Abstract
This paper reports the design, synthesis and characterization of thin films as a platform for studying the separate influences of physical and chemical cues of a matrix on the adhesion, growth and final phenotype of cells. Independent control of the physical and chemical properties of functionalized, swellable hydrogel thin films was achieved using initiated Chemical Vapor Deposition (iCVD). The systematic variation in crosslink density is demonstrated to control the swelling ability of the iCVD hydrogel films based on 2-hydroxyethyl methacrylate (HEMA). At the same time, the incorporation of controllable concentrations of the active ester pentafluorophenyl methacrylate (PFM) allows easy immobilization of aminated bioactive motifs, such as bioactive peptides. Initial cell culture results with Human Umbilical Vein Endothelial Cells (HUVEC) indicated that the strategy of using PFM to immobilize a cell-adhesion peptide motif onto the hydrogel layers promotes proper HUVEC growth and enhances their phenotype.
Keywords: iCVD, functionalized hydrogel, cell signalling
1. Introduction
In living tissues, cells are subjected to a high variety of physiological signaling, both chemical and mechanical. With regards to the chemical signaling, cells communicate with one another by synthesizing and usually secreting molecules (ligands) to the extracellular matrix (ECM), basically proteins of the ECM and growth factors, which are recognized by a specific cellular receptor. This recognition ligand-receptor causes a cascade of intracellular signals that alters cell behavior. Nowadays, biomimetic matrices are increasingly designed to provide the environment with these chemical signals.[1] One strategy could be the development of matrices able to release growth factors that would promote specific biological processes; for instance, the on time supply of a growth factor, such as EGF (epithelial growth factor), that induces cell motility on certain cells. Another strategy could be the functionalization of the artificial matrix with signaling molecules that would be recognized by receptors, which helps to the physical interaction between the cell and the material. One classical example is the matrix modification with the canonical RGD peptide motif from fibronectin, which is well known to promote cellular adhesion since 1984.[2]
However, along with the chemical signals, the mechanical forces are critical for many tissues, since they are constantly suffering tension, shear, loading, etc.[3] Essentially, the cell signaling exerted by forces is transduced through receptors that are in intimate contact with the matrix. Therefore, the main consequence of this receptor-matrix interaction is that cells and matrix are mechanically coupled, so that matrix deformation is considered the main cause of the mechanical signaling. By mimicking these mechanical forces in tissue engineered constructs, it would be possible to obtain more physiological environments and thus a more physiological cell response.
Hence, after many decades of focusing research on chemical signaling, including soluble growth factors and insoluble matrix components, now mechanical interactions are known also to have a central role in tissue development, remodeling as well as degeneration. The tension exerted by cells when pulling and pushing the environment and the ability of the ECM to balance this stress depends on the inherent properties of the matrix and specifically on its stiffness. In general, a relatively stiff substrate will resist cellular forces more than a soft one, causing the cell to spread better on the surface and grow; while softer matrices promote cell retraction, turn off growth and could switch on differentiation.[4,5] Actually, several studies in the last decade reported distinct cell behaviors on stiff and soft substrates. Fibroblasts and epithelial cells, for instance, were observed to spread better on stiff substrates than on softer ones, where they used to adopt a more spherical shape.[6] On the contrary, neurons appear to survive better on soft surfaces, as they are able to develop more extensive and branched neurites on them than on rigid ones.[7] Therefore, it is increasingly clear that substrate stiffness plays an essential role in cell behavior and the effects are so important that it is even possible to distinguish between cell phenotypes depending on the compliance of the surface; for example, cell growth on soft agar gels is used to identify cancer cells.[8] Furthermore, Engler et al. proved that matrix stiffness is able to drive mesenchymal stem cell differentiation in a more selective way than the soluble induction factors: soft gels that mimic brain induce neurogenic differentiation, stiffer ones that mimic muscle lead to myocytes and more rigid matrices that mimic bone are osteogenic.[9]
Several systems in two and three dimensions have been developed in order to study the specific effects of the physical properties of the matrix on different cell functions. Studies in 3D matrices frequently use hydrogels based on natural proteins, such as fibrin, collagen and other more complex gels such as Matrigel™ (a commercial gelatinous protein mixture)[10], algae-derived polysaccharides such as alginate and agarose[11], synthetic bioengineered peptides such as self-assembling peptide hydrogels[12-14]. Their compliance may be controlled basically by adjusting the polymer mass,[15] but also the addition of other factors such as fibronectin into collagen gels, salts into fibrin and calcium ions into alginate may finely tailor the material stiffness[11]. The same hydrogels can also be gelled forming flat surfaces to perform simple experiments in 2D, that are easier to interpret but are less comparable with the in vivo situation, which in general operates in 3D. However, 2D substrates made of synthetic polymers like polyacrylamides present very interesting properties. On one hand, their crosslink degree and thus their stiffness can be easily tuned with the amount of crosslink agent. On the other hand, these polymers, unlike natural matrices, are chemically stable and are considered not to adhere proteins of the serum, so that the mechanical signals can be separated from the chemical ones. Nevertheless, the inertness of these materials might turn into an inconvenience, as it is harder to covalently attach molecules to act as ligands for cells.[16] So far, these polymers have been used with a layer of collagen deposited on it in order to promote cell adhesion.[6,9,17]
So if the mechanical properties of a substrate could be modified in a controlled manner, it is expected that the mechanical modulation of the cellular behavior may be influenced. Thus, developing a platform where the crosslink density and swelling may be easily tailored could become a powerful tool. It has already been documented that these changes of molecular structure lead to changes of the stiffness of the material,[18] which could allow to study, systematically, the effect of mechanical signaling on cell behavior. However, since cells also respond to chemical stimuli, a question arises at the moment: is it possible to study the chemical and mechanical signals independently? It is not an easy problem to solve. On one hand, few biocompatible materials enable to undergo systematic changes of their stiffness or chemical signaling. On the other hand, the techniques to evaluate these properties in aqueous media are still under development,[19-22] although some interesting results have been recently described by Van Vliet et al.[23]
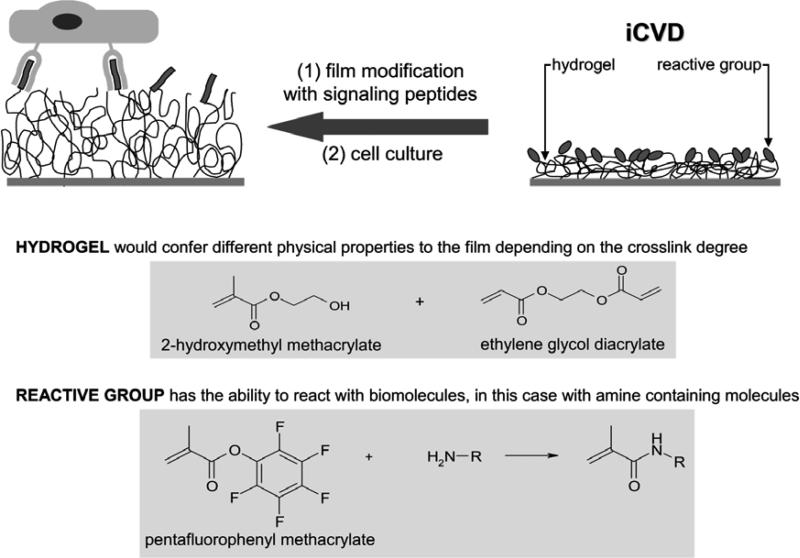
Our group have worked for many years to develop functional thin films by initiated Chemical Vapor Deposition iCVD.[24-27] We have already described in a previous paper the use of iCVD to produce 2-hydroxymethyl methacrylate (HEMA) based hydrogels with tunable swelling properties depending on the amount of incorporated crosslinking agent.[18] Also we have described the use of pentafluophenyl methacrylate (PFM) as reactive monomer for attaching biomolecules through nucleophilic substitution.[26,28,29] Combining both ideas, here we report the development of a new platform that combines a polymeric hydrogel thin film, with controlled crosslink degree and swelling ability, with a reactive molecule (PFM) for the potential functionalization of the material with specific biologically active motifs (see Fig. 1).
