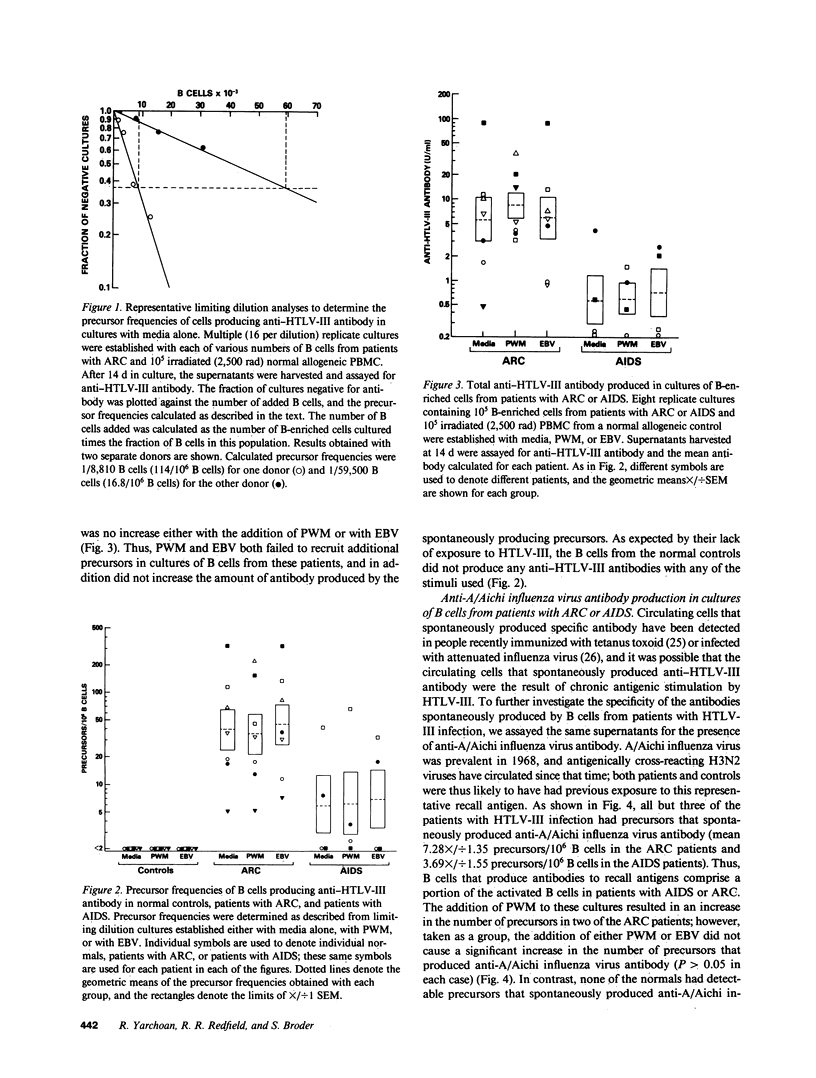
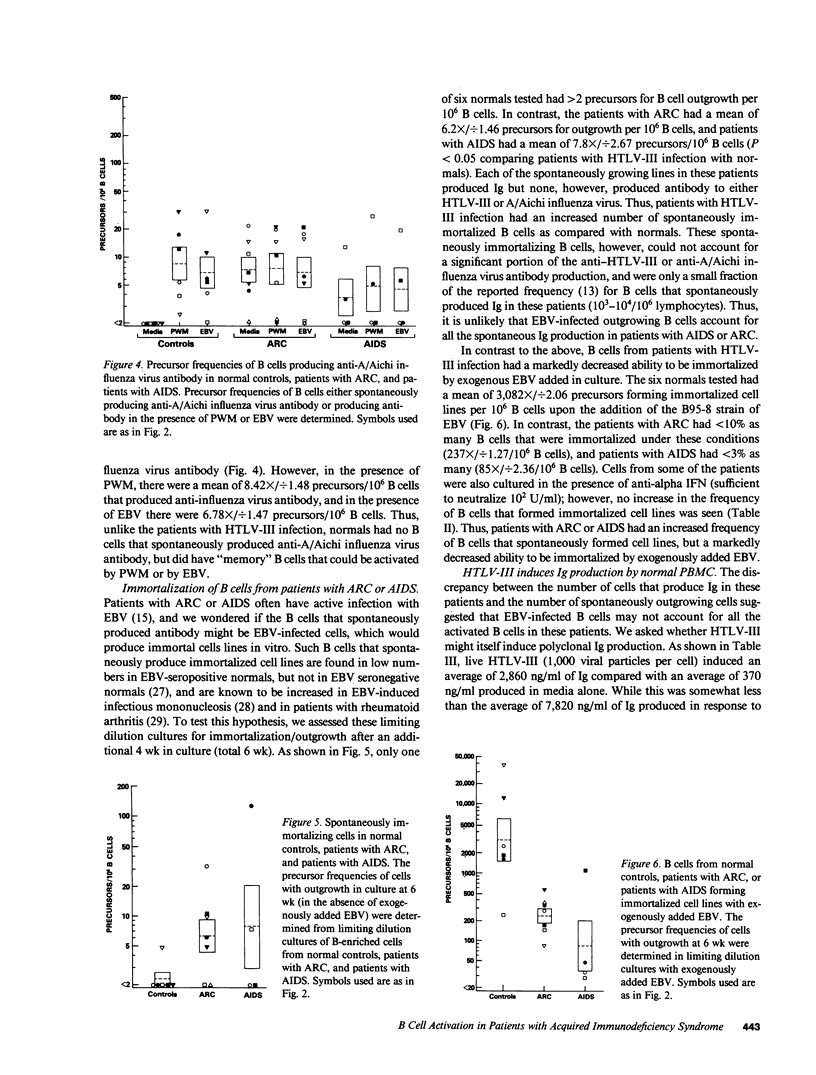
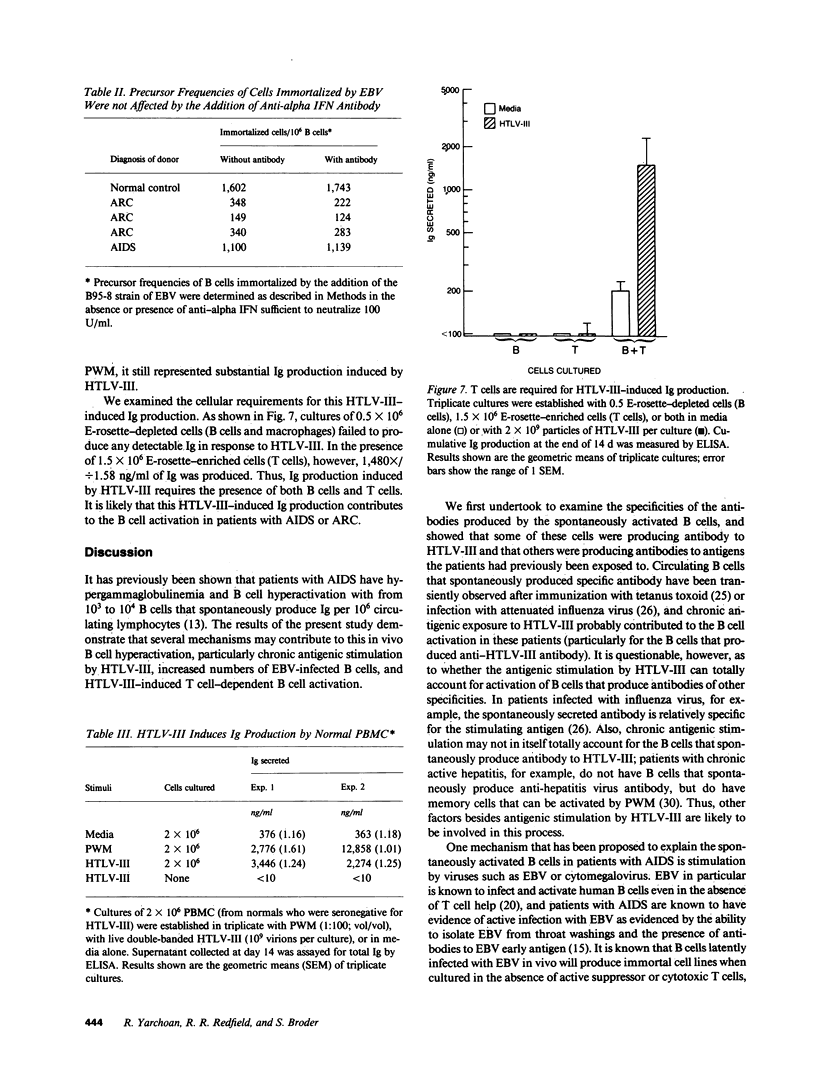
Abstract
Patients with acquired immunodeficiency syndrome (AIDS) and AIDS-related complex (ARC) have hyperimmunoglobulinemia and increased numbers of circulating immunoglobulin-secreting cells. In this paper, we studied the basis for this B cell hyperactivity. Limiting dilution studies of B cells from seven patients with ARC and four with AIDS revealed that some B cells spontaneously produced antibodies to human T cell lymphotropic virus, type III/lymphadenopathy-associated virus (HTLV-III/LAV) (39:10(6) and 7:10(6) B cells, respectively), suggesting that chronic antigenic stimulation by HTLV-III/LAV was one contributing factor. The patients also had an increased number of spontaneously outgrowing B cells than did normals (6:10(6) vs. less than 2:10(6) B cells), suggesting that they had an increased number of Epstein-Barr virus (EBV)-infected B cells. However, fewer B cells from patients were immortalized by exogenously added EBV than were B cells from normals. In additional studies, HTLV-III/LAV induced immunoglobulin secretion (mean 2,860 ng/ml) by peripheral blood mononuclear cells from normals; this HTLV-III/LAV-induced immunoglobulin secretion required the presence of both B and T cells. Thus, antigenic stimulation by HTLV-III/LAV, increased numbers of EBV-infected B cells, and HTLV-III/LAV-induced T cell-dependent B cell activation all contribute to the B cell hyperactivity in patients with HTLV-III/LAV disease.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aman P., Ehlin-Henriksson B., Klein G. Epstein-Barr virus susceptibility of normal human B lymphocyte populations. J Exp Med. 1984 Jan 1;159(1):208–220. doi: 10.1084/jem.159.1.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ammann A. J., Schiffman G., Abrams D., Volberding P., Ziegler J., Conant M. B-cell immunodeficiency in acquired immune deficiency syndrome. JAMA. 1984 Mar 16;251(11):1447–1449. [PubMed] [Google Scholar]
- Broder S., Gallo R. C. A pathogenic retrovirus (HTLV-III) linked to AIDS. N Engl J Med. 1984 Nov 15;311(20):1292–1297. doi: 10.1056/NEJM198411153112006. [DOI] [PubMed] [Google Scholar]
