Abstract
There has been a significant amount of research done on liposomes and nanoparticles as drug carriers for protein drugs. Proteins and enzymes have been used both as targeting moieties and for their therapeutic potential. High specificity and rapid reaction rates make proteins and enzymes excellent candidates for therapeutic treatment, but some limitations exist. Many of these limitations can be addressed by a well studied nanotechnology based delivery system. Such a system can provide a medium for delivery, stabilization of the drugs, and enable site specific accumulation of drugs. Nanomedicines such as these have great potential to revolutionize the pharmaceutical industry and improve healthcare worldwide.
Keywords: Protein therapeutics, nanoparticles, liposomes, drug delivery, nanomaterials
1. Inroduction
There is a large emphasis on healthcare and improving the quality of life in the United States. The amount of money spent on healthcare soared to 17.3% of the Gross Domestic Product, or 2.5 trillion dollars, in 2009 and continues to the rise according to USA Today.1 According to the same article, 246 billion dollars of this total was spent on prescription drugs. These figures illustrate the demand for effective healthcare which is largely met by the development of effective pharmaceuticals.
Drugs have been developed which show efficacy in treating many major pathologies worldwide, but there are severe limitations to many of these drugs. Side effects plague many prescription drug users, and high doses only aggravate this problem. Despite high doses, low concentration of drug at a diseased site is also a consistent drawback to many drugs that have the potential to be effective therapeutics. Another major limitation of many drugs is a low circulation time. The body is effective at removing foreign molecules, including drugs, which results in a short time for the drug to reach its target. Most therapeutics that rely on the circulation for drug delivery see the vast majority of the drug cleared from the body in just a few hours. These are just a few of the limitations of modern drugs, others exist, many of which are specific to each individual drug.
The limitations listed can be addressed, to a certain degree, by the modern advances of nanotechnology. Nanotechnology has gained global attention for its unique ability to hurdle some of the barriers that have limited innovation. The application of nanotechnology to drug delivery has seen dramatic success in this area which seemed unattainable in the recent past. While drug delivery has seen success, many other areas of nanotechnology research are viewed as fantastically as ever, with many still holding out hope for more dramatic applications.
As will be discussed in this review, liposomes and nanoparticles as drug delivery systems have answered many of the common drug limitations. Some of the earliest research was dedicated to delivering small molecules to a target site with as much specificity and as long a circulation lifetime as possible. A renewed excitement for drug delivery by liposomes and nanoparticles was seen over the past fifteen years as a formulation for each saw FDA approval. A barrage of research to improve these approved delivery devices began and still continues with attempts to actively target and increase efficacy.
Protein therapeutics have also received a lot of attention since the first protein drug received approval just over two decades ago. Enzymes especially, have been the subject of much scrutiny into their therapeutic applications. This has resulted in many drugs developed for the treatment of disease, many of which had not seen any treatment prior to protein therapeutics. The high specificity of proteins and fast reaction rates of enzymes along with several other advantages have led to the expansion of research in this area.
The combination of the abilities of the nanocarriers described and the advantages of proteins has resulted in a field which has seen significant success. Proteins were first applied to nanoparticles and liposomes as a means to actively target the carried drug to the diseased site. Immune system proteins have been the most popular protein because of their relative specificity to a variety of different targets. The use of nanocarriers has also been applied to proteins which have shown therapeutic ability and has seen some success. While there is a significant amount of research that has been done, there is still much that remains to be done for these systems with significant potential to be utilized. When they have been characterized and tested, they could greatly increase the quality of life and change the course of healthcare worldwide. The goal of this review is to provide a thorough survey of the research using nanoparticles and proteins for therapeutic treatment of disease with discussion of the barriers and holes in this field of research.
2. Background
Proteins are biological polymers built of amino acids that are organized in a way that allows them to have a specific role in the body. Proteins offer a variety of distinct functions from transport and signaling to enzymatic reactions and direct involvement in the immune response. Because proteins play such a vital role in the normal function of the body and their absence causes significant problems, it stands to reason that they can also assist in the treatment of diseases. One of the most logical applications of protein therapy is in the case of a genetic disease which results in the underproduction of a necessary enzyme. In this case, the enzyme is applied in therapeutically relevant doses to reduce or prevent that diseases presentation in the body. Many proteins, especially enzymes, have the therapeutic ability to treat diseases, many of which are genetic, that have no other available treatment regime. This unique niche has resulted in many of these treatments being granted Orphan Drug status. The Orphan Drug Act was approved in the United States to expedite the availability of these special drugs.
All proteins used in medicine fall into one of two categories: enzymatic therapeutics and proteins involved in biological recognition and signaling. Enzyme treatments are useful for catalyzing the reactions for which they were designed. Enzymes can perform several different types of reactions, many of which are useful in treating disease. There classes of enzymatic reactions are: reduction, oxidation, hydrolysis, transfer of groups, isomerization, covalent bond breakage, and group addition to a double bond. In this review we will discuss enzymes that utilize oxidation, reduction, hydrolysis, and covalent bond breakage to exercise their medicinal activities. Other non-enzymatic proteins are useful in medicine. These proteins exploit cell signaling pathways and cellular recognition, either by receptor recognition or target specificity in the protein itself.
There are a wide variety of pathologies that can be treated by protein therapeutics. These range from major pathologies such as cancer, to genetic diseases that affect a small portion of the population. Cancer is a leading killer in the United States, taking the lives of 23.1% of Americans according to a Center for Disease Control (CDC) study in 2006.2 The same study calculated that more than 500,000 American lives were claimed by cancer in 2006 alone. Modern medicine has improved cancer treatment, resulting in a small decline in the mortality over the past two decades, but despite a concentrated effort, there is still no effective treatment. Proteins also have the potential to treat many other diseases which we will explore in this review. Nanotechnology does have answers to some of the problems that have a hindrance to the progress of effective treatment of cancer as well as other diseases.
For the past 30 years there has been expansive research performed in the area of nanotechnology and drug delivery. Modern technology has allowed for the specific engineering of uniformly sized nanoparticles made from a variety of materials. Metals, polymers, and some ceramics are being formed into spheres ranging in size from 1nm up to 1000nm, commonly referred to as nanoparticles. While nanotechnology specifically refers to anything in the 10−9m scale, it colloquially refers to an even more narrow range, often to a scale from 1–100nm. The most prominent area of medical nanotherapeutic research and controlled release of drug is in the area of cancer therapeutics.
Research on controlled drug delivery has expanded greatly since the first controlled release study done by Dr. Folkman at Harvard in 1964.3 Research in the area of controlled release has advanced from patches to microparticles and now it merges with the field of nanotechnology to attempt to revolutionize medicine once more.4 The invention of nanoparticles and liposomes (lipid based nanoparticles) that carry chemotherapeutic drugs and their subsequent FDA approval in recent years has shed some light on the potential that current research could have on future medicine.
3. Effects of Size
The size of these nanoparticles is of the utmost importance. Particles under 1 micron are necessary to increase the surface contact to the external system which allows increased loading in the case of surface attachment.5 It is also imperative to have nanoparticles that are able to travel through capillaries which are as small as several microns. This demand has resulted in the explosion in the field of nanotechnology. The biodistribution of liposomes, or where they find their final resting place in the body, is a factor of the size.5 Smaller particles are cleared from the body within minutes by the kidneys. Many larger particles are taken up by the reticuloendothelial System (RES) which is made up of tissue macrophages such as liver macrophages, called Kupffer cells, and spleen macrophages.
The ability of a delivered drug to reach a tissue is dependent on the fenestration of the epithelial lining of the blood vessels. There must be a high fenestration size in the epithelial lining to allow nanoparticles into the interstitial space.6 This high fenestration size occurs when vessels become leaky to allow more nutrients into the interstitial space which happens in damaged tissue. Leaky vessels are common in cancerous regions as well as at an inflammation site. In normal, healthy tissues, the drug can still reach the interstitial space and exert its pharmacological action, but only when the free drug is able to move across the vessel lining. This occurs much less often in controlled release carriers as compared to a simple injection of free drug. There are studies that have measured the different fenestration sizes of organs in different animals and there appears to be some consistency in the fenestration size of different diseased organs.6, 7 This could provide some guidelines to researchers attempting to target a specific organ by using nanoparticles designed with a size and targeting moiety for that diseased organ.
4. Nanoscale Protein Delivery Vehicles
4.1 Nanoparticles
Nanoparticles are nanoscale spheres capable of carrying drug loaded into the bulk or loaded onto the surface. Nanoparticles can be polymeric, metallic, or ceramic but are most commonly polymeric in the case of controlled release drug delivery by a biodegradable particle. Metallic particles have been studied for their unique properties as well.
Gold nanoparticles are the earliest nanoparticles, and have been studied for a variety of applications. Iron oxide nanoparticles are of interest because they can act as magnetic resonance imaging (MRI) contrast agents and, as we will mention later, have gained much attention in the area of hyperthermic cancer treatment.
4.2 Liposomes
Nanoparticles constructed from lipids that form bilayer micelles are called liposomes and have a unique set of properties that makes them ideal for certain delivery applications. Liposomes are made up of lipids which have a structure that mimics the cell membrane, but lack the numerous embedded proteins present in cell membranes. The properties of the liposomes depend on their lipid composition. Liposomes can be unilamellar (single membrane layer) or multilamellar (multiple membrane layers), but the latter are rarely employed for biomedical applications. Liposomes were first proposed as drug delivery devices in 1974 by Gregoriadis et al. and have since gone through many changes to bring liposome technology where it is today.8 All liposomes contain cholesterol which provides fluidity to the membrane just as it does in the cell membrane. As new breakthroughs have shown how these carriers can be effective, it became necessary to add other components to liposomes to increase their ability to reach the therapeutic site. Amphiphilic molecules with polyethylene glycol9 chains on the end were used to limit opsonization in the body and increase circulation half-life. There has also been attachment of certain targeting moieties10 to liposomes to facilitate incorporation into malignant cells at the therapeutic site.
Site specific delivery and controlled release are the main two goals for nanoparticles and liposomes as nanotherapeutics.
4.3 PEGylated liposomes and nanoparticles
A major breakthrough in liposome and nanoparticle technology was made when polyethylene glycol was chemically attached to the particle surface. The human body is adept at removing foreign objects to prevent invasion of pathogens. When any foreign object enters the body, it is immediately coated by proteins (opsonized) that mark it for uptake by natural killer cells. This process occurs when nanoparticles or liposomes are introduced into circulation limiting their half-life in blood stream to several minutes. Thus, it becomes important for any drug carrier to be resistant to opsonization. Hydrophobic surfaces are more prone to protein adsorption and are therefore undesirable on drug carriers. This becomes a problem for particles with hydrophobic surfaces, and results in their rapid removal from the blood stream. Thus, the challenge to develop a protein-resistant coating for hydrophobic particles to prevent rapid clearance was presented. This call was answered by researchers Alexander Klibanov and Vladimir Torchilin,9 and several other groups simultaneously,11, 12 who applied a principle for surface coating that had been developed at Rutgers in the 1960’s.13 This technology utilizes a polyethylene glycol (PEG) polymer chain attached to the surface of the liposome or nanoparticle. This PEG chain is extremely hydrophilic and can prevent protein adsorption. Lack of opsonization limits the immune systems rapid recognition and uptake of the PEGylated nanocarriers resulting in a longer circulation time. Other hydrophilic spacers, such as polyHEMA have been applied to nanoparticle systems with similar success.
5. Liposomal Drug Loading
5.1 Conventional liposome drug loading
The conventional method of liposome drug loading involves passive encapsulation.14 Passive encapsulation occurs when drug dissolved in solution is captured by the lipid film folding into the three dimensional liposome structure. This method of drug loading results in low encapsulation efficiency.15 There have been a wide variety of loading encapsulations reported by different groups from just a few percent to just over half of the drug loaded into the interior of the liposome when using this method.16, 17 These encapsulation efficiencies did not come close to the efficiency that was achieved in the early 90’s by researchers looking at gradient driven, or remote, loading.
5.2 Remote liposome drug loading
One of the most important discoveries was that of the ability to utilize remote loading to increase the encapsulation efficiency dramatically over traditional loading. The first salt used was ammonium sulfate by the Barenholz group in 1993.18 The high ammonium sulfate concentration on the inside of the liposome drew amine groups into the bulk of the liposome, which then precipitates out of solution. This study showed a significant increase in encapsulation efficiency. This loading method resulted in as high as a 100 fold difference between internal and external concentration. This resulted in an encapsulation of greater than 90%. An increase is seen from just a few percent loading efficiency to greater than 90% loading.