Figure 1. General strategy.
iCVD polymerization of a biomimetic surface which combines a hydrogel, with tunable crosslink densities and swelling ability, with a monomer that allows aminated-molecules attachment.
2. Results and discussion
2.1. Initial exploratory approach: iCVD polymerization of the ternary system HEMA-co-EGDA-co-PFM
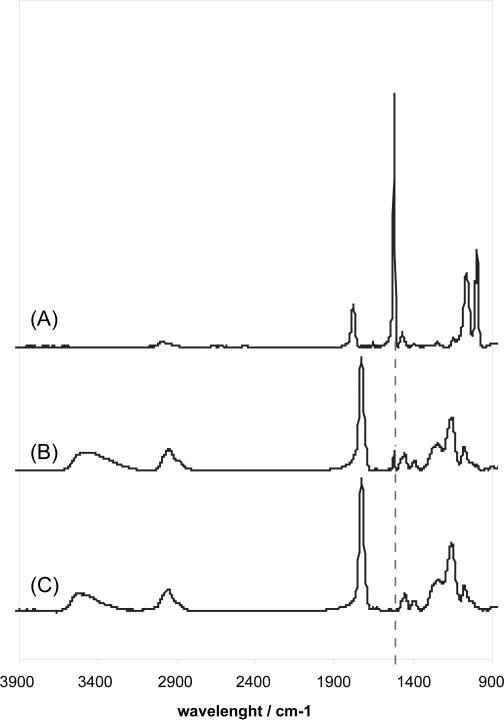
In order to polymerize this ternary system, PFM was heated and introduced into the reactor chamber together with HEMA and EGDA monomers. Fig. 2 proves that all three components are polymerized together, as the aromatic stretching peak appears in the terpolymer, indicating the presence of PFM, among the HEMA and EGDA characteristic signals (O-H stretching at 3700-3050 cm−1, C-H stretching at 3050-2700 cm−1, C=O stretching at 1750-1690 cm−1, C-H bending 1500-1350 cm−1 and C-O stretching at 1300-1200 cm−1). In addition, the XPS measurements revealed a F percentage that oscillates between 2.2 and 2.8 %.
Figure 2. FTIR spectra comparison showing the PFM incorporation into the hydrogel.
FTIR spectra of (A) PFM homopolymer, (B) HEMA-co-EGDA-co-PFM terpolymer and (C) HEMA-co-EGDA hydrogel. In the terpolymer (B), the characteristic aromatic stretching vibration of PFM at 1525 cm−1 is clearly distinguished among the HEMA and EGDA signals.
In order to ensure the possibility to polymerize hydrogels with tunable swelling capacities in this three-monomer system, a series of PFM containing hydrogels with different crosslink agent amounts was prepared. The flow rate of HEMA, PFM and TBPO (initiator) were kept constant, while flow rate of EGDA was varied, as observed in Table 1, which summarizes the experimental conditions. The total flow rate was always maintained at 2 sccm using a patch flow of nitrogen and the reactor pressure was kept at 200 mTorr. These arrangements ensured a constant residence time in the reactor of 10 s for all the experiments. The thickness of the samples was approximately 600 nm.
Table 1.
Details of experiments runs of HEMA-co-EGDA-co-PFM polymerization.
| flow rates / sccm |
partial pressure / mTorr |
|||||||
|---|---|---|---|---|---|---|---|---|
| sample | HEMA | EGDA | PFM | TBPO | N2 | HEMA | EGDA | PFM |
| HE4P | 0.30 | 0.11 | 0.04 | 0.15 | 1.40 | 38 | 14 | 5 |
| HE2P | 0.30 | 0.08 | 0.04 | 0.15 | 1.45 | 38 | 10 | 5 |
| HE1P | 0.30 | 0.06 | 0.04 | 0.15 | 1.47 | 38 | 8 | 5 |
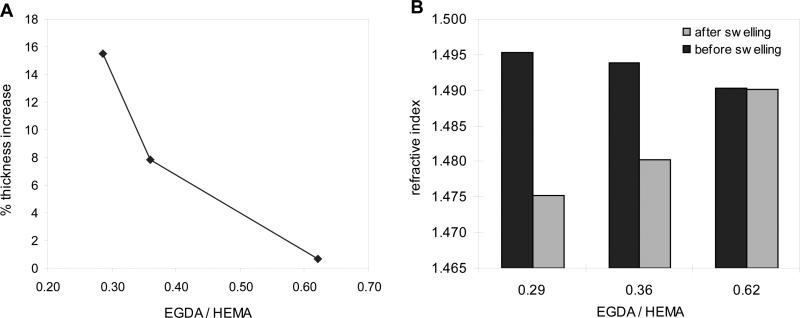
The resulting hydrogels, which include 1 % of the hydrophobic pentafluorophenyl monomer, were seen to behave in the same way as the HEMA-co-EGDA hydrogels in terms of swelling in aqueous media. Their swelling capacity is expressed as the percentage of thickness increase after soaking the dry films in PBS. Figure 3 shows how the swelling decreases as the polymers are more crosslinked, as crosslinking hinders the incorporation of water into the structure (Fig. 3a). Not only the thickness but also the refractive index of the wet films respond to the water uptake. Since water has a much lower refractive index than the polymer itself, the refractive index values dramatically drop when the films swell more (Fig. 3b). This effect has been already described for the HEMA-co-EGDA hydrogels.[24] Actually, even the chemical changes in the polymer itself (dry film) are noticeable by means of the refractive index, which slightly decreases when EGDA ratio increases.
Figure 3. Crosslink degree and swelling properties of HEMA-co-EGDA-co-PFM films.
(A) Changes in the film thickness as function of the crosslink degree. (B) Refractive index of the samples with different EGDA concentration before and after swelling in PBS.
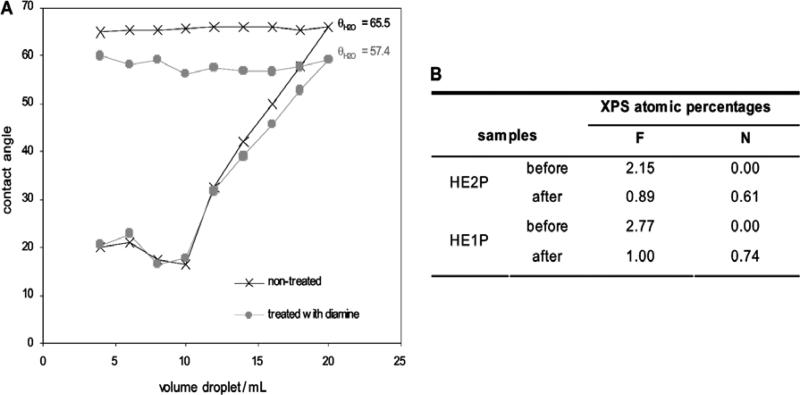
The retention of the pentafluorophenyl labile ring during the polymerization enables the easy functionalization of the films with amines through a nucleophilic attack. At the same time, the presence of the crosslinker not only allows the swelling tailoring, but also avoids the dissolution of the film, both in hydrophobic media, such as ethanol or even N,N-dimethylformamide/ethanol (10:90 v/v), and hydrophilic media, such as water or physiological saline medium. In order to prove the reactivity of the PFM embedded into the hydrogel, the films were made react with 1,6-hexyldiamine in N,N-dimethylformamide / ethanol (10:90 v/v). The advancing contact angles of the sample before and after the treatment provide evidence of the success of the reaction, since the modified film is clearly more hydrophilic (Fig. 4a), as expected after the substitution of a highly hydrophobic moiety such as the pentafluorophenyl for the more hydrophilic diamine molecule. The advancing angles report how the hydrophobic character of the film changes between samples, meanwhile the receding angles represent the hydrophilic part of the samples, the hydrogel, and thus both the modified and non-modified samples follow the same behavior. This topic is more extensively discussed further on. Since PFM is been proved to react faster with amines than with water,[29] the reactivity of the samples was also tested in aqueous solution. This environment is indicative of our final biological applications and the interaction of water and the film is enhanced by the presence of the hydrogel. XPS after the treatment (Fig. 4b) shows the decrease of the fluorine content, together with the correspondent N signal increase coming from the attached amine. Nevertheless, the C-C stretching adsorption in the FTIR is not noticeably changed after the reaction (data not shown), which means that that most of the PFM in the bulk hydrogel remains unreacted .
