Abstract
Background
The prevalence of obesity is increasing globally and will, if left unchecked, have major implications for both population health and costs to health services.
Objectives
To assess the effectiveness of strategies to change the behaviour of health professionals and the organisation of care to promote weight reduction in overweight and obese people.
Search methods
We updated the search for primary studies in the following databases, which were all interrogated from the previous (version 2) search date to May 2009: The Cochrane Central Register of Controlled Trials (which at this time incorporated all EPOC Specialised Register material) (The Cochrane Library 2009, Issue 1), MEDLINE (Ovid), EMBASE (Ovid), CINAHL (EBSCO), and PsycINFO (Ovid). We identified further potentially relevant studies from the reference lists of included studies.
Selection criteria
Randomised controlled trials (RCTs) that compared routine provision of care with interventions aimed either at changing the behaviour of healthcare professionals or the organisation of care to promote weight reduction in overweight or obese adults.
Data collection and analysis
Two reviewers independently extracted data and assessed study quality.
Main results
We included six RCTs, involving more than 246 health professionals and 1324 overweight or obese patients. Four of the trials targeted professionals and two targeted the organisation of care. Most of the studies had methodological or reporting weaknesses indicating a risk of bias.
Meta-analysis of three trials that evaluated educational interventions aimed at GPs suggested that, compared to standard care, such interventions could reduce the average weight of patients after a year (by 1.2 kg, 95% CI −0.4 to 2.8 kg); however, there was moderate unexplained heterogeneity between their results (I2 = 41%). One trial found that reminders could change doctors’ practice, resulting in a significant reduction in weight among men (by 11.2 kg, 95% CI 1.7 to 20.7 kg) but not among women (who reduced weight by 1.3 kg, 95% CI −4.1 to 6.7 kg). One trial found that patients may lose more weight after a year if the care was provided by a dietitian (by 5.6 kg, 95% CI 4.8 to 6.4 kg) or by a doctor-dietitian team (by 6 kg, 95% CI 5 to 7 kg), as compared with standard care. One trial found no significant difference between standard care and either mail or phone interventions in reducing patients’ weight.
Authors’ conclusions
Most of the included trials had methodological or reporting weaknesses and were heterogeneous in terms of participants, interventions, outcomes, and settings, so we cannot draw any firm conclusions about the effectiveness of the interventions. All of the evaluated interventions would need further investigation before it was possible to recommend them as effective strategies.
Medical Subject Headings (MeSH): Body Weight, Controlled Clinical Trials as Topic, Delivery of Health Care [organization & administration; standards], Obesity [psychology; *therapy], Overweight [psychology; therapy], Patient Education as Topic, Professional Practice [organization & administration; *standards], Randomized Controlled Trials as Topic, Weight Loss
MeSH check words: Adult, Female, Humans, Male
BACKGROUND
Description of the condition
The prevalence of obesity is increasing, both in the developed and developing world and if left unchecked, it will have huge implications for population health and for health services expenditure in coming decades (WHO 1998; WHO 2004). Benefits of weight loss for obese people have been shown in short-term studies, as measured by reduction in cardiovascular risk factors (for example lipids, insulin, and blood pressure) and improvement in psychological status (Garrow 2000).
Description of the intervention
Information on the effectiveness of interventions to promote weight loss in patients is available. Although there are gaps in the evidence, a number of potentially effective weight loss interventions have been identified: diet, exercise, and behavioural strategies for adults, in combination where possible; the use of maintenance strategies such as continued therapist contact; selected use of pharmaceutical interventions in conjunction with strategies to change lifestyle; and surgery for selected morbidly obese patients (Colquitt 2005; Curioni 2006; NICE 2006; Padwal 2003; Shaw 2005; Shaw 2006; Thomas 2007).
The extent to which health professionals deliver such interventions within routine healthcare is uncertain. In the past, health professionals’ application of effective patient weight loss strategies may have been limited because of an abundance of research of variable quality with no consistent or clear conclusions, other than an apparent pessimism about the long-term effectiveness of treatments overall. Even with the availability of reviews stating the effectiveness of patient interventions (Douketis 1999; EHCB 1997; Glenny 1997; NHLBI 1998; O’Meara 2000), health professionals may be inconsistent in their application of such guidelines in routine care, often citing barriers such as lack of time, lack of access to the guidelines, or lack of confidence in the guidelines’ conclusions and their relevance to their clinical practice (Cabana 1999). Other potential barriers to effective management of obesity may include lack of access to appropriate support services and a lack of motivation to work with this patient group due to negative perceptions of overweight and obese people or of the efficacy of treatments (Frank 1993; HEA 1995; Price 1987; Puhl and Brownell 2001; Puhl and Heuer 2009; Summerbell 1998).
Interventions aimed at improving the way healthcare professionals work to reduce the weight of people who are obese or overweight can be divided into those targeted at the health professionals themselves and those targeted at the organisation of care.
Why it is important to do this review
In the UK the proportion of obese people is increasing rapidly and, if this trend continues, the UK could be a predominantly obese society by 2050 (Foresight 2007). It is essential to develop and implement effective strategies to prevent and treat obesity at the level of the individual, family, and healthcare provider, as well as in the environment (Foresight 2007). The purpose of this review is to evaluate the effectiveness of interventions at the provider level, including both interventions targeted directly at health professionals and interventions targeted at the organisation of care.
OBJECTIVES
To assess the effectiveness of strategies to change either or both the behaviour of health professionals and the organisation of care to promote weight reduction in overweight and obese people.
METHODS
Criteria for considering studies for this review
Types of studies
Randomised controlled trials (RCTs).
Types of participants
Fully qualified health professionals, working with overweight or obese adults.
Due to variability in the classification of overweight and obesity in primary studies, we included all trials enrolling adults described as overweight or obese. We used definitions based on body mass index (BMI - in kilogram/metre2): overweight was defined as a BMI over 25 but less than 30 and obesity as a BMI of 30 or over (EHCB 1997; NHLBI 1998).
We included studies of patients if a reduction in weight was specified as an objective of the intervention and outcome weight data were provided for the overweight or obese subpopulations within these patient groups. Thus, all patients in an included study had to be overweight or obese, or results from the overweight or obese subpopulation had to be provided separately.
Settings of studies
Healthcare organisations, defined as organisations that had health care as their primary objective. All patients in an included study had to be recruited in the context of a healthcare setting .
Types of interventions
We included any intervention that aimed to help a health professional implement an intervention targeting weight reduction in overweight or obese people. These interventions can be divided into two main categories according to the EPOC taxonomy (see Appendix 1) as one of the following.
i. Interventions targeting health professionals
Interventions aimed at improving the effectiveness of health professionals working to reduce the weight of overweight or obese people. This category includes strategies such as providing professionals with information or training on appropriate practice.
ii. Interventions targeting the organisation of care
Interventions aimed at changing the organisation of care directed at reducing the weight of overweight or obese people. This category includes interventions that were predominantly about changes in organisational systems, such as the introduction of multi-disciplinary teams, changes in skill mix, or in the setting of service delivery.
Comparators
We included only studies that had standard care as the comparator arm of the study.
We planned the following comparisons:
interventions targeting health professionals versus standard care;
interventions targeting the organisation of care versus standard organisation of care.
The standard care comparator groups had to meet either of these two criteria:
study participants receiving routine weight management service(s) in the context of their normal healthcare provision and setting, or:
study participants being informed of the availability of routine weight management service(s) in the context of their normal healthcare provision and setting.
Excluded studies
We excluded the following types of studies.
Studies that varied the clinical content or intensity of care, or both, of the intervention aimed at reducing weight, without a normal care control group. Therefore we excluded studies comparing the effectiveness of different durations of follow up, intervention, or frequency of consultation with obese or overweight people.
Studies that reported neither patients’ weight nor body mass index.
Studies that reported only knowledge or attitudes of health professionals or patient satisfaction, with no objective measure of professional performance or patient outcomes.
Types of outcome measures
We included any objective measure of provider performance consistent with EPOC guidelines (EPOC 2002) or patient outcomes. We also planned to report any available cost data.
Main outcomes
Patient’s body weight.
Other outcomes
Patient outcomes
body mass index (BMI); satisfaction with provider practice or healthcare provision; psychological outcomes (self-esteem, stress, depression, dietary restraint); morbidity (measures of disease status and sick leave); measures of body fat; effects on risk factors (differences in cholesterol levels, blood pressure); patient behaviour (attendance levels at weight management or physical exercise programmes); the number of withdrawals from treatment.
Health professional outcomes
measures of health practitioners’ behaviour, knowledge, attitudes, or satisfaction.
Search methods for identification of studies
-
For the first version of this review:
We utilised expertise used to develop search strategies for EPOC (EPOC 2009a) and the Effective Health Care Bulletin on obesity (EHCB 1997) to develop a search strategy for this review.
We searched the following databases : MEDLINE Ovid CD-ROM (1966 - 1/1998), PsycLit Silverplatter CD-ROM (1974 - 12/1997), EMBASE (Ovid via Bids) (1979 - 12/1997), Cinahl ARC Service (WinSPIRS online) (1982 - 11/1997), SIGLE Blaiseline (1980 - 11/1997), Sociofile ARC service (WinSPIRS online) (1974 - 10/1997), Dissertation Abstracts Dialog Corporation Dialog service (1861 - 1/1998), Conference Papers Index Dialog Corporation Dialog service (1973 - 1/1998), Resource Database in Continuing Medical Education (searched 6/1997). Copies of the full search strategies are available on request from the first author.
We also searched the following Cochrane Review Group specialised registers using ‘overweight’ and ‘obesity’ as the basis for key terms (EPOC 2009a): EPOC (5/1997), Cochrane Depression, Anxiety and Neurosis Group (8/1997), Cochrane Diabetes Group Register (8/1997). We also searched The Cochrane Controlled Trials Register (CCTR) (9/1997).
We undertook the following searches of key journals according to Cochrane criteria: International Journal of Obesity (1977 - 12/1997), European Journal of Clinical Nutrition (1988 - 12/1997), Journal of Human Nutrition and Dietetics (1988 - 12/1991), Human Nutrition: Clinical Nutrition (1982 - 12/1987), Human Nutrition: Applied Nutrition (1982 - 12/1987), Health Psychology (1993 - 12/1997), Obesity Research (1993 - 1994). We contacted experts in this field through the Association for the Study of Obesity (ASO), the British Dietetic Association (BDA), and the Journal of the American Dietetic Association (JADA) and asked them to notify us of potentially relevant papers. EPOC colleagues undertaking a review of preventive care identified further potentially relevant studies from their searches and the reference lists of included studies.
-
For the second version of this review:
For this update, we searched the following databases using the original search strategies: Medline Ovid CD-ROM Database (1/1997 - 4/2000), EMBASE Ovid via BIDS web (1/1998 - 2/2000), Cinahl Ovid CD-ROM Database (11/1997 - 2/2000), PsycLit Ovid Online (1/1997 - 5/2000), Sigle Blaiseline (1980 - 4/2000). We also searched the EPOC register and pending databases (4/2000). We did not search additional databases that were searched for the original production of the review again due to their low yield of relevant studies and the cost of accessing these databases.
-
For the third version of this review:
We updated the search for primary studies in the following databases, which were all interrogated from the previous (version 2) search date to May 2009:
The Cochrane Central Register of Controlled Trials (CENTRAL) (which at this time incorporated all EPOC Specialised Register material) (The Cochrane Library 2009, Issue 1), MEDLINE (Ovid), EMBASE (Ovid), CINAHL (EBSCO), PsycINFO (Ovid). We did not search SIGLE at this time as it had ceased being updated.
Search strategies for primary studies incorporate the methodological component of the EPOC search strategy combined with selected index terms and free text terms. We translated the MEDLINE search strategy into the other databases using the appropriate controlled vocabulary as applicable. We identified further potentially relevant studies from the reference lists of included studies.
We have included full search strategies for all databases in Appendix 2, Appendix 3, Appendix 4, and Appendix 5.
Data collection and analysis
Selection of studies
We searched for randomised controlled trials (RCTs) that compared routine provision of care with interventions aimed either at changing the behaviour of health professionals or the organisation of care to promote weight reduction in overweight and obese adults. We downloaded all titles and abstracts retrieved by electronic searching to the reference management database Endnote and removed duplicates. Two review authors (from KD, ME, GF, SK, TP, HA, JB) then examined the remaining references independently. We excluded those studies which clearly did not meet the inclusion criteria and obtained copies of the full text of remaining references. Two review authors (from KD, ME, GF, SK, TP, HA, JB) assessed the eligibility of these papers independently. Two authors (from KD, ME, GF, SK) resolved disagreements by discussion. We documented reasons for exclusion.
Data extraction and management
Two reviewers (from KD, ME, SK, TP, HA, JB, HD, FB) independently extracted data on study design, patient characteristics, interventions, outcomes, and risk of bias to a form specially designed for the review (Appendix 6). We noted the length of follow up for outcome measurement because short-term studies may be misleading, given that patients do not always maintain their initial weight losses (EHCB 1997).
Assessment of risk of bias in included studies
We used The Cochrane Collaboration’s tool for assessing risk of bias (Higgins 2008) on six standard criteria: adequate sequence generation, concealment of allocation, blinded or objective assessment of primary outcome(s), adequately addressed incomplete outcome data, free from selective reporting, and free of other risk of bias. We used three additional criteria specified by EPOC (EPOC 2009b): similar baseline characteristics, reliable primary outcome measures, and adequate protection against contamination.
Measures of treatment effect
Where possible, we extracted the mean weight of participants in each arm at the end of the study and the standard deviation of this mean. We calculated the difference between the intervention and control arms in the final mean weight of participants, and its standard error, to summarise the effect of treatment. Some studies presented the overall treatment effect and its standard error, but not the final weight in each arm and, in these cases, we used these reported treatment effects directly.
If final mean weights in each arm and their SDs (or the difference in final mean weight and its SE) were not reported, we extracted the mean change in weight between baseline and the end of the study in each arm, and its standard deviation; hence we calculated the difference between the intervention and control arms in the mean change in weight of participants, and its standard error, to summarise the effect of treatment.
For one study (Rogers 1982), final weight was not reported but the average amount that patients were overweight was reported by treatment arm at the end of the study, so we used this as the primary outcome.
If results were presented at more than one time point, we used the results for the longest duration of follow up in our primary meta-analysis.
Unit of analysis issues
We noted whether studies randomised patients or healthcare providers (either GPs or GP practices). If analysis did not allow for clustering of patients within healthcare providers, we recorded a unit of analysis error, as such analysis tends to over-estimate the precision of the effect of treatment ( Goldstein 2003).
Dealing with missing data
If primary outcome data were missing, or only imputed data were reported, we contacted trial authors to request data on the outcomes among participants who were assessed.
Assessment of heterogeneity
We assessed heterogeneity between studies by visual inspection of forest plots, by estimation of the percentage of heterogeneity between trials which could not be ascribed to sampling variation (Higgins 2003), by a formal statistical test of the significance of the heterogeneity (Deeks 2001) and, when possible, by sub-group analyses (see below). If there was evidence of substantial heterogeneity, we investigated and reported the possible reasons for this.
Assessment of reporting biases
We had planned to examine funnel plots corresponding to meta-analysis of the primary outcome in order to assess the potential for small study effects such as publication bias. However, as we only found six included studies, three of which had data suitable for meta-analysis, we did not examine funnel plots.
Data synthesis
We have summarised the outcome data extracted from papers in Analysis 1.1.
The mean differences between the patient’s weight (or change in weight) in the intervention and standard care arms at the end of the each trial are presented in separate forest plots for educational interventions (Cohen 1991; Martin 2006; Moore 2003), reminders (Rogers 1982) and organisational interventions (Pritchard 1999; Sherwood 2006). For both educational interventions and organisational interventions, we used the generic inverse variance facility of Review Mananager 5 (RevMan 5) (RevMan 2008), because the trials of Moore 2003 and Pritchard 1999 reported the final weight (or change in weight) in the intervention arms relative to the standard care arm, rather than the final weight (or change in weight) in both intervention and standard care arms.
For the three trials that considered educational interventions, we pooled results in a meta-analysis using the mean difference method (Deeks 2008). We used a random-effects model with inverse variance weighting (DerSimonian 1986).
Subgroup analysis and investigation of heterogeneity
We performed sub-group analyses and grouped trials based on whether they reported final values of patients’ weight or the change from baseline. We considered factors such as the type of intervention, whether it was evidence-based, and the length of follow up in interpretation of any heterogeneity.