Another study by the same group in 2009 summarizes their work in gradient loading over the previous 15 years.19 Nine different small molecule drugs were tested and a model was developed to predict their loading using the different gradient methods. In 91% of cases, the model predicted well the outcome. This study showed an increase from low loading (defined as 0–40% loading) to high loading (defined as 70–100%) in most cases. Another method of loaded shown in this study uses a calcium acetate gradient to achieve similar success.
6. Protein therapies Utilizing Nanocarriers
6.1 Cancer
Current cancer chemotherapy agents have been shown to be cytotoxic and have many side effects in vivo. These side effects include some more familiar symptoms like hair loss, nausea, as well as more hazardous pathologies such as neutropenia and kidney failure.20 This inherent cytotoxicity limits the doses of these therapeutic drugs that can be administered to patients. This systemic cytotoxicity is caused by the drug acting on all cells in the body, not just the on cancer cells. Even if new chemotherapy agents could be identified, there are still the overarching issues of systemic toxicity, high doses, and drug resistance that can build up over time. Thus, if a carrier could be designed that would deliver the drug only to the cancer site it would severely limit the action of the cytotoxic drug on healthy tissue and restrict its action to the cancer cells. This directed delivery is often called ‘targeting’ and may provide effective solutions for the major hurdles in oncology. Overcoming these barriers is the chief goal of oncologic nanomedicine. The ability to design these targeted nanocarriers is a distinct advantage of nanotechnology which allows consistent low drug doses to be delivered to the targeted site.
6.1.1 Passive targeting
Earlier in this review, the leaky vessels present in malignant tumors were referenced. These leaky vessels are the result of fenestrated epithelium and pressure gradients between the vessels and the interstitium. This leakiness causes an increased flow to the cancerous site which results in an accumulation of nanoparticles being transported in the blood.21 Due to the rapid production of tissue vasculature at malignant sites, there is an inadequate lymphatic system presence which results in further accumulation due to lack of drainage. This is where PEGylated particles have a comparative advantage because of their extended circulation time.21 The outcome of which is a relatively high concentration of engineering particles at the cancer site compared to normal, healthy tissues. This effect has been aptly named the enhanced permeation and retention effect (EPR) by the researcher who first described it in 2001.22 Maeda also noted that there was a higher concentration of the particles in the tumor tissues than the plasma. The EPR effect resulted in a very popular method of liposome delivery due to its convenience as well as it highly effective nature. This method of delivery has been called passive targeting or passive diffusion.
There are two nanoparticle formulations approved by the United Stated Food and Drug Administration (FDA) that are designed to carry small molecule cancer treatments by passive diffusion with several more currently in clinical trials. The first nanoparticle to be FDA approved is called Doxil and is a liposomal formulation for the treatment of cancer. Doxil first received approval in 1995 for cancer treatment and received full approval after a several years in accelerated approval in 2003. Doxil is now approved for treatment of several types of cancer.23, 24 The second, titled Abraxane, is a nanoparticle built from the protein albumin and was approved in 2005.25, 26 Abraxane is discussed in more detail in the section devoted to use of blood carrier proteins in cancer therapy.
Several proteins have been proposed for treatment of cancer utilizing passive targeting mechanism. One such protein is tissue necrosis factor alpha (TNF-α). TNF-α is involved in a complex cell signaling cascade which is involved in the regulation of cell proliferation, survival, and apoptosis. In the presence of malignant tissue, TNF-α binds to its receptor and initiates the process of apoptosis. TNF-α has been of interest to the cancer research community for years but has not been able to reach the potential with which it was initially credited due to systemic toxicity.27 The ability to preferentially target the drug only to the tumor site has revived this protein therapeutic by loading in liposomes and on nanoparticles. One study loaded TNF-α onto the surface of PEGylated colloidal gold nanoparticles to determine the effect on tumor necrosis.28 They showed that the protein did effectively prevent tumor growth in a mouse model and increased the percent survival from 33% with native TNF-α to 100% with the nanoparticle delivered form.
Another study used recombinant TNF loaded into liposomes designed to release their contents upon thermal treatment.29 This allows the release of the recombinant protein to be controlled by heat. The study showed that an increase in local temperature from 37°C to 42°C resulted in an increase in liposomal release from nearly no release to almost 60%. Thermal induced release five minutes and one hour after injection of the drug shows an increase in tumor growth suppression over time. A study on TNF-α loaded into PEGylated liposomes suggests that the passively delivered drug can augment the effects of radiation, but does little to prevent cancer growth on its own.30 Limited or no toxicity findings of the drug were reported in the study.
One study used L-asparaginase encapsulated in a liposome to treat malignant tumors that are sensitive to asparaginase.31 L-asparaginase is thought to have anti-cancer activity due to its ability to lower blood asparagine, which is needed by lymphatic and leukemic cells.32 This study showed that liposomal asparaginase was a more effective treatment than free enzyme at the same concentration. Treatment with liposomal asparaginase also has an important feature of being non-immunogenic.10, 33 There is not a production of antibodies against asparaginase when liposomal enzyme is used therapeutically.
Another study investigated the apoptotic abilities of liposome encapsulated membrane proteins.34 Two membrane proteins, voltage-dependent anion channel (VDAC) and pro-apoptotic Bak, were incorporated into liposomes individually and in combination. Uptake by cells in vitro was observed by fluorescent tagging of the encapsulated proteins and subsequent apoptotic activity was observed by following cytosolic cytochrome c concentration and apoptotic intermediates via western blotting. This study showed that the proteins induced apoptosis individually, but when delivered in tandem there was a significantly greater effect. This initial study sheds some light on the therapeutic efficacy of membrane proteins.
6.1.2 Antibody directed targeting
A different class of nanocarriers utilizes highly specific interactions of biorecognition molecules (“targeting ligands”) with cancer-specific biomarkers to achieve drug delivery to a tumor. This therapeutic methodology is referred to as ‘active’ targeting. This approach heavily utilizes proteins as the most natural targeting ligands.
The most common protein targeting agents used in active targeting to malignant tumors are IgG antibodies immobilized on the nanoparticle surface. These immune system proteins have a very high specificity and can selectively and effectively localize the nanocarrier at the tumor site. When antibodies are immobilized on liposome surfaces for targeting the resulting constructs are aptly named ‘immunoliposomes’. Immunoliposomes35–37 have become a very fashionable area of research over the past decade. Attempts to improve currently used liposomal cancer treatments have shown positive results.38, 39
One of the most common targeting agents is the anti-HER2 antibody which targets the p-185, or HER2, receptor over expressed on the surface of many malignant tumor cells.40, 41 Park et al published impressive results in a study comparing passively delivered liposomal doxorubicin (Doxil) and HER2-targeted liposomal doxorubicin in HER2 over-expressing tissues.41 Park et al and others showed that there was no effect of targeting on tissue accumulation, but that tumor cell cytotoxicity increases in the immunoliposome treatment.42 Several of these studies also observed intracellular aggregation of HER2 targeted nanospheres using several different methods including colloidal gold as compared to untargeted colloidal gold showing stromal aggregation without targeting. Park et al published starting results of a 16% cure rate in mice with the immunoliposome system increasing to ~50% with more optimized samples.43 This is impressive especially in comparison to a 0% cure rate in both free doxorubicin and in liposomal doxorubicin. This study by Park et al illustrates the immense potential targeted therapies can achieve if their properties are optimized.
Another popular internalizing antigen for targeting is the CD19 antigen expressed in B-Lymphoid cancers.44 As was the case for the HER2 receptor, the CD19 antigen and all other targets for specialized delivery must result in the internalization of the carrier by endocytosis. The necessity of this cell internalization is illustrated in the targeted versus the untargeted drug’s cancer cell cytotoxicity with the only tangible difference between the two being internalization of the carrier. Liposomes with anti-CD19 have been shown to be three fold more likely to attach to B-lymphoma cells.44, 45, 45,45 Studies comparing the immunoliposome treatment regime to the untargeted liposome revealed that the targeted carrier was more effective at preventing cancer growth.44 The study showed 81% increase in lifespan for free doxorubicin and liposomal doxorubicin with a 159% increase in lifespan for the target and 3 of 7 mice surviving long term (over 100 days.) One immunoliposome which used anti-CD19 antibody fragments as targeting moieties achieved over a 200% increase in life time and 5 of 7 mice surviving long term. Laginha et al compared the anti-cancer ability of identical liposome constructs with different antibody targeting moieties; anti-CD19 and anti-CD20.46 CD20 is a B-cell surface exposed antigen similar to CD19 and drugs targeted to each individually show similar cancer cytotoxicity. When both antibodies are immobilized on the liposome they show increased B-Lymphoma cytotoxicity. Many other liposomal and nanoparticle formulations using anti-CD19, anti-CD20, and proteins to target other Cluster of Differentiation cell surface antigens.
ElBayoumi and Torchilin had success in active targeting of the FDA approved liposomal formulation Doxil using monoclonal autoantibodies.47 Early studies by Torchilin’s group show that monoclonal autoantibody 2C5 specifically recognizes many tumor tissue types but not normal, healthy tissues.48 The biodistribution of the targeted construct was followed by radiolabeling and a small increase in RES uptake was seen over plain Doxil with 55% of injected drug in RES organs as compared with 49% in the untargeted treatment. The result of the targeted treatment was a decrease in tumor size to 25–40% of the Doxil treated group. Another study by Torchilin and Gupta showed a similar 2C5 targeted Doxil therapy effectively treating brain tumors in mice.38 They again showed a significant decrease in tumor to ~50% the size of the Doxil regime 24 days after the treatment began. This and other work by Torchilin sets the stage for continuing development of antibody targeted liposomes to be the standard for cancer treatment in the near future.
Nanoparticles are also targeted to tumors by antibodies but are not as ubiquitous as liposomal treatments. The main reasons are higher biocompatibility and lower immunogenicity of liposomes, whose composition mimics that of cell membranes. Nanoparticles have also been explored as drug carriers, but have been predominantly utilized for imaging and hyperthermic cancer therapy. Quantum dots for fluorescent imaging of tumors often use antibodies as targeting moieties.49–52 Possible therapeutic uses of quantum dots have been discussed but are questionable because most quantum dots contain highly toxic elements such as Cd or Se and their long term fate in the body is not known. Antibody targeted magnetic nanoparticles are often studied for hyperthermic treatment of cancer, many of which utilize the biological ligands we have discussed.36, 53–55
7. Limitations to Nanoparticle Based Targeting and Drug Delivery
There are several major hurdles that must be overcome for much of this technology to be effective and reach the market.56 Much of the work in nanotechnology has focused on systemic administration and accumulation at diseased sites, but there are common misconceptions in the field which can lead to an unrealistic expectation of the treatment efficacy of many nanoparticulate treatment modalities. Here, we will discuss some of these misconceptions with an eye on the future of nanotechnological development, which we believe has a bright future of effective treatments for a variety of diseases. However, we must be honest about the major barriers in the field in order to make meaningful steps towards future innovation.
7.1 Poor targeting
As can be inferred from the recent work in the field of targeted drug delivery, the main barrier to effective treatment through systemic delivery is poor targeting. Systemic administration of nanoparticulate drugs, most often through intravenous injection, has made great claims of success and of ther future potential of such treatments, but there appears to be little improvement in the efficacy of these treatment options.57 While increased circulation time and targeting moieties have improved accumulation at the target site, therapeutic efficacy for many applications necessitates even better targeting.
While there is an increase in accumulation of nanoparticles in many sites as described by the EPR effect, it is not as large of an increase as it often appears when reading the literature. It has been reported that the amount of drug delivered via nanoparticles which actually reaches the target site is typically less than 5%.58 This indicates that 95% is elsewhere in the body. This is unchanged by targeting moieties on the outside of the particles. Particles in a certain narrow range (already discussed previously in this review) are able to avoid clearance to some degree and accumulate in tissues where leaky vasculature exists. Despite this increase in accumulation, which, no doubt, does occur, the amount of drug reaching the targeted site is far from ideal.