Figure 4. Functionalization of the HEMA-co-EGDA-co-PFM films.
(A) Advancing and receding contact angle of the hydrogel with PFM before and after reaction in a 10 mM diamine solution in DMF:EthOH (10:90 v/v) for 12h at 37 °C. (B) XPS survey scan before and after reaction in 10 mM diamine solution in PBS for 5h at room temperature.
2.2. iCVD polymerization of a two-layer film: hydrogel bulk with a surface reactive polymer
To address the unreacted PFM groups in the bulk of the film, a 2-layer approach was undertaken to optimize the reactivity of the PFM groups. Therefore, instead of synthesizing an homogeneous coating, the new strategy lie in a first polymerization of the HEMA-co-EGDA hydrogel and a consecutive deposition of PFM on top. Thus, the hydrogel bulk with tunable swelling capacity would confer specific physical properties on the film and the higher concentration of the active molecules at the surface would favor the reaction with biomolecules, which in addition will be exclusively located in the outer layer. Moreover, this approach makes possible to reduce, to a large extent, the amount of expensive PFM monomer utilized, and thus reducing the cost of the process.
To synthesize this system, the HEMA-co-EGDA hydrogel was initially polymerized until the interferometer indicates the desired thickness. Afterwards, the HEMA and EGDA valves were closed and simultaneously the PFM valve was opened (in some cases EGDA was also copolymerized with PFM). Meanwhile, the initiator and nitrogen flows and the filament heating kept running. As the new vapor mixture passed the filament, the change in the thermal conductivity and the specific heat results in a change of the heat loss and finally in a modification of the filament temperature. Therefore, the supplied power had to be readjusted after the monomers swap. Once the system stabilized, PFM was let polymerize until the desired thickness was reached.
This strategy allows to form a two-layer film with tunable thicknesses: a hydrogel bulk covalently linked to an outer PFM polymer. Different samples were synthesized varying the PFM layer thickness in an approximate range from few nanometers to hundreds of nanometers. Table 2 shows the ellipsometric analysis using one-layer and two-layer models. The mean square error (MSE) reveals that the more complex two-layer model is superior for samples HP1 and HP2. Furthermore, the addition of the thickness values of the hydrogel and the PFM is consistent with the total thickness obtained with the one-layer model. The index of refraction of the hydrogel layer always presents a higher value than the PFM does, as expected since HEMA-co-EGDA polymers usually show values in the range of 1.480 and 1.510, while the index of refraction of polymerized PFM is in the range of 1.460-1.470. Obviously, the resulting index of refraction may substantially differ due to the presence of the crosslink agent. In the third sample (HP3) it is difficult to distinguish the one- and two- layer models, most likely due to the thinness of the PFM layer.
Table 2.
Spectroscopic ellipsometry analysis of the hydrogels with a PFM layer.
| sample | parameters | 1 layer model | 2 layer model | |
|---|---|---|---|---|
| HP1 | MSE | 17.53 | 10.86 | |
| thickness / nm | 270 ± 0.9 |
HEMA
PFM |
102 ± 3 170 ± 3 |
|
| index of refraction | 1.4884 |
HEMA
PFM |
1.5251 1.4493 |
|
| HP2 | MSE | 8.068 | 3.216 | |
| thickness / nm | 197 ± 0.5 |
HEMA
PFM |
128 ± 4 63 ± 4 |
|
| index of refraction | 1.4810 |
HEMA
PFM |
1.5137 1.4850 |
|
| HP3 | MSE | 14.67 | 14.74 | |
| thickness / nm | 120 ± 0.4 |
HEMA
PFM |
100 ± 34 20 ± 34 |
|
| index of refraction | 1.4989 |
HEMA
PFM |
1.4996 1.4950 |
|
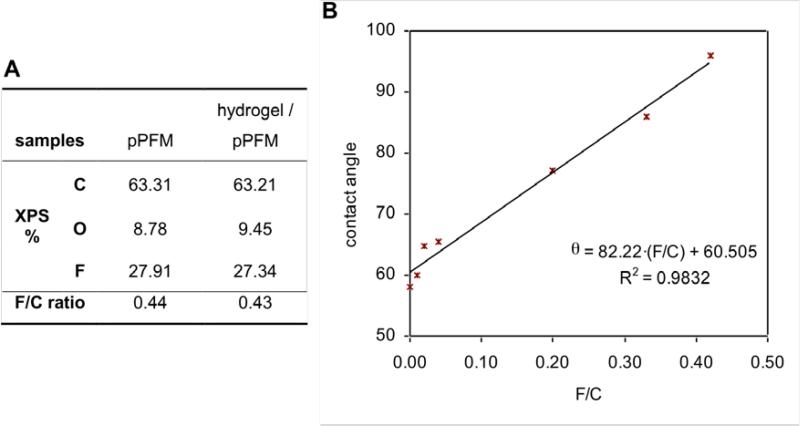
Unlike the ternary hydrogels (HEMA-co-EGDA-co-PFM) previously described, having less than 3 at. % surface fluorine, the two-layer approach makes possible fluorine concentrations on the surface from 0 to nearly 30 at. %, the last one corresponding to the PFM homopolymer. As shown in Fig. 5a, when the PFM layer is thick enough, the XPS atomic percentages of the hydrogel with PFM agreed with the PFM homopolymer, indicating a compact PFM layer on top of the hydrogel. However, it is possible to obtain films with much lower F/C ratios, which mean a lower PFM concentration in the outer layer and consequently indicated a blend between the deposited hydrogel and the subsequent PFM polymer growth. This hypothesis was also confirmed by contact angle measurements, since it was observed that the contact angle increased with increasing the F/C ratio (Fig. 5b). The contact angle value ranged from 58, for a HEMA-co-EGDA hydrogel with no PFM (with an EGDA/HEMA ratio of 0.14), to 96, for a hydrogel with enough PFM on top to achieve a F/C ratio of 0.42, nearly equal to the PFM homopolymer. A simple linear regression analysis could explain the relationship between the contact angle results and the F/C ratios on the surface, with a coefficient of determination (r2) of 0.9832.
Figure 5. Surface characterization of the hydrogels with PFM.
(A) Comparison of the XPS percentages for a polymerized PFM film and a hydrogel with a compact top PFM layer. (B) Sessile contact angle of various hydrogels with different F/C ratio on the surface.
In order to study more accurately the first nanometers at the surface of the two-layer films, angle-resolved X-ray photoelectric spectroscopy (ARXPS) was performed at both 20° and 90°. The variation of the take-off angle allows to study the elemental composition depending on the depth; when the angle is 90°, the X-ray is perpendicular to the surface, so that it penetrates deeper into the film than when it is at an angle of 20° from the surface. Hence, the F/C atomic ratios could be determined as function of the sampling depth for three hydrogels with different PFM content and for a PFM-co-EGDA copolymer. The depth of the analysis (d) was approximated using the expression d=3·λ·sin(θ), where θ is the take-off angle and λ is the photoelectron escape depth, which was estimated to be 4 nm (± 1.5) for hydrophobic polymeric coatings containing fluorine.[30,31] For all the hydrogels with PFM, the F/C ratio in the first 8 nm (take-off angle of 90°) was much higher than the F/C that would correspond to the whole film calculated from the FTIR analysis. When only the first 2.7 nm were analyzed (take-off angle of 20°), the F/C was observed to increase even more. Therefore, these results, summarized in Table 3, revealed an enrichment of fluorinated units towards to the surface and thus the formation of a PFM graded layer. As a control, ARXPS was also performed on a copolymer of PFM and EGDA with an EGDA/PFM ratio of 0.11, since certain degree of segregation of fluorinated monomers to the interface-polymer had been previously reported for simple iCVD copolymers of glycidyl methacrylate with 2,2,3,3,4,4,5,5,6,6,7,7-dodecafluoroheptyl acrylate.[31] In the control, however, a difference of the F/C ratio as function of depth is insignificant, indicating that this phenomenon is a characteristic associated with the two-layer films.