We had also planned to sub-group analyses by overweight and obesity, as they may hold different implications for health and treatment, but this was not possible because the included papers did not distinguish between such patients.
Sensitivity analysis
We examined results after one year’s follow up (or as close as possible to one year) in a sensitivity analysis.
RESULTS
Description of studies
See: Characteristics of included studies; Characteristics of excluded studies.
Results of the search
First version of review: from initial searches of electronic databases and requests for help, EH screened 7193 abstracts. Two authors (EH and AMG) independently assessed and cross-checked any studies that appeared relevant (244) . Two authors (EH and CS or SK) independently assessed 107 full text copies of papers; 95 studies failed to meet the inclusion criteria. We included 12 studies in the review.
Second version of the review: from the searches of electronic databases, EH screened 1612 abstracts. This elicited 33 abstracts for dual screening (potentially relevant studies/number of hits): MEDLINE 4/390, EMBASE 1/422, PsycLit 0/141, Sigle 0/71, CINAHL 0/521, EPOC register 13/21, EPOC pending database 15/46. Two authors (EH and CS) independently assessed and cross-checked these abstracts . In addition, we identified 18 studies through other means (direct notification to the authors, searches for other Cochrane reviews). From these, we retrieved 43 full paper copies which two authors (EH and CS) independently assessed for inclusion criteria.. Of these 43, we agreed to include seven in the review update. However, one of these papers (Haring 1976) was an additional report of an included paper (Rogers 1982), therefore we included only six new studies in the second version of this review. Tne authors resolved all discrepancies easily by discussion. Third version of review: our searches found 12,097 references. We excluded 11,698 of these from a reading of their title and abstract (where available) as they did not meet our inclusion criteria. We retrieved 399 references in full and two authors (from KD, ME, SK, TP, HA, JB) independently assessed these for inclusion. Of these papers, 367 clearly did not fulfil the inclusion criteria. Of the remaining 32 studies, 18 were included in the previous version of the review. Since some of the inclusion and exclusion criteria had changed for the current update (principally study design, routine comparator, and requirement that study be conducted in a healthcare setting), a number of studies previously included were now excluded (Atkinson 1977; Balch 1976; Counterweight Prog 2004; Ferstl 1975; Hagen 1974; Hakala 1994; Jeffery 1979; Jeffery 1982; Levitz 1974, Lindstrom 1976; McDonald 1984; Meyers 1996; Ogden 1997; Perri 1987; Richman 1996; Simkin-Silverman 1997). We excluded eight further studies after detailed inspection; we have reported the reasons for exclusion of these 26 studies in the Characteristics of excluded studies table. We identified six papers that met the inclusion criteria of this review, three of which (Cohen 1991; Pritchard 1999; Rogers 1982) were included in the previous version of the review.
Included studies
See: Characteristics of included studies and Table 1.
Table 1. Summary of methods and participants of included studies.
| Study ID | Methods | Participants | Patients |
|---|---|---|---|
| Cohen 1991 |
Design: CRCT Unit of allocation: Provider Unit of analysis error: Yes Power calculation: No |
Providers: 18 family physicians at one family health center |
Patients: 30 overweight or obese individuals (BMI > 27.3 or 27.8 for males and females respectively) Age: I: 59.3 and C: 59.7 years Gender: 73% females Ethnicity:unclear Diabetes :unclear IHD: all patients were hypertensive |
| Martin 2006 |
Design: CRCT Unit of allocation: Provider Unit of analysis error: No Power calculation: Yes |
Providers:8 physicians from two clinics |
Patients: 144 overweight or obese patients (BMI> 25) Age:I:40.7 (12.6), C:43.0 (11.4) years Gender: 100% female Ethnicity: 100% african-american Diabetes and IHD:unclear |
| Moore 2003 |
Design: CRCT Unit of allocation: Practice Unit of analysis error: No Power calculation: Yes |
Providers: 245 healthcare staff from 44 practices |
Patients: 843 obese patients (BMI>30) Age:I:48.8 (10.9), C:48.8 (12.2) years Gender: I: 75% and C: 73% female Ethnicity: unclear Diabetes and IHD:unclear |
| Pritchard 1999 |
Design: RCT Unit of allocation: Patients Unit of analysis error: No Power calculation: Yes |
Providers: one dietitian and unclear number of GPs at one practice |
Patients: 270 overweight or obese patients (BMI≥25) Age:Unclear Gender: 72% females Ethnicity: unclear Diabetes: unclear IHD:Unclear |
| Rogers 1982 |
Design: RCT Unit of allocation: Unclear Unit of analysis error: Unclear Power calculation: No |
Providers:Unclear number of physicians at cardiac, pulmonary and renal clinics |
Patients: 147 obese patients (whose weight exceeded 20% of their ideal weight) Age:unclear Gender: 77% females Ethnicity: unclear Diabetes: 33.3% IHD:Unclear |
| Sherwood 2006 |
Design: RCT Unit of allocation: Patient Unit of analysis error:No Power calculation: Yes |
Providers: Unclear number of trained nutritionists and/or exercise specialists |
Patients: 1801 overweight or obese patients (23/77) Age:50.7 yrs Gender: 72% females Ethnicity: 91% Caucasian Diabetes 5.5% IHD:Unclear (27.3% on IHD related medication) |
Interventions targeting health professionals
Four of the included studies compared professional interventions with standard care (Cohen 1991; Martin 2006; Moore 2003; Rogers 1982). They evaluated the effects of training and/or giving educational materials on obesity management to GPs alone (Cohen 1991; Martin 2006) or to GPs and their practice teams (Moore 2003), and the effects of reminders to doctors to perform specific preventive actions, such as recommending diets (Rogers 1982).
Cohen 1991 investigated the effect of a brief educational intervention (one session of unspecified length) on obesity management given by behavioural psychologists to GPs. Eighteen GPs were randomised, and 30 patients included. All patients who visited a health clinic and met the inclusion criteria for the study (diagnosed hypertension, a BMI of 27.8 or more in males or 27.3 or more in females, age between 20 and 75 years) were invited to participate. Of the 67 patients who met the inclusion criteria, 31 agreed to participate, one of whom was subsequently excluded due to other health problems. The study compared the effect of this intervention on the advice given by GPs to obese patients to that given with no intervention. No objective measures of provider performance were supplied. All 30 patients were assessed at baseline and six and 12 months after the start of the intervention. Martin 2006 assessed the effect of seven additional hours of education (number of sessions not specified) on obesity management. Eight GPs from two practices were randomised and 144 patients were recruited to the study. Patients who visited their primary care physician, at two family medicine clinics, and met the inclusion criteria for the study (female, age between 18 and 65 years, BMI ≥ 25, low income, attendee at the clinic for at least one year, and absence of any serious or uncontrolled medical condition) were invited to participate. Enrollment in the study continued until each physician had a maximum of 20 patients. The study compared the effect of the additional training and interventions tailored to the character of the overweight and obese patients by a multidisciplinary team delivered over six months in combination with the standard care provided by GPs. Both groups of GPs had received two hours of training on obesity management. No objective measures of provider performance were provided. One hundred and six patients were assessed at six months.
Moore 2003 evaluated the effect of three educational sessions delivered to GPs and their teams by four dietitians who were specifically trained for this study. Forty-four practices were randomised, with a total of 231 health professionals and 843 patients recruited to the study. The practice staff invited consecutively attending obese adults (BMI ≥ 30, 16 to 64 years) to participate in the trial during a six-month recruitment period. An objective measure of provider performance was made available by extracting information about clinician behaviour from medical records. Clinicians’ knowledge of obesity management was also assessed. Patients’ weight and BMI were measured at three, 12 and 18 months after the start of the intervention.
Rogers 1982 evaluated the effectiveness of reminders on hypertension, renal disease and obesity management. 147 overweight patients were randomised. The use of a computerised medical system which recommended the professional to take ‘corrective actions’ according to selected criteria was compared to traditional handwritten medical records. The patients’ weight was assessed at baseline, and after 12 to 15 and 22 to 24 months.
The studies were based in either the USA (family practice in Cohen 1991, primary care clinics in Martin 2006, hospital outpatient cardiac, pulmonary and renal clinics in Rogers 1982) or the UK (general practices Moore 2003). The clinical content of the interventions was explicitly based on research evidence in only one study (Moore 2003). One study included consultation with the health professionals who were targeted (Rogers 1982), but none of the studies included any consumer involvement prior to the intervention, as part of the study design.
The studies differed in the degree to which patients were overweight. One study included patients who were 120% overweight (Rogers 1982), but it was not clear how these percentages were determined. Two studies specified that included patients had to be over a certain BMI, but did not report the baseline values for the sample (Martin 2006; Moore 2003). Cohen 1991 reported average baseline BMI value for their patient population (34.1). None of the studies stated the proportion of patients who were in the overweight and obese categories, and only one study stated the prevalence of diabetes: 33.3% (Rogers 1982). None of the studies gave the rate of ischaemic heart disease in their patient populations, although one study (Cohen 1991) restricted participants to those who were hypertensive.
The mean age of the included patients was reported in three of the studies: 59.5 (Cohen 1991), 41.8 (Martin 2006), and 48.6 years (Moore 2003).The percentages of women were 64% (Cohen 1991), 100% (Martin 2006), and 74% (Moore 2003). Ethnicity was reported only in Martin 2006, in which all of the participants were African-American.
Two studies (Cohen 1991; Rogers 1982) did not allow for clustering of patients within healthcare providers in analysis, whereas the other two studies (Martin 2006; Moore 2003) did.
The outcomes measured varied considerably but all of the studies measured patients’ weight or weight change. Two studies measured some form of physician behaviour change (Moore 2003; Rogers 1982). Outcomes were measured with follow up periods varying from six (Martin 2006) to 24 months (Rogers 1982) from the initiation of intervention.
Two studies reported power calculations (Martin 2006; Moore 2003). No studies provided cost data.
Interventions targeting the organisation of care
Two included studies evaluated organisational interventions (Pritchard 1999; Sherwood 2006). Neither study reported any consultation with the health professionals who were targeted or included any consumer involvement. Neither study was reported to be based on research evidence.
Pritchard 1999 assessed interventions delivered by dietitians alone or in combination with GPs. The study randomised 273 patients and included 270 obese patients. It compared six counselling sessions given either by a dietitian only, or by a doctor-dietitian team, with standard care. Patients’ weight was assessed at baseline and 12 months after the start of the intervention, as were blood pressure, glycated haemoglobin, and medication use.
Sherwood 2006 assessed a series of ten sessions of weight loss advice delivered by mail or phone, compared with standard care, which consisted of informing patient participants of the availability of routine weight management services in the context of their normal healthcare provision. These routine weight management services were available to all three groups and the proportion of patients who took up these services was noted. In total 1801 overweight patients were randomised into three groups. Patients’ weight was assessed at baseline and after 24 months, and was self-reported at six, 12, and 18 months. Participation in weight management programmes was also assessed (i.e. activation of treatment, number of sessions completed, and completion of the whole programme). Both of the organisational interventions were based in the community (Australian general practice in Pritchard 1999; at home or in community clinics in the USA in Sherwood 2006).
The studies differed in the degree to which patients were overweight. One study included only patients over a certain BMI, but did not report the baseline value of BMI for the sample (Pritchard 1999). Sherwood 2006 was the only study that stated the proportion of patients who were in the overweight (27%, BMI 25 to 29.9) and obese (73%, BMI 30to > 40) categories.One study (Sherwood 2006) reported the prevalence of diabetes (5.5%). None of the studies gave the prevalence of Ischaemic heart disease in their patient populations, although Pritchard 1999 reported the prevalence of hypertension (35%), and Sherwood 2006 reported that 27% of the patient population were taking medications for cardiovascular disease.
The mean age of the patient population was reported in one of the studies (50.7 years in Sherwood 2006). Pritchard 1999 reported that 27% of the included patients were over 50 years. The percentages of women were 62% (Pritchard 1999) and 72% (Sherwood 2006). Sherwood 2006 reported that 91% of participants were categorised as “white”.
Both studies randomised patients (Pritchard 1999; Sherwood 2006). One study (Pritchard 1999) did not allow for clustering of patients within healthcare providers in analysis; it was unclear whether Sherwood 2006 did so.
The outcomes measured varied but both studies measured patients’ weight (Pritchard 1999) or weight change (Sherwood 2006). Neither study measured any form of physician behaviour change. Outcomes were measured with follow-up periods varying from 12 months (Pritchard 1999) to 24 months (Sherwood 2006).
Both organisational studies reported power calculations and provided cost data (Pritchard 1999; Sherwood 2006).
Excluded studies
In total, we excluded 26 studies after obtaining full copies of the papers. The main reasons for exclusion were: lack of a standard care arm (eight); patients were not recruited in a healthcare setting (four); not all patients were overweight or obese (three); no objective outcome data were recorded/available for one or both arms (three); study was not an RCT (three); intervention was not led by a healthcare professional (one); non-adult patients (one); and intervention simply added a new component of care (one) (see Characteristics of excluded studies).
Risk of bias in included studies
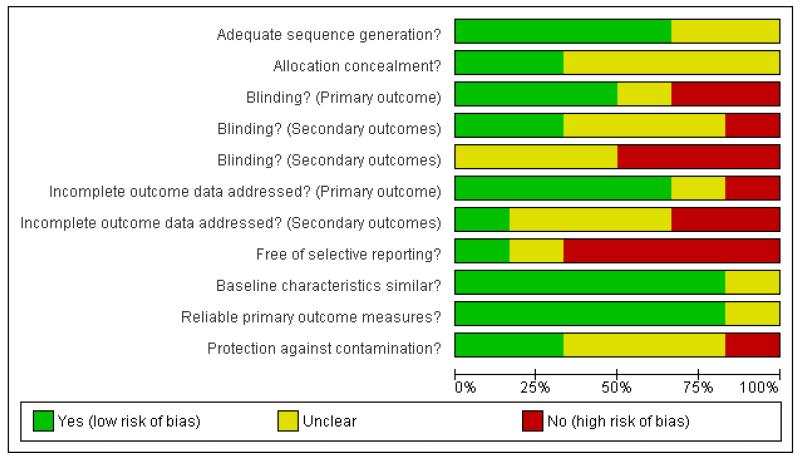
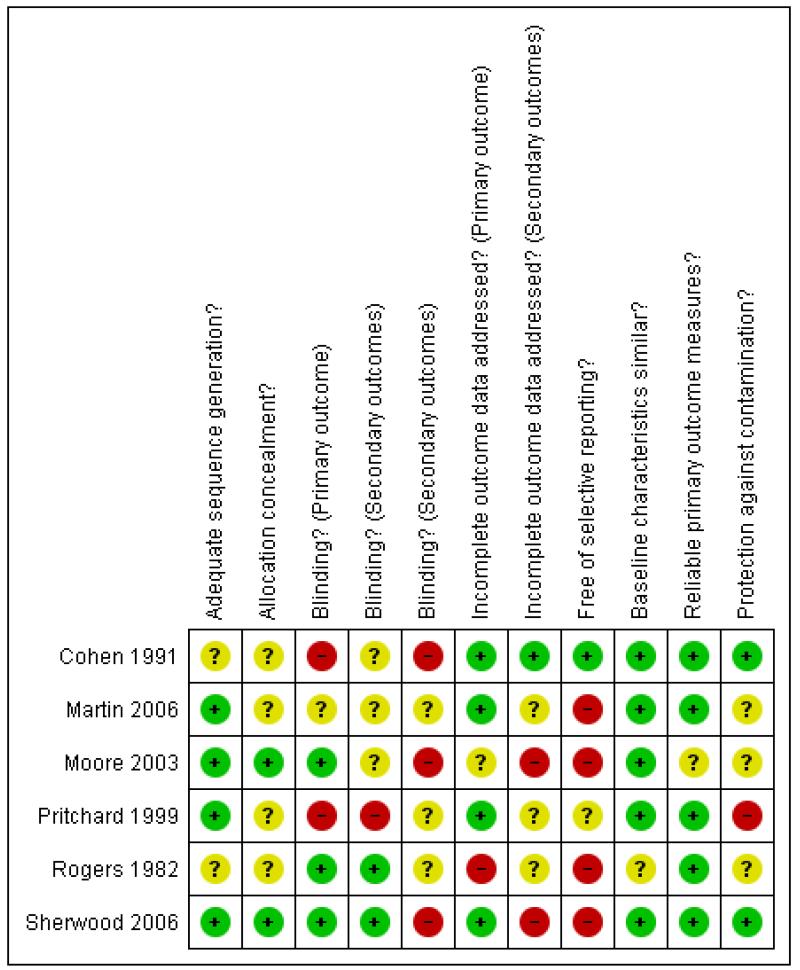
We have described the risk of bias in included studies in the ‘Risk of Bias’ tables within Characteristics of included studies and summarised in Figure 1 and Figure 2.