- Diehl V., Henle G., Henle W., Kohn G. Demonstration of a herpes group virus in cultures of peripheral leukocytes from patients with infectious mononucleosis. J Virol. 1968 Jul;2(7):663–669. doi: 10.1128/jvi.2.7.663-669.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dusheiko G. M., Hoofnagle J. H., Cooksley W. G., James S. P., Jones E. A. Synthesis of antibodies to hepatitis B virus by cultured lymphocytes from chronic hepatitis B surface antigen carriers. J Clin Invest. 1983 May;71(5):1104–1113. doi: 10.1172/JCI110860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eyster M. E., Goedert J. J., Poon M. C., Preble O. T. Acid-labile alpha interferon. A possible preclinical marker for the acquired immunodeficiency syndrome in hemophilia. N Engl J Med. 1983 Sep 8;309(10):583–586. doi: 10.1056/NEJM198309083091003. [DOI] [PubMed] [Google Scholar]
- Fahey J. L., Prince H., Weaver M., Groopman J., Visscher B., Schwartz K., Detels R. Quantitative changes in T helper or T suppressor/cytotoxic lymphocyte subsets that distinguish acquired immune deficiency syndrome from other immune subset disorders. Am J Med. 1984 Jan;76(1):95–100. doi: 10.1016/0002-9343(84)90756-3. [DOI] [PubMed] [Google Scholar]
- Fauci A. S., Macher A. M., Longo D. L., Lane H. C., Rook A. H., Masur H., Gelmann E. P. NIH conference. Acquired immunodeficiency syndrome: epidemiologic, clinical, immunologic, and therapeutic considerations. Ann Intern Med. 1984 Jan;100(1):92–106. doi: 10.7326/0003-4819-100-1-92. [DOI] [PubMed] [Google Scholar]
- Fearon E. R., Kazazian H. H., Jr, Waber P. G., Lee J. I., Antonarakis S. E., Orkin S. H., Vanin E. F., Henthorn P. S., Grosveld F. G., Scott A. F. The entire beta-globin gene cluster is deleted in a form of gamma delta beta-thalassemia. Blood. 1983 Jun;61(6):1269–1274. [PubMed] [Google Scholar]
- Gallo R. C., Salahuddin S. Z., Popovic M., Shearer G. M., Kaplan M., Haynes B. F., Palker T. J., Redfield R., Oleske J., Safai B. Frequent detection and isolation of cytopathic retroviruses (HTLV-III) from patients with AIDS and at risk for AIDS. Science. 1984 May 4;224(4648):500–503. doi: 10.1126/science.6200936. [DOI] [PubMed] [Google Scholar]
