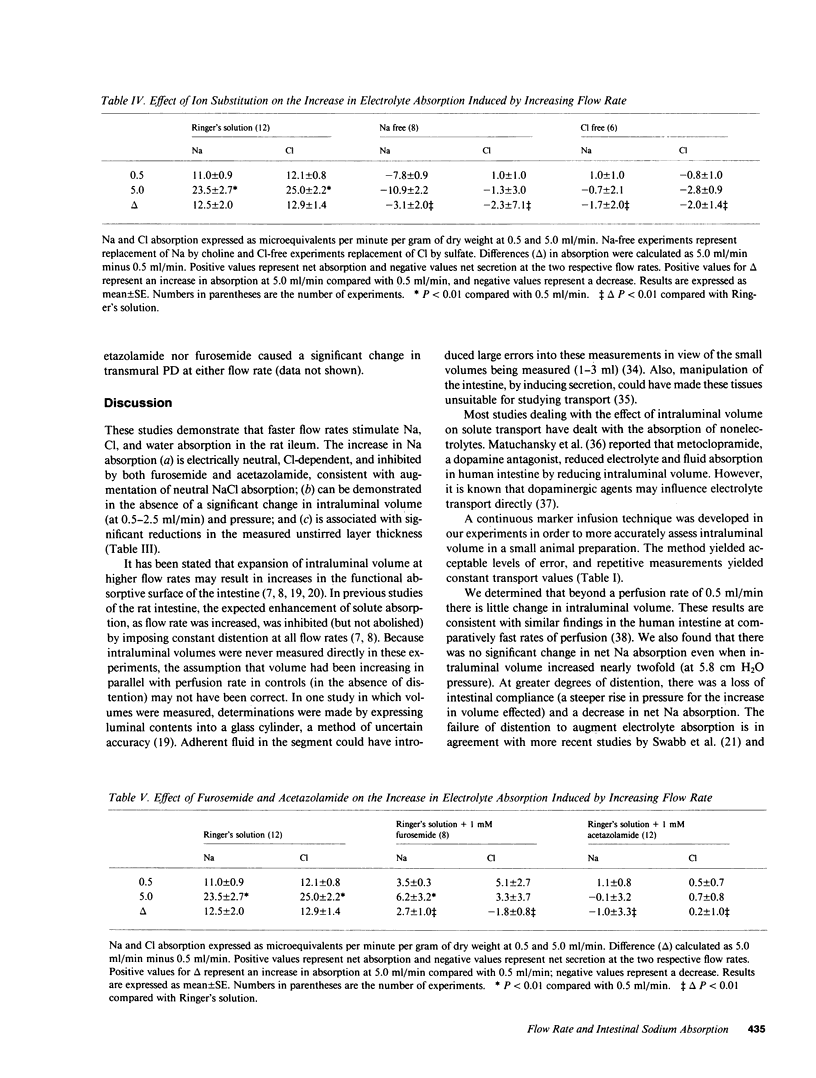
Studies in intact animals have shown that intestinal solute absorption is enhanced with increasing flow rates; the mechanism of this phenomenon has not been explored in detail. We used single pass perfusions of rat ileum to study the effect of higher flow rate on electrolyte absorption. Augmenting perfusion rate from 0.5 to 5.0 ml/min resulted in increased rates of sodium (11.0 +/- 0.9 vs. 23.5 +/- 2.7 mueq/min X g) and chloride (12.1 +/- 0.8 vs. 25.0 +/- 2.2 mueq/min X g) absorption, reduction in the estimated unstirred layer thickness (668 +/- 31 vs. 433 +/- 28 micron), minimal changes in intraluminal pressure and transmural potential difference, and a small, though significant, increase in intraluminal volume (19.4 +/- 8.4%). Removal of sodium from the perfusion medium abolished the effect of increased flow rate on chloride absorption as did removal of chloride on sodium absorption; addition of furosemide or acetazolamide to Ringer's solution also inhibited this effect. In separate experiments, stepwise increases in intraluminal volume were induced by elevating the outflow tubing; no effect on electrolyte transport was observed. These studies demonstrate that neutral sodium chloride absorption is enhanced in rat ileum at higher flow rates, perhaps as a result of a decrease in the thickness of unstirred layers.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alvarado F. D-xylose transport in the chicken small intestine. Comp Biochem Physiol. 1967 Feb;20(2):461–470. doi: 10.1016/0010-406x(67)90261-7. [DOI] [PubMed] [Google Scholar]
- Beubler E., Juan H. PGE-release, blood flow and transmucosal water movement after mechanical stimulation of the rat jejunal mucosa. Naunyn Schmiedebergs Arch Pharmacol. 1978 Oct;305(1):91–95. doi: 10.1007/BF00497010. [DOI] [PubMed] [Google Scholar]
- Boley S. J., Agrawal G. P., Warren A. R., Veith F. J., Levowitz B. S., Treiber W., Dougherty J., Schwartz S. S., Gliedman M. L. Pathophysiologic effects of bowel distention on intestinal blood flow. Am J Surg. 1969 Feb;117(2):228–234. doi: 10.1016/0002-9610(69)90308-0. [DOI] [PubMed] [Google Scholar]
- Bright-Asare P., Binder H. J. Stimulation of colonic secretion of water and electrolytes by hydroxy fatty acids. Gastroenterology. 1973 Jan;64(1):81–88. [PubMed] [Google Scholar]
- CURRAN P. F., SOLOMON A. K. Ion and water fluxes in the ileum of rats. J Gen Physiol. 1957 Sep 20;41(1):143–168. doi: 10.1085/jgp.41.1.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caren J. F., Meyer J. H., Grossman M. I. Canine intestinal secretion during and after rapid distention of the small bowel. Am J Physiol. 1974 Jul;227(1):183–188. doi: 10.1152/ajplegacy.1974.227.1.183. [DOI] [PubMed] [Google Scholar]
- Charney A. N., Donowitz M. Prevention and reversal of cholera enterotoxin-induced intestinal secretion by methylprednisolone induction of Na+-K+-ATPase. J Clin Invest. 1976 Jun;57(6):1590–1599. doi: 10.1172/JCI108429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeSimone J. A. Diffusion barrier in the small intestine. Science. 1983 Apr 8;220(4593):221–222. doi: 10.1126/science.6828892. [DOI] [PubMed] [Google Scholar]
- Debnam E. S., Levin R. J. Effects of fasting and semistarvation on the kinetics of active and passive sugar absorption across the small intestine in vivo. J Physiol. 1975 Nov;252(3):681–700. doi: 10.1113/jphysiol.1975.sp011165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond J. M. A rapid method for determining voltage-concentration relations across membranes. J Physiol. 1966 Mar;183(1):83–100. doi: 10.1113/jphysiol.1966.sp007852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietschy J. M., Sallee V. L., Wilson F. A. Unstirred water layers and absorption across the intestinal mucosa. Gastroenterology. 1971 Dec;61(6):932–934. [PubMed] [Google Scholar]