Figure 1. Risk of bias graph: review authors’ assessment of each risk of bias domain presented as percentages across all included studies.
Figure 2. Risk of bias summary: review authors’ assessment of the risk of bias of the individual domains for each included study.
Sherwood 2006 had a low risk of bias according to all but one of the specified criteria (i.e.“free of selective reporting”). The remaining five studies had several methodological or reporting weaknesses that suggest a risk of bias.
Allocation
Four studies reported their method of sequence generation (Martin 2006 ; Moore 2003 ; Pritchard 1999; Sherwood 2006) and two of them also attempted to conceal the allocation of intervention (Moore 2003; Sherwood 2006).
Blinding
Three studies blinded the assessors of the primary outcome (weight or weight change) (Moore 2003; Rogers 1982; Sherwood 2006) and two also blinded the assessors of at least some of the secondary outcomes (Rogers 1982; Sherwood 2006).
Incomplete outcome data
Four studies appropriately managed incomplete data from the primary outcomes (Cohen 1991; Martin 2006; Pritchard 1999; Sherwood 2006) and one did so for its secondary outcomes as this study had no drop-outs (Cohen 1991). In Pritchard 1999 the drop-out rate was 45% in the dietitian arm and 29% in both the doctor/dietitian and in standard care arms.
Selective reporting
Only one study reported results for all of the outcomes that were specified in the methods section (Cohen 1991).
Other potential sources of bias
Five studies provided baseline data demonstrating that the treatment groups were not significantly different (Cohen 1991; Martin 2006; Moore 2003; Pritchard 1999; Sherwood 2006). Weight was measured in a reliable manner (by a health professional in a clinical setting) in five studies (Cohen 1991; Martin 2006; Pritchard 1999; Rogers 1982; Sherwood 2006). Three studies took steps to ensure that the control group was not contaminated by knowledge or change in practice from the intervention groups (Cohen 1991; Pritchard 1999; Sherwood 2006).
Effects of interventions
The effects of the interventions on both health professionals’ behaviour and patients’ outcomes are reported in the data synthesis tables in Analysis 1.1.
Interventions targeting health professionals versus standard care
Three included studies evaluated educational interventions targeted at GPs.
Cohen 1991 reported that, on average, patients in the intervention group lost more weight than those in the control group - 2.4 kg at six months and 2.2 kg at 12 months - although only the difference at six months was statistically significant (P < 0.05). However, the small number of included patients (n = 30), combined with a potential unit of analysis error, means that the results of this study should be interpreted with care.
Martin 2006 reported that, on average, patients in the intervention group lost significantly more weight than those in the control group at six months (mean difference = 1.69 kg, P < 0.01).
Moore 2003 found evidence of a change in clinicians’ behaviours: those receiving the intervention were more likely to discuss weight, record weight, record a target weight, and have a dietary target than those in the control group. The results also suggested that professionals’ knowledge of obesity management had improved. Nevertheless, for about half the patients recruited to the trial, medical records showed no indication that the patients were counselled about their weight, and the study found no statistically significant difference between the intervention and control groups in either weight or BMI at three, 12, or 18 months.
As Cohen 1991, Martin 2006, and Moore 2003 evaluated similar interventions, we combined their results in a meta-analysis using a random effects model (Analysis 2.1), and results at the longest follow up available: 12, six, and 18 months respectively. Pooling the studies showed that patients whose GPs received the intervention lost on average, 1.2 kg more than patients receiving standard care, but this difference in weight between the groups was not statistically significant (95% CI ranged from 0.4 kg more weight lost in the control group to 2.8 kg more weight lost in the intervention group). The findings of the three studies showed moderate heterogeneity (I2 = 41%), largely because the study of Moore 2003 found the intervention had little effect, whereas the two other studies found that it helped patients lose weight. Sensitivity analysis (Analysis 2.2), using results from 12, six, and 12 months respectively, gave similar pooled results.
The study of Cohen 1991 did not allow for clustering of patients within GPs in analysis of their findings; and therefore it probably under-estimated the standard deviation of the patients’ weight change and hence received undue weight in the meta-analysis. However, correct standard deviations would have made little difference to the overall pooled result;, they would simply change simply changing it towards no difference between the intervention and control groups.
As recommended (Higgins 2008), we subgrouped studies by those that reported change in weight between baseline and the end of the study (Cohen 1991; Martin 2006) and those that reported weight at the end of the study (Moore 2003). Analysis of change from baseline is sometimes conducted in order to allow for differences between the groups at baseline despite randomisation. In the studies of Cohen 1991 and Moore 2003, the weights of patients in the intervention and control groups were similar at baseline, so the method of analysis should make little difference to the findings. In the study of Martin 2006, patients receiving standard care tended to be slightly heavier, so analysis of the patients’ final weight would have shown a more marked effect of the intervention than the analysis shown. This might have resulted in the pooled estimate of the effect showing a statistically significant reduction in weight in patients in the intervention group, but with greater heterogeneity between studies.
We do not present a funnel plot because results were available from only three studies.
One study evaluated the effects of reminders to doctors to perform specific actions (Rogers 1982). Rogers 1982 reported that reminders led to significantly more diet advice (13.5%) being given or diets being reviewed over two years. Men comprised about a quarter of the patients and men and women were analysed separately. At 10-15 months, men and women in the intervention group had lost 5.3 kg and 1.4 kg more weight respectively than those receiving standard care, but the difference between intervention and standard care was not statistically significant for either men or women. At 22-24 months, men in the intervention group had a net loss of 11.2 kg compared to standard care, whereas women had a net loss of 1.3 kg, but the differences between intervention and standard care were statistically significant only for men (Analysis 3.1). Although men lost more weight than women, the difference between men and women was not statistically significant.
Interventions targeting the organisation of care versus standard organisation of care
Pritchard 1999 compared interventions delivered by GPs and dietitians. The study found that, after one year, patients who received an intervention delivered by a doctor and dietitian lost 6.7 kg (95% CI, 5.9 to 7.5 kg) more weight than patients in the standard care group; those who received an intervention delivered by a dietitian lost 5.6 kg (95% CI, 4.8 to 6.4 kg) more weight than patients in the standard care group. However, 34% of randomised patients dropped out of the study and these results were based on analysis that assumed that patients’ weight remained unchanged after they dropped out of the study. For patients in the doctor-dietitian and dietitian-only groups, the cost of each additional kilogram lost over and above the weight change in the control group was $7.3 and $9.8 respectively (Australian dollars 1999). Patients in both the doctor/dietitian group and the dietitian group showed significant decreases in mean blood pressure compared to the standard care group, with net reductions of 12 mmHg (95% CI, 9 to 15 mmHg) and 7 mmHg (95% CI, 4 to 10 mmHg) respectively, and with a significantly greater decrease in the doctor/dietitian group. The drop-out from the weight loss program was 20% lower in the doctor/dietitian group and in the standard care group than in the dietitian-only group.
Sherwood 2006 assessed the method of delivery of a counselling intervention (mail or phone) to encourage weight loss. The study reported that although mail interventions were significantly more successful in encouraging patients to start on a weight loss programme, phone interventions were more successful in encouraging them to stay on the programme and to complete it. Despite this, the study found no significant differences between the groups in terms of change in weight between baseline and either 18 or 24 months. This may have been partly because a high proportion of participants did not start the 10-session weight reduction programme (phone 35%; mail 55%) and partly because 44% of randomised patients did not have their weight measured at the end of the study and analysis assumed no weight loss among these patients. Phone counselling was less cost effective than mail counselling or standard care, with an additional cost of $60/kilogram of weight loss.
We present the findings of these studies (Pritchard 1999; Sherwood 2006), after 12, 12, 24 and 24 months follow up respectively, in a forest plot (Analysis 4.1), but we did not combine their results in a meta-analysis as the interventions were clinically heterogeneous. Sensitivity analysis (Analysis 4.2), using results from 12, 12, 18 and 18 months respectively, gave similar results.
We do not present a funnel plot as no meta-analysis was performed.
DISCUSSION
Summary of main results
Six trials met our inclusion criteria: four evaluated educational or reminder interventions targeted at GPs (Cohen 1991; Martin 2006; Moore 2003; Rogers 1982) and two evaluated organisational interventions (Pritchard 1999; Sherwood 2006).
Interventions targeting health professionals
Meta-analysis of the three trials that evaluated educational interventions (Cohen 1991; Martin 2006; Moore 2003) found that these interventions were associated with a small reduction in patients’ weight. The random effects model that we used assumes that the pooled studies differ - for example in the type of population studied, or in the intervention assessed, or in the outcome measured - but that although the effect of the intervention therefore differs between studies, these effects are similar and cluster around a mean (Higgins 2009).
This estimated mean was a reduction in patients’ weight of 1.2 kg among patients whose GPs received educational interventions, compared with standard care; however, the uncertainty in our estimate of this mean was such that it might be anywhere between a weight loss of 2.8 kg and a weight gain of 0.4 kg in patients. This can be interpreted to suggest that in most populations and for most types of educational interventions, the average reduction in weight of patients will probably be greater if they receive care from a GP who received an educational intervention than if they received standard care.
One study (Rogers 1982) found that reminders could change doctors’ practice, but this did not result in a significant reduction in patients’ weight although the level of implementation was high (79%).
Interventions targeting the organisation of care
One study (Pritchard 1999) found that patients may benefit if dietitians only or doctor-dietitian teams delivered weight-loss counselling instead of doctors’ standard care.
One study (Sherwood 2006) found no evidence that mail or phone interventions were better than standard care in reducing patients’ weight.
Overall completeness and applicability of evidence
The included studies were heterogeneous in terms of participants, interventions, outcomes, and settings. In addition, considering the repertoire of interventions that may be employed to improve practice or the organisation of care (EPOC 2002), only a small number of different interventions have been evaluated rigorously. For example none of the interventions evaluated strategies to change health professionals’ attitudes towards overweight and obese people per se, weight loss counselling, or their beliefs on treatment efficacy. The low level of implementation of interventions found in Moore 2003 may reflect health professionals’ negative attitudes. These attitudes may constitute significant barriers to improving the effectiveness of weight reduction programmes (Frank 1993; HEA 1995; Price 1987; Puhl and Brownell 2001; Puhl and Heuer 2009; Summerbell 1998). Omission of the health professionals’ attitudes towards overweight or obese people is a limitation of the studies included in this review.
In the UK, US, and Australia, where the interventions were conducted, the prevalence of obesity is similar among men and women (Australian Bureau of Statistics 2008; Ogden 2006; Rennie 2005). However, in all included studies, samples were dominated by women (62%-100%), which may represent selection bias. If the imbalance was due to men’s reluctance to seek health care or their unwillingness to participate, it is possible that only highly motivated men were included. This may explain the greater effects on weight loss among men in Rogers 1982. However, the motivation of participants is unknown.
It is difficult to determine the extent to which the weight change strategies used in the included studies reflect what is currently known about good practice. Studies that are not based on good evidence run the risk of implementing changes that are not effective. Good evidence about patient interventions (for example Douketis 1999; EHCB 1997; Glenny 1997; NHLBI 1998; NICE 2006; O’Meara 2000) was not available when some of the studies in this review were published. However, of the three studies conducted and published after 2000 (Martin 2006; Moore 2003; Sherwood 2006), only one was explicitly evidence-based (Moore 2003).
In the reminder study (Rogers 1982), the recommendations for preventive actions were computer printouts attached to the patient’s chart. At that time GPs did not usually have their own computer. It is unclear whether point of care on-screen reminders (Shojania 2009) on obesity care would be more effective than the printed reminders evaluated in this study.
Only one of the included studies developed the intervention in consultation with the health professionals involved (Rogers 1982). This has the potential to improve uptake as professionals ‘buy-in’ to the guidelines. None of the included studies were developed in consultation with patients, which could have affected not only the focus of the intervention but also the drop-out rate.
A weight loss of ≥ 5% (or 3-5 kilograms) in obese people is reported to positively affect health outcomes, for example, by decreasing blood pressure, and is therefore considered clinically significant (CRD 1997; NHLBI 1998). In this review, the effect of the intervention on mean weight loss, if any, was modest (< 2%) (Cohen 1991; Martin 2006; Moore 2003; Sherwood 2006), with the exception of Pritchard 1999 and Rogers 1982 (only for the males in the intervention group) in which the mean weight loss exceeded the 5% or five- kilogrammes limit. Some studies (Martin 2006; Pritchard 1999; Sherwood 2006) reported not only mean weight loss but also percentage of participants who lost > 5% and/ or 10% of body weight, which may be a good measure of the success of an intervention from a clinical point of view.
Part of the clinical reasoning behind encouraging overweight and obese people to lose weight is that weight loss is suggested to reduce the risk factors for cardiovascular disease (for example high blood pressure, lipid, and insulin levels) and thereby decrease mortality. The benefits of weight loss for overweight and obese people may be measured by reduction in these risk factors. However, none of the studies included in this review evaluated the effects of interventions on lipid or insulin levels. Only two evaluated the effects of weight loss on blood pressure (Cohen 1991; Pritchard 1999) and only one (Pritchard 1999) found a significant reduction in blood pressure which was associated with a clinically significant weight loss.
Quality of the evidence
This review included six RCTs that involved more than 246 health professionals and 1324 overweight or obese patients and evaluated a variety of types of interventions. Most studies had methodological shortcomings, which weakens the reliability and generalisability of their findings. The heterogeneity of interventions, small sample sizes, high drop-out rates among patients, and sometimes low level of implementation make it difficult to draw firm conclusions on how the management of weight loss in obese patients might be improved.
In one study (Pritchard 1999) the weight of patients who dropped out was imputed, assuming their weight remained unchanged after they dropped out of the study. Since the drop-out rates varied substantially between the intervention groups (29%, 29%, and 45% respectively), the analyses may have yielded erroneously positive results for weight loss, since previous studies of long-term weight changes (that is ≥ 12 months) have shown that participants tend to regain their former weight after initial weight loss (EHCB 1997).
In one trial (Cohen 1991) the analysis did not allow for clustering of patients within healthcare providers, which is likely to overestimate the precision of the effect of treatment ( Goldstein 2003), and hence give the study undue weight in a meta-analysis. However, even if this study were given much less weight, the results of the meta-analysis of the two studies of educational interventions would be little changed as Cohen’s findings were consistent with those of the larger study of Martin 2006.
In the only evidence-based intervention included in this review, the level of implementation was very low (Moore 2003);only half of the patients were counselled about their weight and no conclusions about the effectiveness of the intervention could be drawn.
Potential biases in the review process
Although a comprehensive search was performed, including a search of the grey literature, the possibility of having missed relevant studies cannot be excluded. All references found by the search were sifted and data extracted by two reviewers independently. We included only RCTs in the review, as they generally provide the strongest level of evidence of causation available (Higgins 2008). Hence we have attempted to reduce bias in the review process.
The results of the meta-analysis must be interpreted with some caution. Firstly, the fact that the three included studies included all had different end-points (six, 12, and 18 months) could have biased the results due to the short-term character of weight loss. The significant effect reported at six months in Martin 2006 might have vanished if the intervention would have continued for yet another six months. Secondly, the allocation and randomisation processes were unclear in both studies that reported a significant weight loss, which may have resulted in an upward bias in effect of the meta-analysis (Egger 2003; Moher 1998; Schulz 1995). In addition, lack of clarity in allocation is problematic because, while interventions were aimed at the providers, characteristics of the providers were not compared at baseline, so we cannot tell if randomisation was effective at the provider level. Finally, even if the three studies were relatively similar (short educational intervention targeting GPs), the intervention provided in Martin 2006 was somewhat different since it also included considerably individualised intervention for patients.
The study of the effects of reminders (Rogers 1982) was likewise at risk of bias because of poor methodological quality. In particular, the study failed to report adequate methods of randomisation or concealment of allocation.