Table 3.
Angle-resolved XPS of hydrogels with PFM.
| F/C |
XPS sampling depth / nm | |||
|---|---|---|---|---|
| sample | % PFM | bulk | surface | |
| HEMA-co-EGDA / PFM | 0.1 | 0.003 | 0.15 | 2.7 |
| 0.08 | 8.0 | |||
| 0.6 | 0.009 | 0.38 | 2.7 | |
| 0.26 | 8.0 | |||
| 20 | 0.259 | 0.47 | 2.7 | |
| 0.43 | 8.0 | |||
| PFM-co-EGDA | 45 | 0.504 | 0.43 | 0.42 |
| 2.7 | 8.0 | |||
2.4. Behavior of the graded hydrogels in aqueous medium
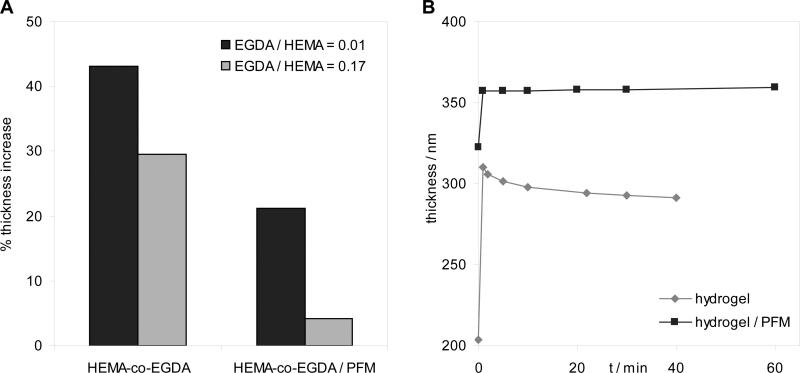
Initially, the main concern regarding the final application of the two-layer approach was the possible inability of the hydrogel to swell, due to the large concentration of hydrophobic units in the interface with water, that could prevent water from penetrating into the hydrogel. However, the hydrogels with PFM at the surface did swell in water but, as expected, increase of thickness shown in the hydrogels with PFM was lower than in the homopolymer hydrogels with the same crosslink density as depicted in Fig. 6a, indicating a clear dependence of the degree of swelling on the surface percentage of fluorine. Although the presence of PFM restricts the swelling capability, dynamic studies of the thickness variation in aqueous media reveal that both hydrogels with and without PFM swell up to their maximum thickness within the first minute of soaking the sample in water and then is maintained (Fig. 6b). This indicates that the equilibrium state is quickly reached, regardless of the presence of the fluorinated groups. In the case of the hydrogel without PFM, the plateau zone points out a slight decrease of the thickness with time that it was probably due to a partial dissolution of the film, caused by a very low crosslink density. The constant plateau observed in the other sample indicates that it is stable against dissolution.
Figure 6. Thickness increase of the two-layer hydrogels with PFM in water compared with the HEMA-co-EGDA hydrogel.
(A) Decrease of the swelling ability for two samples with different crosslink degrees, EGDA/HEMA = 0.01 and 0.17. (B) Thickness increase as
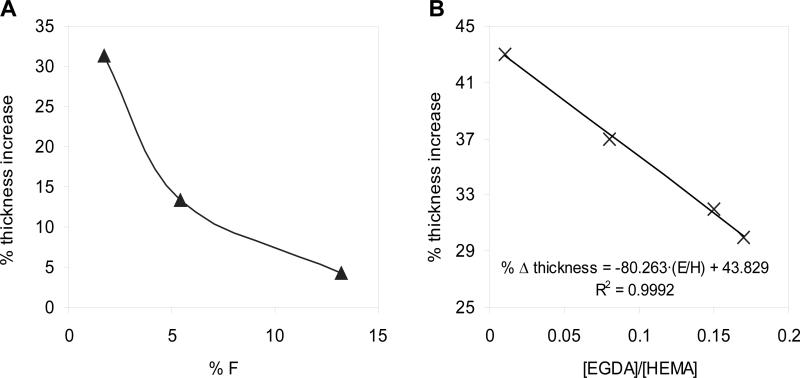
Figure 7a reveals that the thickness increase upon swelling in water is inversely correlated to the measured fluorine content on the surface. Although the swelling is clearly reduced, the values are not fully comparable, since the crosslink densities for the three hydrogels are not the same and this factor plays an essential role in the swelling capability. Specifically, the film with a fluorine amount of 5.4 % was synthesized with an EGDA/HEMA ratio of 0.13, whereas the other two have a crosslink degree of 0.17. For hydrogels with no PFM, these crosslink densities would imply an estimated thickness increase of 33 % and 30 %, respectively. However, the measured differences of thickness increase among the different PFM-containing samples shown in the figure are clearly higher than the 3 % variation coming from the deviation in the crosslink degree. Therefore, the trend followed by the swelling ability as function of the fluorine amount is confirmed. It is worth mentioning that the values of the thickness increase of the HEMA-co-EGDA hydrogels were estimated based on a previous swelling study of hydrogels with different EGDA percentages (Fig. 7b), where it was observed that the thickness increase and the EGDA/HEMA ratios were related by a linear tendency for crosslink densities in the approximate range from 0.01 to 0.2. Beyond these limits, the relation adopted a hyperbolic behavior.[24]
Figure 7. Swelling behavior of the two-layer hydrogels and the HEMA-co-EGDA without PFM.
(A) Thickness increase percentage for three HEMA-co-EGDA with a PFM layer, maintaining nearly the same crosslink degree as function of the amount of fluorine on the surface (XPS). (B) Linear regression of the thickness increase percentage of the HEMA- co-EGDA copolymers versus the EGDA/HEMA ratio.
The restriction of the swelling ability of the hydrogels caused by the PFM is not desirable, because the thickness increase is pursued. However, a subsequent functionalization of the PFM could affect the properties of the film in water, as the interface will change completely. Consequently, it was decided to study the reactivity of the two-layer hydrogels with a more hydrophilic aminated molecule: 2-(2-aminoethoxy)ethanol. The reaction was performed at a concentration of 100 mM in ethanol during 12 h at 37 °C. The two-layer polymer sample before funcationalization had a thickness increase in water as low as 4 % with a EGDA/HEMA of 0.17. After the reaction, the FTIR analysis of the resulting sample showed no C-F stretching peak and the contact angle was reduced from 84° to 67°. Regarding the XPS measurements, the F percentage decreased from 13.19 % to 1.71 %, whereas N increased up to a 0.5 %. Finally, the swelling degree rose from the aforementioned 4 % to a 31 %, which is approximately the thickness increase underwent by a hydrogel containing no PFM with the same crosslink density. Therefore, the samples seemed to be able to recover the intrinsic ability of the hydrogels to swell, once nearly all the PFM has been removed. However, more experiments should be performed to ensure that this effect is not due to a partial coating dissolution.
