Abstract
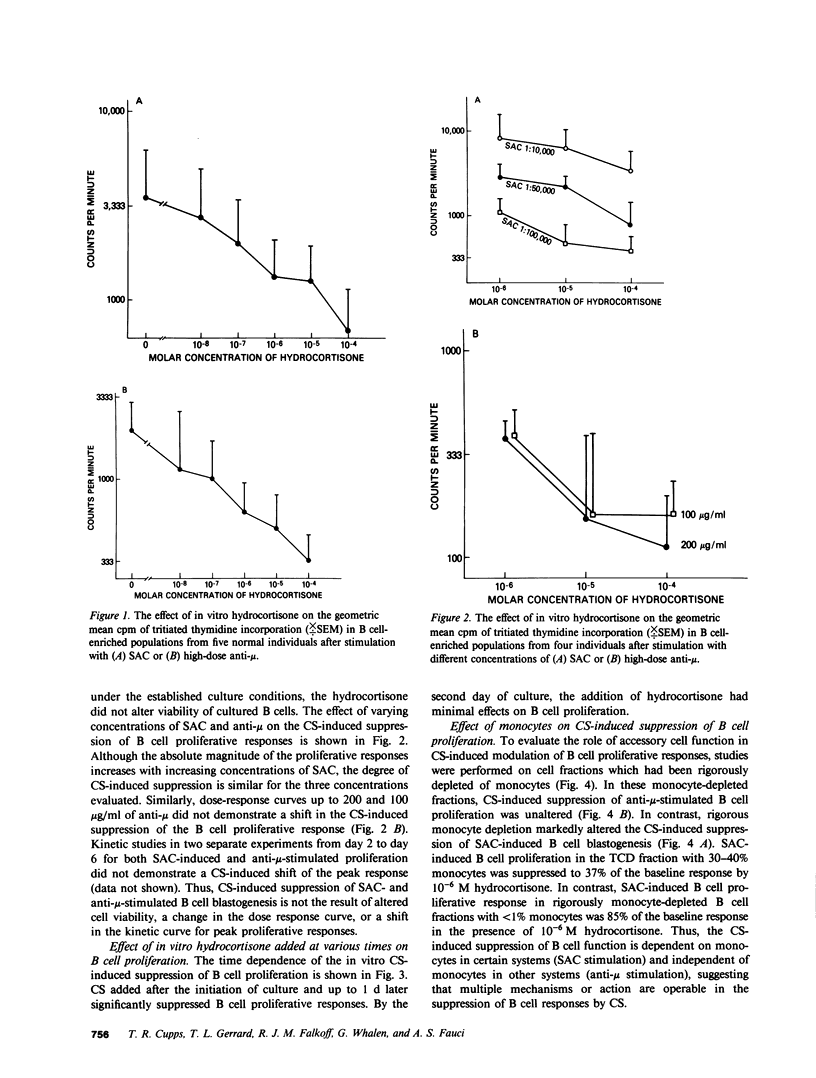
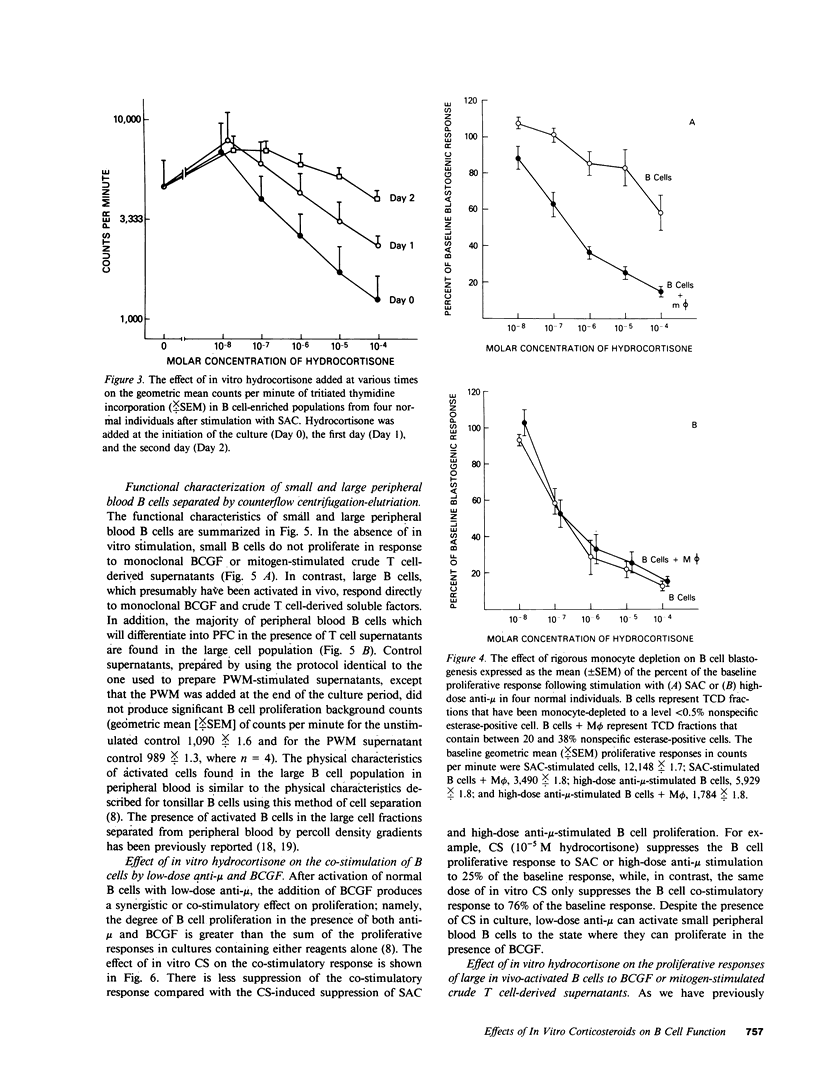
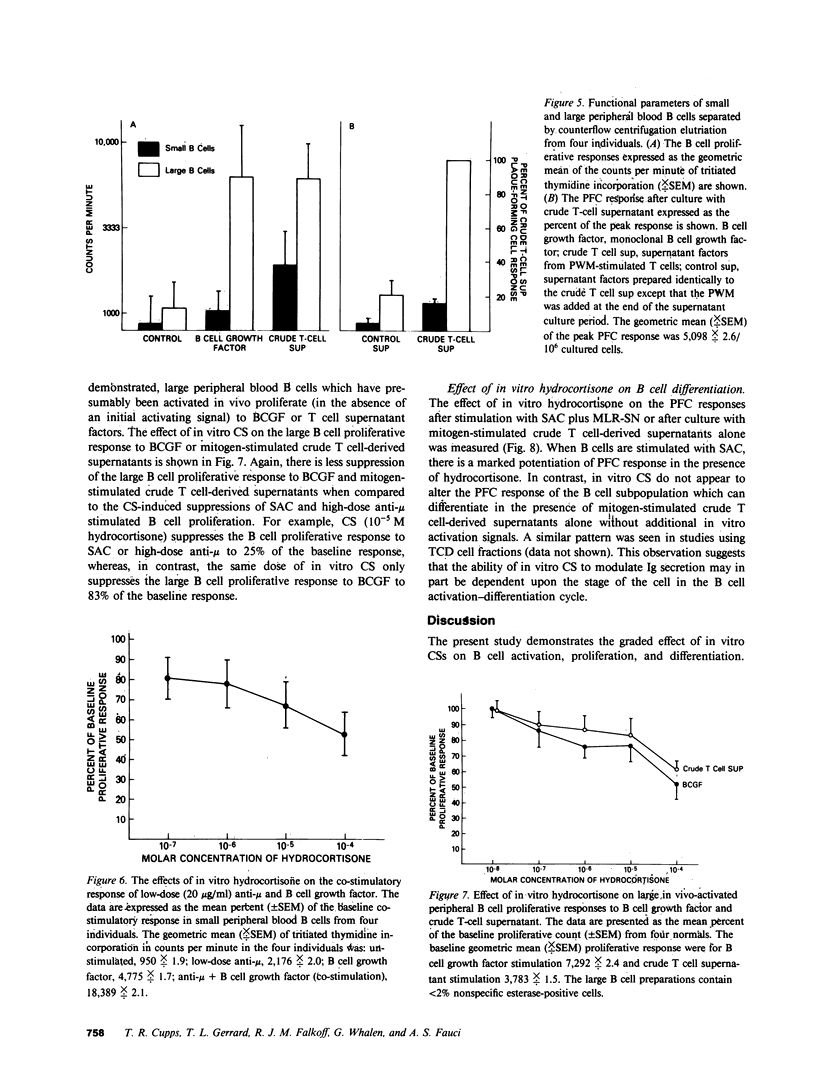
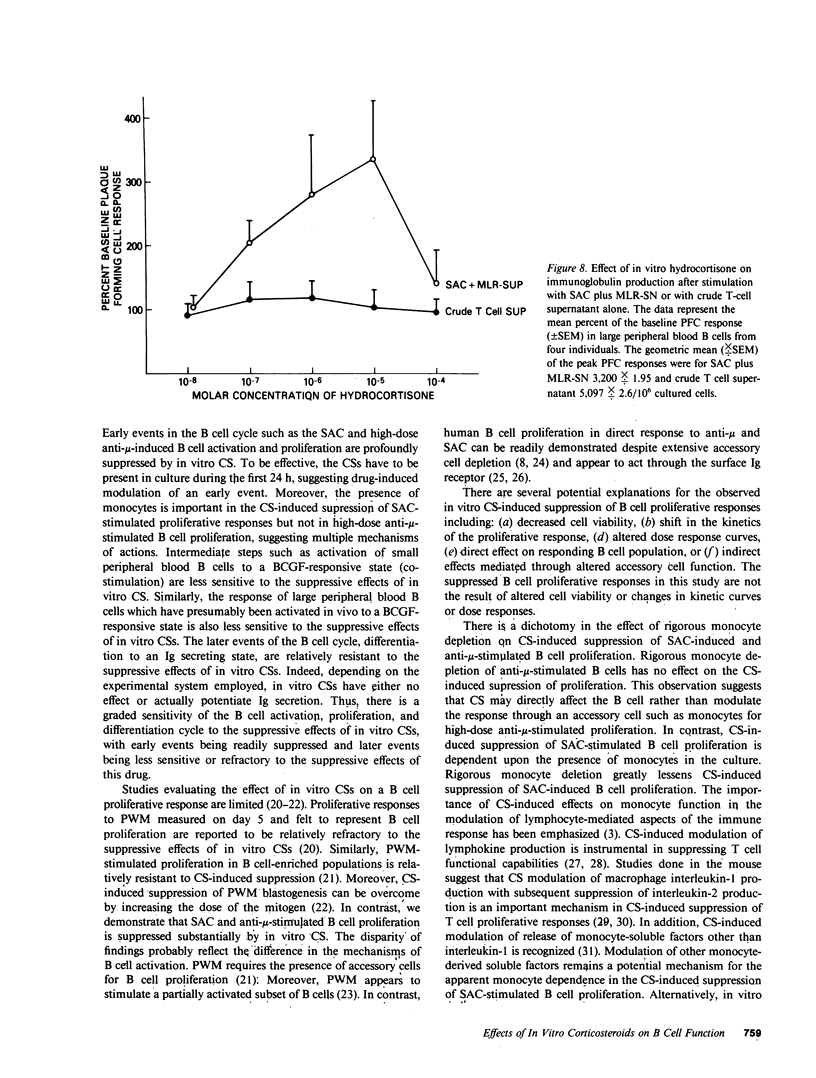
The present study demonstrates the graded effect of in vitro corticosteroids (CSs) on the different phases of B cell activation, proliferation, and differentiation. Early events such as activation and proliferation of high-dose anti-mu or Staphylococcus aureus-stimulated B cells are profoundly suppressed by the presence of in vitro CSs. The suppressed proliferative response may be mediated by a direct effect on B cells and/or modulation of accessory cell function. Later events in the B cell cycle such as the proliferative response to B cell growth factor after either in vivo or in vitro activation are less sensitive to the suppressive effects of in vitro CSs. The final events in the B cell cycle; namely, the differentiation to the immunoglobulin-producing state, is not suppressed by in vitro CSs. Indeed, depending on the systems employed, there is either no effect or enhancement of immunoglobulin secretion by the presence of in vitro CSs. The graded effect of in vitro CSs on the discrete phases of the B cell activation, proliferation, and differentiation cycle provide new insights into the complex nature of CS-induced modulation of human B cell responses.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blomgren H., Andersson B. Steroid sensitivity of the PHA and PWM responses of fractionated human lymphocytes in vitro. Exp Cell Res. 1976 Feb;97(2):233–240. doi: 10.1016/0014-4827(76)90612-1. [DOI] [PubMed] [Google Scholar]