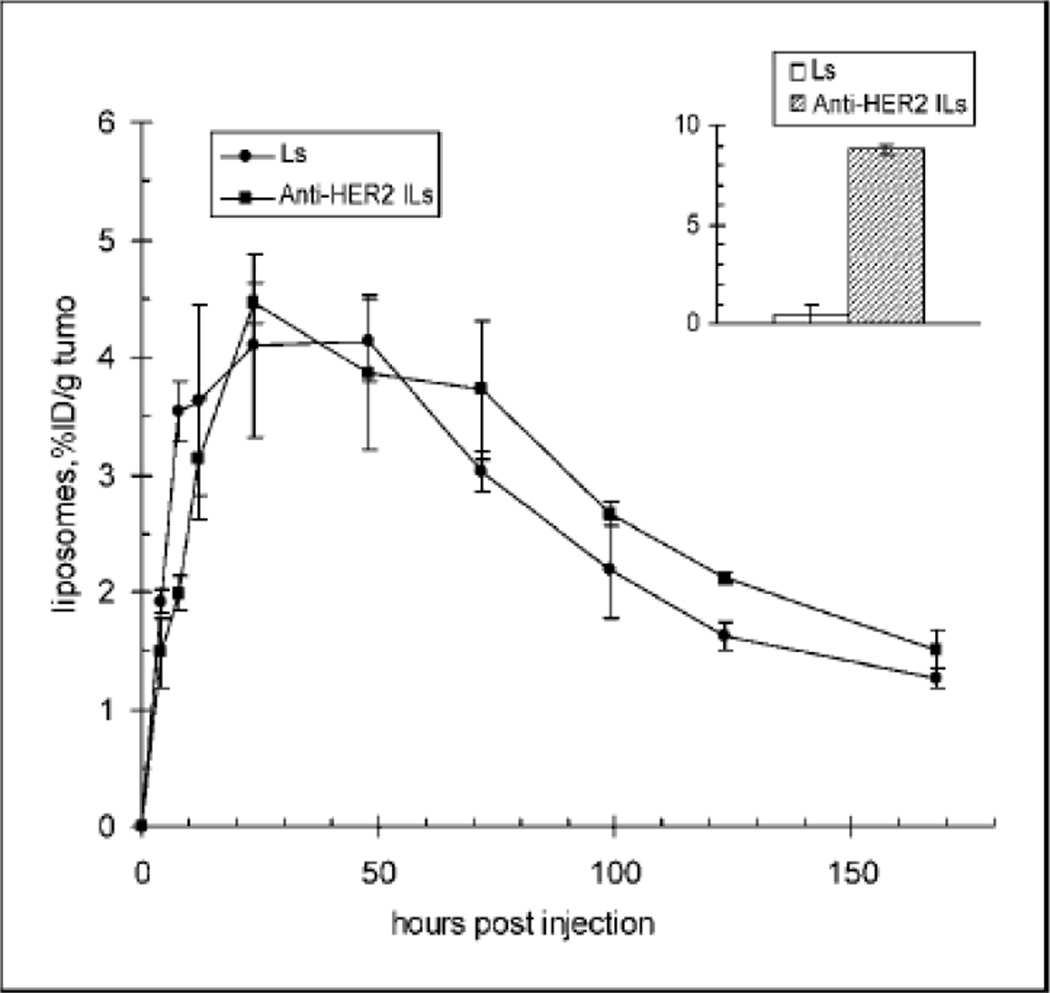
Active targeting is thought by many, largely because of a body of misleading literature, to be a technology which can effectively corral the nanoparticles to the site where they play their therapeutic role. However, ‘decorated’ nanoparticles are subjected to the direction of the blood flow and follow the circulation in the same way as undecorated particles.59 The targeting moiety, or ligand, can only interact with its receptor if the distance between them is less than a half a nanometer.58 This distance is extremely limiting. However, once the particles decorated with targeting moieties reach these tissues which possess the receptor, the targeting agent can anchor them and even increase cellular uptake of these particles, potentially increasing efficacy. An example of this, which has been referenced in a review on some of the limitations discussed herein, is shown in Figure 2.59 This figure shows no major difference in accumulation of antibody targeted liposomes compared with untargeted in the tumor. There is an increased uptake of the anti-HER2 antibody surface decorated liposome though, as can be seen in the inset in the Figure.42 This is a typical response as if there is a reported increase in accumulation due to targeting it is typically small and not significantly different from the untargeted particle.59
Figure 1.
The percentage of the radio-labeled immunoliposomes accumulated in the tumor is not significantly different from liposomes which are not targeted with the anti-HER2 protein. However, the inset shows internalization of the liposomes into the cancer cells. Here, the antibody modification makes a large difference. Image taken from Kirpotin et al.60
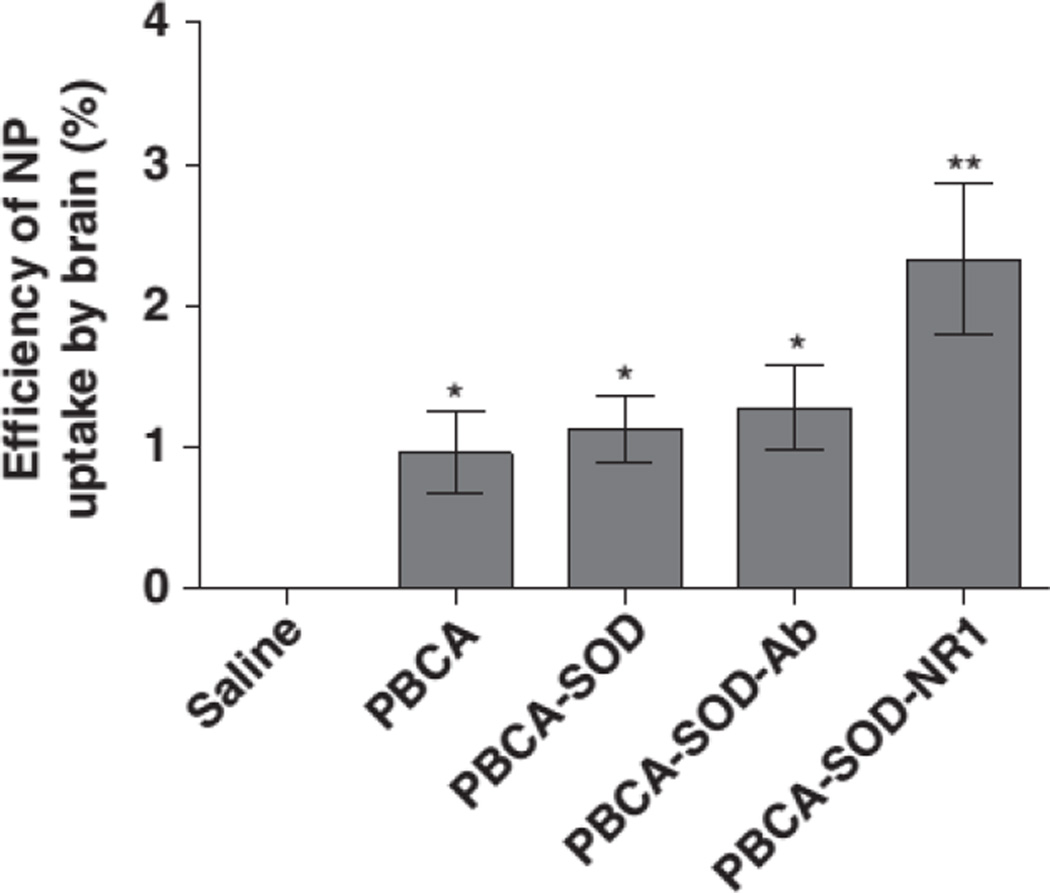
However, recent work in our group has shown that targeting can increase the localization of a drug delivery system. It was shown that targeting with anti-NR1 antibody significantly increased the efficiency of particle uptake into the brain. Figure 3 shows the trend of increasing uptake as the targeting antibody is attached. This paradoxical data appears to point to contradicting principles, but it could simply illustrate the physiological complexity of human transport processes and disease. Targeting will likely have drastically different effects depending on the region of the body, the targeting agent, the size of the particles, and many other factors.
Figure 3.
The efficiency with which PBCA nanoparticles are taken up by the brain is significantly improved by targeting with anti-NR1 antibody. NR1 is an anti NMDA (N-methyl-D-aspartate) receptor antibody.102
A discussion of these limitations does not invalidate the discourse on advances which are being made, both in so-called passive targeting and in active targeting. There have been two FDA approved nanoparticle delivery systems (discussed earlier is this review). These both utilize passive delivery based on the EPR effect, but there has been work on increasing efficacy through active targeting. We are not attempting to discredit or cast doubt on either the work done in the recent past or on the potential that these particles have to effectively treat a variety of diseases. Many of these diseases pose complex pathologies which make them difficult to treat with any other therapies, and nanotechnology appears uniquely positioned to provide treatment. However, it is vital to have an understanding of the limitations present in the field so that meaningful advances can be made.
One answer to the limitations seen in the ability to target drugs to the site where they are needed is the use of local delivery. There are several therapies have been approved utilizing controlled release of drugs at a specific site.61–65 If the injury or disease site is known and is accessible, direct administration of a drug delivery system that can deliver a therapeutic dose over time is beneficial, especially in contrast to the potential of a systemically delivered drug. There are only certain situations where such an application is appropriate, but if applicable, many of the limitations discussed for systemic delivery are not present when local delivery is achieved.
7.2 Surface modification and immune response
Another necessity for systemically delivered drugs is increased circulation time which is an imperative for efficient delivery. The clearance of foreign materials from both the circulation and the tissue is efficient and is a major barrier to effective treatment, especially when targeting is necessary.56 Questions as to protein and enzyme stability and efficacy in vivo once applied to a nanoparticle system also remain. Other limitations include the ability to add the modified PEG groups after drug formation, which is an area that has seen much improvement over the last few years.56 The maintenance of attached targeting ligands once drugs are in the circulation is also an issue limiting the application of these treatment options.56 If the targeting ligands are lost before localization at the tissue then there is reduced internalization and thereby, reduced efficacy.
Another area that limits the application these treatments from reaching the market is the ensuing immune response. This is related to the surface modification limitation because the immune response and clearance rate of the particles are directly related to their surface, as that is the chemistry with which the body interacts. Methods to reduce the immunogenicity, such as PEGylation and the use of liposomes as carriers, have made strides toward this goal, but it remains a significant barrier.66
7.3 Sterile formulations and manufacturing limitations
Another major barrier is the preparation of sterile formulations for treatment.67 Many of the sterilization methods compromise the integrity of the therapeutic or polymer scaffold.68 There are methods that can be used, such as irradiation, which remains one of the most popular methods of sterilization for polymeric delivery vehicles, but questions remain as to the efficacy of the sterilization treatment and its potential effect on many proteins and polymer delivery systems.67–69 The ability to scale up the manufacture of nanoparticles for marketing once a formulation is approved is also a limitation.70
8. Blood Transport Proteins for Therapy and Directed Targeting
While antibodies are the most common protein used for active targeting blood transport proteins such as transferrin and albumin, come in close behind. Transferrin, a protein that transports iron in the blood, is the most common non-immune targeting agent used in cancer treatment. Transferrin is a useful targeting moiety because it preferentially binds to malignant tumor cells because of increased presentation of transferrin receptors on the cell surface. The high specificity of transferring and the high levels of endocytosis make this mechanism a popular method for targeted drug delivery. Both nanoparticles and liposomes have used this targeting mechanism and have shown good results.
Transferrin directed targeting to malignant tumors has been performed by many groups. Liposomal doxorubicin is again the drug of choice for many studies targeting malignant tumors via the transferrin endocytotic pathway. One recent study showed more than a five-fold increase in tumor doxorubicin concentration over the free drug and a 44% increase over the PEGylated liposomal doxorubicin delivery system.71 This study did show an increase in liver uptake of the transferring targeted drug, but other RES organs saw a decreased concentration. This study also shows a similar circulation time to the untargeted drug. Another study loaded paclitaxel inside nanoparticles and targeted them using transferrin on the nanoparticle surface.72 Mice that were treated by untargeted paclitaxel nanoparticles showed increasing tumor volume after ~50 days but at 80 days the tumor volume had decreased slightly in the targeted group. This study showed increased lifetime of mice compared with controls with the only death coming after 3 months of treatment and was seemingly unrelated to cancer. Several reviews that cover transferrin as a targeting agent for drug delivery are available.72–75 Transferrin has also been utilized to mediate endocytosis for nanomaterials specializing in gene delivery.76 Lactoferrin is a related enzyme in the transferrin family that was recently used as a targeting protein to achieve gene delivery of nanoparticles to the central nervous system (CNS).77 Comparison of lactoferrin-based targeting to transferrin-based targeting showed a 5.2 times increase in CNS delivery over unmodified nanoparticles and a 2.3 times increase over transferrin modified nanoparticles.78 Several quality reviews have been published in the area of gene delivery and can be consulted for further information on this elegant medicinal treatment regime.79–81
Albumin is a serum protein that is commonly linked to small molecules for delivery to tumors where it preferentially accumulates, but until recently, it has not been used in association with nanotechnology.82 Novel nanoparticle constructs built from albumin for the delivery of paclitaxel that did not cause hypersensitivity reactions was designed by American Bioscience and titled Abraxane.83 Paclitaxel is encapsulated inside the albumin nanoparticle shell and delivered to the tumor site. Once at the tumor site, Abraxane binds to albumin binding proteins dubbed SPARC (Secreted protein, acidic and rich in cysteine), which are over expressed in certain cancers.84 This binding results in endocytosis and increased therapeutic efficacy over the previously used delivery via Cremaphor (non-aqueous polyethylated castor oil).85 Delivery in the non aqueous solvent is thought to have inhibited the ability of albumin to bind cell surface proteins and thereby prevent endocytosis and reduce the drugs impact.86
9. Proteins for Oxygen Delivery
Blood transport proteins have not only been used for cancer. One protein that has received attention as a useful therapeutic is hemoglobin. Hemoglobin is a blood transport protein that functions to deliver oxygen systemically via red blood cells. Hemoglobin is of interest as a potential red blood cell replacement treatment for cases of high blood loss to prevent hemorrhagic shock due to low oxygen in the blood.87 Hemoglobin, when injected into circulation without a carrier, is quickly cleared from the system (0.5 – 1.5 hours).88 The use of a carrier has shown the ability to increase circulation time and provided a viable means of preventing hypoxic shock.