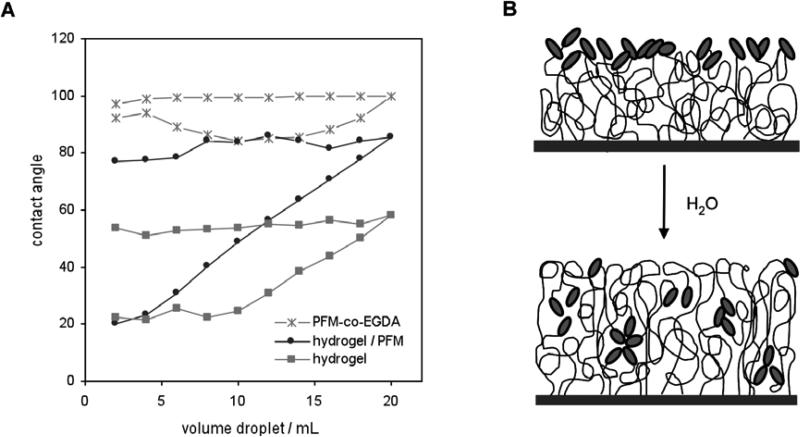
Information regarding surface interaction with different media may be extracted from the contact angle analysis, specially from the advancing and receding mode. In these measurements most of the surfaces show some kind of hysteresis, which is the difference between the advancing (θadv) and the receding (θrec) contact angles, typically due to an effect of roughness, chemical heterogeneity or mobility.[32] In the case of hydrogels and more specifically of pHEMA, the fundaments of the hysteresis phenomenon have been already reported for films polymerized by different techniques: redox-initiated polymerization,[33,34] photografting[35] and iCVD.[24] Basically, they agreed that the extreme hysteresis shown by these hydrogels is caused by chain mobility, which allows the polymer to restructure depending on the environment (air or water). Therefore, the addition of a crosslink agent would reduce polymer mobility and thus hysteresis.[24,34] However, the effect of the presence of such a hydrophobic polymer as polymerized PFM on top of a hydrogel remains unknown. A hydrogel with a PFM layer was tested for the advancing and receding contact angle as function of the droplet volume, compared to a HEMA-co-EGDA hydrogel and a PFM-co-EGDA polymer (Fig. 8a). The advancing contact angles for each surface remain approximately constant and the values are higher when the amount of fluorine increases. A hydrogel with a PFM layer with a F/C of 0.20 shows a mean advancing contact angle of 82, while the absence of PFM involves a mean contact angle of 55 and a crosslinked PFM film with F/C ratio of 0.42 shows a contact angle of 99. Initially, surfaces are in equilibrium with air, so that the hydrophobic groups (both CH3 and CH2, but specially pentafluorophenyl rings from PFM) are basically exposed, which leads to an advancing angle typically sensitive to hydrophobic domains. As the surfaces get in contact with water, they establish a new equilibrium with the aqueous environment, which involves a chain reorientation. Now mainly the hydrophilic moieties, like the hydroxyl groups of HEMA for our films, are located in the interface. On one hand, while increasing the droplet volume, the contact angle is held approximately constant, since water always interacts with part of the surface that has not been wet yet and thus has not suffered chain reorientation. Small variations in the contact angle values are expected, specially in the hydrogels with PFM, due to chemical inhomogeneities. On the other hand, once the whole testing surface is wet, the more hydrophilic configuration acts holding back water, which flattens the droplet as volume decreases. Therefore, the receding contact angle gradually drops until the true contact angle for the new surface is reached. Interestingly, once wet the presence of PFM on the hydrogel does not seem to alter the hydrophilic behavior of the surface in water, since the hydrogel both with and without a PFM layer (with the same crosslink degree) reach the same low receding angle (θrec = 22° - 24°). Then, for hydrogels with the same crosslink densities, the degree of hysteresis will be different depending on the amount of PFM at the surface. On the contrary, the absence of the hydrogel leads to a very low hysteresis and a completely different template. A schematic representation of the structure of the interface of a two-layer hydrogel with PFM both with air and water is drawn in Fig. 8b. Actually, similar structures have been recently proposed for poly(ethylene glycol) with fluoroalkyl endgroups.[36]
Figure 8. Behavior in aqueous medium of the hydrogels with a PFM layer.
(A) Advancing and receding contact angle as function of the droplet volume of a HEMA-co- EGDA hydrogel (EGDA to HEMA ratio of 0.14), a HEMA-co-EGDA with a graded PFM layer with F/C = 0.13 and EGDA/HEMA = 0.14 and a PFM-co-EGDA. (B) Schematic representation of the polymeric surface before (top picture) and after soaking in water (bottom picture).
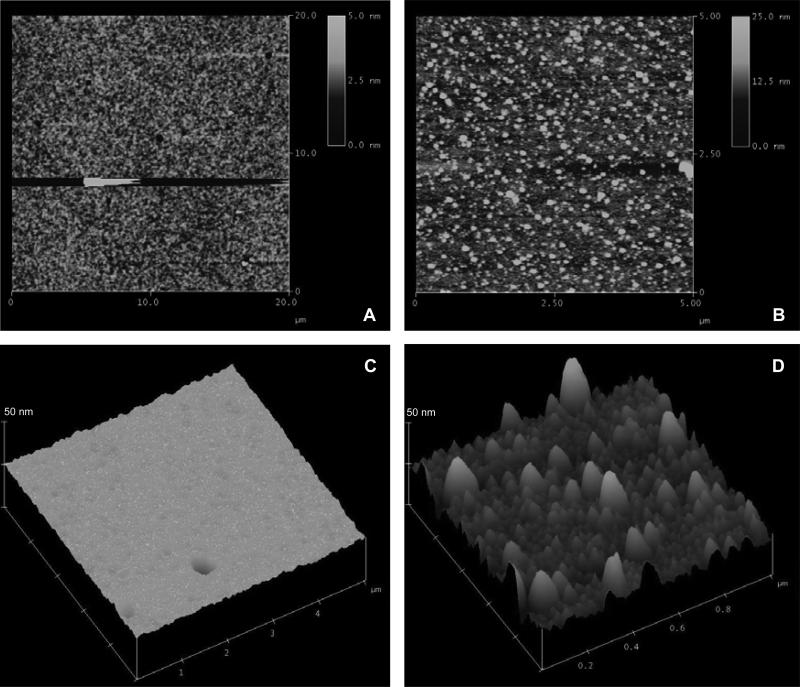
Once in water, the swelling and the reorganization of the polymer chains in the two-layer hydrogels with PFM could imply also a change in the topography of the surfaces at the micrometric level. In order to study this phenomenon, different samples were analyzed by atomic force microscopy (AFM) in their dry state and also in water. Fig. 9 shows the AFM micrographs of a hydrogel with a graded layer of PFM (with a 18 % of F according to the XPS results and a crosslink density of 0.10). The two images on the left correspond to the dry sample at 2D and 3D and different scanning dimensions, whereas the images on the right are from the polymer in water, also at different scanning dimensions. Both in 2D and 3D is clearly observed that the sample underwent topographical changes once into the water. Some kind of protrusions appeared on the surface, with very variable heights ranging from 10 to 40 nm and diameters that could reach the 180 nm. These peaks probably correspond to the hydrogel zones that have not been covered by PFM and, in contact with water, they swell. The hydrogels with no PFM do not present this behavior, all the surface swell equally, maintaining nearly the same topological features in the dry and the wet state.
Figure 9. AFM topological micrographs of a hydrogel with a PFM layer on top.
Two-dimensional views of the (A) dry sample and the (B) sample in water. Three-dimensional views at the same z-scale (50 nm) of the (C) dry sample and the (D) sample in water.
2.5. Experiments to test the biological behavior of the graded hydrogels with PFM
Preliminary studies on cell morphology were performed on PFM films functionalized with an adhesion peptide motif in order to ensure that the working surfaces were biomimetic enough for the cells to attach properly. Thus, cells were cultured on crosslinked PFM coatings with and without a laminin-derived peptide motif (RYVVLPR) known to promote cell binding.[37] In addition, the experiments were performed in presence and absence of serum to see whether or not the serum proteins interfere with the recognition of the attached peptide. Specifically, mouse embryonic fibroblasts (MEF) were used for these initial experiments, since they are well known to be a robust cell system able to secrete proteins and conditioning factors. Therefore, if these cells do not survive or present an unusual phenotype, it would mean that the testing environment is really hostile.
After 2 hours post-seeding (results not shown), all cells cultured with serum adopt a normal phenotype and no significant differences were observed among substrates (also considering a control plastic dish), meaning that serum proteins attach on the surfaces as expected, preventing cells to recognize the coupled peptide. On the contrary, the effect of the RYVVLPR peptide was observed when MEF where cultured without serum, as cells seeded on the peptide-modified surface present a better morphology than the ones on the polymer itself, as well as the cells on plastic dish. Hence, this suggests that not only the cells are attached to the peptide on the surface of the material, but also the recognition is sufficient to enhance cell morphology. After 12 hours of incubation, however, no morphological differences were observed among the various substrates neither in presence or absence of serum. Therefore, these results suggested that the cells that survived the initial contact with the surfaces are able to properly attach after 12 h, as they have already “conditioned” their environment by secreting their own matrix on the material surfaces and by producing soluble factors (such as growth factors and cytokines). For this reason, cell behavior experiments on substrates should be carefully designed to avoid the biological effect of the cells on changing the properties of the surface material and the environment in general.