One of the two studies of organisational interventions was at low risk of bias. Sherwood 2006 - which reported that the intervention had virtually no effect on participants’ weight - satisfied most methodological criteria but Pritchard 1999 - which reported a very large and statistically significant effect - failed to confirm adequate concealment of allocation or blinding.
In summary, the beneficial effects of the intervention reported by Cohen 1991, Martin 2006, Pritchard 1999, and Rogers 1982 may be influenced by bias consequent to poor methodological quality. The better quality studies (Moore 2003; Sherwood 2006) showed little effect of the intervention.
There is the additional threat of publication bias: studies reporting a beneficial effect of the intervention or a larger effect size may be published, while a similar amount of data pointing in the other direction may remain unpublished (Hopewell 2009). Unfortunately, we were unable to assess publication bias in this review because of the small number of included studies and the heterogeneity of the interventions assessed. Although a comprehensive search was performed, including a search of the grey literature, we cannot be sure that we did not miss some relevant studies.
Agreements and disagreements with other studies or reviews
The low level of implementation of the weight loss intervention found in one of the studies (Moore 2003) is in accordance with studies showing that health professionals quite often fail to recommend or give advice on weight loss (Galuska 1999; Wadden 2000).
We are not aware of any other reviews of the evidence for interventions to change professional behaviour or the organisation of delivery of care for overweight or obese people.
AUTHORS’ CONCLUSIONS
Implications for practice
Health professionals, particularly primary care providers, have the potential to influence large numbers of patients. We currently have little evidence about how clinical practice or the organisation of care might be improved to help obese and overweight patients achieve weight loss.
Implications for research
Previous systematic reviews have shown that diet, exercise, and behavioural approaches in combination are effective strategies to manage overweight and obesity, at least in the short-term (CRD 1997; NHLBI 1998). Since obesity is such a major public health problem and resources for health care are limited, evidence-based and cost-effective healthcare interventions to improve the management of obese patients are urgently needed. The review highlighted the paucity of information about how clinical practice or the organisation of care for overweight and obese people might be improved. Although the evidence regarding brief training interventions and reminder systems was equivocal, these interventions may be worth further investigation, as is the inclusion of a dietitian in the care team.
Men and women are equally affected by the obesity epidemic, and research populations should be representative of the overweight and obese patients found in the healthcare setting under examination.The criteria for inclusion should be clear and participants adequately described (age, sex, weight, BMI, ethnicity, diseases, medication, blood pressure, etc). Characteristics of the patients that may modify the effects of interventions - for example, the degree of overweight or obesity classified according to international classifications (WHO 2005), should be documented, as should the patients’ readiness to change. Likewise, the characteristics of the health professionals targeted by the interventions could be documented. Characteristics could include their attitudes towards overweight or obese people, weight counselling behaviours, and their beliefs on the efficacy of treatment. This information would aid in the interpretation and the generalisability of the results and facilitate the appropriate targeting of interventions to the various sub-populations.
The review revealed only a small number of well-designed RCTs, which generally constitute the best available evidence of effectiveness (Higgins 2008). Of the studies assessed for inclusion in the review, few studies included a standard care arm, and few of these included explicitly reported that the intervention was evidence based. In undertaking new studies, care should be taken to ensure that innovative interventions are always compared to ‘standard care,’ in order to assess whether any improvements of health professionals’ practice or patient outcomes are above what would be expected from current practice. As far as possible, future studies should be based on effective evidence-based patient interventions (CRD 1997; NHLBI 1998).To ensure high methodological quality of studies, the following aspects should be given particular attention: power calculations should be performed in order to ensure a sample size that is adequate to detect a clinically important reduction in weight; and study designs should include adequate follow up of both patients and providers. Further, interventions should be designed in consultation with the health professionals to whom they are targeted, because this has the potential to improve uptake as they ‘buy-in’ to the guidelines, and healthcare consumers should also be consulted in order to ensure that the intervention has the right focus and is acceptable to them (The Counterweight Project Team 2008).
The randomisation process should be clearly described and the sequence adequately generated, and attempts should be made to conceal the allocation of professionals or patients, or both, to the intervention or control group. Primary outcome(s) should be blindly or objectively assessed, incomplete outcome data should be adequately addressed, and the study should be free from selective reporting and of other risk of bias (Higgins 2008). Furthermore, care should be taken to ensure comparability between groups at baseline or analysis should include the baseline value of the outcome as a covariate, or both (Senn 1994). Care should be taken to ensure that the primary outcome measures are reliable and that the study design adequately protects against contamination (EPOC 2009b). Analysis should be by intention-to-treat and allow for clustering within healthcare professionals. Guidelines for the reporting of clinical trials should be followed to ensure that a fair appraisal can be made of the points in the trial design at which bias could have been introduced (for example CONSORT 2001; CONSORT CRCT 2004).
There is a need for investigators to adopt standard measures for assessing patient outcomes. To allow easier comparison of effectiveness across different interventions, mean weight (kg) and BMI are recommended measures (EHCB 1997; Glenny 1997; NHLBI 1998). Reporting the proportions of participants with weight loss greater than 5% or 5 kg may be a good additional measure of the success of an intervention from a clinical point of view. Studies also need to focus on objective process and patient outcomes related to cardiovascular risk factors because of the relationship between obesity and cardiovascular disease, and therefore assessment of beneficial effects of interventions should take into account changes in these outcomes.
Since clear anti-fat attitudes and discrimination against overweight people have been documented in the healthcare sector (Puhl and Brownell 2001: Puhl and Heuer 2009), future interventions to improve weight loss management could usefully seek to address health professionals’ negative attitudes. Negative attitudes in health professionals may also result in obese people failing to seek health care (Brownell and Puhl 2003).
PLAIN LANGUAGE SUMMARY.
Interventions to change the behaviour of health professionals and the organisation of care to promote weight reduction in overweight and obese adults
Although obesity used to be confined largely to high income countries, the proportion of people who are overweight or obese is now increasing globally. Obesity is a major risk factor for a number of chronic diseases, which have negative consequences for individuals, populations, and health service costs.
We searched the scientific literature for randomised controlled trials that compared routine care with interventions that aimed to change either the way health professionals worked to achieve weight loss in overweight and obese people or interventions that aimed to change the organisation of care for them. We examined the effects of interventions targeting the behaviour of health professionals or the way care is organised, with the aim of improving the management of overweight and obese people in primary care, outpatient and community settings. Our review found six relevant trials, assessing more than 246 health professionals and 1324 patients. One of these trials reported that issuing doctors with reminders about weight management strategies helped to reduce their patients’ weight; one trial found that dietitian or doctor plus dietitian led weight-loss programmes were more efficient than routine care. One trial found no evidence that either mail or phone interventions were better than standard care in reducing patients’ weight.Three trials looked at brief training packages for doctors, but their findings were not consistent. All the included studies varied in terms of participants, interventions, outcomes, and settings. Consequently, we cannot draw any firm conclusions about the effectiveness of these interventions.
ACKNOWLEDGEMENTS
For the current version of this review we would like to acknowledge the help and advice of the following people: Managing editor Alain Mayhew, Lisa Bero, editor of the EPOC Group, and the peer reviewers (Donna Odierna, Nancy Santesso, Michelle Fiander and Russ Gruen) for their valuable comments and support in producing the review. Thanks are also due to Jannie Hedegaard and Elad Goldberg for translation of articles, and to Ann-Marie Glenny and Emma Harvey for their work on previous versions of the review.
We thank Doug Salzwedel for developing and running the search strategy.
For previous versions of this review the acknowledgements are as follows.
Many people helpfully gave their time for activities associated with this review. Particular thanks go to Julie Glanville (NHS Centre for Review and Dissemination, University of York) for her invaluable input in devising and running most of the electronic search strategies. Thanks also go to Cynthia Fraser (EPOC) for her invaluable work on the EMBASE and CCTR searches, and to Janette Colclough (JMB Morrell Library, University of York) for her input in devising the EMBASE strategy and for always being helpful and approachable with regard to our Information Resource needs. We are grateful to Pat Spoor (Cochrane Diabetes Group), Hugh McGuire (Cochrane Depression, Anxiety and Neurosis Group), Anne Taylor Vaisey (McMaster University), and Marlies Hulscher for their help in searching for studies. We are especially indebted to those people that helped with assessing non-English language papers for inclusion and providing translations of papers: Felix Gurtner and Eric Jensen (formerly of Arcovita, Switzerland), JP Glutting (Iberoamerican Cochrane Centre) and Shaun Treweek (Folkehelsa, Norway).
Thanks also are due to Mary Ann Thomson O’Brien and Jeremy Grimshaw for their invaluable advice in scoping the review; to the EPOC editorial group and peer reviewers (Mona Britton, Cindy Mulrow, and Brenda Wilson); and to Andrew Hill at the University of Leeds - all of whom provided us with useful suggestions for improving the protocol and the review. Last but not least, thanks go to Claire Allen and Graham Mowatt for their practical and administrative support in preparing the manuscripts for publication.
Emma Harvey formulated the idea for the review, and took the lead role in searching, identifying, and assessing studies, and in writing up the first version of the review. Anne-Marie Glenny, Sara Kirk, and Carolyn Summerbell helped to screen studies for inclusion and quality, checked data extraction, and provided comments and advice on drafts of the original protocol and review. Carolyn Summerbell took the lead in writing the first update of the review.
SOURCES OF SUPPORT
Internal sources
University of York, UK.
University Dental Hospital of Manchester, UK.
Nuffield Institute for Health, Leeds, UK.
Leeds Metropolitan University, UK.
University of Teesside, UK.
Newcastle University, Newcastle upon Tyne, UK.
External sources
UK NIHR Cochrane Programme Grant, UK.
CHARACTERISTICS OF STUDIES
Characteristics of included studies [ordered by study ID]
| Methods |
Design: CRCT Unit of allocation: Provider: Family practice residents were randomly assigned to either an experimental or a control group (Pg 25/ Col 1/ Para 1) Unit of analysis: Patient: (Table 2/ Pg 27). But no attempt was made to account for the clustering effect: Analysis of covariance was used to compare weight change and blood pressure change between the experimental and control groups and to compare blood pressure change between the weight losers and weight gainers, adjusting for initial values. The unpaired T test (two-tailed) was used to compare the experimental and control groups and the weight gainers and losers with respect to baseline age, weight, BMI, mean arterial pressure, number of medications, and number of visits to the physician. The Mann-Whitney U test was used to compare the experimental and control groups and the weight losers and gainers with regard to change in the number of medications. The Spearman rank correlation coefficient was used to assess the correlation between change in number of medications and change in blood pressure. (Pg 26 /Col 1/ para 7) Power calculation: No power calculation |
|
| Participants |
The number randomised into the trial:
Episodes of care: Number of visits to family practitioner: 9.7 (SD 3.0) intervention group; 5.2 (SD 2.4) control group, (Table 2/pg 27) Patients: 30 (Pg 26/ Col 2/ Para 1) (31 patients originally randomised but data for one patient who was excluded due to another health problem is not presented) Providers: 18 (Pg 26/ Col 2/ Para 1) Int: 10; Control: 8 Practices: 1 (The Lawrenceville Family Health Center, Pg 25/ Col 2/ Para2) Hospitals: N/A Communities or regions: N/A Characteristics of participating healthcare providers: Profession: Physicians: 18 family practice physicians (Pg 26/ Col 2/ Para1) Level of training: In post-graduate training: Residents (Pg 25/ Col 2/ Para 2) Age of health professional: Unclear Years since graduation or in practice: Unclear Proportion of eligible providers (or allocation units) who participated in the evaluation: Unclear Characteristics of the participating patients: Clinical problem(s) of participating patients: Overweight (BMI ≥ 25 but ≤ 30): Unclear: To be included participants had to be obese defined by a body mass of 27.8 or more in males and 27.3 in females. (Pg 25 /Col 2/ Para 3). Obese (BMI ≥ 30): Unclear: To be included participants had to be obese defined by a body mass of 27.8 or more in males and 27.3 in females. (Pg 25/ Col 2/ Para 3). Mean BMI 34.2 (int) and 34.0 (controls) (Table 1/ pg 26) But no distinction between overweight and obese populations provided. Diabetes: Unclear: Ischemic heart disease: All patients were hypertensive (systolic blood pressure > 139 or diastolic blood pressure > 89) (Pg 25/ col 2/ para 3) Other characteristics of participating patients: Age: Mean: 59.3 years (intervention group) 59.7 years (control group) (Table 1/pg 26) Baseline Weight /BMI Intervention: 91.8 kg /34.2 Control: 91.7 kg /34.0 Gender: 22 females, 8 males, equally distributed between the two groups (Pg 26/ Col 2/ Para 1) Ethnicity: Unclear Other: All hypertensive. Diagnosis of hypertension based on an average systolic BP of 140mm Hg or more on 2 or more readings or an average diastolic BP of 90mm Hg on 2 or more readings recorded in the FHC record. (Pg 25/ Col 2/ Para 3) Mean arterial pressure 105.6 (intervention group), 105.9 (control group) (Table 1/pg 26) Setting: Reimbursement system: Unclear Setting of care: General practice or community-based: The Lawrenceville Family Health Center, (Pg 25/ Col 2/ Para 2) Academic status of the setting of care: University (teaching) hospital: The University of Pittsburgh, St Margaret Memorial Hospital (Pg 25/ Col 1/ Para 3). The Lawrenceville Family Health Centre is the model family practice unit for the family practice residents at St Margaret Memorial Hospital. (Pg 25/ Col 2/ Para 2) Country: USA: Pittsburgh, PA (Pg 25/ Col 1/ Para 3) |
|
| Interventions |
Professional intervention:
Intervention group: At a residents physicians’ meeting all residents were informed of the broad principles of the trial; details that would influence the status of experimental or control groups were excluded. Physicians assigned to the experimental group were taught about the importance of weight reduction in managing hypertension and were provided with information about the effects of specific foods on body weight. The teaching session was conducted by a behavioural psychologist who has special interest and expertise in weight reduction. During the teaching session the physicians were questioned about their knowledge of the caloric content of foods and were given practical strategies for changing the dietary habits of their patients. The goal of the dietary advice was to reduce the caloric content of the diet without radically changing the patient’s life style. Methods of encouraging patients, such as reinforcement, were also discussed. The residents were given an instruction sheet that included low-calorie alternatives to high calorie foods. Other key strategies included seeing patients monthly and reviewing the previous day’s food intake with the patient. (Pg 25/ Col 2/ Para 4 - Pg 26/ Col 1/ Para 1) Control group: At a residents physicians meeting all residents were informed of the broad principles of the trial; details that would influence the status of experimental or control groups were excluded. The physicians in the control group received no special instructions or materials (Pg 25/ Col 2/ Para 4) Timing of intervention: Proximity to clinical decision-making: Remote educational sessions: A single training session was provided at the start of the trial (Pg 25/ Col 2/ Para 4 - Pg 26/ Col 1/ Para 1) Frequency/number of intervention events: A single training session was provided at the start of the trial (Pg 25/ Col 2/ Para 4 - Pg 26/ Col 1/ Para 1) Duration of intervention: A single training session of unknown duration was provided at the start of the trial (Pg 25/ Col 2/ Para 4 - Pg 26/ Col 1/ Para 1) Healthcare professional recipient: Intervention group: A single training session was provided to the experimental group physicians at the start of the trial (Pg 25/ Col 2/ Para 4 - Pg 26/ Col 1/ Para 1) Control group: The physicians in the control group received no special instructions or materials (Pg 25/ Col 2/ Para 4) Intervention deliverer: Intervention group: The teaching session was conducted by a behavioural psychologist who has special interest and expertise in weight reduction (Pg 25/ Col 2/ Para 5) Control group: N/A Types of targeted behaviour of the health professionals: To use the practical strategies taught to change their patient’s dietary habits (Pg 25/ Col 2/ Para 4 - Pg 26/ Col 1/ Para 1) Development of the intervention: Consultation with professional recipients: Unclear Evidence base of intervention: Unclear Consumer involvement: Not specified Barriers to change: Not done Source of funding for study: This study was conducted as part of Dr Cohen’s fellowship at St. Margaret Memorial Hospital (Pg 25/ Col 1/ Para 3) but the source of the funds wasn’t stated Ethical approval: Not reported |