A study performed by Phillips et al exhibited that PEGylated liposomal hemoglobin had a 3 fold longer circulation time than unmodified liposomal hemoglobin.88 They also showed that over half (51.3%) of liposomal hemoglobin was still in circulation 48 hours after injection. Other groups have shown recent advances in PEGylation which improve the circulation time and loading efficiency of the liposomal hemoglobin. Post-insertion of PEG groups resulted in a longer circulation time and a ten times greater encapsulation efficiency of hemoglobin.89 Hemoglobin in liposomes was also delivered with recombinant albumin to rats under induced hemorrhagic shock (half of blood removed) and provided restoration.90 The dose of hemoglobin applied to the circulation by this method can be very large, up to the equivalent of several liters of blood given by transfusion.91 Liposomal hemoglobin is stable for over a year when stored at room temperature, which makes it more appealing as a replacement therapy.92
There are, however, some barriers that must be overcome before such as system can be used therapeutically. Peripheral blood monocytes mature to macrophages which phagocytize the hemoglobin carriers and remove them from the circulation.93 The same study showed blocking of phagocytosis with the use of antibodies illustrating an opsonin-independent pathway for phagocytosis. Effective avoidance of uptake must be realized before liposomal hemoglobin will be a viable red blood cell replacement. Similar barriers including immunogenicity and RES uptake affect all targeted nanocarriers.
10. Enzymes for the Relief of Oxidative Stress
Reactive oxygen species (ROS) are a common presence in all living things. It has been hypothesized that the lifespan of a biological species is determined by the amount of ROS produced during metabolism.94 ROS have been found to be involved in the pathogenesis of inflammation, shock, ischemia/reperfusion and trauma induced cell death95. They have thus been the subject of extensive research in pharmacology.96 Since the inception of the field of enzymatic therapeutics, there has been research attempting to find an effective use of antioxidant enzymes, such as Superoxide Dismutase (SOD), an enzyme which transforms the ROS superoxide (O2−) into much less toxic hydrogen peroxide and catalase (CAT) or glutathione reeducates (GLR), which in turn degrade hydrogen peroxide to give water and oxygen.97 Again, a carrier may be desired for the delivery of these enzymes to the therapeutic site because the relatively large enzyme is cleared in less than thirty minutes without a carrier.98
Early studies incorporating SOD into liposomes for relief of oxidative stress showed success.96, 99 One study showed a reduction in brain infarct sizes in a rat model of 33% in the anterior, 25% in the middle and 18% in the posterior brain samples.96 Several recent publications by Reddy et al have confirmed therapeutic reduction of oxidative stress in the brain by using PLGA nanoparticles with loaded SOD.100, 101 They showed a 65% reduction in infarct size in the nanoparticle formulation versus a 25% increase in size when treated with free SOD, both compared to a saline regime.100
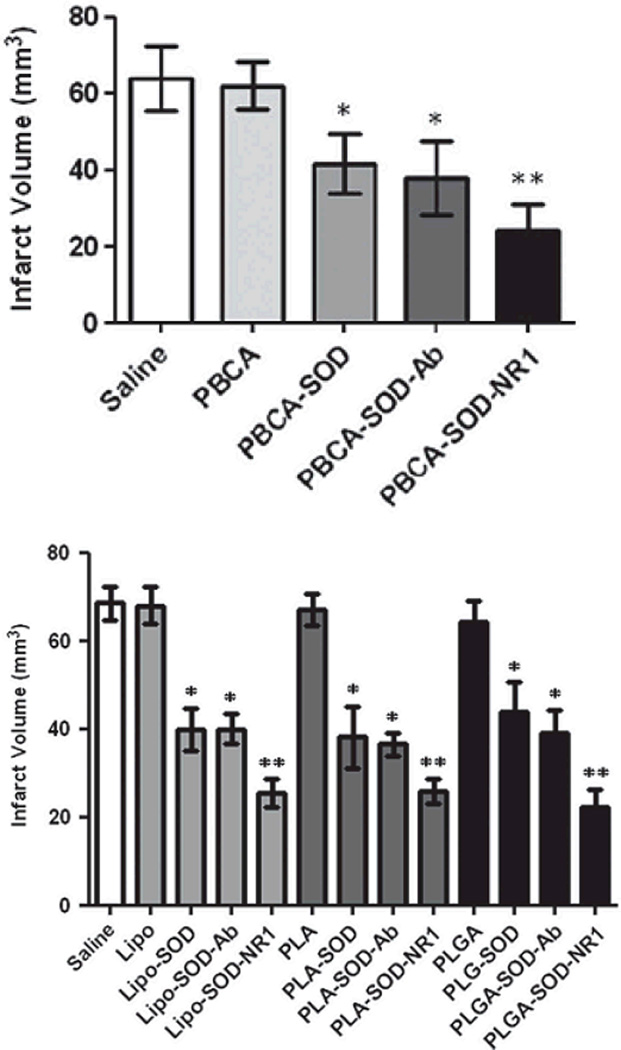
In light of the referenced studies by Reddy et al which loaded SOD into the bulk of PLGA nanoparticles, our group has investigated the therapeutic effect of SOD loaded onto the surface of nanoparticles.102 Targeting antibody and SOD were both loaded onto the surface of poly (butyl cyanoacrylate) (PBCA) nanoparticles and investigated targeting potential and effect of ischemic protection. Ischemia was induced in mice for one hour followed by 24 hours of reperfusion to mimic cerebral ischemia and reperfusion injury. The results indicated that targeting had a significant effect on both specific brain localization of the particles and resulted in a significant decrease in infarct volume (Figure 4). Our results correlated closely with a control mimicking the particles prepared by Reddy et al. This work again illustrates the efficacy of the enzyme in protecting from oxidative stress.
Figure 4.
Effect of modification and targeting of various drug delivery vehicles for treatment of brain injury. Upper graph shows a significantly decreased infarct volume after treatment with PBCA nanoparticles modified with SOD and a further decrease when these particles were targeted with NR1 antibody on the particle surface. In the lower graph, a similar trend was observed with liposomes (lipo), poly(lactic acid) (PLA) nanoparticles, and PLGA nanoparticles with bulk loaded SOD.102
Another important application of the SOD therapeutic system has been in the treatment of arthritis. Gaspar et al showed that liposomal SOD effectively reduced edema at the inflammatory site and had preferential accumulation due to the extended circulation of the PEGylated carrier. Another study looking into SOD as an anti-fibrotic agent also showed success.103 SOD incorporated into liposomes acted as an antagonist toward TGF-β1 which resulted in the dedifferentiation of myofibroblasts at the wound site. Another review by our group has shown the application of SOD towards arthritis as well as respiratory disease, wound healing, and the central nervous system in more detail than we will discuss here.104
Anionic liposomes were shown in vitro to increase the cellular SOD by 108% versus ~85% in neutral formulations and cationic liposomes, which have been shown to be more cytotoxic than neutral or negatively charged liposome formulations.105 The presence of SOD has also been shown to induce apoptosis of neutrophils in vivo when added endogenously.98, 106 Several studies have also been performed in an attempt to elucidate an effective method of SOD delivery.107, 108 These studies show increased protection from inactivation, increased activity, and, as expected, increased resistance from uptake by reticuloendothelial system.
There is still much work to be done in this area to understand the biochemistry and physiological role of SOD. The largest challenges that face researchers in this field emerge from a lack of knowledge of the pathogenesis of many of the pathologies associated with oxidative stress.109 Free radicals play a vital role in normal physiology and everyday function of the body, but are also associated with a variety of diseases as is illustrated above.97 The difficult task set before researchers is to determine the specific role that ROS play in these diseases and then apply the drug in appropriate dose at the time of maximum therapeutic efficacy.109 CAT is another antioxidant enzyme that can reduce oxidative stress. This enzyme works in cooperation with SOD to eliminate the hydrogen peroxide formed by SOD. Hydrogen peroxide, which still has the potential to cause oxidative damage, is transformed into water and oxygen.97 Because of this cooperative antioxidant action, these enzymes were utilized together to increase therapeutic efficacy.110–112 CAT has been shown to be effective as an antioxidant which can be stabilized by a carrier and have increased activity for therapeutic applications.113 One recent study has shown the effective antioxidant activity of catalase by incorporating the enzyme into iron oxide nanoparticles for magnetic delivery to endothelial cells.114 Another study targeting catalase containing latex nanoparticles to endothelium showed antioxidant protection for over two hours after cell uptake.115 Other antioxidants and strategies for selective delivery to endothelial cells are thoroughly reviewed elsewhere.116, 117
11. Antithrombolytic Protein Therapeutics
Tissue plasminogen activator (tPA) is an enzyme which activates plasminogen to plasmin, which is capable of breaking down fibrin clots. tPA was among the first enzymes to be approved for use by the FDA as a drug and, along with SOD, has gained much of the attention given to enzymology as a field wrought with potential therapeutics.118 The enzyme approved was a recombinant form of tPA termed Alteplase for use as an antithrombolytic after myocardial infarction and acute ischemic stroke.119–121 This has brought tPA much attention but its application is limited due to systemic activation of plasminogen in the circulation which can result in hemorrhage.122, 123 When this drug is applied to patients after myocardial infarction there is a high mortality rate of 6.3% within 30 days of treatment with 1.5% experiencing fatal cerebral hemorrhage.124 This high mortality rate indicates the need for a specific carrier with the ability to target fibrin clots without affecting the systemic wound healing ability.120
One study observed liposomal tPA showing comparable activity to that of free tPA at a four-fold lower concentration.125 A more recent study with magnetically targeted nanoparticles surface modified with Alteplase showed 82% of blood flow was restored to a blocked vessel compared with no therapeutic advantage in free Alteplase and free nanoparticle.126 Another study looked into PLGA nanoparticles loaded with tPA surface modified with chitosan grafted with cell adhesion peptide RGD. This setup resulted in an increase in ~41% increase in the weight of digested clot compared with free tPA. The particle without RGD grafts showed the highest clot lysis times decrease to ~39% compared to the free drug.127
Several studies have also looked into liposomes as a delivery vehicle for tPA. One group used liposomes release their contents which signaled by an external auditory trigger. They showed a 49.5% improvement in thrombosis over a no ultrasound control.128 A similar study showed that the rtPA (recombinant tPA, Alteplase) carried by the liposomes showed the same level of activity as free enzyme after ultrasound triggered release.129 A third study showed a similar level of activity between free tPA and activity of loaded echogenic liposomes before the ultrasound signal. Once the liposomes were ruptured, an increase from 48% fractional clot loss to 89% was observed.121 All three studies had ~50% loading of tPA into the liposomal carriers. A study looking at antiactin targeted liposomes containing tPA found that hemorrhagic volumes decreased ~38% when compared to untargeted liposomes.37 Other groups have looked into a different plasminogen activator called streptokinase. One group studied streptokinase loaded into perfluorocarbon nanoparticles in an in vitro study and saw a rapid fibrinolysis and with as little as 1% surface binding there was a 29% drop in clot volumes.130 Another study using liposomes and several other vesicular delivery systems loaded with streptokinase saw an increase in accumulation at the site of thrombosis most notable in the liposome treatment.131 Other liposome studies for the delivery of antithrombolytic agents have been reviewed thoroughly.132
12. Signaling Protein Molecules for Therapeutic Applications
There are many signaling protein molecules that have potential therapeutic ability such as insulin, growth factors, and cytokines. One apparent application of insulin is in treatment of type I diabetes. Many studies utilizing the large surface area of the lungs for aerosolized delivery have proven effective.133, 134 One of these studies showed a homogenous delivery of insulin to the lungs and a decrease in side effects outside of the lungs which has better efficacy due to increased loading efficiency, low inflammation and immunogenicity.133 It has also been shown that small liposome size results in better pulmonary delivery.134 Increased delivery with smaller liposomes do to a decrease in alveolar macrophage uptake has also been exhibited.134 Insulin has also been loaded into liposomes that are sensitive to blood glucose levels.135, 136 This elegant system proposes to use pH sensitive liposomes with surface bound glucose oxidase which will bind glucose and form acidic groups. This change in pH will signal the release of loaded insulin and a corresponding reduction in circulation glucose concentration.136 Kim et al have shown this liposomal system to hold onto contents with no glucose present and to release their contents when exposed to glucose.135 However, although improvements to this system have been shown, this system is unable to fully distinguish the difference between normal levels of glucose and elevated levels of glucose in the system indicative of diabetes.137 An appropriate sensitivity physiologically relevant glucose concentrations must be achieved before the system can be used.