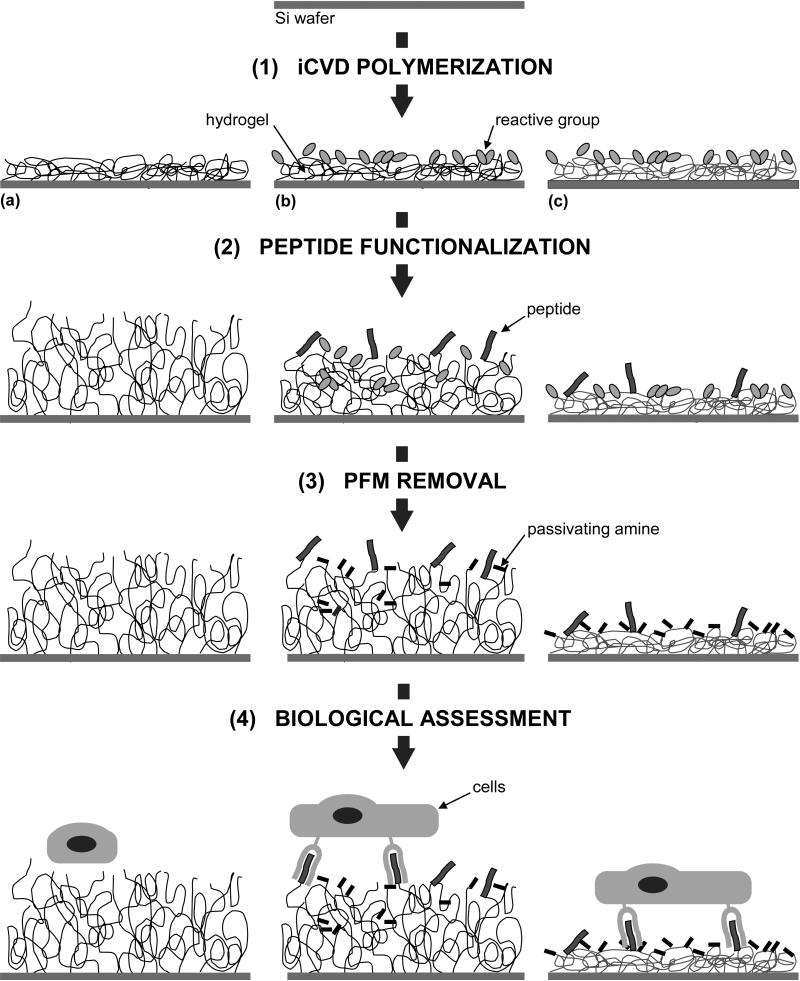
Once the experimental conditions were set and it was proved (in our experimental system) that cells could recognize the attached peptide, a series of experiments was designed in order to study the cell phenotype on a film combining hydrogel (HEMA-co-EGDA) with a PFM layer on top. In comparison, the same experiments were performed on a substrate that do not contain the hydrogel and a substrate with the hydrogel alone. The strategy followed is schematically represented in Fig. 10 and consists of four steps: iCVD polymerization of the three samples (a hydrogel, a graded hydrogel with PFM and a crosslinked PFM film); functionalization of the polymers with the linear peptide GRGDSPY, containing the integrin receptor-binding motif RGD, which is derived from fibronectin (and the corresponding non-active peptide GRADSPY where the residue Glysine [G] was changed by an Alanine [A]); removal of the remaining pentafluorophenyl groups with another amine 2-(2-aminoethoxy)ethanol; and finally, the actual cell attachment. In this case, Human Umbilical Vein Endothelial Cells (HUVEC) were used for the cultures, as they are known to intimately interact with surfaces while they naturally tend to develop into monolayers. These cells were cultured for 2 h on all the conditions described above without serum and growth factors in order to promote the specific recognition of cell surface receptors with the cell-adhesion peptide and to avoid interference due to cell binding mediated by serum proteins, as previously described for the fibroblast system.
Figure 10. Scheme of the strategy to test the biological activity of iCVD polymerized films.
It consists of 4 steps: (1) the polymerization itself of different substrates, in this case, (a) HEMA-co-EGDA, (b) HEMA-co-EGDA with a PFM layer and (c) PFM-co-EGDA, (2) functionalization of the PFM-containing films with peptides, (3) passivation of the remaining PFM with an amine and (4) the cellular culture on the functionalized films.
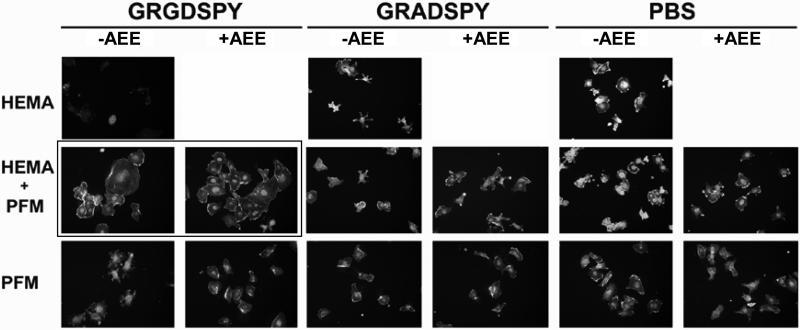
Fig. 11 depicts HUVEC morphology acquired by fluorescent microscopy on all the differently modified films. Significant differences on cell morphology were observed between GRGDSPY- and GRADSPY-modified surfaces only from the two-layer film that combines a hydrogel bulk with the attached peptides on top. When the RGD motif is present, cells adopt a normal phenotype and seem to start forming a monolayer, in contrast to the non-signaling RAD motif and the control surface with no peptide attached. This behavior is not observed on the substrates which do not contain the hydrogel. In fact, in these cases cell morphology is quite similar to the one on the RAD-modified surfaces or on the hydrogels with no PFM, where no peptide recognition and thus no specific attachment through integrin receptors occur. These results suggest that the presentation of the active signaling peptide in the right configuration promotes cells to adopt a normal phenotype. The presence of the hydrogel might be the reason for this favored cell-material interaction. However, the nature of the physical interaction in terms of stiffness of the hydrogel substrate or topological presentation, or both, still remains unclear.
Figure 11. HUVEC morphology at 2 h of incubation.
Cells were cultured on three different substrates: the HEMA-based hydrogel (first raw), the hydrogel with a PFM layer (second raw) and the PFM polymer (third raw). The films were functionalized with GRGDSPY (first and second column) and GRADSPY (third and forth column) and, as controls, other samples were immersed in PBS under the same conditions (fifth and sixth column). ‘+AEE’ and ‘-AEE’ indicate whether or not the samples were treated with the passivating amine.
3. Conclusions
The synthesis of a novel functional surface by initiated chemical vapor deposition has been reported here. The films, consisting of a hydrogel (HEMA-co-EGDA) and a reactive monomer (PFM), may be synthesized as a homogeneous hydrogel or in a two graded layer due to the excellent versatility of the technique. By adjusting the crosslink to monomer ratio, the crosslink degree and, thus, the swelling ability of the hydrogels are easily tailored, which has a direct effect on the physical properties of the material. Moreover, the presence of reactive esters allows coupling any molecule presenting a free amine, specially biologically active peptides, even in water. Hence, these surfaces are a platform where both the physical properties and the surface chemistry may be easily tailored. Preliminary biological experiments suggested that cells are able to recognize peptidic motifs anchored on this kind of surfaces, leading to an enhanced cellular phenotype. Therefore, these films may be considered as a potential candidate to study the influence of the mechanical and chemical signaling in cell behavior.