|
| Outcomes |
Outcomes measured:
Weight change Blood pressure change (Change in mean arterial pressure change in mm Hg) Change in the number of medications Number of visits to the physician (Tables 2 - 3/ Pg 27) Length of time outcomes measured after initiation of the intervention: All outcomes measured at baseline, 6 months and 12 months (Table 1/ Pg 27) Ceiling effect: Identified by investigator: Unclear Identified by reviewer: No, potential for weight loss in population clear and demonstrated. (Table 1/ Pg 27). However, it was not clear to what extent physicians were already doing the intervention behaviours Losses to follow up: Number randomised: Intervention group: 15 (Pg 26/ Col 2/ Para 1) Control group: 15 (Pg 26/ Col 2/ Para 1) Number completing follow up: Intervention group: 15. Over the entire 12-month period of study there were no dropouts from the experimental group (Pg 26/ Col 2/ Para 2) Control group: 15. Over the entire 12-month period of study there were no drop-outs from the control group (Pg 26/ Col 2/ Para 2) Reasons for loss to follow up: Intervention group: N/A Control group: N/A Economic variables: Costs of the intervention: Not reported Changes in direct healthcare costs as a result of the intervention: Not reported Changes in non-healthcare costs as a result of the intervention: Not reported Costs associated with the intervention linked with provider or patient outcomes in an economic evaluation: Not reported |
|
| Notes | Unit of analysis error: Results were analysed without allowing for clustering of patients within physicians (page 26/Col1/Bottom para) | |
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Adequate sequence generation? | Unclear | The residents were stratified by residency year and randomly assigned to either control or experimental groups (Pg 25/ Col 2/ Para 4) |
| Allocation concealment? | Unclear | The residents were stratified by residency year and randomly assigned to either control or experimental groups (Pg 25/ Col 2/ Para 4) |
| Blinding? Primary outcome |
No | Weight change: At each visit the patients weight was recorded and any weight change noted (Pg 26/ Para 1/ Col 4) |
| Blinding? Secondary outcomes |
Unclear |
Mean arterial pressure change: At baseline the blood pressure was measured by a nurse who had been trained in accordance with recommendations of the American Heart Association (Pg 26/ Col 1/ Para 2) But no information was presented on later measurements. Number of visits: No information presented |
| Blinding? Secondary outcomes |
No | Change in number of antihypertensive medications: Management of the patient’s hypertension medication was left to the resident (Pg 26/ Para 1/ Col 4) |
| Incomplete outcome data addressed? Primary outcome |
Yes | Weight change: Over the entire 12 month period of the study there were no drop-outs from either experimental or control groups (Pg 26/ Col 2/ Para 2) |
| Incomplete outcome data addressed? Secondary outcomes |
Yes | Mean arterial pressure change / Number of visits / Change in number of antihypertensive medications: Over the entire 12 month period of the study there were no drop-outs from either experimental or control groups (Pg 26/ Col 2/ Para 2) |
| Free of selective reporting? | Yes | The authors state they intend to measure changes in weight, arterial blood pressure, number of antihypertensive agents prescribed, number of visits, variables which are all presented in the paper (Table 1 and Table 3/ Pg 27) |
| Baseline characteristics similar? | Yes | Table 1/pg26 |
| Reliable primary outcome measures? Average weight change |
Yes | Weight change: At baseline the patient’s weight was measured by a nurse who had been trained in accordance with recommendations of the American Heart Association (Pg 26/ Col 1/ Para 2). At 6 and 12 months the patient’s weight were noted by the same trained nurse (Pg 26/ Col 1/ Para 6) |
| Protection against contamination? | Yes | The physicians in the control group received no special instructions or materials. Physicians in the experimental group were asked not to share information from the educational sessions or special materials with control physicians. (Pg 25/ Col 2/ Para 4) There was no evidence of contamination between the experimental and control groups during the 12 months of the study. Chart audit revealed no use of the educational materials by control residents and interviews with them disclosed no awareness of information from the teaching session. (Pg 26/ Col 2/ Para 1) |
| Methods |
Design: CRCT Unit of allocation: Provider: Clinician: Individualised (stratified per clinic, balanced, nested design) (Pg 1413/ Col 2/ Para 3) Unit of analysis: The analysis of the primary response variable, weight change at 6 months, was effected with a mixed linear model that included treatment group (two levels) and clinic (four physician practices were recruited from each of two clinics) as fixed effects in a factorial arrangement. An additional random effect was introduced to account for sampling variability among physician practices and to provide the appropriate test statistic for the treatment effect, due to the nesting of subjects within practice. (Pg 1415/ Col 2/ Para 2) Power calculation: The sample size of 20 participants per physician was chosen based on a power analysis indicating that 16 subjects per physician (128 patients total) would give 80% power to detect a difference of 23% in success proportion under a one-tailed hypothesis. The final target sample size of 20 subjects per physician was judged adequate to allow for attrition and other sources of exclusion. The power analysis was conducted using a binomial model for a proportion of success of 5 lb or 2.27 kg within a physician practice in achieving weight loss. (Pg 1414/ Col 1/ Para 2) |
|
| Participants |
The number randomised into the trial:
Episodes of care: Tailored intervention group received six monthly active treatment visits during which their physician delivered the intervention. Each visit lasted ~15 minutes. (Pg 1414/ Col 2/ Para 3) Unclear for standard care participants - received no special instructions and were seen, as needed, for regular medical care. (Pg 1415/ Col 1/ Para 2) Patients: 144 (Fig 1 / Pg 1416) Providers: 8 (Pg 1413 / Col 2/ Para 3) Practices: 2 (Pg 1413 / Col 2/ Para 3) Hospitals: N/A Communities or regions: N/A Characteristics of participating healthcare providers: Profession: 8 physicians: 4 clinicians in each group, from 2 clinics (Pg 1413/ Col 2/ Para 3) Level of training: Fully trained (presumed rather than stated) (Pg 1413/ Col 2/ Para 3) Age of health professional: Unclear Years since graduation or in practice: Unclear Proportion of eligible providers (or allocation units) who participated in the evaluation: Unclear Characteristics of the participating patients: Clinical problem(s) of participating patients: pg refs needed Overweight (BMI ≥ 25 but ≤ 30): Not stated, all patients BMI ≥ 25 (Pg 1413/ Col 2/ Para 2) Obese (BMI ≤ 30): Not stated, all patients BMI ≥ 25 (Pg 1413/ Col 2/ Para 2) Diabetes: Unclear Ischemic heart disease: Unclear Other characteristics of participating patients: Age: Intervention group: Mean 40.69 years, SD 12.59 (n = 73). Control group: Mean 42.97 years, SD 11.38 (n = 71). (Table 1/ Pg 1415) Range: Inclusion criteria 18 to 65 years (Pg 1413/ Col 2/ Para 2) Baseline Weight kg(SD) Intervention (n = 71): 103.0 (17.95) Control (n = 73): 100.86 (20.8) Gender: 100% female (Pg 1413/ Col 2/ Para 2) Ethnicity: 100% African American (Pg 1413/ Col 2/ Para 2) Other: All low income. All with no serious or uncontrolled medical condition (Pg 1413/ Col 2/ Para 2) Setting: Reimbursement system: Fee for service: each physician received a $35.00 reimbursement for each office visit, which was the amount reimbursable under state Medicaid rules for similar office visits.” (Pg 1414/ Col 1/ Para 3) Setting of care: General practice-based (Pg 1413/ Col 2/ Para 2) Academic status of the setting of care: Non-teaching or university affiliated (Pg 1413/ Col 2/ Para 2) Unclear Country: USA, Baton Rouge, LA (Pg 1413/ Col 2/ Para 2) |
|
| Interventions |
Professional intervention:
Intervention group: Physicians from both groups initially received 2 hours of instruction on general obesity treatment, as outlined by the National Heart, Lung, and Blood Institute clinical practice guideline on the Identification, Evaluation, and Treatment of Overweight and Obesity in Adults (36). The four physicians providing tailored interventions then received an additional 7 hours of training, which addressed the assessment of stage of change, motivational interviewing, and techniques for the behavioral treatment of obesity. This training also included instruction on appropriate dietary recommendations, such as ways to reduce dietary fat intake, appropriate fruit and vegetable intake, how to read food labels, and how to modify recipes. Tailored Intervention Patients in the tailored intervention group received six monthly active treatment visits during which their physician delivered the intervention. Each visit lasted 15 minutes. Physicians received protocols for each monthly visit, and participants received both oral recommendations from their physician and handouts summarising the focus of each visit. The treatment materials delivered by the physician were individually prepared and tailored to each patient by a multidisciplinary research team consisting of the physician, a health psychologist, a registered dietitian, and an exercise physiologist. Physicians provided feedback and input to the multidisciplinary team, although the actual materials were written and prepared by the other research team members. Physicians had the option of either delivering the treatment using a prepared script or delivering the intervention with the assistance of an outline of main points to be covered. The content of the tailored interventions was obtained from the information provided by participants during the baseline assessment visit. Based on current eating practices and preferences, a dietitian provided recommendations to assist each participant in making healthier food choices and provided meal preparation tips. The exercise physiologist provided tailored physical activity recommendations based on the participant’s current activity levels, activity preferences, and any barriers to activity reported (e.g., medical conditions, lack of social support, unsafe neighbourhoods). A health psychologist developed tailored behavioral change recommendations based on Social Cognitive Theory, the Transtheoretical Model, and behavioral principles that targeted constructs such as self-efficacy, motivational readiness to change, social support, pros/cons of behavior change, self-reinforcement, realistic goal setting, stimulus control, and contingency management. The recommendations written by each expert were incorporated into the tailored intervention materials that were presented by the physician to the patient. In addition, the recommendations were tailored to the cultural and socioeconomic status backgrounds of the participants by taking cultural preferences into account when formulating dietary and exercise plans, providing educational materials prepared specifically for African Americans, and giving low-cost alternatives when making diet and physical activity recommendations. Topics of the monthly meetings included introductory information on weight loss, ways to decrease dietary fat, ways to increase physical activity, dealing with barriers to weight loss, healthy alternatives when eating out and shopping, and ways to staymotivated during weight loss efforts. (Pg 1414/ Col 2/ Para 2 - Pg 1415/ Col 1/ Para 1) Control group: “All physicians, regardless of treatment condition, initially received 2 hours of instruction on general obesity management, as outlined by the National Heart, Lung and Blood Institute clinical practice guideline on the Identification, Evaluation and Treatment of Overweight and Obesity in Adults.” (Pg 1414/ Col 2/ Para 2). “Standard care physicians were instructed to provide their usual obesity management conducted during a typical office visit. Standard care participants received no special instructions and were seen, as needed, for regular medical care. Information provided by standard care participants during the initial assessment was not used during any subsequent office visit.” (Pg 1415/ Col 1/ Para 2) Timing of intervention: Proximity to clinical decision-making: Remote educational sessions (Pg 1414/ Col 2/ Para 2) Frequency/number of intervention events: Unclear (Pg 1414/ Col 2/ Para 2) Duration of intervention: 7 hours of additional training (Pg 1414/ Col 2/ Para 3) Healthcare professional recipient: Intervention group: Unclear - simply states all physicians received 2 hours of training and those providing tailored intervention received an additional 7 hours of training. (Pg 1414/ Col 2/ Para 2) Control group: Unclear - simply states all physicians received 2 hours of training (Pg 1414/ Col 2/ Para 2) Intervention deliverer: Intervention group: Unclear who delivered the training session. (Pg 1414/ Col 2/ Para 2) The treatment materials delivered by the physician were individually prepared and tailored to each patient by a multidisciplinary research team consisting of the physician, a health psychologist, a registered dietitian, and an exercise physiologist. (Pg 1414/ Col 2/ Para 3) Control group: Unclear (Pg 1414/ Col 2/ Para 2) Types of targeted behaviour of the health professionals: Tailored intervention to patients during six, monthly active treatment visits. (Pg 1414/ Col 2/ Para 3-4) Development of the intervention: Consultation with professional recipients: Unclear Evidence-base of intervention: Unclear -Although the two hours of training provided to all the physicians was evidence-based: 2 hours of instruction on general obesity treatment, as outlined by NHLBI 1998. (Pg 1414/ Col 2/ Para 2) The additional 7 hours training for the intervention group was not referenced. Consumer involvement: Unclear Barriers to change: Not done Source of funding for study: The study was supported by The National Institute of Diabetes and Digestive and Kidney Diseases (Grant R01 DK57476) and co-sponsored by the Centers for Disease Control and Prevention, the Centre for Chronic Disease Prevention and Health Promotion, Division of Nutrition and Physical Activity, and by the Office of Research on Women’s Health (Pg 1419/ Col 1/ Para 3) Ethical approval: Unclear if the boards that approved the study were ethics boards: The study was approved by the institutional review boards of the Pennington Biomedical Research Centre, the Louisiana State University Health Sciences Centre, and the Baton Rouge Medical Centre (Baton Rouge, LA). (Pg 1414/ Col 1/ Para 3) |
|
| Outcomes |
Outcomes measured:
Weight change BMI Length of time outcomes measured after initiation of the intervention: Baseline and 6 months (Pg 1414/ Col 1/ Para 4) Ceiling effect: Identified by investigator: Unclear Identified by reviewer: Unclear Losses to follow up: Number randomised: Intervention group: 71 (Fig 1/ Pg 1416) Control group: 73 (Fig 1/ Pg 1416) Number completing follow up: Intervention group: 48 (6 months) (Fig 1/ Pg 1416) Control group: 58 (6 months) (Fig 1/ Pg 1416) Reasons for loss to follow up: Intervention group: 8 lost to follow up (1 died, 7 lost contact). 4 missed 6-month appointment. 3 no longer met medical inclusion criteria. (Fig 1/ Pg 1416) Control group: 19 lost to follow up (5 scheduling conflicts, 14 lost contact). 1 missed 6-month appointment. 3 no longer met medical inclusion criteria. (Fig 1/ Pg 1416) Economic variables: Costs of the intervention: Not reported Changes in direct healthcare costs as a result of the intervention: Not reported Changes in non-healthcare costs as a result of the intervention: Not reported Costs associated with the intervention linked with provider or patient outcomes in an economic evaluation: Not reported |
|
| Notes | No unit of analysis error. See Page 1415/Col2/“Statistcial analysis” section | |
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Adequate sequence generation? | Yes | (Pg 1414/ Col 1/ Para 1) |
| Allocation concealment? | Unclear | Not reported |
| Blinding? Primary outcome |
Unclear | Patient weight change. Blinding not referred to. |
| Blinding? Secondary outcomes |
Unclear | BMI |
| Blinding? Secondary outcomes |
Unclear | N/A |
| Incomplete outcome data addressed? Primary outcome |
Yes | Patient weight change: Good explanation of missing data and an intent-to-treat (ITT)1 analysis using baseline values carried forward for dropouts was completed. (Pg 1417/ Col 1/ Para 2). Overall 20% attrition rate; more drop outs from the intervention group (23 of 71 in the intervention group and 15 of 73 in the control group) . Also “Participants who dropped out of the study differed from study completers in that they tended to be younger, 35.4 (11.6) vs. 43.3 (11.6) years (P <= 0.01). In addition, the dropouts among the intervention group tended to have smaller waist circumferences (P < 0.01) and to be younger (P < 0.05) than the standard care dropouts. (Pg 1416/ Col 1/ Para 1) |
| Incomplete outcome data addressed? Secondary outcomes |
Unclear | Not reported |
| Free of selective reporting? | No | Participants’ weight and BMI were obtained at baseline and 6 months (Pg 141 / Col 1/ Para 4) but only weight change was reported in Table 1/ Pg 1416 |