- Dobbins J., Racusen L., Binder H. J. Effect of D-alanine methionine enkephalin amide on ion transport in rabbit ileum. J Clin Invest. 1980 Jul;66(1):19–28. doi: 10.1172/JCI109830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donowitz M., Elta G., Battisti L., Fogel R., Label-Schwartz E. Effect of dopamine and bromocriptine on rat ileal and colonic transport. Stimulation of absorption and reversal of cholera toxin-induced secretion. Gastroenterology. 1983 Mar;84(3):516–523. [PubMed] [Google Scholar]
- Elsenhans B., Zenker D., Caspary W. F. Guaran effect on rat intestinal absorption. A perfusion study. Gastroenterology. 1984 Apr;86(4):645–653. [PubMed] [Google Scholar]
- FISHER R. B., PARSONS D. S. The gradient of mucosal surface area in the small intestine of the rat. J Anat. 1950 Jul;84(3):272–282. [PMC free article] [PubMed] [Google Scholar]
- HAKIM A. A., LIFSON N. UREA TRANSPORT ACROSS DOG INTESTINAL MUCOSA IN VITRO. Am J Physiol. 1964 Jun;206:1315–1320. doi: 10.1152/ajplegacy.1964.206.6.1315. [DOI] [PubMed] [Google Scholar]
- Hollander D. Intestinal absorption of vitamins A, E, D, and K. J Lab Clin Med. 1981 Apr;97(4):449–462. [PubMed] [Google Scholar]
- Humphreys M. H. Inhibition of NaCl absorption from perfused rat ileum by furosemide. Am J Physiol. 1976 Jun;230(6):1517–1523. doi: 10.1152/ajplegacy.1976.230.6.1517. [DOI] [PubMed] [Google Scholar]
- JACOBSON E. D., BONDY D. C., BROITMAN S. A., FORDTRAN J. S. Validity of polyethylene glycol in estimating intestinal water volume. Gastroenterology. 1963 Jun;44:761–767. [PubMed] [Google Scholar]
- Lee J. S. Effects of stretching and stirring on water and glucose absorption by canine mucosal membrane. J Physiol. 1983 Feb;335:335–341. doi: 10.1113/jphysiol.1983.sp014537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J. S. Intraluminal distension pressure on intestinal lymph flow, serosal transudation and fluid transport in the rat. J Physiol. 1984 Oct;355:399–409. doi: 10.1113/jphysiol.1984.sp015426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin R. J., Mitchell M. A. Problems involved in correlating changes of functional diffusive and anatomical surface areas of the upper and lower chicken small intestine during fasting. Br Poult Sci. 1984 Jan;25(1):27–31. doi: 10.1080/13632758408454839. [DOI] [PubMed] [Google Scholar]
- Levin R. J. Techniques, terminology and parameters in intestinal absorption. Br Med Bull. 1967 Sep;23(3):209–212. doi: 10.1093/oxfordjournals.bmb.a070557. [DOI] [PubMed] [Google Scholar]
- Levitt M. D., Aufderheide T., Fetzer C. A., Bond J. H., Levitt D. G. Use of carbon monoxide to measure luminal stirring in the rat gut. J Clin Invest. 1984 Dec;74(6):2056–2064. doi: 10.1172/JCI111629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis L. D., Fordtran J. S. Effect of perfusion rate on absorption, surface area, unstirred water layer thickness, permeability, and intraluminal pressure in the rat ileum in vivo. Gastroenterology. 1975 Jun;68(6):1509–1516. [PubMed] [Google Scholar]
- Lucas M. L. Estimation of sodium chloride diffusion coefficient in gastric mucin. Dig Dis Sci. 1984 Apr;29(4):336–345. doi: 10.1007/BF01318520. [DOI] [PubMed] [Google Scholar]