- Butler J. L., Muraguchi A., Lane H. C., Fauci A. S. Development of a human T-T cell hybridoma secreting B cell growth factor. J Exp Med. 1983 Jan 1;157(1):60–68. doi: 10.1084/jem.157.1.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper D. A., Duckett M., Petts V., Penny R. Corticosteroid enhancement of immunoglobulin synthesis by pokeweed mitogen-stimulated human lymphocytes. Clin Exp Immunol. 1979 Jul;37(1):145–151. [PMC free article] [PubMed] [Google Scholar]
- Cupps T. R., Edgar L. C., Fauci A. S. Suppression of human B lymphocyte function by cyclophosphamide. J Immunol. 1982 Jun;128(6):2453–2457. [PubMed] [Google Scholar]
- Cupps T. R., Fauci A. S. Corticosteroid-mediated immunoregulation in man. Immunol Rev. 1982;65:133–155. doi: 10.1111/j.1600-065x.1982.tb00431.x. [DOI] [PubMed] [Google Scholar]
- Falkoff R. J., Muraguchi A., Hong J. X., Butler J. L., Dinarello C. A., Fauci A. S. The effects of interleukin 1 on human B cell activation and proliferation. J Immunol. 1983 Aug;131(2):801–805. [PubMed] [Google Scholar]
- Falkoff R. J., Zhu L. P., Fauci A. S. Separate signals for human B cell proliferation and differentiation in response to Staphylococcus aureus: evidence for a two-signal model of B cell activation. J Immunol. 1982 Jul;129(1):97–102. [PubMed] [Google Scholar]
- Falkoff R. M., Peters M., Fauci A. S. T cell enrichment and depletion of human peripheral blood mononuclear cell preparations. Unexpected findings in the study of the functional activities of the separated populations. J Immunol Methods. 1982;50(1):39–49. doi: 10.1016/0022-1759(82)90302-7. [DOI] [PubMed] [Google Scholar]
- Fauci A. S., Dale D. C., Balow J. E. Glucocorticosteroid therapy: mechanisms of action and clinical considerations. Ann Intern Med. 1976 Mar;84(3):304–315. doi: 10.7326/0003-4819-84-3-304. [DOI] [PubMed] [Google Scholar]