- Gottlieb M. S., Schroff R., Schanker H. M., Weisman J. D., Fan P. T., Wolf R. A., Saxon A. Pneumocystis carinii pneumonia and mucosal candidiasis in previously healthy homosexual men: evidence of a new acquired cellular immunodeficiency. N Engl J Med. 1981 Dec 10;305(24):1425–1431. doi: 10.1056/NEJM198112103052401. [DOI] [PubMed] [Google Scholar]
- Ihle J. N., Lee J. C. Possible immunological mechanisms in C-type viral leukemogenesis in mice. Curr Top Microbiol Immunol. 1982;98:85–101. doi: 10.1007/978-3-642-68369-5_7. [DOI] [PubMed] [Google Scholar]
- Lane H. C., Masur H., Edgar L. C., Whalen G., Rook A. H., Fauci A. S. Abnormalities of B-cell activation and immunoregulation in patients with the acquired immunodeficiency syndrome. N Engl J Med. 1983 Aug 25;309(8):453–458. doi: 10.1056/NEJM198308253090803. [DOI] [PubMed] [Google Scholar]
- Masur H., Michelis M. A., Greene J. B., Onorato I., Stouwe R. A., Holzman R. S., Wormser G., Brettman L., Lange M., Murray H. W. An outbreak of community-acquired Pneumocystis carinii pneumonia: initial manifestation of cellular immune dysfunction. N Engl J Med. 1981 Dec 10;305(24):1431–1438. doi: 10.1056/NEJM198112103052402. [DOI] [PubMed] [Google Scholar]
- Montagnier L., Gruest J., Chamaret S., Dauguet C., Axler C., Guétard D., Nugeyre M. T., Barré-Sinoussi F., Chermann J. C., Brunet J. B. Adaptation of lymphadenopathy associated virus (LAV) to replication in EBV-transformed B lymphoblastoid cell lines. Science. 1984 Jul 6;225(4657):63–66. doi: 10.1126/science.6328661. [DOI] [PubMed] [Google Scholar]
- Nicholson J. K., McDougal J. S., Spira T. J., Cross G. D., Jones B. M., Reinherz E. L. Immunoregulatory subsets of the T helper and T suppressor cell populations in homosexual men with chronic unexplained lymphadenopathy. J Clin Invest. 1984 Jan;73(1):191–201. doi: 10.1172/JCI111190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsson K., Klein G. Phenotypic and cytogenetic characteristics of human B-lymphoid cell lines and their relevance for the etiology of Burkitt's lymphoma. Adv Cancer Res. 1982;37:319–380. doi: 10.1016/s0065-230x(08)60886-6. [DOI] [PubMed] [Google Scholar]
- Pahwa S. G., Quilop M. T., Lange M., Pahwa R. N., Grieco M. H. Defective B-lymphocyte function in homosexual men in relation to the acquired immunodeficiency syndrome. Ann Intern Med. 1984 Dec;101(6):757–763. doi: 10.7326/0003-4819-101-6-757. [DOI] [PubMed] [Google Scholar]