| Baseline characteristics similar? | Yes | Patients in the intervention group are lighter, younger and have smaller waist circumferences but authors state that the differences are not statistical significant (Table 1/ Pg 1415 and Pg 1415 / Col 2/ Para 3) |
| Reliable primary outcome measures? Average weight change |
Yes | Patient weight: Standard measurement method by single clinician (Pg 414/ Col 1/ Para 4) |
| Protection against contamination? | Unclear | Clinicians in the intervention and control group worked at the same two clinics so communication likely |
| Methods |
Design: CRCT Unit of allocation: Practice: We have evaluated, in a cluster randomised trial, a training programme (the intervention) promoting the evidence based treatment of obesity, delivered to general practice teams (unit of randomisation). (Pg 1/ Col 2/ Para 3) Unit of analysis: No unit of analysis error: See Page 33/Col1/“sample size and analysis” section: “We analysed …. using Stata to account for both within cluster and between cluster variation.” Power calculation: A clinically significant effect of intervention can be achieved with as little as 5% (or 3-5 kg) weight loss in obese people. We designed the study to have 80% power to detect a mean difference in weight between treatment arms of approximately 3-5 kg, assuming 5% significance and a within practice correlation coefficient of 0.05. Allowing for withdrawal and loss to follow up of 15%, this gave a required number of patients per treatment arm of approximately 660, equivalent to 22 practices recruiting 30 patients each. (Pg3/ Col 1/ Para 4) |
|
| Participants |
The number randomised into the trial:
Episodes of care: Not clear Patients: Number invited unknown, 991 returned consent form, 843 completed baseline assessment and randomised. (Fig 2 / Pg 1086). Providers: 245 (Fig 1/ Pg 1086). Practices: 44 practices randomised (Fig 1/ Pg 1086) Hospitals: N/A Communities or regions: N/A Characteristics of participating healthcare providers: Profession: Unclear: 245 staff (elsewhere referred to as practitioners; a mix of GPs and practice nurses) in 44 general practices were randomised. (Fig 1/ Pg 1086). Level of training: Unclear (information not available) Age of health professional: Unclear (information not available) Years since graduation or in practice: Unclear (information not available) Proportion of eligible providers (or allocation units) who participated in the evaluation: From Figure 1/ Pg 1086, 161 practices invited to participate, 46 agreed and 44 were randomised. All 44 practices completed the trial. One practice (allocated to the intervention group) declined the training intervention but agreed to continue with outcome assessment, and one would only consent to the training if two of the three sessions were combined. Page 3 column 1, start of results section Characteristics of the participating patients: Clinical problem(s) of participating patients: Overweight (BMI ≥ 25 but ≤ 30): 0 Obese (BMI ≥ 30): The study protocol required practice staff to invite consecutively attending obese adults (body mass index equal to or greater than 30 kg/m2) aged 16 to 64 years to participate in the trial over a defined six-month recruitment period. (Pg 1086/ Col 1/ Para 2) Diabetes: Unclear Ischemic heart disease: Unclear Other characteristics of participating patients: All numbers from patients who completed baseline data collection and were randomised. 415 intervention; 428 control, 843 overall. Age: Mean (SD), Intervention group 48.8 (10.9); Control group 48.8 (12.2) years (Table 1/ Pg 1087) Gender: N (%) male; Intervention group 104 (25%); Control group 116 (27%). Overall 220 (Table 1/ Pg 1087) Ethnicity: Unclear Other: Mean (SD) weight: Intervention group 100.8 (18.1); Control group 100.2 (17. 4) Mean (SD) BMI: Intervention group 37.0 (5.7); Control group 36.9 (5.8). (Table 1/ Pg 1087) Setting: Reimbursement system: Unclear: UK NHS Primary Care; not described in terms of reimbursement system. Setting of care: General practice or community-based: We recruited practices from four health authority areas in the Northern and Yorkshire region of England during a four month period. (Pg 1085/ Col 2/ Para 5). Academic status of the setting of care: Unclear Country: UK: We recruited practices from four health authority areas in the Northern and Yorkshire region of England during a four-month period. (Pg 1085/ Col 2/ Para 5) |
|
| Interventions |
Professional intervention:
Intervention group: We delivered three 90-minute sessions, intended to be delivered at intervals of no less than one week and no more than two weeks apart, to the 22 intervention practices. We asked all general practitioners and practice nurses to attend all three sessions. Four dietitians were trained in the standardised delivery of the training and then delivered the programme to small group, multidisciplinary general practice teams. The programme promoted a model approach to obesity treatment, which incorporated best evidence and was perceived to be brief enough that primary care staff could deliver it to their patients. The training covered information on the clinical benefit of weight loss and effective treatment options, including reduction of dietary energy intake, increased physical activity, and pharmaceutical intervention. The model of obesity management entailed practitioners seeing patients regularly (about every two weeks) until they had lost 10% of their original body weight and then less regularly (about every one to two months) for maintenance of weight over a sustained period. Current and target weight and dietary and activity targets were to be recorded in the patients’ records to facilitate continuity of support across practice teams. Prescription of a moderate energy deficit diet was advocated, as recommended by the Scottish Intercollegiate Guidelines Network. A “ready reckoner” was produced to allow practitioners to estimate a patient’s daily energy requirement and then to calculate a daily 500 kcal (2.5 MJ) deficit. Diet sheets and supporting written resources facilitated the dietary prescription to patients. At the end of the three training sessions, practices devised individualised weight management protocols based on the model and were encouraged to implement this with patients recruited to the study. (Pg 1086/ Col 2/ Para 1-2) Control group: Control practices were asked to provide standard care to their patients. (Pg 1087/ Col 1/ Para 1). Note: Control practices still had to engage with patient recruitment. The study protocol required practice staff to invite consecutively attending obese adults (body mass index ≥ 30 kg/m2) aged 16 to 64 years to participate in the trial over a defined six-month recruitment period. Patients were asked to return a consent form to the practice by stamped addressed envelope or on their next visit. The recruitment strategy was extended to include assistance from study personnel and mail shots. Towards the end of the recruitment period, a researcher accessed the list of patients who had been recruited in the early stages and invited them to attend for collection of baseline data, so that all patients had been weighed within two months of randomisation. (Pg 1086/ Col 1/ Para 2) Timing of intervention: Proximity to clinical decision-making: Remote educational sessions: We delivered three 90 minute sessions, intended to be delivered at intervals of no less than one week and no more than two weeks apart, to the 22 intervention practices. We asked all general practitioners and practice nurses to attend all three sessions. (Pg 1086/ Col 2/ Para 1) Frequency/number of intervention events: 3 sessions (Pg 1086/ Col 2/ Para 1) Duration of intervention: Three 90 minutes sessions over 4 weeks (Pg 1086/ Col 2/ Para 1) Healthcare professional recipient: Intervention group: Group/practice: Four dietitians were trained in the standardised delivery of the training and then delivered the programme to small group, multidisciplinary general practice teams. “We asked all general practitioners and practice nurses to attend all three sessions” (Pg 1086/ Col 2/ Para 1 and Pg 1087/ Col1/ Para 1) Control group: None: Control practices were asked to provide standard care to their patients. Note: Control practices still had to engage with patient recruitment. (Pg 1086/ Col 1/ Para 2 and Pg 1087/ Col1/ Para 1) Intervention deliverer: Intervention group: Four dietitians were trained in the standardised delivery of the training and then delivered the programme. (Pg 1086/ Col 2/ Para 1) Control group: None. Types of targeted behaviour of the health professionals: The programme promoted a model approach to obesity treatment, which incorporated best evidence and was perceived to be brief enough that primary care staff could deliver it to their patients. (Pg 1086/ Col 2/ Para 1) Development of the intervention: Consultation with professional recipients: No: The educational strategy was based on a previous nutrition training programme. (Pg 1086/ Col 2/ Para 1) Evidence base of intervention: Yes: The programme promoted a model approach to obesity treatment, which incorporated best evidence and was perceived to be brief enough that primary care staff could deliver it to their patients. (Pg 1086/ Col 2/ Para 1) Consumer involvement: Not specified Barriers to change: Not clear Source of funding for study: NHS Executive, Northern and Yorkshire. (Pg 1089/ Col 2/ Para 2) Ethical approval: The Northern and Yorkshire regional medical research ethics committee and five local research ethics committees approved the study. (Pg 1089/ Col 2/ Para 4) |
|
| Outcomes |
Outcomes measured:
Weight and change in weight Clinician behaviour Clinician knowledge Length of time outcomes measured after initiation of the intervention: Weight and change in weight at three, 12, and 18 months: The primary outcome measure was difference in mean weight of patients between intervention and control practices 12 months after the intervention. We also measured difference in weight at three months and 18 months post-intervention. (Pg 1087 / Col 1/ Para 2) Clinician behaviour: Researchers extracted information from the medical records of those patients still participating in the trial, in both arms, one year after the intervention. (Pg 1087/ Col 1/ Para 3) Clinician knowledge: After the intervention (time point unclear): We measured knowledge of obesity management and self-reported behaviour in obesity management consultations for all practice staff before and after the intervention. (Pg 1087 / Col 1/ Para 2) Ceiling effect: Identified by investigator: Unclear: Implied but not stated Identified by reviewer: No: Plenty of room for weight loss in the patient population. However, unclear how frquently health care professionals are advising about weight loss Losses to follow up: Number randomised: Staff: Of 245 staff, 14 didn’t complete baseline assessment and don’t appear in the numbers randomised (Fig 1/ Pg 1086) Patients - 991 returned consent form; 148 of these were lost prior to completing baseline assessment; 843 randomised (Fig 2/ Pg 1086) Intervention group: 22 practices (Fig 1/ Pg 1086) 116 staff (Fig 1/ Pg 1086) 415 patients (Table 1/ Pg 1087) Control group: 22 practices (Fig 1/ Pg 1086) 115 staff (Fig 1/ Pg 1086) 428 patients (Table 1/ Pg 1087) Number completing follow up: Intervention group: 22 practices (Fig 1/ Pg 1086) 95 staff (at follow up) (Fig 1/ Pg 1086) 331 patients at 3 months (Fig 2/ Pg 1086) 279 patients at 12 months (Fig 2/ Pg 1086) 256 patients at 18 months (Fig 2/ Pg 1086) Control group: 22 practices (Fig 1/ Pg 1086) 97 staff (at follow up) (Fig 1/ Pg 1086) 333 patients at 3 months (Fig 2/ Pg 1086) 286 patients at 12 months (Fig 2/ Pg 1086) 275 patients at 18 months (Fig 2/ Pg 1086) Reasons for loss to follow up: Intervention group: Practices: N/A Staff: None given Patients: None given Control group: Practices: N/A Staff: None given Patients: None given Economic variables: Costs of the intervention: No Changes in direct healthcare costs as a result of the intervention: No Changes in non-healthcare costs as a result of the intervention: No Costs associated with the intervention linked with provider or patient outcomes in an economic evaluation: No |
|
| Notes | No unit of analysis error: See Page 33/Col1/“sample size and analysis” section | |
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Adequate sequence generation? | Yes | Raab and Butcher did the randomisation, using the method they described in 2001 (Raab 2001 - see also below), in which patient level characteristics (body mass index at recruitment, age, and sex) and practice level characteristics (practice size, socioeconomic status, and existence of dietetic service) were used to inform randomisation. One permutation of treatment allocation with acceptable balance was randomly selected, a method that ensured equal numbers of practices and approximately equal numbers of patients in both treatment arms. Researchers collecting baseline data contacted a distant member of the project team to ascertain intervention status. (Pg 1086/ Col 1/ Para 3) We initially considered randomisation stratified by Health Authority area and practice size. This would have ensured acceptable balance at the practice level and, in practical terms, would have meant that 50 per cent of each dietitian’s local practices would require intervention. In the long run, such a procedure could be expected to yield approximately equal distribution of patient characteristics at baseline, but for an individual trial balancing of allocation on baseline practice and patient-level characteristics in the design becomes more important as the number of clusters decreases. Since we perceived the number of clusters to be relatively small in each Health Authority area (for example, six in Scarborough), we felt it was particularly important to ensure good balance on these characteristics within each Health Authority area. Owing to patient recruitment occurring prior to practice allocation, it is possible to use additional information on patient-level as well as practice-level characteristics to balance the practice allocation. We will use the method described by Raab 2001 to randomly select one permutation of treatment allocation with acceptable balance. This method will ensure approximately equal numbers of patients and practices in both treatment arms. It will also balance practice and patient level characteristics thought to be important predictors of outcome: practice size, socioeconomic status and existence of a practice dietitian at the practice level; age, sex and body mass index at the patient level. (Moore 2001 Pg 337/ Col 1/ Para 3-4) |
| Allocation concealment? | Yes | Researchers collecting baseline data contacted a distant member of the project team to ascertain intervention status. (Pg 1086/ Col 1/ Para 3) |
| Blinding? Primary outcome |
Yes | Weight and change in weight: Patients were not aware of the intervention status of their practice, and researchers collecting outcome measurements from patients were blind to the intervention status of the practices, both before and after the intervention. Double blinding was not possible in this trial, as practice staff were inevitably aware of whether or not they had been trained. (Pg 1087/ Col 1/ Para 5) |
| Blinding? Secondary outcomes |
Unclear | Clinician behaviour: As above. It is unclear whether the research staff were blind to allocation for this outcome measure. (Pg 1087/ Col 1/ Para 5) |
| Blinding? Secondary outcomes |
No | Practitioners knowledge: As above. It is unclear whether the research staff were blind to allocation for this outcome measure. (Pg 1087/ Col 1/ Para 5) |
| Incomplete outcome data addressed? Primary outcome |
Unclear |
Weight and change in weight: Not specifically stated. Fig 2/ Pg 1086 shows the losses of patients to the study. These are of a similar proportion but were not formally statistically tested. Attritions not alluded to but high - 991 patients gave consent but only 843 attended for randomisation - of these, 664 attended 3 monthly follow up, 565 12 months follow up and 531 18 months follow up (Table 1 / Pg 1087) and it is not stated what proportion of missing patients were from the randomised vs. control groups |
| Incomplete outcome data addressed? Secondary outcomes |
No |
Clinician behaviour: Doesn’t state whether data collection for this outcome variable was blinded. Also, although data were collected for 670 patient records (Pg 1087/ Col 2/ Para 4) , the results reported are for lesser numbers and the number of omissions from each group are not reported (Table 4/ Pg 1088). Practitioners knowledge: 95% completed the baseline questionnaire and 83% the post-intervention assessment but again it is not stated what proportions of intervention vs control practices were represented. (Pg 1087/ Col 2/ Para 3). Also clinicians could be more likely to respond if they know the answers |
| Free of selective reporting? | No | In Moore 2001 the authors state they intend to measure “change in indicators of patients’ food choice” and “measures of patient psychological and physical well being will be measured using validated questionnaires” (Pg 338/ Col 1/ Para 5 of Moore 2001) and cite references for The Hospital Anxiety and Depression Scale and EuroQoL. These data are not reported in the primary report of the study |
| Baseline characteristics similar? | Yes | (Table 1/ Pg 1087) but no statistical testing of the differences reported |
| Reliable primary outcome measures? Average weight change |
Unclear | Weight and change in weight measured but who measured these outcomes and the method used was not clearly stated |
| Protection against contamination? | Unclear | As stated earlier, in an effort to further eliminate contamination, we offered training only to general practitioners and practice nurses. In reality, enforcing this research condition was difficult, and many additional practice staff, including district nurses and health visitors, showed up for the training. We detected no evidence of contamination between intervention groups, but this cannot be ruled out. (Pg 1088/ Col 2/ Para 2) |
| Methods |
Design: RCT Unit of allocation: Patients: Immediately after screening, the study dietitian used a table of random numbers to allocate each consecutive patient (Pg 312/ Col 2/ Para 3) Unit of analysis: Patients: A Chi2 test was used to compare the demographic composition of the study groups. Confidence intervals for differences in means were used to compare groups with respect to outcome measurements. (Pg 313/ Col 1/ Para 4) Power calculation: Based on an expected 5% weight reduction in the dietitian group and 10% in the doctor/ dietitian group, a minimum of 35 overweight patients per group was required to achieve a power of 0.9 that the null hypothesis would be rejected at the 0.5 level. (Pg 312/ Col 2/ Para 3) Number expected = 35 × 3 groups = 105 (Pg 312/ Col 2/ Para 3) Number recruited = 273 (Pg 313/ Col 2/ Para 4) Number overweight recruited = 270 (Table 1/ Pg 313) |