While applications of insulin as a diabetic treatment are familiar, it has also recently been used as a targeting moiety for liposomes to malignant hepatocarcinoma cells which over-express insulin receptors.138 In vitro studies showed a high level of uptake of the insulin modified liposomes in hepatocarcinoma cells. In vivo studies revealed a nearly ten-fold improvement in circulation time. These findings indicate the potential of this system to treat insulin receptor overexpressing cancers.
Growth factors are also a class of small molecules that are of interest for drug targeting applications. One of the main areas of research applying nanocarriers to growth factors has been for delivery across the blood-brain barrier (BBB). Epidermal Growth Factor has been used as a nanoparticle targeting moiety to EGF receptors. They can penetrate the BBB to treat a specific type of brain cancer.139 Nerve Growth Factor (NGF) is necessary for the survival of particular brain neurons which are conspicuously dying when an individual has Alzheimer’s disease. Thus, a carrier is designed to deliver NGF to the brain via poly (butyl cyanoacrylate) (PBCA) nanoparticles which have been shown in clinical trials to be effective.140 Effective transport was shown in a study by Kurakhmaeva et al by observing an increase in NGF inside the mouse brain.141 A study using heparin coated nanoparticles loaded with Vascular Endothelial Growth Factor (VEGF) showed slow release over one month in an in vitro release study.142 VEGF is a cytokine that promotes neo-vascularization which can be very useful in tissue engineering and wound healing.142
Cytokines have shown much potential in vitro, but very few have shown effectiveness in vivo because of related toxicity, but liposomes have the ability to shield and deliver cytokines effectively.143 The most common cytokine for studied for its therapeutic ability is TNF-α, which we have already discussed. Interleukin-2 (IL-2) is another cytokine has been loaded into liposomes effectively and shown to treat malignant tumors better than free IL-2.144 Later studies of IL-2 loaded liposomes in combination with liposomal albumin shows the potential of a better therapeutic ability to treat viral disease and cancer.145 Another liposomal IL-2 potential application is as an influenza vaccine. It was shown to provide 70–90% protection in young adults with a lower 50% protection in the elderly with causing an immune response.146
13. Conclusion
Many nanoparticle and liposomal systems for protein mediated therapy and delivery have been discussed herein. Liposomes are emerging as a potentially revolutionary delivery mechanism that has seen significant success and has an FDA approved formulation. Proteins and enzymes have been utilized for therapeutic treatment in tandem with nanoscale delivery device, many of which have seen increased efficacy due to delivery. The wide variety of protein types and diseases illustrates the versatility and potential therapeutic efficacy of these proteins in combination with nanoparticles for delivery, protection, and stabilization. While much is known about liposomal protein delivery, there is still much to learn in order for them to be broadly applied clinically. We do not claim to have reviewed every protein applied to nanotechnology, for the field is extensive. But we do hope to offer a summary of some of the major works in the field in order to give an idea of what groundbreaking new therapies and technologies are just over the horizon. Future directions of research are bright, with hopes for increased circulation times, enhanced sensitivity to stimuli, better release profiles, better nanomaterials production methods, greater targeting ability. As the body of research grows, the technologies discussed are expected to revolutionize medicine and produce effective therapeutics for the treatment of a variety of diseases.
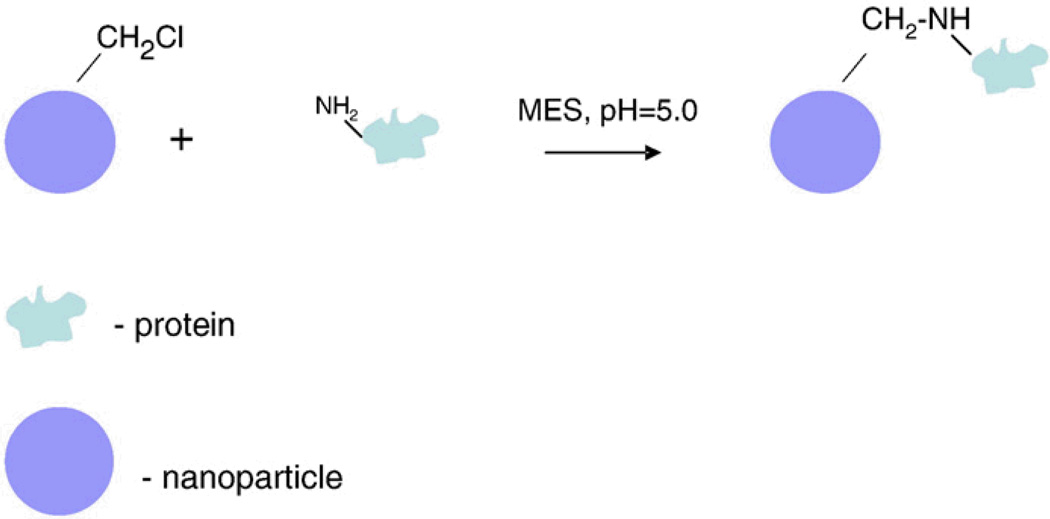
Figure 1.
A simple diagram illustrating a common method of protein immobilization on a nanoparticles surface is shown on the left. A chloromethyl reactive group is attached to the surface of the nanoparticle and this group reacts with amine groups on the surface of the protein resulting in conjugation.
Contributor Information
John N. Barry, Department of Bioengineering, Clemson University, 301 Rhodes Hall, Clemson, SC 29634, United States, johnb@clemson.edu
Alexey A. Vertegel, Department of Bioengineering, Clemson University, 301 Rhodes Hall, Clemson, SC 29634, United States, vertege@clemson.edu
References
- 1.John Fritze UT. Medical expenses have 'very steep rate of growth'. USA Today. 2010 [Google Scholar]
- 2.Centers for Disease Control and Prevention. National Health Interview Survey Public Use Data File 2005. 2006 [Google Scholar]
- 3.Folkman J, Long DM. The use of silicone rubber as a carrier for prolonged drug therapy. J Surg Res. 1964;4:139–142. doi: 10.1016/s0022-4804(64)80040-8. [DOI] [PubMed] [Google Scholar]
- 4.Hoffman AS. The origins and evolution of “controlled” drug delivery systems. J Controlled Release. 2008;132:153–163. doi: 10.1016/j.jconrel.2008.08.012. [DOI] [PubMed] [Google Scholar]
- 5.Gaumet M, Vargas A, Gurny R, Delie F. Nanoparticles for drug delivery: The need for precision in reporting particle size parameters. European Journal of Pharmaceutics and Biopharmaceutics. 2008;69:1–9. doi: 10.1016/j.ejpb.2007.08.001. [DOI] [PubMed] [Google Scholar]
- 6.Nagayasu A, Uchiyama K, Kiwada H. The size of liposomes: A factor which affects their targeting efficiency to tumors and therapeutic activity of liposomal antitumor drugs. Adv Drug Deliv. Rev. 1999;40:75–87. doi: 10.1016/s0169-409x(99)00041-1. [DOI] [PubMed] [Google Scholar]
- 7.Hirano A, Kawanami T, Llena JF. Electron microscopy of the blood-brain barrier in disease. Microsc Res Tech. 1994;27:543–556. doi: 10.1002/jemt.1070270609. [DOI] [PubMed] [Google Scholar]
- 8.Satishkumar R, Vertegel A. Charge-directed targeting of antimicrobial protein-nanoparticle conjugates. Biotechnol Bioeng. 2008;100:403–412. doi: 10.1002/bit.21782. [DOI] [PubMed] [Google Scholar]
- 9.Gregoriadis G, Swain CP, Wills EJ, Tavill AS. Drug-Carrier Potential of Liposomes in Cancer Chemotherapy. The Lancet. 1974;303:1313–1316. doi: 10.1016/s0140-6736(74)90682-5. [DOI] [PubMed] [Google Scholar]
- 10.Klibanov AL, Maruyama K, Torchilin VP, Huang L. Amphipathic polyethyleneglycols effectively prolong the circulation time of liposomes. FEBS Lett. 1990;268:235–237. doi: 10.1016/0014-5793(90)81016-h. [DOI] [PubMed] [Google Scholar]
- 11.Torchilin VP. Recent advances with liposomes as pharmaceutical carriers. Nat Rev Drug Discov. 2005;4:145–160. doi: 10.1038/nrd1632. [DOI] [PubMed] [Google Scholar]
- 12.Allen TM, Hansen C, Martin F, Redemann C, Yau-Young A. Liposomes containing synthetic lipid derivatives of poly(ethylene glycol) show prolonged circulation half-lives in vivo. Biochimica et Biophysica Acta (BBA) - Biomembranes. 1991;1066:29–36. doi: 10.1016/0005-2736(91)90246-5. [DOI] [PubMed] [Google Scholar]
- 13.Senior J, Delgado C, Fisher D, Tilcock C, Gregoriadis G. Influence of surface hydrophilicity of liposomes on their interaction with plasma protein and clearance from the circulation: Studies with poly(ethylene glycol)-coated vesicles. Biochimica et Biophysica Acta (BBA) - Biomembranes. 1991;1062:77–82. doi: 10.1016/0005-2736(91)90337-8. [DOI] [PubMed] [Google Scholar]
- 14.Davis FF. The origin of pegnology. Adv Drug Deliv Rev. 2002;54:457–458. doi: 10.1016/s0169-409x(02)00021-2. [DOI] [PubMed] [Google Scholar]
- 15.Mayer LD, Bally MB, Hope MJ, Cullis PR. Techniques for encapsulating bioactive agents into liposomes. Chem Phys Lipids. 1986;40:333–345. doi: 10.1016/0009-3084(86)90077-0. [DOI] [PubMed] [Google Scholar]
- 16.Barenholz Y, Haran G. Method of Amphipathic Drug Loading in Liposomes by pH Gradient. 1989:413, 037. [Google Scholar]
- 17.Herman EH, Rahman A, Ferrans VJ, Vick JA, Schein PS. Prevention of chronic doxorubicin cardiotoxicity in beagles by liposomal encapsulation. Cancer Research. 1983;43:5427–5432. [PubMed] [Google Scholar]
- 18.Oh Y, Nix D, Straubinger R. Formulation and efficacy of liposome-encapsulated antibiotics for therapy of intracellular mycobacterium avium infection. Antimicrob Agents Chemother. 1995;39:2104–2111. doi: 10.1128/aac.39.9.2104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Haran G, Cohen R, Bar LK, Barenholz Y. Transmembrane ammonium sulfate gradients in liposomes produce efficient and stable entrapment of amphipathic weak bases. Biochimica et Biophysica Acta (BBA) - Biomembranes. 1993;1151:201–215. doi: 10.1016/0005-2736(93)90105-9. [DOI] [PubMed] [Google Scholar]
- 20.Zucker D, Marcus D, Barenholz Y, Goldblum A. Liposome drugs' loading efficiency: A working model based on loading conditions and drug's physicochemical properties. J Controlled Release. 2009;139:73–80. doi: 10.1016/j.jconrel.2009.05.036. [DOI] [PubMed] [Google Scholar]
- 21.Malam Y, Loizidou M, Seifalian AM. Liposomes and nanoparticles: Nanosized vehicles for drug delivery in cancer. Trends Pharmacol Sci. 2009;30:592–599. doi: 10.1016/j.tips.2009.08.004. [DOI] [PubMed] [Google Scholar]
- 22.Oku N, Namba Y, Okada S. Tumor accumulation of novel RES-avoiding liposomes. Biochimica et Biophysica Acta (BBA) - Lipids and Lipid Metabolism. 1992;1126:255–260. doi: 10.1016/0005-2760(92)90238-q. [DOI] [PubMed] [Google Scholar]
- 23.Maeda H. The enhanced permeability and retention (EPR) effect in tumor vasculature: The key role of tumor-selective macromolecular drug targeting. Adv Enzyme Regul. 2001;41:189–207. doi: 10.1016/s0065-2571(00)00013-3. [DOI] [PubMed] [Google Scholar]
- 24.Gordon AN, Tonda M, Sun S, Rackoff W. Long-term survival advantage for women treated with pegylated liposomal doxorubicin compared with topotecan in a phase 3 randomized study of recurrent and refractory epithelial ovarian cancer. Gynecol Oncol. 2004;95:1–8. doi: 10.1016/j.ygyno.2004.07.011. [DOI] [PubMed] [Google Scholar]