- Polyclonal activation of B cells in homosexual men. N Engl J Med. 1984 Aug 23;311(8):536–537. doi: 10.1056/NEJM198408233110814. [DOI] [PubMed] [Google Scholar]
- Popovic M., Flomenberg N., Volkman D. J., Mann D., Fauci A. S., Dupont B., Gallo R. C. Alteration of T-cell functions by infection with HTLV-I or HTLV-II. Science. 1984 Oct 26;226(4673):459–462. doi: 10.1126/science.6093248. [DOI] [PubMed] [Google Scholar]
- Popovic M., Sarngadharan M. G., Read E., Gallo R. C. Detection, isolation, and continuous production of cytopathic retroviruses (HTLV-III) from patients with AIDS and pre-AIDS. Science. 1984 May 4;224(4648):497–500. doi: 10.1126/science.6200935. [DOI] [PubMed] [Google Scholar]
- Rickinson A. B., Wallace L. E., Epstein M. A. HLA-restricted T-cell recognition of Epstein-Barr virus-infected B cells. Nature. 1980 Feb 28;283(5750):865–867. doi: 10.1038/283865a0. [DOI] [PubMed] [Google Scholar]
- Robert-Guroff M., Nakao Y., Notake K., Ito Y., Sliski A., Gallo R. C. Natural antibodies to human retrovirus HTLV in a cluster of Japanese patients with adult T cell leukemia. Science. 1982 Feb 19;215(4535):975–978. doi: 10.1126/science.6760397. [DOI] [PubMed] [Google Scholar]
- Rocchi G., Felici A., Ragona G., Heinz A. Quantitative evaluation of Epstein-Barr-virus-infected mononuclear peripheral blood leukocytes in infectious mononucleosis. N Engl J Med. 1977 Jan 20;296(3):132–134. doi: 10.1056/NEJM197701202960302. [DOI] [PubMed] [Google Scholar]
- Sarngadharan M. G., Popovic M., Bruch L., Schüpbach J., Gallo R. C. Antibodies reactive with human T-lymphotropic retroviruses (HTLV-III) in the serum of patients with AIDS. Science. 1984 May 4;224(4648):506–508. doi: 10.1126/science.6324345. [DOI] [PubMed] [Google Scholar]
- Shaw G. M., Harper M. E., Hahn B. H., Epstein L. G., Gajdusek D. C., Price R. W., Navia B. A., Petito C. K., O'Hara C. J., Groopman J. E. HTLV-III infection in brains of children and adults with AIDS encephalopathy. Science. 1985 Jan 11;227(4683):177–182. doi: 10.1126/science.2981429. [DOI] [PubMed] [Google Scholar]