- Fauci A. S., Pratt K. R., Whalen G. Activation of human B lymphocytes. IV. Regulatory effects of corticosteroids on the triggering signal in the plaque-forming cell response of human peripheral blood B lymphocytes to polyclonal activation. J Immunol. 1977 Aug;119(2):598–603. [PubMed] [Google Scholar]
- Fauci A. S., Whalen G., Burch C. Activation of human B lymphocytes XVI. Cellular requirements, interactions, and immunoregulation of pokeweed mitogen-induced total-immunoglobulin producing plaque-forming cells in peripheral blood. Cell Immunol. 1980 Aug 15;54(1):230–240. doi: 10.1016/0008-8749(80)90204-x. [DOI] [PubMed] [Google Scholar]
- Fothergill J. J., Wistar R., Jr, Woody J. N., Parker D. C. A mitogen for human B cells: anti-Ig coupled to polyacrylamide beads activates blood mononuclear cells independently of T cells. J Immunol. 1982 May;128(5):1945–1949. [PubMed] [Google Scholar]
- Gerrard T. L., Fauci A. S. Activation and immunoregulation of antigen-specific human b lymphocyte responses: multifaceted role of the monocyte. J Immunol. 1982 May;128(5):2367–2372. [PubMed] [Google Scholar]
- Gerrard T. L., Jurgensen C. H., Fauci A. S. Differential effect of monoclonal anti-DR antibody on monocytes in antigen- and mitogen-stimulated responses: mechanism of inhibition and relationship to interleukin 1 secretion. Cell Immunol. 1983 Dec;82(2):394–402. doi: 10.1016/0008-8749(83)90172-7. [DOI] [PubMed] [Google Scholar]
- Gillis S., Crabtree G. R., Smith K. A. Glucocorticoid-induced inhibition of T cell growth factor production. I. The effect on mitogen-induced lymphocyte proliferation. J Immunol. 1979 Oct;123(4):1624–1631. [PubMed] [Google Scholar]
- Gillis S., Crabtree G. R., Smith K. A. Glucocorticoid-induced inhibition of T cell growth factor production. II. The effect on the in vitro generation of cytolytic T cells. J Immunol. 1979 Oct;123(4):1632–1638. [PubMed] [Google Scholar]