- 25.Holkova B, Takeshita K, Cheng DM, et al. Effect of highly active antiretroviral therapy on survival in patients with AIDS-associated pulmonary kaposi's sarcoma treated with chemotherapy. J Clin Oncol. 2001;19:3848–3851. doi: 10.1200/JCO.2001.19.18.3848. [DOI] [PubMed] [Google Scholar]
- 26.Gradishar WJ, Tjulandin S, Davidson N, et al. Phase III trial of nanoparticle albumin-bound paclitaxel compared with polyethylated castor oil-based paclitaxel in women with breast cancer. J Clin Oncol. 2005;23:7794–7803. doi: 10.1200/JCO.2005.04.937. [DOI] [PubMed] [Google Scholar]
- 27.Ibrahim NKN. Multicenter phase II trial of ABI-007, an albumin-bound paclitaxel, in women with metastatic breast cancer. J Clin Oncol. 2005;23:6019–6026. doi: 10.1200/JCO.2005.11.013. [DOI] [PubMed] [Google Scholar]
- 28.Carswell EA, Old LJ, Kassel RL, Green S, Fiore N, Williamson B. An endotoxin-induced serum factor that causes necrosis of tumors. Proceedings of the National Academy of Sciences of the United States of America. 1975;72:3666–3670. doi: 10.1073/pnas.72.9.3666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Paciotti GF, Kingston DG, Tamarkin L. Colloidal gold nanoparticles: A novel nanoparticle platform for developing 19 multifunctional tumor-targeted drug delivery vectors. Drug Dev Res. 2006;67:47–54. [Google Scholar]
- 30.Yuyama Y, Tsujimoto M, Fujimoto Y, Oku N. Potential usage of thermosensitive liposomes for site-specific delivery of cytokines. Cancer Lett. 2000;155:71–77. doi: 10.1016/s0304-3835(00)00410-9. [DOI] [PubMed] [Google Scholar]
- 31.Kim DW, Andres ML, Li J, et al. Liposome-encapsulated tumor necrosis factor-α enhances the effects of radiation against human colon tumor xenografts. Journal of Interferon & Cytokine Research. 2001;21:885–897. doi: 10.1089/107999001753289497. [DOI] [PubMed] [Google Scholar]
- 32.Gaspar MM, Perez-Soler R, Cruz MEM. Biological Characterization of L-Asparaginase Liposomal Formulations. Cancer Chemotherapy and Pharmacology. 1996;38:373–377. doi: 10.1007/s002800050497. [DOI] [PubMed] [Google Scholar]
- 33.Miller DS, Laszlo J, Lyon G, Kelso H, Miller D, McCarty KS. Reduction of Human Blood Asparagine by Hemodialysis. Cancer. 1972;29:1347–1351. doi: 10.1002/1097-0142(197205)29:5<1347::aid-cncr2820290533>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 34.Pisal DS, Kosloski MP, Balu-Iyer SV. 4 Delivery of therapeutic proteins. J Pharm Sci. 2010;99:2557–2575. doi: 10.1002/jps.22054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liguori L, Marques B, Villegas-Mendez A, Rothe R, Lenormand J. Liposomes-mediated delivery of pro-apoptotic therapeutic membrane proteins. J Controlled Release. 2008;126:217–227. doi: 10.1016/j.jconrel.2007.12.004. [DOI] [PubMed] [Google Scholar]
- 36.Kozlowska D, Foran P, MacMahon P, Shelly MJ, Eustace S, O'Kennedy R. Molecular and magnetic resonance imaging: The value of immunoliposomes. Adv Drug Deliv Rev. 2009;61:1402–1411. doi: 10.1016/j.addr.2009.09.003. [DOI] [PubMed] [Google Scholar]
- 37.Ito A, Kuga Y, Honda H, et al. Magnetite nanoparticle-loaded anti-HER2 immunoliposomes for combination of antibody therapy with hyperthermia. Cancer Lett. 2004;212:167–175. doi: 10.1016/j.canlet.2004.03.038. [DOI] [PubMed] [Google Scholar]
- 38.Asahi M, Rammohan T, Sumii T, et al. Antiactin-targeted immunoliposomes ameliorate tissue plasminogen activator-induced hemorrhage after focal embolic stroke. Journal of Cerebral Blood Flow and Metabolism. 2003;23:895–899. doi: 10.1097/01.WCB.0000072570.46552.DF. [DOI] [PubMed] [Google Scholar]
- 39.Gupta B, Torchilin V. Monoclonal Antibody 2C5-Modified Doxorubicin-Loaded Liposomes with significantly Enhanced Therapeutic Activity Against Intracranial Human Brain U-87 MG Tumor Xenografts in Nude Mice. Cancer Immunology, Immunotherapy. 2007;56:1215–1223. doi: 10.1007/s00262-006-0273-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lukyanov AN, Elbayoumi TA, Chakilam AR, Torchilin VP. Tumor-targeted liposomes: Doxorubicin-loaded long-circulating liposomes modified with anti-cancer antibody. J Controlled Release. 2004;100:135–144. doi: 10.1016/j.jconrel.2004.08.007. [DOI] [PubMed] [Google Scholar]
- 41.Park JW, Hong K, Carter P, et al. Development of anti-p185HER2 immunoliposomes for cancer therapy. Proceedings of the National Academy of Sciences of the United States of America. 1995;92:1327–1331. doi: 10.1073/pnas.92.5.1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Park JW, Kirpotin DB, Hong K, et al. Tumor targeting using anti-her2 immunoliposomes. J Controlled Release. 2001;74:95–113. doi: 10.1016/s0168-3659(01)00315-7. [DOI] [PubMed] [Google Scholar]
- 43.Kirpotin DB, Drummond DC, Shao Y, et al. Antibody targeting of long-circulating lipidic nanoparticles does not increase tumor localization but does increase internalization in animal models. Cancer Research. 2006;66:6732–6740. doi: 10.1158/0008-5472.CAN-05-4199. [DOI] [PubMed] [Google Scholar]
- 44.Park JW, Hong K, Kirpotin DB, et al. Anti-HER2 immunoliposomes. Clinical Cancer Research. 2002;8:1172–1181. [PubMed] [Google Scholar]
- 45.Cheng WWK, Allen TM. Targeted delivery of anti-CD19 liposomal doxorubicin in B-cell lymphoma: A comparison of whole monoclonal antibody, fab′ fragments and single chain fv. J Controlled Release. 2008;126:50–58. doi: 10.1016/j.jconrel.2007.11.005. [DOI] [PubMed] [Google Scholar]
- 46.Ishida T, Iden DL, Allen TM. A combinatorial approach to producing sterically stabilized (stealth) immunoliposomal drugs. FEBS Lett. 1999;460:129–133. doi: 10.1016/s0014-5793(99)01320-4. [DOI] [PubMed] [Google Scholar]
- 47.Laginha K, Mumbengegwi D, Allen T. Liposomes targeted via two different antibodies: Assay, B-cell binding and cytotoxicity. Biochimica et Biophysica Acta (BBA) - Biomembranes. 2005;1711:25–32. doi: 10.1016/j.bbamem.2005.02.007. [DOI] [PubMed] [Google Scholar]
- 48.ElBayoumi TA, Torchilin VP. Tumor-targeted nanomedicines: Enhanced antitumor efficacy in vivo of doxorubicin-loaded, long-circulating liposomes modified with cancer-specific monoclonal antibody. Clinical Cancer Research. 2009;15:1973–1980. doi: 10.1158/1078-0432.CCR-08-2392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Iakoubov L, Rokhlin O, Torchilin V. Anti-nuclear autoantibodies of the aged reactive against the surface of tumor but not normal cells. Immunol Lett. 1995;47:147–149. doi: 10.1016/0165-2478(95)00066-e. [DOI] [PubMed] [Google Scholar]
- 50.Tada H, Higuchi H, Wanatabe TM, Ohuchi N. In vivo real-time tracking of single quantum dots conjugated with monoclonal anti- HER2 antibody in tumors of mice. Cancer Research. 2007;67:1138–1144. doi: 10.1158/0008-5472.CAN-06-1185. [DOI] [PubMed] [Google Scholar]
- 51.Jayagopal A, Russ PK, Haselton FR. Surface engineering of quantum dots for in vivo vascular imaging. Bioconjug Chem. 2007;18:1424–1433. doi: 10.1021/bc070020r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gao J, Chen X, Cheng Z. Near-infrared quantum dots as optical probes for tumor imaging. Current Topics in Medicinal Chemistry. 2010;10:1147–1157. doi: 10.2174/156802610791384162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gao X, Cui Y, Levenson RM, Chung LWK, Nie S. In vivo cancer targeting and imaging with semiconductor quantum dots. Nat Biotech. 2004;22:969–976. doi: 10.1038/nbt994. [DOI] [PubMed] [Google Scholar]
- 54.Huang X, Jain PK, El-Sayed IH, El-Sayed MA. Determination of the minimum temperature required for selective photothermal destruction of cancer cells with the use of immunotargeted gold nanoparticles. Photochem Photobiol. 2006;82:412–417. doi: 10.1562/2005-12-14-RA-754. [DOI] [PubMed] [Google Scholar]
- 55.Koh I, Wang X, Varughese B, Isaacs L, Ehrman SH, English DS. Magnetic iron oxide nanoparticles for biorecognition: evaluation of surface coverage and activity. The Journal of Physical Chemistry B. 2006;110:1553–1558. doi: 10.1021/jp0556310. [DOI] [PubMed] [Google Scholar]
- 56.Ito A, Shinkai M, Honda H, Kobayashi T. Medical application of functionalized magnetic nanoparticles. Journal of Bioscience and Bioengineering. 2005;100:1–11. doi: 10.1263/jbb.100.1. [DOI] [PubMed] [Google Scholar]
- 57.Gabizon A, Shmeeda H, Zalipsky S. Pros and cons of the liposome platform in cancer drug targeting*. J Liposome Res. 2006;16:175–183. doi: 10.1080/08982100600848769. [DOI] [PubMed] [Google Scholar]
- 58.Ruenraroengsak P, Cook JM, Florence AT. Nanosystem drug targeting: Facing up to complex realities. J Controlled Release. 2010;141:265–276. doi: 10.1016/j.jconrel.2009.10.032. [DOI] [PubMed] [Google Scholar]
- 59.Bae YH, Park K. Targeted drug delivery to tumors: Myths, reality and possibility. J Controlled Release. 2011;153:198–205. doi: 10.1016/j.jconrel.2011.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kwon IK, Lee SC, Han B, Park K. Analysis on the current status of targeted drug delivery to tumors. J Controlled Release. 2012;164:108–114. doi: 10.1016/j.jconrel.2012.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kirpotin DB, Drummond DC, Shao Y, et al. Antibody targeting of long-circulating lipidic nanoparticles does not increase tumor localization but does increase internalization in animal models. Cancer Research. 2006;66:6732–6740. doi: 10.1158/0008-5472.CAN-05-4199. [DOI] [PubMed] [Google Scholar]
- 62.Lee P. Treatment of Central Precocious Puberty with Depot Lupron. New York; New York: Raven Press; 1993. [Google Scholar]
- 63.Dlugi A, Miller J, Knittle J. Lupron depot (leuprolide acetate for depot suspension) in the treatment of endometriosis - a randomized, placebo-controlled, double-blind-study. Fertil Steril. 1990;54:419–427. doi: 10.1016/s0015-0282(16)53755-8. [DOI] [PubMed] [Google Scholar]