4. Experimental
iCVD polymerization
Films were deposited on 100-mm-diameter silicon (Si) substrates in a custom-built vacuum reactor. The cylindrical reactor (height of 3.3 cm and radius of 12 cm) was covered with a quartz top that allowed laser interferometry (633 nm He-Ne laser source, JDS Uniphase) to monitor the depositions. The reactor was equipped with a Nichrome filament (80% Ni / 20% Cr, AWG26, Omega Engineering) that was resistively heated (280 °C) and a backside-cooled stage (32 °C). Pressure in the vacuum chamber was varied depending on the experiment (200 - 250 mTorr) using a butterfly valve. The monomers HEMA (Aldrich) and PFM (Polysciences) and the cross-linker EGDA (Polysciences) were heated and delivered into the reactor by using regulated needle valves. Meanwhile the initiator TBPO (Aldrich) was vaporized at room temperature and metered into the chamber through a mass-flow controller, as well as the gas N2. HEMA was always maintained at 65 ± 1 °C, whereas the EGDA temperature was varied from 50 to 60 °C, according to the degree of crosslink desired. PFM was heated up from 50 to 70 °C, depending on the flow needed. All vapors were mixed together before entering the reactor through a side port.
Chemical characterization of the surfaces
X-ray photoelectric spectrometry (XPS) was carried out on a Kratos Axis Ultra spectrometer using a monochromatized Al Kα source. Spectra were recorded using a 70° take-off angle relative to the sample surface, except for the angle-resolved XPS (ARXPS) measurements, where take-off angles of 20° and 90° were set. The deconvolutions of the signals were performed in CASA XPS software, with relative sensitivity factors (RSF) of 0.278 for C, 0.780 for O, 1.000 for F and 0.477 for N. Fourier transform infrared spectroscopy (FTIR) measurements were done on a Nicolet Nexus 870 spectrometer in normal transmission mode using a deuterated triglycine sulfate (DTGS) KBr detector over the range of 400-4000 cm−1 at a 4 cm−1 resolution. All spectra were baseline- corrected. The crosslink degree of the HEMA-co-EGDA hydrogels, expressed as the ratio of crosslink agent to monomer (EGDA/HEMA), was calculated from the FTIR analysis. The calculation relates the C=O area ascribed to EGDA to the one correspondent to HEMA, under the assumption that the carbonyl group from all acrylic monomers had the same adsorption coefficient, as already proved for the iCVD HEMA-co-EGDA system.[24] The area contributed by the EGDA carbonyl units is the total C=O area (AC=O) less the area correspondent to the carbonyls of HEMA and PFM. HEMA and PFM carbonyl adsorptions were calculated in relation to two unique adsorption peaks for each one (the aromatic C-C stretching for PFM at 1525 cm−1, ACar, and the OH bond for HEMA at 3700-3000 cm−1, AOH). The ratios of the carbonyl area to these adsorptions are denoted as rPFM and rHEMA, respectively. Specifically, the following equation was used for the crosslink density calculation.
| (1) |
Film thickness, optical properties and swelling analysis: Variable-angle spectroscopic ellipsometry
The thickness and optical properties of the polymerized films were analyzed on a J.A. Woollam M-2000 spectroscopic ellipsometer with a xenon light source. Data were acquired at three angles (65°, 70°, and 75°) and 225 wavelengths, and the Cauchy-Urbach model was used to fit the data. For the two-layer model, the Cauchy-Urbach model was set to treat two layers independently using as starting point the optical properties obtained for the separate polymers and the approximate thicknesses given by the interferometry. Ellipsometry was also used to determine the swelling properties of the surface hydrogels. For this purpose a simple liquid cell was placed on to the M-2000 ellipsometer stage, for which the cell was designed. Each film-coated substrate was secured in the cell and the measurements were made before (air/solid interface) and after the cell was filled with PBS (solution/solid interface). The data from the dry and swollen films were fit with the Cauchy - Urbach model, considering always the film as a unique material.
Film wettability characterization
Contact angle measurements, performed on a goniometer equipped with an automatic dispenser (Model 500, Ramé-Hart), were used to evaluate the wettability of the films. The contact angle was determined by placing a drop of water on the surface and measuring the angle between the plane of the surface and the tangent to the drop at the point of contact with the substrate (measurement known as sessile drop). Dynamic contact angles measurements (advancing and receding) were performed on the surfaces, by gradually increasing and decreasing the volume of the water droplet and measuring the contact angle each time.
Film topography analysis
Atomic force microscopy (AFM) images were acquired with a Nanoscope III Extended Multimode Atomic Force Microscope (Digital Instruments, Santa Barbara, CA), with a lateral resolution of 5 nm and vertical of 1 Å, by tapping mode. The analysis was performed by the Nanoscope III v4.22 software for Nanoscope microscope.
Sample modification
PFM-containing samples were functionalized with various aminated molecules: 1,6-diaminohexane (Aldrich), 2-(2-aminoethoxy)ethanol (Aldrich) and peptides with the following sequences: RYVVLPR, GRGDSPY-FITC and GRADSPY-FITC (Biopolymer Facility at MIT), the last two carrying a fluorescein label (FITC) at the C-terminal. Three solvents were used: Phosphate Buffered Saline (PBS, Aldrich), N,N-dimethylformamide (DMF, Aldrich) and ethanol (Aldrich). The samples were immersed in the corresponding solution, left on a shaker, rinsed twice in ethanol and water and dried. The conditions of the different reactions are summarized in Table 4. The reaction of PFM with a fluorescent label was verified under a Nikon Epi-Fluorescence microscope TE300 (Nikon, NY).
Table 4.
Conditions of the functionalization of the PFM-containing samples.
| reagents | media | concentration | temperature | time |
|---|---|---|---|---|
| 1,6-diaminohexane | PBS and DMF / ethanol (10:90 v/v) | 10 mM | 37 °C and 60 °C | 12 h and 5 h |
| 2-(2-aminoethoxy) ethanol | ethanol | 100 mM | 37 °C | 12 h |
| peptides | PBS | 0.7 - 1 mM | 37 °C | 6 h |
Biological assessment
crosslinked PFM films were functionalized with an adhesion peptide (RYVVLPR) and were equilibrated in Dulbecco's Modified Eagles Medium (DMEM, Gibco) for 1 h in a tissue culture incubator (37 °C, 5 % CO2), using a non-functionalized film and a plastic dish as controls. Mouse embryonic fibroblasts (MEF, ATCC) cultures in DMEM with 10 % Foetal Bovine Serum (FBS) were trypsinized, washed in DMEM without serum, counted and used to seed on samples at a concentration of 20,000 cells / cm2. Next, two sets of experiments were performed, in presence and absence of serum. Both experiments were carried out both at 2 and 12 h to study cell binding and morphology. After that, the cells were fixed with 2 % (w/v) paraformaldehyde for 1 h, washed and stained with DAPI for nuclear staining (Molecular Probes) and Phallodin-Rhodamin for actin cytoskeleton (Sigma). These two dyes were visualized on a Nikon inverted microscope with DAPI and rhodamine filters.
Similar subsets of experiments were carried out in presence of Human Umbilical Vein Endothelial Cells (HUVEC, Lonza). For these experiments, three different iCVD-polymerized films were compared: a HEMA-co-EGDA hydrogel with a 30 % swelling degree, a hydrogel with PFM polymer layer on top with an initial 21 % of swelling, that could be further increased after the PFM removal, and a PFM-co-EGDA film. Some samples were functionalized with the linear peptide sequence GRGDSPY and others with the corresponding permutated peptide GRADSPY. Both sequences carried a fluorescein label (FITC) in order to easily check the peptide attachment by fluorescent microscopy. Furthermore, the same films were immersed in PBS at the same conditions, as controls. After peptide attachment, some of the samples were incubated in 2-(2-aminoethoxy)ethanol in PBS to remove the remaining PFM. In comparison, the same substrates without the passivating amine were also tested; they were incubated only in PBS under the same conditions. The resulting series of samples and controls were incubated in endothelial basal medium (Lonza), without serum and growth factors, for 1 h in a tissue culture incubator (37 °C, 5 % CO2). Human Umbilical Vein Endothelial Cells were cultured in EGM-2 BulletKit (endothelial basal medium plus growth supplements, Lonza), trypsinized and suspended in endothelial basal medium (in the absence of growth factors and serum). After counting cells, those were seeded on the surfaces, placed in 24-well multiplates, at 20,000 cells / cm2 and let attach for 2 h. Afterwards, cells were fixed with 2 % (w/v) paraformaldehyde for 1 h, washed and stained with DAPI for nuclear staining (Molecular Probes) and Phallodin-Rhodamin for actin cytoskeleton (Sigma) and visualized under a fluorescence microscope.