- Gordon D., Nouri A. M. Comparison of the inhibition by glucocorticosteroids and cyclosporin A of mitogen-stimulated human lymphocyte proliferation. Clin Exp Immunol. 1981 May;44(2):287–294. [PMC free article] [PubMed] [Google Scholar]
- Grayson J., Dooley N. J., Koski I. R., Blaese R. M. Immunoglobulin production induced in vitro by glucocorticoid hormones: T cell-dependent stimulation of immunoglobulin production without B cell proliferation in cultures of human peripheral blood lymphocytes. J Clin Invest. 1981 Dec;68(6):1539–1547. doi: 10.1172/JCI110408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heilman D. H., Gambrill M. R., Leichner J. P. The effect of hydrocortisone on the incorporation of tritiated thymidine by human blood lymphocytes cultured with phytohaemagglutinin and pokeweed mitogen. Clin Exp Immunol. 1973 Oct;15(2):203–212. [PMC free article] [PubMed] [Google Scholar]
- Hirano T., Kuritani T., Kishimoto T., Yamamura Y. In vitro immune response of human peripheral lymphocytes. I. The mechanism(s) involved in T cell helper functions in the pokeweed mitogen-induced differentiation and proliferation of B cells. J Immunol. 1977 Oct;119(4):1235–1241. [PubMed] [Google Scholar]
- Karsh J., Klippel J. H., Plotz P. H., Decker J. L., Wright D. G., Flye M. W. Lymphapheresis in rheumatoid arthritis. A randomized trial. Arthritis Rheum. 1981 Jul;24(7):867–873. doi: 10.1002/art.1780240701. [DOI] [PubMed] [Google Scholar]
- Kishimoto T., Miyake T., Nishizawa Y., Watanabe T., Yamamura Y. Triggering mechanism of B lymphocytes. I. Effect of anti-immunoglobulin and enhancing soluble factor on differentiation and proliferation of B cells. J Immunol. 1975 Nov;115(5):1179–1184. [PubMed] [Google Scholar]