- 64.Westphal M, Hilt DC, Bortey E, et al. A phase 3 trial of local chemotherapy with biodegradable carmustine (BCNU) wafers (gliadel wafers) in patients with primary malignant glioma. Neuro-oncology. 2003 Apr;5:79–88. doi: 10.1215/S1522-8517-02-00023-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Westphal M, Ram Z, Riddle V, Hilt D, Bortey E. Executive Comm Gliadel Study Grp. Gliadel (R) wafer in initial surgery for malignant glioma: Long-term follow-up of a multicenter controlled trial. Acta Neurochir. 2006;148:269–275. doi: 10.1007/s00701-005-0707-z. [DOI] [PubMed] [Google Scholar]
- 66.Tice T. Delivering With Depot Formulations. [Accessed Jan. 29, 2013];Drug Development & Delivery, Drug Development and Delivery. 2004 < http://www.drugdeliverytech.com/ME2/dirmod.asp?sid=&nm=&type=Publishing&mod=Publications%3A%3AArticle&mid=8F3A7027421841978F18BE895F87F791&tier=4&id=281C4870DD59497489C9203 05CCF1695>. [Google Scholar]
- 67.Barenholz Y. Liposome application: Problems and prospects. Current Opinion in Colloid & Interface Science. 2001;6:66–77. [Google Scholar]
- 68.Shearer H, Ellis MJ, Perera SP, Chaudhuri JB. Effects of common sterilization methods on the structure and properties of poly(D,L lactic-co-glycolic acid) scaffolds. Tissue Eng. 2006;12:2717–2727. doi: 10.1089/ten.2006.12.2717. [DOI] [PubMed] [Google Scholar]
- 69.Igartua M, Hernandez RM, Eduardo Rosas J, Elkin Patarroyo M, Luis Pedraz J. Gamma-irradiation effects on biopharmaceutical properties of PLGA microspheres loaded with SPf66 synthetic vaccine. Eur J Pharm Biopharm. 2008;69:519–526. doi: 10.1016/j.ejpb.2007.12.014. [DOI] [PubMed] [Google Scholar]
- 70.Lue J, Wang X, Marin-Muller C, et al. Current advances in research and clinical applications of PLGA-based nanotechnology. Expert Rev Mol Diagn. 2009;9:325–341. doi: 10.1586/erm.09.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Crommelin DJA, Park K, Florence A. Pharmaceutical nanotechnology: Unmet needs in drug delivery. J Controlled Release. 2010;141:263–264. doi: 10.1016/j.jconrel.2009.11.019. [DOI] [PubMed] [Google Scholar]
- 72.Li X, Ding L, Xu Y, Wang Y, Ping Q. Targeted delivery of doxorubicin using stealth liposomes modified with transferrin. Int J Pharm. 2009;373:116–123. doi: 10.1016/j.ijpharm.2009.01.023. [DOI] [PubMed] [Google Scholar]
- 73.Sahoo SK, Ma W, Labhasetwar V. Efficacy of transferrin-conjugated paclitaxel-loaded nanoparticles in a murine model of prostate cancer. International Journal of Cancer. 2004;112:335–340. doi: 10.1002/ijc.20405. [DOI] [PubMed] [Google Scholar]
- 74.Li HH. Transferrin/transferrin receptor-mediated drug delivery. Med Res Rev. 2002;22:225–250. doi: 10.1002/med.10008. [DOI] [PubMed] [Google Scholar]
- 75.Visser CC, Stevanović S, Voorwinden LH, et al. Targeting liposomes with protein drugs to the blood–brain barrier in vitro. European Journal of Pharmaceutical Sciences. 2005;25:299–305. doi: 10.1016/j.ejps.2005.03.008. [DOI] [PubMed] [Google Scholar]
- 76.Wagner E, Curiel D, Cotten M. Delivery of drugs, proteins and genes into cells using transferrin as a ligand for receptor-mediated endocytosis. Adv Drug Deliv Rev. 1994;14:113–135. [Google Scholar]
- 77.Qian ZM, Li H, Sun H, Ho K. Targeted drug delivery via the transferrin receptor-mediated endocytosis pathway. Pharmacological Reviews. 2002;54:561–587. doi: 10.1124/pr.54.4.561. [DOI] [PubMed] [Google Scholar]
- 78.Huang R, Ke W, Liu Y, Jiang C, Pei Y. The use of lactoferrin as a ligand for targeting the polyamidoamine-based gene delivery system to the brain. Biomaterials. 2008;29:238–246. doi: 10.1016/j.biomaterials.2007.09.024. [DOI] [PubMed] [Google Scholar]
- 79.Huang RR. Neuroprotection in a 6-hydroxydopamine-lesioned parkinson model using lactoferrin-modified nanoparticles. J Gene Med. 2009;11:754–763. doi: 10.1002/jgm.1361. [DOI] [PubMed] [Google Scholar]
- 80.Merdan T, Kopecek J, Kissel T. Prospects for cationic polymers in gene and oligonucleotide therapy against cancer. Adv Drug Deliv Rev. 2002;54:715–758. doi: 10.1016/s0169-409x(02)00046-7. [DOI] [PubMed] [Google Scholar]
- 81.Schroeder A, Levins CG, Cortez C, Langer R, Anderson DG. Lipid-based nanotherapeutics for siRNA delivery. J Intern Med. 2010;267:9–21. doi: 10.1111/j.1365-2796.2009.02189.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Panyam J, Labhasetwar V. Biodegradable nanoparticles for drug and gene delivery to cells and tissue. Adv Drug Deliv Rev. 2003;55:329–347. doi: 10.1016/s0169-409x(02)00228-4. [DOI] [PubMed] [Google Scholar]
- 83.Kratz F. Albumin as a drug carrier: Design of prodrugs, drug conjugates and nanoparticles. J Controlled Release. 2008;132:171–183. doi: 10.1016/j.jconrel.2008.05.010. [DOI] [PubMed] [Google Scholar]
- 84.Micha JP, Goldstein BH, Birk CL, Rettenmaier MA, Brown JV., III Abraxane in the treatment of ovarian cancer: The absence of hypersensitivity reactions. Gynecol Oncol. 2006;100:437–438. doi: 10.1016/j.ygyno.2005.09.012. [DOI] [PubMed] [Google Scholar]
- 85.Green MR, Manikhas GM, Orlov S, et al. Abraxane®, a novel cremophor®-free, albumin-bound 23 particle form of paclitaxel for the treatment of advanced non-small-cell lung cancer. Annals of Oncology. 2006 Aug;17:1263–1268. doi: 10.1093/annonc/mdl104. [DOI] [PubMed] [Google Scholar]
- 86.Hawkins MJ, Soon-Shiong P, Desai N. Protein nanoparticles as drug carriers in clinical medicine. Adv Drug Deliv Rev. 2008;60:876–885. doi: 10.1016/j.addr.2007.08.044. [DOI] [PubMed] [Google Scholar]
- 87.Gradishar WJ, Tjulandin S, Davidson N, et al. Phase III trial of nanoparticle albumin-bound paclitaxel compared with polyethylated castor Oil–Based paclitaxel in women with breast cancer. Journal of Clinical Oncology. 2005;23:7794–7803. doi: 10.1200/JCO.2005.04.937. [DOI] [PubMed] [Google Scholar]
- 88.Torchilin VP. Multifunctional nanocarriers. Adv Drug Deliv Rev. 2006;58:1532–1555. doi: 10.1016/j.addr.2006.09.009. [DOI] [PubMed] [Google Scholar]
- 89.Phillips WT, Klipper RW, Awasthi VD, et al. Polyethylene glycol-modified liposome-encapsulated hemoglobin: A long circulating red cell substitute. Journal of Pharmacology and Experimental Therapeutics. 1999;288:665–670. [PubMed] [Google Scholar]
- 90.Awasthi VD, Garcia D, Klipper R, Goins BA, Phillips WT. Neutral and anionic liposome-encapsulated hemoglobin: Effect of postinserted poly(ethylene glycol)-distearoylphosphatidylethanolamin e on distribution and circulation kinetics. Journal of Pharmacology and Experimental Therapeutics. 2004;309:241–248. doi: 10.1124/jpet.103.060228. [DOI] [PubMed] [Google Scholar]
- 91.Sakai H, Masada Y, Horinouchi H, et al. Hemoglobin-vesicles suspended in recombinant human serum albumin for resuscitation from hemorrhagic shock in anesthetized rats. Critical Care Medicine. 2004;32:539–545. doi: 10.1097/01.CCM.0000109774.99665.22. [DOI] [PubMed] [Google Scholar]
- 92.Sakai H, Sou K, Tsuchida E. Methods in Enyzmology Liposomes, Pt G. 525 B Street, Suite 1900, San Diego, CA 92101-4495 USA: Elsevier Academic Press Inc; 2009. Hemoglobin-Vesicles as an Artificial Oxygen Carrier; pp. 363–384. [DOI] [PubMed] [Google Scholar]
- 93.Sakai H, Tomiyama K, Sou K, Takeoka S, Tsuchida E. Poly(ethylene glycol)-conjugation and deoxygenation enable long-term preservation of hemoglobin-vesicles as oxygen carriers in a liquid state. Bioconjug Chem. 2000;11:425–432. doi: 10.1021/bc990173h. [DOI] [PubMed] [Google Scholar]
- 94.Shibuya-Fujiwara N, Hirayama F, Ogata Y, Ikeda H, Ikebuchi K. Phagocytosis in vitro of polyethylene glycol-modified liposome-encapsulated hemoglobin by human peripheral blood monocytes plus macrophages through scavenger receptors. Life Sci. 2001;70:291–300. doi: 10.1016/s0024-3205(01)01392-3. [DOI] [PubMed] [Google Scholar]
- 95.Finkel T, Holbrook NJ. Oxidants, oxidative stress and the biology of ageing. Nature. 2000;408:239–247. doi: 10.1038/35041687. [DOI] [PubMed] [Google Scholar]
- 96.Cuzzocrea S, Riley DP, Caputi AP, Salvemini D. Antioxidant therapy: A new pharmacological approach in shock, inflammation, and Ischemia/Reperfusion injury. Pharmacological Reviews. 2001;53:135–159. [PubMed] [Google Scholar]
- 97.Imaizumi S, Woolworth V, Fishman R, Chan P. Liposome-entrapped superoxide dismutase reduces cerebral infarction in cerebral ischemia in rats. Stroke. 1990;21:1312–1317. doi: 10.1161/01.str.21.9.1312. [DOI] [PubMed] [Google Scholar]
- 98.MatÉs JM, Pérez-Gómez C, De Castro IN. Antioxidant enzymes 24 and human diseases. Clin Biochem. 1999;32:595–603. doi: 10.1016/s0009-9120(99)00075-2. [DOI] [PubMed] [Google Scholar]
- 99.Yasui K, Baba A. Therapeutic potential of superoxide dismutase (SOD) for resolution of inflammation. Inflammation Res. 2006;55:359–363. doi: 10.1007/s00011-006-5195-y. [DOI] [PubMed] [Google Scholar]
- 100.Stanimirovic DB, Markovic M, Micic DV, Spatz M, Mrsulja BB. Liposome-entrapped superoxide dismutase reduces ischemia/reperfusion ‘oxidative stress’ in gerbil brain. Neurochem Res. 1994;19:1473–1478. doi: 10.1007/BF00968993. [DOI] [PubMed] [Google Scholar]
- 101.Reddy MK, Labhasetwar V. Nanoparticle-mediated delivery of superoxide dismutase to the brain: An effective strategy to reduce ischemia-reperfusion injury. FASEB J. 2009;23:1384–1395. doi: 10.1096/fj.08-116947. [DOI] [PubMed] [Google Scholar]
- 102.Yun X, et al. Nanoparticles for targeted delivery of antioxidant enzymes to the brain after cerebral ischemia and reperfusion injury. J Cereb Blood Flow Metab. 2013;33(4):583–592. doi: 10.1038/jcbfm.2012.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Reddy M, Wu L, Kou W, Ghorpade A, Labhasetwar V. Superoxide Dismutase-Loaded PLGA Nanoparticles Protect Cultured Human Neurons Under Oxidative Stress. Applied Biochemistry and Biotechnology. 2008;151:565–577. doi: 10.1007/s12010-008-8232-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Vozenin-Brotons M, Sivan V, Gault N, et al. Antifibrotic action of Cu/Zn SOD is mediated by TGF-β1 repression and phenotypic reversion of myofibroblasts. Free Radical Biology and Medicine. 2001;30:30–42. doi: 10.1016/s0891-5849(00)00431-7. [DOI] [PubMed] [Google Scholar]
- 105.Maximov V, Reukov V, Vertegel A. Targeted delivery of therapeutic enzymes. Journal of Drug Delivery Science and Technology. 2009;19:311–320. [Google Scholar]