Acknowledgments
The authors acknowledge support of the NIH, under funding contract # 1-RO1-EB003805-01A1 to CES, contract #NO1-NS2-2347, and support in part by the U.S. Army through the Institute for Soldier Nanotechnologies, under Contract DAAD-19-02-D-0002 with the U.S. Army Research Office to KKG. Also, the authors would like to thank the Generalitat de Catalunya for the Research Consolidated Group grant: SGR-2005 to Grup d'Enginyeria de Materials (GEMAT). NMB acknowledges financial support from DURSI (Generalitat de Catalunya) and the European Social Fund.
References
- 1.Lutolf MP, Hubbell JA. Nat. Biotechnol. 2005;23:47. doi: 10.1038/nbt1055. [DOI] [PubMed] [Google Scholar]
- 2.Pierschbacher MD, Ruoslahti E. Nature. 1984;315:30. doi: 10.1038/309030a0. [DOI] [PubMed] [Google Scholar]
- 3.Chen CS, Tan J, Tien J. Annual Review of Biomedical Engineering. 2004:275. doi: 10.1146/annurev.bioeng.6.040803.140040. [DOI] [PubMed] [Google Scholar]
- 4.Schwarz US, Balaban NQ, Riveline D, Bershadsky A, Geiger B, Safran SA. Biophys. J. 2002;89:1380. doi: 10.1016/S0006-3495(02)73909-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ingber DE. Proc. Natl. Acad. Sci. U. S. A. 2003;100:1472. doi: 10.1073/pnas.0530201100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pelham RJ, Wang YL. Proc. Natl. Acad. Sci. U. S. A. 1997;94:13661. doi: 10.1073/pnas.94.25.13661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Flanagan LA, Ju YE, Marg B, Osterfield M, Janmey PA. Neuroreport. 2002;13:2411. doi: 10.1097/01.wnr.0000048003.96487.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Welch DR, Sakamaki T, Pioquinto R, Leonard TO, Goldberg SF, Hon Q, Erikson RL, Rieber M, Rieber MS, Hicks DJ, Bonventre JV, Alessandrini A. Cancer Res. 2000;60:1552. [PubMed] [Google Scholar]
- 9.Engler AJ, Sen S, Sweeney HL, Discher DE. Cell. 2006;133:677. doi: 10.1016/j.cell.2006.06.044. [DOI] [PubMed] [Google Scholar]
- 10.Ratner BD, Hoffman AS, Schoen FJ, Lemons JE. Biomaterials Sciene: An Introduction of Materials Science in Medicine. second edition Elsevier Academic Press; San Diego USA: 2004. [Google Scholar]
- 11.Smith AM, Harris JJ, Shelton RM, Perrie Y. J Control Release. 2007;119:94. doi: 10.1016/j.jconrel.2007.01.011. [DOI] [PubMed] [Google Scholar]
- 12.Zhang SG, Semino CE. Tissue Engineering, Stem Cells and Gene Therapies in Advances in Experimental Medicine and Biology. 2003;534:147. doi: 10.1007/978-1-4615-0063-6_12. [DOI] [PubMed] [Google Scholar]
- 13.Genove E, Shen C, Zhang SG, Semino CE. Biomaterials. 2005;26:3341. doi: 10.1016/j.biomaterials.2004.08.012. [DOI] [PubMed] [Google Scholar]
- 14.Kris AS, Kamm RD, Sieminski AL. Biochem. Biophys. Research Com. 2008;371:134. doi: 10.1016/j.bbrc.2008.07.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mackintosh FC, Kas J, Janmey PA. Phys. Rev. Lett. 1995;75:4425. doi: 10.1103/PhysRevLett.75.4425. [DOI] [PubMed] [Google Scholar]
- 16.Georges PC, Janmey PA. J. Appl. Physiol. 2005;98:1547. doi: 10.1152/japplphysiol.01121.2004. [DOI] [PubMed] [Google Scholar]
- 17.Engler AJ, Griffin MA, Sen S, Bonnetnann CG, Sweeney HL, Discher DE, Cell Biol J. 2004;166:877. doi: 10.1083/jcb.200405004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Berg MC, Yang SY, Hammond PT, Rubner MF. Langmuir. 2004;20:1362. doi: 10.1021/la0355489. [DOI] [PubMed] [Google Scholar]
- 19.Mermut O, Lefebvre J, Gray DG, Barrett CJ. Macromolecules. 2003;36:8819. [Google Scholar]
- 20.Engler AJ, Richert L, Wong JY, Picart C, Discher DE. Surf. Sci. 2004;571:142. [Google Scholar]
- 21.Richert L, Engler AJ, Discher DE, Picart C. Biomacromolecules. 2004;5:1908. doi: 10.1021/bm0498023. [DOI] [PubMed] [Google Scholar]
- 22.Thompson MT, Berg MC, Tobias IS, Rubner MF, Van Vliet KJ. Biomaterials. 2005;26:6836. doi: 10.1016/j.biomaterials.2005.05.003. [DOI] [PubMed] [Google Scholar]
- 23.Thompson MT, Berg MC, Tobias IS, Lichter JA, Rubner MF, Van Vliet KJ. Biomacromolecules. 2006;7:1990. doi: 10.1021/bm060146b. [DOI] [PubMed] [Google Scholar]
- 24.Chan K, Gleason KK. Langmuir. 2005;19:8930. doi: 10.1021/la051004q. [DOI] [PubMed] [Google Scholar]
- 25.Mao Y, Gleason KK. Langmuir. 2004;20:2484. doi: 10.1021/la0359427. [DOI] [PubMed] [Google Scholar]
- 26.O'Shaughnessy WS, Marí-Buyé N, Borrós S, Gleason KK. Macromolecular Rapid Communications. 2007;28:1877. [Google Scholar]
- 27.Lau KK, Gleason KK. Macromol. Biosci. 2007;7:429. doi: 10.1002/mabi.200700017. [DOI] [PubMed] [Google Scholar]
- 28.Francesch L, Garreta E, Balcells M, Edelman ER, Borros S. Plasma Processes and Polymers. 2005;2:605. [Google Scholar]
- 29.Francesch L, Borros S, Knoll W, Forch R. Langmuir. 2007;23:3927. doi: 10.1021/la062422d. [DOI] [PubMed] [Google Scholar]
- 30.Schmidt DL, Coburn CE, Dekoven BM, Potter GE, Meyers GF, Fischer DA. Nature. 1994;372:39. [Google Scholar]
- 31.Mao Y, Gleason KK. Macromolecules. 2006;39:3895. [Google Scholar]
- 32.Rupp F, Scheideler L, Geis-Gerstorfer J. Chemical Engineering & Technology. 2002;25:877. [Google Scholar]
- 33.Holly FJ. Journal of Biomedical Materials Research. 1975;9:315. doi: 10.1002/jbm.820090307. [DOI] [PubMed] [Google Scholar]
- 34.Karlsson JO, Gatenholm P. Macromolecules. 1999;32:7594. [Google Scholar]
- 35.Morra M, Occhiello E, Garbassi F. Journal of Adhesion Science and Technology. 1992;6:653. [Google Scholar]
- 36.Mackel MJ, Sanchez S, Kornfield JA. Langmuir. 2007;23:3. doi: 10.1021/la0617762. [DOI] [PubMed] [Google Scholar]
- 37.Skubitz APN, Mccarthy JB, Qi Z, Yi XY, Furcht LT. Cancer Res. 1990;50:7612. [PubMed] [Google Scholar]