- Kuritani T., Cooper M. D. Human B cell differentiation. II. Pokeweed mitogen-responsive B cells belong to a surface immunoglobulin D-negative subpopulation. J Exp Med. 1982 May 1;155(5):1561–1566. doi: 10.1084/jem.155.5.1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuritani T., Cooper M. D. Human B cell differentiation. II. Pokeweed mitogen-responsive B cells belong to a surface immunoglobulin D-negative subpopulation. J Exp Med. 1982 May 1;155(5):1561–1566. doi: 10.1084/jem.155.5.1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuritani T., Cooper M. D. Human B cell differentiation. IV. Effect of monoclonal anti-immunoglobulin M and D antibodies on B cell proliferation and differentiation induced by T cell factors. J Immunol. 1983 Sep;131(3):1306–1311. [PubMed] [Google Scholar]
- Mayernik D. G., Ul-Haq A., Rinehart J. J. Differentiation-associated alteration in human monocyte-macrophage accessory cell function. J Immunol. 1983 May;130(5):2156–2160. [PubMed] [Google Scholar]
- Muraguchi A., Butler J. L., Kehrl J. H., Falkoff R. J., Fauci A. S. Selective suppression of an early step in human B cell activation by cyclosporin A. J Exp Med. 1983 Sep 1;158(3):690–702. doi: 10.1084/jem.158.3.690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muraguchi A., Butler J. L., Kehrl J. H., Fauci A. S. Differential sensitivity of human B cell subsets to activation signals delivered by anti-mu antibody and proliferative signals delivered by a monoclonal B cell growth factor. J Exp Med. 1983 Feb 1;157(2):530–546. doi: 10.1084/jem.157.2.530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muraguchi A., Kasahara T., Oppenheim J. J., Fauci A. S. B cell growth factor and T cell growth factor produced by mitogen-stimulated normal human peripheral blood T lymphocytes are distinct molecules. J Immunol. 1982 Dec;129(6):2486–2489. [PubMed] [Google Scholar]