- 106.Rengel RG, Filipović-Grčić J, Čepelak I, Žanić-Grubišić T, Barišić K. The effect of liposomes with superoxide dismutase on A2182 cells. European Journal of Pharmaceutics and Biopharmaceutics. 2005;60:47–51. doi: 10.1016/j.ejpb.2004.12.002. [DOI] [PubMed] [Google Scholar]
- 107.Ghio AJ, Suliman HB, Carter JD, Abushamaa AM, Folz RJ. Overexpression of extracellular superoxide dismutase decreases lung injury after exposure to oil fly ash. Am J Physiol Lung Cell Mol Physiol. 2002;283:L211–L218. doi: 10.1152/ajplung.00409.2001. [DOI] [PubMed] [Google Scholar]
- 108.Nagami H, Yoshimoto N, Umakoshi H, Shimanouchi T, Kuboi R. Liposome-assisted activity of superoxide dismutase under oxidative stress. Journal of Bioscience and Bioengineering. 2005;99:423–428. doi: 10.1263/jbb.99.423. [DOI] [PubMed] [Google Scholar]
- 109.Lo Y, Tsai J, Kuo J. Liposomes and disaccharides as carriers in spray-dried powder formulations of superoxide dismutase. J Controlled Release. 2004;94:259–272. doi: 10.1016/j.jconrel.2003.09.019. [DOI] [PubMed] [Google Scholar]
- 110.Valko M, Leibfritz D, Moncol J, Cronin MTD, Mazur M, Telser J. Free radicals and antioxidants in normal physiological functions and human disease. Int J Biochem Cell Biol. 2007;39:44–84. doi: 10.1016/j.biocel.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 111.Turrens JF, Crapo JD, Freeman BA. Protection against oxygen toxicity by intravenous injection of liposome-entrapped catalase and superoxide dismutase. J Clin Invest. 1984;73:87–95. doi: 10.1172/JCI111210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Jubeh TT, Nadler-Milbauer M, Barenholz Y, Rubinstein A. Local treatment of experimental colitis in the rat by negatively charged liposomes of catalase, TMN and SOD. J Drug Target. 2006;14:155–163. doi: 10.1080/10611860600648429. [DOI] [PubMed] [Google Scholar]
- 113.Yusa T, Crapo JD, Freeman BA. Liposome-mediated augmentation of brain SOD and catalase inhibits CNS O2 toxicity. J Appl Physiol. 1984;57:1674–1681. doi: 10.1152/jappl.1984.57.6.1674. [DOI] [PubMed] [Google Scholar]
- 114.Yoshimoto M, Sakamoto H, Yoshimoto N, Kuboi R, Nakao K. Stabilization of quaternary structure and activity of bovine liver catalase through encapsulation in liposomes. Enzyme Microb Technol. 2007;41:849–858. [Google Scholar]
- 115.Chorny M, Hood E, Levy RJ, Muzykantov VR. Endothelial delivery of antioxidant enzymes loaded into non-polymeric magnetic nanoparticles. J Controlled Release. 2010;146:144–151. doi: 10.1016/j.jconrel.2010.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Muro S, Cui X, Gajewski C, Murciano J, Muzykantov VR, Koval M. Slow intracellular trafficking of catalase nanoparticles targeted to ICAM-1 protects endothelial cells from oxidative stress. Am J Physiol Cell Physiol. 2003;285:C1339–C1347. doi: 10.1152/ajpcell.00099.2003. [DOI] [PubMed] [Google Scholar]
- 117.Muro S, Muzykantov VR. Targeting of antioxidant and antithrombotic drugs to endothelial cell adhesion molecules. Curr Pharm Des. 2005;11:2383–2401. doi: 10.2174/1381612054367274. [DOI] [PubMed] [Google Scholar]
- 118.Muzykantov VR. Targeting of superoxide dismutase and catalase to vascular endothelium. J Controlled Release. 2001;71:1–21. doi: 10.1016/s0168-3659(01)00215-2. [DOI] [PubMed] [Google Scholar]
- 119.Weintraub MI. Thrombolysis (tissue plasminogen activator) in stroke: A medicolegal quagmire. Stroke. 2006;37:1917–1922. doi: 10.1161/01.STR.0000226651.04862.da. [DOI] [PubMed] [Google Scholar]
- 120.Yurko Y, Maximov V, Andreozzi E, Thompson GL, Vertegel AA. Design of biomedical nanodevices for dissolution of blood clots. Materials Science and Engineering: C. 2009;29:737–741. [Google Scholar]
- 121.Collen D. Fibrin-selective thrombolytic therapy for acute myocardial infarction. Circulation. 1996;93:857–865. doi: 10.1161/01.cir.93.5.857. [DOI] [PubMed] [Google Scholar]
- 122.Shaw GJ, Meunier JM, Huang S, Lindsell CJ, McPherson DD, Holland CK. Ultrasound-enhanced thrombolysis with tPA-loaded echogenic liposomes. Thromb Res. 2009;124:306–310. doi: 10.1016/j.thromres.2009.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Haber E, Quertermous T, Matsueda G, Runge M. Innovative approaches to plasminogen activator therapy. Science. 1989;243:51–56. doi: 10.1126/science.2492113. [DOI] [PubMed] [Google Scholar]
- 124.Weitz J, Stewart R, Fredenburgh J. Mechanism of action of plasminogen activators. Thrombosis and. Haemostasis. 1999;82:974–982. [PubMed] [Google Scholar]
- 125.Huber K, Runge M, Bode C, Gulba D. Thrombolytic therapy in acute myocardial infarction - update 1996. Annals of Hematology. 1996;73:S29–S38. [PubMed] [Google Scholar]
- 126.Heeremans J, Prevost R, Bekkers M, et al. Thrombolytic Treatment with Tissue Type Plasminogen-Activator (t-PA) Containing Liposomes in Rabbits - A Comparison with Gree t-PA and. Haemostasis. 1995;73:488–494. [PubMed] [Google Scholar]
- 127.Ma Y, Wu S, Wu T, Chang Y, Hua M, Chen J. Magnetically targeted thrombolysis with recombinant tissue plasminogen activator bound to polyacrylic acid-coated nanoparticles. Biomaterials. 2009;30:3343–3351. doi: 10.1016/j.biomaterials.2009.02.034. [DOI] [PubMed] [Google Scholar]
- 128.Chung T, Wang S, Tsai W. Accelerating thrombolysis with chitosan-coated plasminogen activators encapsulated in poly-(lactide-co-glycolide) (PLGA) nanoparticles. Biomaterials. 2008;29:228–237. doi: 10.1016/j.biomaterials.2007.09.027. [DOI] [PubMed] [Google Scholar]
- 129.Tiukinhoy-Laing SD, Huang S, Klegerman M, Holland CK, McPherson DD. Ultrasound-facilitated thrombolysis using tissue-plasminogen activator-loaded echogenic liposomes. Thromb Res. 2007;119:777–784. doi: 10.1016/j.thromres.2006.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Smith DAB, Vaidya SS, Kopechek JA, et al. Ultrasound-triggered release of recombinant tissue-type plasminogen activator from echogenic liposomes. Ultrasound Med Biol. 2010;36:145–157. doi: 10.1016/j.ultrasmedbio.2009.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Marsh JJ. Fibrin-targeted perfluorocarbon nanoparticles for targeted thrombolysis. Nanomedicine (London, England) 2007;2:533–543. doi: 10.2217/17435889.2.4.533. [DOI] [PubMed] [Google Scholar]
- 132.Erdoğan SS. Thrombus localization by using streptokinase containing vesicular systems. Drug Deliv. 2006;13:303–309. doi: 10.1080/10717540600559544. [DOI] [PubMed] [Google Scholar]
- 133.Elbayoumi TA, Torchilin VP. Liposomes for targeted delivery of antithrombotic drugs. Expert Opinion on Drug Delivery. 2008;5:1185–1198. doi: 10.1517/17425240802497457. [DOI] [PubMed] [Google Scholar]
- 134.Huang Y, Wang C. Pulmonary delivery of insulin by liposomal carriers. J Controlled Release. 2006;113:9–14. doi: 10.1016/j.jconrel.2006.03.014. [DOI] [PubMed] [Google Scholar]
- 135.Chono S, Fukuchi R, Seki T, Morimoto K. Aerosolized liposomes with dipalmitoyl phosphatidylcholine enhance pulmonary insulin delivery. J Controlled Release. 2009;137:104–109. doi: 10.1016/j.jconrel.2009.03.019. [DOI] [PubMed] [Google Scholar]
- 136.Jo S, Lee H, Kim J. Glucose-sensitive liposomes incorporating hydrophobically modified glucose oxidase. Lipids. 2008;43:937–943. doi: 10.1007/s11745-008-3223-0. [DOI] [PubMed] [Google Scholar]
- 137.Jin-Chul K, Seong-Min J, Hyang-Hee J, Jae-Hyung C, Mi-Ran H. Development of self regulated insulin delivery system: Glucose sensitive liposome. J Biotechnol. 2007;131:S49–S50. [Google Scholar]
- 138.Jo S, Lee HY, Kim J. Glucose-sensitivity of liposomes incorporating conjugates of glucose oxidase and poly(N-isopropylacrylamide-co-methacrylic acid-co-octadecylacrylate) Int J Biol Macromol. 2009;45:421–426. doi: 10.1016/j.ijbiomac.2009.06.008. [DOI] [PubMed] [Google Scholar]
- 139.Yang ZZ. Preparation and characterization of zedoary turmeric oil–loaded insulin-modified sterically stabilized liposomes. J Liposome Res. 2010;20:9–15. doi: 10.3109/08982100903015017. [DOI] [PubMed] [Google Scholar]
- 140.Tsutsui Y, Tomizawa K, Nagita M, et al. Development of bionanocapsules targeting brain tumors. J Controlled Release. 2007;122:159–164. doi: 10.1016/j.jconrel.2007.06.019. [DOI] [PubMed] [Google Scholar]
- 141.Zhou Q, Sun X, Zeng L, Liu J, Zhang Z. A randomized multicenter phase II clinical trial of mitoxantrone-loaded nanoparticles in the treatment of 108 patients 27 with unresected hepatocellular carcinoma. Nanomedicine: Nanotechnology, Biology and Medicine. 2009;5:419–423. doi: 10.1016/j.nano.2009.01.009. [DOI] [PubMed] [Google Scholar]
- 142.Kurakhmaeva KBK. Brain targeting of nerve growth factor using poly(butyl cyanoacrylate) nanoparticles. J Drug Target. 2009;17:564–574. doi: 10.1080/10611860903112842. [DOI] [PubMed] [Google Scholar]
- 143.Chung Y, Tae G, Hong Yuk S. A facile method to prepare heparin-functionalized nanoparticles for controlled release of growth factors. Biomaterials. 2006;27:2621–2626. doi: 10.1016/j.biomaterials.2005.11.043. [DOI] [PubMed] [Google Scholar]
- 144.ten Hagen TLM. Liposomal Cytokines in the Treatment of Infectious Diseases and Cancer. In: Nejat Duzgunes., editor. Methods in Enzymology. Academic Press; 2005. pp. 125–145. [DOI] [PubMed] [Google Scholar]
- 145.Kedar E, Rutkowski Y, Braun E, Emanuel N, Barenholz Y. Delivery of Cytokines by Liposomes. 1. Preparation and Characterization of Interleukin-2 Encapsulated in Long-Circulating Sterically Stabilized Lipsomes. Journal of Immunotherapy. 1994;16:47–59. doi: 10.1097/00002371-199407000-00005. [DOI] [PubMed] [Google Scholar]
- 146.Neville ME, Boni LT, Pflug LE, Popescu MC, Robb RJ. Biopharmaceutics of Liposomal Interleukin-2, Oncolipin. Cytokine. 2000;12:1691–1701. doi: 10.1006/cyto.2000.0769. [DOI] [PubMed] [Google Scholar]
- 147.Ben-Yehuda A, Joseph A, Barenholz Y, et al. Immunogenicity and safety of a novel IL-2-supplemented liposomal influenza vaccine (INFLUSOME-VAC) in nursing-home residents. Vaccine. 2003;21:3169–3178. doi: 10.1016/s0264-410x(03)00251-2. [DOI] [PubMed] [Google Scholar]