- Siegal F. P., Lopez C., Hammer G. S., Brown A. E., Kornfeld S. J., Gold J., Hassett J., Hirschman S. Z., Cunningham-Rundles C., Adelsberg B. R. Severe acquired immunodeficiency in male homosexuals, manifested by chronic perianal ulcerative herpes simplex lesions. N Engl J Med. 1981 Dec 10;305(24):1439–1444. doi: 10.1056/NEJM198112103052403. [DOI] [PubMed] [Google Scholar]
- Stevens R. H., Macy E., Morrow C., Saxon A. Characterization of a circulating subpopulation of spontaneous antitetanus toxoid antibody producing B cells following in vivo booster immunization. J Immunol. 1979 Jun;122(6):2498–2504. [PubMed] [Google Scholar]
- Thiele C. J., Morrow C. D., Stevens R. H. Multiple subsets of anti-tetanus toxoid antibody-producing cells in human peripheral blood differ by size, expression of membrane receptors, and mitogen reactivity. J Immunol. 1981 Mar;126(3):1146–1153. [PubMed] [Google Scholar]
- Thorley-Lawson D. A., Chess L., Strominger J. L. Suppression of in vitro Epstein-Barr virus infection. A new role for adult human T lymphocytes. J Exp Med. 1977 Aug 1;146(2):495–508. doi: 10.1084/jem.146.2.495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorley-Lawson D. A. The transformation of adult but not newborn human lymphocytes by Epstein Barr virus and phytohemagglutinin is inhibited by interferon: the early suppression by T cells of Epstein Barr infection is mediated by interferon. J Immunol. 1981 Mar;126(3):829–833. [PubMed] [Google Scholar]
- Tosato G., Blaese R. M., Yarchoan R. Relationship between immunoglobulin production and immortalization by Epstein Barr virus. J Immunol. 1985 Aug;135(2):959–964. [PubMed] [Google Scholar]
- Tosato G., Magrath I. T., Blaese R. M. T cell-mediated immunoregulation of Epstein Barr virus- (EBV) induced B lymphocyte activation in EBV-seropositive and EBV-seronegative individuals. J Immunol. 1982 Feb;128(2):575–579. [PubMed] [Google Scholar]
- Tosato G., Steinberg A. D., Yarchoan R., Heilman C. A., Pike S. E., De Seau V., Blaese R. M. Abnormally elevated frequency of Epstein-Barr virus-infected B cells in the blood of patients with rheumatoid arthritis. J Clin Invest. 1984 Jun;73(6):1789–1795. doi: 10.1172/JCI111388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weissman I. L., McGrath M. S. Retrovirus lymphomagenesis: relationship of normal immune receptors to malignant cell proliferation. Curr Top Microbiol Immunol. 1982;98:103–112. doi: 10.1007/978-3-642-68369-5_8. [DOI] [PubMed] [Google Scholar]