- Rinehart J. J., Wuest D., Ackerman G. A. Corticosteroid alteration of human monocyte to macrophage differentiation. J Immunol. 1982 Oct;129(4):1436–1440. [PubMed] [Google Scholar]
- Romagnani S., Giudizi M. G., Biagiotti R., Almerigogna F., Maggi E., Del Prete G., Ricci M. Surface immunoglobulins are involved in the interaction of protein A with human B cells and in the triggering of B cell proliferation induced by protein A-containing Staphylococcus aureus. J Immunol. 1981 Oct;127(4):1307–1313. [PubMed] [Google Scholar]
- Sjöberg O., Kurnick J. Conditions for induction of specific and polyclonal antibody production by Cowan 1 bacteria and by pokeweed mitogen. Scand J Immunol. 1980;11(1):47–51. doi: 10.1111/j.1365-3083.1980.tb00207.x. [DOI] [PubMed] [Google Scholar]
- Smith K. A. T-cell growth factor. Immunol Rev. 1980;51:337–357. doi: 10.1111/j.1600-065x.1980.tb00327.x. [DOI] [PubMed] [Google Scholar]
- Smith R. S., Sherman N. A., Middleton E., Jr Effect of hydrocortisone on immunoglobulin synthesis and secretion by human peripheral lymphocytes in vitro. Int Arch Allergy Appl Immunol. 1972;43(6):859–870. doi: 10.1159/000230903. [DOI] [PubMed] [Google Scholar]
- Snyder D. S., Unanue E. R. Corticosteroids inhibit murine macrophage Ia expression and interleukin 1 production. J Immunol. 1982 Nov;129(5):1803–1805. [PubMed] [Google Scholar]
- Thorn G. W. Clinical considerations in the use of corticosteroids. N Engl J Med. 1966 Apr 7;274(14):775–781. doi: 10.1056/NEJM196604072741406. [DOI] [PubMed] [Google Scholar]
- Werb Z. Biochemical actions of glucocorticoids on macrophages in culture. Specific inhibition of elastase, collagenase, and plasminogen activator secretion and effects on other metabolic functions. J Exp Med. 1978 Jun 1;147(6):1695–1712. doi: 10.1084/jem.147.6.1695. [DOI] [PMC free article] [PubMed] [Google Scholar]