- Mathias J. R., Carlson G. M., DiMarino A. J., Bertiger G., Morton H. E., Cohen S. Intestinal myoelectric activity in response to live Vibrio cholerae and cholera enterotoxin. J Clin Invest. 1976 Jul;58(1):91–96. doi: 10.1172/JCI108464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matuchansky C., Huet P. M., Mary J. Y., Rambaud J. C., Bernier J. J. Effects of cholecystokinin and metoclopramide on jejunal movements of water and electrolytes and on transit time of luminal fluid in man. Eur J Clin Invest. 1972 Mar;2(3):160–175. doi: 10.1111/j.1365-2362.1972.tb00586.x. [DOI] [PubMed] [Google Scholar]
- Mothes T., Remke H., Müller F. Na dependence of monosaccharide absorption in isolated rabbit small intestine, perfused through lumen and vascular bed. Pflugers Arch. 1981 Nov;392(1):13–16. doi: 10.1007/BF00584575. [DOI] [PubMed] [Google Scholar]
- Pfeiffer C. J. Experimental analysis of hydrogen ion diffusion in gastrointestinal mucus glycoprotein. Am J Physiol. 1981 Feb;240(2):G176–G182. doi: 10.1152/ajpgi.1981.240.2.G176. [DOI] [PubMed] [Google Scholar]
- Read N. W., Barber D. C., Levin R. J., Holdsworth C. D. Unstirred layer and kinetics of electrogenic glucose absorption in the human jejunum in situ. Gut. 1977 Nov;18(11):865–876. doi: 10.1136/gut.18.11.865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Read N. W. The relationships between colonic motility and transport. Scand J Gastroenterol Suppl. 1984;93:35–42. [PubMed] [Google Scholar]
- Rey F., Drillet F., Schmitz J., Rey J. Influence of flow rate on the kinetics of the intestinal absorption of glucose and lysine in children. Gastroenterology. 1974 Jan;66(1):79–85. [PubMed] [Google Scholar]
- Schiller L. R., Davis G. R., Santa Ana C. A., Morawski S. G., Fordtran J. S. Studies of the mechanism of the antidiarrheal effect of codeine. J Clin Invest. 1982 Nov;70(5):999–1008. doi: 10.1172/JCI110711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smithson K. W., Millar D. B., Jacobs L. R., Gray G. M. Intestinal diffusion barrier: unstirred water layer or membrane surface mucous coat? Science. 1981 Dec 11;214(4526):1241–1244. doi: 10.1126/science.7302593. [DOI] [PubMed] [Google Scholar]
- Soergel K. H., Whalen G. E., Harris J. A. Passive movement of water and sodium across the human small intestinal mucosa. J Appl Physiol. 1968 Jan;24(1):40–48. doi: 10.1152/jappl.1968.24.1.40. [DOI] [PubMed] [Google Scholar]
- Turnberg L. A., Bieberdorf F. A., Morawski S. G., Fordtran J. S. Interrelationships of chloride, bicarbonate, sodium, and hydrogen transport in the human ileum. J Clin Invest. 1970 Mar;49(3):557–567. doi: 10.1172/JCI106266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westergaard H., Dietschy J. M. Delineation of the dimensions and permeability characteristics of the two major diffusion barriers to passive mucosal uptake in the rabbit intestine. J Clin Invest. 1974 Sep;54(3):718–732. doi: 10.1172/JCI107810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White J. F. Intracellular potassium activities in Amphiuma small intestine. Am J Physiol. 1976 Oct;231(4):1214–1219. doi: 10.1152/ajplegacy.1976.231.4.1214. [DOI] [PubMed] [Google Scholar]
- Wilson F. A., Dietschy J. M. The intestinal unstirred layer: its surface area and effect on active transport kinetics. Biochim Biophys Acta. 1974 Aug 21;363(1):112–126. doi: 10.1016/0005-2736(74)90010-8. [DOI] [PubMed] [Google Scholar]
- Winne D. Rat jejunum perfused in situ: effect of perfusion rate and intraluminal radius on absorption rate and effective unstirred layer thickness. Naunyn Schmiedebergs Arch Pharmacol. 1979 Jul;307(3):265–274. doi: 10.1007/BF00505943. [DOI] [PubMed] [Google Scholar]
- Yuasa H., Miyamoto Y., Iga T., Hanano M. A laminar flow absorption model for a carrier-mediated transport in the intestinal tract. J Pharmacobiodyn. 1984 Aug;7(8):604–606. doi: 10.1248/bpb1978.7.604. [DOI] [PubMed] [Google Scholar]