- Whang-Peng J., Lee E. C., Sieverts H., Magrath I. T. Burkitt's lymphoma in AIDS: cytogenetic study. Blood. 1984 Apr;63(4):818–822. [PubMed] [Google Scholar]
- Yachie A., Tosato G., Straus S. E., Blaese R. M. Immunostimulation by cytomegalovirus (CMV): helper T cell-dependent activation of immunoglobulin production in vitro by lymphocytes from CMV-immune donors. J Immunol. 1985 Aug;135(2):1395–1400. [PubMed] [Google Scholar]
- Yarchoan R., Guo H. G., Reitz M., Jr, Maluish A., Mitsuya H., Broder S. Alterations in cytotoxic and helper T cell function after infection of T cell clones with human T cell leukemia virus, type I. J Clin Invest. 1986 May;77(5):1466–1473. doi: 10.1172/JCI112459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yarchoan R., Murphy B. R., Strober W., Clements M. L., Nelson D. L. In vitro production of anti-influenza virus antibody after intranasal inoculation with cold-adapted influenza virus. J Immunol. 1981 Nov;127(5):1958–1963. [PubMed] [Google Scholar]
- Yarchoan R., Schneider H. S., Wray B. B., Nelson D. L. Specific anti-influenza virus antibody production in vitro by lymphocytes from a subset of patients with hypogammaglobulinemia. J Clin Invest. 1983 Jun;71(6):1720–1727. doi: 10.1172/JCI110926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yarchoan R., Tosato G., Blaese R. M., Simon R. M., Nelson D. L. Limiting dilution analysis of Epstein-Barr virus-induced immunoglobulin production by human B cells. J Exp Med. 1983 Jan 1;157(1):1–14. doi: 10.1084/jem.157.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziegler J. L., Beckstead J. A., Volberding P. A., Abrams D. I., Levine A. M., Lukes R. J., Gill P. S., Burkes R. L., Meyer P. R., Metroka C. E. Non-Hodgkin's lymphoma in 90 homosexual men. Relation to generalized lymphadenopathy and the acquired immunodeficiency syndrome. N Engl J Med. 1984 Aug 30;311(9):565–570. doi: 10.1056/NEJM198408303110904. [DOI] [PubMed] [Google Scholar]
- Zoon K. C., Smith M. E., Bridgen P. J., zur Nedden D., Anfinsen C. B. Purification and partial characterization of human lymphoblast interferon. Proc Natl Acad Sci U S A. 1979 Nov;76(11):5601–5605. doi: 10.1073/pnas.76.11.5601. [DOI] [PMC free article] [PubMed] [Google Scholar]