|
| Participants |
The number randomised into the trial:
Episodes of care: Dietitian group: 6 sessions (Pg 312/ Col 2/ Para 5) Dietitician/GP group: 6 sessions with dietitian plus 3 sessions with GP (Pg 312/ Col 2/ Para 7) Control group: Baseline and endpoint assessment plus standard care (Pg 313/ Col 1/ Para 1) Patients: 270 (plus 3 patients who had hypertension and/or diabetes but who were not overweight) (Table 1/ Pg 313) Providers: 1 Dietitcian (Pg 312/ Col 1/ Para 4). Unclear numbers for GPs Practices: 1 GP practice (Pg 312/ Col 1/ Para 4) Hospitals: N/A Communities or regions: N/A Characteristics of participating healthcare providers: Profession: Physicians (general practitioners) but numbers not stated. 1 Dietitician (Pg 312/ Col 1/ Para 4) Level of training: Fully trained general practitioners and nutritionist (Pg 312 / Col 1/ Para 4) Age of health professional: Unclear Years since graduation or in practice: Unclear Proportion of eligible providers (or allocation units) who participated in the evaluation: Unclear: Only a single site was used (a university general practice) but the number and proportion of general practitioners in this group practice that participated was not stated (Pg 312/ Col 1/ Para 4) Characteristics of the participating patients: Clinical problem(s) of participating patients: Overweight (BMI ≥ 25 but ≤ 30): Unclear: Patients with a body mass index (BMI) of more than 25 were diagnosed as overweight. (Pg 312/ Col 1/Para 6). Total n = 270 overweight participants (Table 1/ Pg 313). Number who were overweight vs obese was not stated. Obese (BMI ≥ 30): Unclear: Patients with a body mass index (BMI) of more than 25 were diagnosed as overweight (312/1/6). Total n = 270 overweight participants (Table 1/ Pg 313). But number overweight vs number obese not stated. Diabetes: Unclear: N = 17 (but some of these may not have been overweight) (Table 1/ Pg 313). Ischemic heart disease: IHD not stated, but 97 had hypertension. Some of these may not have been overweight (Table 1/ Pg 313) Other characteristics of participating patients: NB these data based only all 273 participating patients of whom 3 were not overweight and therefore not included in the results for this review Age: Unclear: 73% of patients were less than 50 years old (Pg 313/ Col 2/ Para 4) Baseline Weight (kg), mean (no SD provided): Doctor/dietitian group (n = 92): 91.7 Dietitian (n = 88): 85.5 Usual care (n = 90): 89.1 Baseline hypertension (mean blood pressure = diastolic BP + (systolic BP − diastolic BP)/3, in mm Hg), mean (no SD provided): Doctor/dietitian group (n = 33): 112 Dietitian (n = 30): 109 Usual care (n = 34):110 Baseline type 2 diabetes (% glycated haemoglobin), mean (no SD provided): Doctor/dietitian group: (n = 6) 8.0 Dietitian (n = 5): 8.2 Usual care (n = 6): 7.7 Gender: 75 men and 198 women (Pg 313/ Col 2/ Para 4) Ethnicity: Unclear Other: Socio-economic status quartile: 58% most disadvantaged, 20% more disadvantaged, 2% least disadvantaged. Occupation: 56% home duties (84% female), 20% driver/trade/labourer, 6% unemployed. 14% clerical/sales, 4% manager/professional. 22% without partners; 78% married or de facto. 31% had hypertension. (Pg 313/ Col 2/ Para 5-6) Setting : Reimbursement system: Unclear Setting of care: General practice (Pg 312/ Col 1/ Para 4) Academic status of the setting of care: Non-teaching or university affiliated: a university general practice (Pg 312/ Col 1/ Para 4) Country: Australia (Lockridge, near Perth, Western Australia (Pg 312/ Col 1/ Para 4) |
|
| Interventions |
Professional intervention:
Intervention groups: Dietitian group: Patients allocated to the dietitian group were invited to join the study by the dietitian at the time of screening. The dietitian conducted six individual counselling sessions, spaced equally, with the last session 12 months after recruitment. The initial session occupied 45 minutes, with 15 minutes for later sessions. Measurements were repeated at all sessions under similar conditions. Counselling focused on principles of good nutrition and exercise. The dietitian questioned life style and dietary patterns to identify problem areas. Counselling included advice on food shopping and cooking methods, food selection, meal planning, and exercise programmes. Patient kept food records and diet history was used in the counselling sessions to provide individual advice. Recommendations included restriction of total dietary energy, reduction of the fat component to no more than 30%, with carbohydrate contributing 50% or more and protein the balance. Smoking was discouraged. Alcohol consumption of no more than two standard drinks a day for women and four for men was recommended, with at least two alcohol-free days a week. (Pg 312/ Col 2/ Para 5-6) GP and dietitian group: After screening, the dietitian flagged the patient record to request the general practitioner, with whom the patient had made an appointment, to invite the patient to join the study. Patients saw the same general practitioner on two other occasions during the 12 months to encourage the patient and monitor progress. The dietitian coordinated the follow-up appointments and flagged the patient record with progress measurements to enable the general practitioner to discuss progress with the patient. Five minutes of general practitioner time was allocated to these tasks. Otherwise, treatment was the same as for the dietitian group. (Pg 312/ Col 2/ Para 7-8) Control group: Standard care group: The control group received the results of the initial measurements and if they had queries were advised to discuss these with the doctor with whom they had made an appointment. No counselling was given by the dietitian. If patients asked the doctor about the measurements, they were treated as any other patient attending the practice. The fact that they were in the control group did not prevent the doctor from providing care usually provided for such conditions. This could include monitoring, advice and prescriptions, but not referral to the study’s dietitian. After 12 months, they received one mailed invitation to attend for reassessment of the initial measurements. In accordance with protocol, doctors were never informed about who was in the control and the dietitian groups. If a patient who was not in the doctor/dietitian asked about screening results, the doctor would not know to which group, if any, the patient belonged. (Pg 312/ Col 2/ Para 9 - Pg 313/ Col 1/ Para 2) Timing of intervention: Proximity to clinical decision-making: Dietitian group: Patients allocated to the dietitian group were invited to join the study by the dietitian at the time of screening. The dietitian conducted six individual counselling sessions, spaced equally, with the last session 12 months after recruitment. (Pg 312/ Col 2/ Para 5-6) GP and dietitian group: After screening, the dietitian flagged the patient record to request the general practitioner, with whom the patient had made an appointment, to invite the patient to join the study. Patients saw the same general practitioner on two other occasions during the 12 months to encourage the patient and monitor progress. The dietitian coordinated the follow up appointments and flagged the patient record with progress measurements to enable the general practitioner to discuss progress with the patient. Five minutes of general practitioner time was allocated to these tasks. (Pg 312/ Col 2/ Para 7-8) Frequency/number of intervention events: Unclear: Dietitians saw the patients on 2 additional occasions but unclear how often the dietitian flagged the patient records for the clinicians attention (Pg 312/ Col 2/ Para 7-8) Duration of intervention: “Five minutes of general practitioner time was allocated to these tasks” (i.e. to read notes flagged by dietitian and to discuss them with the patient) (Pg 312/ Col 2/ Para 7-8). The course of the intervention ran over 12 months (Pg 312/ Col 2/ Para 5-6) Healthcare professional recipient: Intervention group: N/A - organisation of care intervention Control group: N/A - organisation of care intervention Intervention deliverer: Intervention group: N/A - organisation of care intervention Control group: N/A - organisation of care intervention Types of targeted behaviour of the health professionals: N/A - organisation of care intervention Development of the intervention: Consultation with professional recipients: Not specified Evidence base of intervention: Not specified Consumer involvement: Not specified Barriers to change: Not done Source of funding for study: The research was funded by a grant from the Western Australian Health Promotion Foundation. (Pg 315/ Col 2/ Para 7) Ethical approval: Ethics approval was obtained from the Committee of Human Rights, The University of Western Australia. (315/2/8) |
|
| Outcomes |
Outcomes measured:
Changes in weight Blood pressure Glycated haemoglobin Cardiovascular medications use Costs Length of time outcomes measured after initiation of the intervention: Baseline and 12 months (Pg 313/ Col 2/ Para 1) Ceiling effect: Identified by investigator: Unclear Identified by reviewer: No (potential for weight loss clear and demonstrated) Losses to follow up: Number randomised (Table 1/ Pg 314) Intervention groups: Dietitian: 88 GP + dietitian: 92 Control group: 90 Number (%) completing follow up at 12 months (Table 1/ Pg 314) Intervention groups: Dietitian: 48 (55%) GP + dietitian: 65 (71%) Control group: 64 (71%) Reasons for loss to follow up: Intervention groups: Unclear Control group: Unclear Economic variables: Costs of the intervention: Yes (Table 3/ Pg 314) Total cost per group Cost per patient Additional cost per patient Additional cost per kg lost Costs included were dietitian and clinician time, materials, room use and usual practice overheads. (Pg 313/ Col 1/ Para 6) Changes in direct healthcare costs as a result of the intervention: No Changes in non-healthcare costs as a result of the intervention: No Costs associated with the intervention linked with provider or patient outcomes in an economic evaluation: Yes (Table 3/ Pg 314): Additional cost per patient Additional cost per kg lost |
|
| Notes | No unit of analysis error: See page 313/Col1/“outcome and statistical methods” section | |
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Adequate sequence generation? | Yes | Used a table of random numbers to allocate each consecutive patient (Pg 312/ Col 2/ Para 4) |
| Allocation concealment? | Unclear | Immediately after screening, the study dietitian used a table of random numbers to allocate each consecutive patient (Pg 312/ Col 2/ Para 4) |
| Blinding? Primary outcome |
No |
Changes in weight: “doctors were never informed about who was in the control and the dietitian groups” (Pg 313/ Col 2/ Para 1) However, doctors were obviously not blinded to those who were in the doctor/dietitian versus dietitian group. No reference is made to blinding of outcome assessors |
| Blinding? Secondary outcomes |
No |
Hypertension: “doctors were never informed about who was in the control and the dietitian groups” (Pg 313/ Col 2/ Para 1) However, doctors were obviously not blinded to those who were in the doctor/dietitian versus dietitian group. No reference is made to blinding of outcome assessors. Costs: The dietitian maintained a record of activities for two periods of two weeks during the study. Time spent on the study tasks of screening, arranging appointments, changing appointments, drawing patient files, data entry, and counselling was recorded. The time was coasted at $20 per hour for the dietitian. Time spent by the patient with the doctor was coasted at $82 per hour, which was the equivalent cost of bulk billing four standard consultations. Materials used by the dietitian, roomrental and usual practice overheads were coasted and distributed according to the number of counselling sessions taken up by patients in the three groups. The cost effectiveness analysis was used to determine a cost for each intervention in terms of weight change over and above that of the screening group. (Pg 313/Col 1/ Para 6)The assessor of time taken and equipment used was not blinded |
| Blinding? Secondary outcomes |
Unclear |
Random glucose level: For diabetic patients, an Ames pen was used to obtain a capillary sample that was read with an Ames-3 glucometer to obtain a random glucose glucose level. (Pg 312/ Col 1/ Para 8) Glycated haemoglobin: Venous blood was also taken from diabetic patients for glycated haemoglobin at the beginning and the end of the study (Pg 312/ Col 2/ Para 1) No reference is made to blinding of outcome assessors but obviously they could have easily been blinded for these two outcomes |
| Incomplete outcome data addressed? Primary outcome |
Yes | Changes in weight: Missing data have been imputed using appropriate methods: The main outcomes evaluated were changes in weight and mean blood pressure (diastolic pressure + (systolic-diastolic pressure)/ 3) for each of the three groups. These outcomes were subjected to analysis by intention- to-treat, which assumed that a patient’s measurements remained unchanged after the patient dropped out of the study. Thus a patient’s last measurement was used to populate all subsequent missing data values (Pg 313/ Col 1/ Para 4) |
| Incomplete outcome data addressed? Secondary outcomes |
Unclear |
Changes in blood-pessure: Missing data have been imputed using appropriate methods i.e intention to treat analysis Changes in glycated haemoglobin: see above Changes in medication use: |
| Free of selective reporting? | Unclear | All of the study’s pre-specified primary outcomes (weight and blood pressure) have been reported (Table 1/ Pg 314): |
| Baseline characteristics similar? | Yes | Authors state that there were no significant differences between intervention and control groups with respect to sex or age or socioeconomic status quartiles or occupation. (Pg 313 / Col 2/ Para 4-5 and Table 1/ Pg 313) |
| Reliable primary outcome measures? Average weight change |
Yes | Collected by individual: screened opportunistically by the study dietitian (312/1/4). Body weight and height were measured with patients wearing only light indoor clothing. Body weight was measured on digital balance scales to the nearest 0.1 kg with the patient wearing no shoes. (Pg 312/ Col 1/ Para 6) |
| Protection against contamination? | No | The same GP could have delivered care to patients in an intervention group and to patients receiving standard care |
| Methods |
Design: RCT Unit of allocation: Unclear Of the eligible patients 484were randomly selected and assigned to either an intervention or control group (Pg 64/ Col 2/ Para 1). Physicians participating in the study were randomly divided into three groups: 1) those who were to see only patients with automated records available; 2) those who were to see patients without automated records; and 3) those whose patient load was approximately half with and half without automated records (Pg 64/ Col 2/ Para 2).The relationship between physician groupings and intervention and control group is not explained Unit of analysis: Unclear.“The analysis of variance and the analysis of covariance were used to compare the experimental and control conditions on blood pressure and weight measurements” (Pg 65/Col 2/Para 1) Power calculation: No power calculation. |
|
| Participants |
The number randomised into the trial:
Episodes of care: Not available (Table 1/ Pg 67) Patients: 147 obese patients (Table 1/ Pg 67) Providers: Unclear - number of physicians not stated (Pg 64/ Col 2/ Para 2) Practices: N/A Hospitals: 1 - The Cardiac Pulmonary and Renal Clinics of the Northwestern University (Pg 64/ Col 1/ Para 3) Communities or regions: N/A Characteristics of participating healthcare providers: Profession: Physicians but number not stated (Pg 64/ Col 2/ Para 2) Level of training: Not stated (Pg 64/ Col 2/ Para 2) Age of health professional: Not stated (Pg 64/ Col 2/ Para 2) Years since graduation or in practice: Not stated (Pg 64/ Col 2/ Para 2) Proportion of eligible providers (or allocation units) who participated in the evaluation: Not stated (Pg 64/ Col 2/ Para 2) Characteristics of the participating patients: Clinical problem(s) of participating patients: Overweight (BMI ≥ 25 but ≤ 30): Unclear: (Table 1/ Pg 66), Patients whose weight exceeded 20%of their ideal weight were classified as obese - but breakdown not available Obese (BMI ≥ 30): Unclear: (Table 1/ Pg 66), Patients whose weight exceeded 20% of their ideal weight were classified as obese - but breakdown not available Diabetes: (Table 1/ Pg 67): 48/147 obese i.e. 33.3% Ischemic heart disease: Not available Other characteristics of participating patients: Age: Not available (Table 1/ Pg 67) Gender: 88 F, 26M (77% female) (Table 5/ Pg 71) Ethnicity: Not available (Table 1/ Pg 67) Other: N/A Setting: Reimbursement system: Unclear Setting of care: The Cardiac Pulmonary and Renal Clinics of the Northwestern University (Pg 64/ Col 1/ Para 3) Academic status of the setting of care: University (teaching) hospital: The Cardiac Pulmonary and Renal Clinics of the Northwestern University (Pg 64/ Col 1/ Para 3). Country: USA: Michigan (Pg 63/ Col 1/ Para 5) |
|
| Interventions |
Professional intervention:
Intervention group: In the experimental group patients had available a computer printout of a current NUCRSS summary in addition to the traditional medical record. (Pg 64/ Col 2/ Para 1) A computerised medical record system (NUCRSS) was developed to provide physicians with concise and current information on patient’s problems, to identify omissions in recording of observations and treatment recommendations, to show ordered procedures that were not carried out, to record deficiencies in medical reasoning, and most importantly, to recommend corrective actions according to selected criteria. These criteria of “good care” were established by consensus of the physicians providing care at our university (Pg 64/ Col 1/ Para 3) Control group: The control group had available only the handwritten traditional medical record (Pg 64/ Col 2/ Para 1) Timing of intervention: Proximity to clinical decision-making: Immediately proximate to clinical decision making. In the experimental group patients had available a computer printout of a current NUCRSS summary in addition to the traditional medical record (Pg 64/ Col 2/ Para 1). The control group had available only the handwritten traditional medical record (Pg 64/ Col 2/ Para 1) Frequency/number of intervention events: Not stated Duration of intervention: N/A Intervention deliverer: Intervention groups: (i) Computer system (NUCRSS) (Pg 64/ Col 1/ Para 3) and (ii) 50% Computer system (NUCRSS) and 50% handwritten traditional medical record Control group: The control group had available only the handwritten traditional medical record (Pg 64/ Col 2/ Para 1) Types of targeted behaviour of the health professionals: The NUCRSS keeps track of weight loss progress and reminds physicians to review or change diets (Pg 72/ Col 2/ Para 1) Development of the intervention: Consultation with professional recipients: Yes: The criteria (used by NUCRSS) of good care were established by consensus of the physicians providing care at our university (Pg 64/ Col 1/ Para 3) Evidence-base of intervention: Explicitly not evidence-based: These criteria of “good care” were established by consensus of the clinicians providing care at our university (Pg 64/ Col 1/ Para 3) Consumer involvement: Not specified Barriers to change: Not done Source of funding for study: The major support for this project was provided by Grant- Number HS02649 from the National Centre for Health Services Researcgh, HRA. Initial data collection and analysis were made possible by DHEW Grant number H500674-04 and USPAS Grant number RR05370 (NIH). (Pg 63/ Col 1/ Para 4) Ethical approval: Not reported |
|
| Outcomes |
Outcomes measured:
Pounds overweight (patients) Failure to give or review diet (clinicians) Length of time outcomes measured after initiation of the intervention: Baseline Year 1 (10-15 months) Year 2 (22-24 months) (Table 4/ Pg 69) Ceiling effect: Identified by investigator: No Identified by reviewer: No - see Table 3/ Pg 68 Losses to follow up: NB - data not available for all patients at 1 year and 2 year follow up time points Number randomised: Intervention group: 68 (Table 1/ Pg 66) Control group: 79 (Table 1/ Pg 66) Number completing follow up: Intervention group: 62 (at end of study) (Table 1/ Pg 66) Control group: 62 (at end of study) (Table 1/ Pg 66) Reasons for loss to follow up: Intervention group: 1 dead, 5 moved (at end of study) (Table 1/ Pg 66) Control group: 7 dead, 10 moved (at end of study) (Table 1/ Pg 66) Economic variables: Costs of the intervention: Not reported Changes in direct healthcare costs as a result of the intervention: Not reported Changes in non-healthcare costs as a result of the intervention: Not reported Costs associated with the intervention linked with provider or patient outcomes in an economic evaluation: Not reported |
|
| Notes | Unclear whether there was a unit of analysis error: See Page 65/Col 2/Top para | |
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Adequate sequence generation? | Unclear | Pg 62/ Col 2/ Para 1-2 |
| Allocation concealment? | Unclear | Pg 62/ Col 2/ Para 1-2 |
| Blinding? Primary outcome |
Yes | Weight loss: Blind retrospective chart reviews were done for both experimental and control patients (Pg 64/ Col 2/ Para 3) |
| Blinding? Secondary outcomes |
Yes | Dietary advice: Blind retrospective chart reviews were done for both experimental and control patients (Pg 64/ Col 2/ Para 3) |
| Blinding? Secondary outcomes |
Unclear | N/A |
| Incomplete outcome data addressed? Primary outcome |
No |
Weight loss: Table 5/ Pg 71. Obese patients entered into study: 68 computer assisted, 79 handwritten notes Baseline: 55 computer group, 59 handwritten group 12 months: 55 computer group, 57 handwritten group 24 months: 46 computer group, 44 handwritten group Dropout info (Table 1/ Pg 66) does not even cover all of the dropouts by baseline |
| Incomplete outcome data addressed? Secondary outcomes |
Unclear | Dietary advice: Table 3/ Pg 68 does not state how many patients still in study at each time point, just number who were given advice |
| Free of selective reporting? | No | Not all of the study’s pre-specified primary outcomes have been reported (Pg 64/ Col 2/ Para 3): The database consisted of: 1) items related to the utilisation of services and to the overall quality of care (e.g. number of clinic visits, yearly routine physical examinations etc) 2) more detailed information such as the presence or absence of recommended laboratory examinations for patients with obesity 3) answers to a questionnaire concerning patients’ views on their own health and on the care received i.e. only some of 1) is reported, its is unclear what tests 2) were to be ordered for obesity, and 3) is not reported at all |
| Baseline characteristics similar? | Unclear | The number of males and females is not given in the baseline Table 1/ Pg 67 but sex is used to divide the results later. Also information not given for the proportion of obese patients with diabetes and length of prior clinic attendance |
| Reliable primary outcome measures? Average weight change |
Yes | Weight loss: Blind retrospective chart reviews were done for both experimental and control patients (Pg 64/ Col 2/ Para 3) |
| Protection against contamination? | Unclear | No information was provided to assure us that there were no mis-allocated patients, or that computerised records were always available |
| Methods |
Design: RCT Unit of allocation: Patient: Following baseline, the Project Manager randomized participants using an automated computer system to one of three conditions: mail intervention, phone intervention, and standard care (Pg 1566/ Col 1/ Para 6) Unit of analysis: Patient: Power calculation: The primary outcomes examined in this study are changes in body weight from baseline to 18 and 24 months. A required sample size of 500 participants was determined using calculations to have 90% power (α = 0.05, two-tailed) to detect a small effect size for intent-to-treat analyses. (Pg 1567/ Col 2/ Para 3) Number expected to be recruited: 500 per group i.e. 1500 total Number actually recruited: Mail: 600 Phone: 601 Usual care: 600 i.e. 1801 total (Fig 1/ Pg 1568) |
|
| Participants |
The number randomised into the trial:
Episodes of care: Up to 10 sessions in mail and phone groups. Number of sessions actually received by patients described in Figure 1/ Pg 1568 1-10 Weigh to be course Mail 268/600 (44.7%) Phone 392/601 (65.3%) Usual care: 0/600 (0%) 10+ Weigh to be course Mail 62/600 (10.3%) Phone 227/601 (37.8%) Usual care 0/600 (0%) 1-10 Centre for Health promotion (CHP) weight-related encounters Mail 170/600 (28.3%) Phone 356/601 (59.2%) Usual care 114/600 (19%) 10+ CHP weight related encounters Mail 55/600 (9%) Phone 245/601 (40.8%) Usual care 60/600 (10%) Patients: 1801 (Figure 1/ Pg 1568) Providers: Unclear Practices: 4 clinics (Pg 1566/ Col 1/ Para 2) Hospitals: N/A Communities or regions: N/A Characteristics of participating healthcare providers: Profession: Counsellors were staff members of the CHP and were trained nutritionists and/or exercise specialists but numbers not specified (Pg 1566/ Col 2/ Para 3) Level of training: Fully trained: Counsellors were staff members of the CHP and were trained nutritionists and/or exercise specialists (Pg 1566/ Col 2/ Para 3) Age of health professional: Unclear Years since graduation or in practice: Unclear Proportion of eligible providers (or allocation units) who participated in the evaluation: Unclear Characteristics of the participating patients: Clinical problem(s) of participating patients: Overweight (BMI ≥ 25 but ≤ 30): Mail: 25.3% of 600 i.e. 152 Phone: 27.8% of 601 i.e. 167 Control: 27.4% of 600 i.e. 164 Total: 483 (Table 1/ Pg 1569) Obese (BMI ≥ 30): Mail 74.7% of 600 i.e. 448 Phone 72.2% of 601 i.e. 434 Control: 72.6% of 600 i.e. 436 Total 1318 (Table 1/ Pg 1569) Diabetes: % on medications for diabetes: Mail 4.7% of 600 i.e. 28 Phone 6.5% of 601 i.e. 39 Control 5.3% of 600 i.e. 32 Total 99 (Table 1/ Pg 1569) Ischemic heart disease: % on medication for CVD-related: Mail: 26.0% of 600 i.e. 156 Phone: 27.6% of 601 i.e. 166 Control: 28.3% of 600 i.e. 170 Total 492 (Table 1/ Pg 1569) Other characteristics of participating patients: Age : Mean (Standard error) (Table 1/ Pg 1569) Mail: 50.6 years (0.5) Phone: 50.7 years (0.5) Control 50.8 years (0.5) Total mean age = 50 years, SD 12 (Pg 1568/ Col 1/ Para 2) Baseline BMI (kg/m2), mean (standard error): Mail (n = 600): 34.1 (0.2) Phone (n = 601): 33.5 (0.2) Usual care (n = 600): 34.0 (0.2) Gender: Female n = 1293, 72% (Pg 1568/ Col 1/ Para 2) Ethnicity: Caucasian n = 1639, 91% (Pg 1568/ Col 1/ Para 2) Other: Well educated n = 899, 50% college or graduate degree (Pg 1568/ Col 1/ Para 2) Setting: Reimbursement system: Mixed: HealthPartners is a mixed model managed care organization (MCO) (Pg 1566/ Col 1/ Para 2) Setting of care: Community/home based interventions. Participants recruited from 4 clinics (Pg 1566/ Col 1/ Para 2) Academic status of the setting of care: Non-teaching or university affiliated. Participants recruited from 4 clinics (Pg 1566/ Col 1/ Para 2). Country: USA |
|
| Interventions |
Professional intervention:
Intervention group: To measure relative interest in the two treatment conditions, participants were asked to notify the study when they wished to begin their program. Mail intervention individuals were asked to indicate their readiness by sending a postcard to the study office. Phone treatment individuals were given a phone number to call to activate treatment. Once activated, the two weight loss interventions proceeded in parallel formats. Both comprised 10 interactive lessons designed to be completed in sequence with feedback between each lesson from a health counselor. Each lesson included instructional material describing a rationale for a specific behavior change strategy, behaviour change goals related to that strategy, and homework to be completed before beginning the next lesson. Lesson topics included nutrition, physical activity, and behavior management techniques (e.g., behavioral assessment, goal setting, stimulus control, social support, and self-motivation). The primary homework assignment was to keep a food and exercise log. Weight management lessons were designed to be completed as rapidly as one lesson per week. However, study participants were encouraged to proceed at a pace comfortable for them. For phone intervention individuals, all 10 lessons and homework assignment materials were mailed at the beginning of the program. A series of calls was scheduled between the participant and a phone counselor to provide guidance through each lesson and feedback about progress. Phone counselors were staff members of the CHP and were trained nutritionists and/or exercise specialists. During an introductory telephone call, program format and expectations were explained and subsequent calls were scheduled. These calls comprised discussion of behavioural strategies tried since the last session, discussion of content and activities for the lesson, counselor advice about how to improve/maintain lifestyle behaviors, goal setting, and counselor description of the rationale and behavioral assignment for the next lesson. The average length of calls was 19 min. Mail intervention used the same 10 written lessons, behavioral assignments, and counseling protocol and staff. However, interactions between counseling staff and participants were entirely by mail. Participants were first mailed a course manual with two lessons and two feedback forms and were instructed to complete the first lesson and return a progress report. Progress report information included behaviour change goals, perceived progress, and action steps taken to achieve goals. When this progress report was received by the counselor, she reviewed it and made comments in writing, which were forwarded, along with the next session, by return mail. This sequence was repeated for each lesson until the course was completed. (Pg 1566/ Col 2) Follow up intervention options were available to both the phone and the mail groups after completion of the 10-lesson course. These comprised individual follow up on topics of the participant’s choosing. Resources available to the counselor included a wide range of educational resources on lifestyle topics related to weight management maintained by the CHP. Participants could also enroll in other CHP health-related courses. Additionally, participants could repeat all or any part of the WTB intervention. Participants who discontinued contact with their counselor prior to course completion were contacted at 1- , 2-, and then 6-month intervals for up to 2 years to encourage intervention resumption. Individuals who did not activate their assigned intervention were also contacted at 6- month intervals to encourage engagement. (Pg 1567/ Col 1/ Para 1) Control group: Usual care participants had access only to weight management services generally available to members of HealthPartners. After randomisation, they were sent a resource sheet detailing MCO and community weight management options including free general phone counseling, a structured weight management phone course, or a group class offered at several MCO clinics. The phone course and group classes required a modest fee of $25. Similar to participants in the treatment groups, standard care participants could enroll in other CHP health-related courses. (Pg 1567/ Col 1/ Para 2) Timing of intervention: Proximity to clinical decision-making: N/A - organisation of care intervention Frequency/number of intervention events: N/A - organisation of care intervention Duration of intervention: N/A - organisation of care intervention Healthcare professional recipient: Intervention group: N/A - organisation of care intervention Control group: N/A - organisation of care intervention Intervention deliverer: Intervention group: N/A - organisation of care intervention Control group: N/A - organisation of care intervention Types of targeted behaviour of the health professionals: N/A - organisation of care intervention Development of the intervention: Consultation with professional recipients: Unclear Evidence base of intervention: Not specified Consumer involvement: Not specified Barriers to change: Not clear Source of funding for study: Not specified Ethical approval: Not reported |
|
| Outcomes |
Outcomes measured:
Weight loss Costs Number of CHP Weight-Related Encounters Number of sessions taken up on Weigh-To-Be Course Length of time outcomes measured after initiation of the intervention: Baseline, 6, 12, 18 and 24 months (Fig 2/ Pg 1569) Ceiling effect: Identified by investigator: Yes - ‘standard care’ was unusually potent in this study. The CHP is unique in its offering of relatively low cost weight management services to members. Many members, however, are probably not aware of these services and thus do not use them. Usual care participants in this study were explicitly made aware of these member services and participated in them at relatively high rates, about 1 person in 3. As a result, significant weight loss observed in our ‘control’ group may have lessened our ability to detect effects in our active treatments. (Pg1571/ Col 2 / Para 1) Identified by reviewer: No - overall potential for weight loss demonstrated Losses to follow up: Number randomised: (Fig 1/ Pg 1568) Intervention groups: Mail: 600 Phone: 601 Control group: 600 Number completing follow up: (Fig 1/ Pg 1568) Intervention groups: Mail: 381 (24 months) Phone: 404 (24 months) Control group: 410 (24 months) Reasons for loss to follow up: Intervention groups: Not stated Control group: Not stated Economic variables: Costs of the intervention: Yes: Counseling/subject Program development/subject Materials and supplies/subject Overhead/subject Total cost/subject (Table 5/ Pg 1571) Changes in direct healthcare costs as a result of the intervention: No Changes in non-healthcare costs as a result of the intervention: No Costs associated with the intervention linked with provider or patient outcomes in an economic evaluation: Cost/weight loss of 1 kg (Table 5/ Pg 1571) |
|
| Notes | No unit of analysis error: See Page 1567/Col 2/“Analysis” section | |
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Adequate sequence generation? | Yes | Following baseline, the Project Manager randomised participants using an automated computer system to one of three conditions: mail intervention, phone intervention, and standard care. The randomisation scheme consisted of blocks of 15 with the numbers 1-3 to indicate treatment group (phone, mail and standard care) (Pg 1566/ Col 1/ Para 3 - Pg 1566/ Col 2/ Para1) |
| Allocation concealment? | Yes | The randomisation sequence was concealed until after interventions were assigned. (Pg 1566/ Col 2/ Para1) |
| Blinding? Primary outcome |
Yes | Weight: At baseline and 24 months, clinic visits were held at which body weight was measured and self-report measures were completed. Measurement staff were blind to study condition. (Pg 1567/ Col 1/ Para 3) |
| Blinding? Secondary outcomes |
Yes | Self reported measures: No blinding, but the review authors judge that the outcome and the outcome measurement are not likely to be influenced by lack of blinding |
| Blinding? Secondary outcomes |
No | Participation measures. Blinding not possible. |
| Incomplete outcome data addressed? Primary outcome |
Yes | Weight: These analyses used an intent-totreat approach in which baseline values for body weight (0 weight loss) were used for individuals who did not complete follow up surveys. (Pg 1567/ Col 2/ Para 3) |
| Incomplete outcome data addressed? Secondary outcomes |
No | Not applicable. |
| Free of selective reporting? | No | The protocol was published in Jeffery 2004, which stated that the following outcomes would be measured: Questionnaire on Eating and Weight-Revised, A 24-item dietary fat screener, Paffenbarger Activity Questionnaire, Frequency of weighing one-self on a monthly basis. None of these are reported in Sherwood 2006. |
| Baseline characteristics similar? | Yes | Table 1/ Pg 1569 and Pg 1568 / Col 1/ Para 2 Treatment groups differed significantly on only one baseline variable. Phone group participants were more likely to report taking depression medication than those in the other groups (P < 0.013) |
| Reliable primary outcome measures? Average weight change |
Yes | Weight: At baseline and 24 months, clinic visits were held at which body weight was measured and self-report measures were completed. Measurement staff were blind to study condition. (Pg 1567/ Col 1/ Para 3) |
| Protection against contamination? | Yes | Unlikely that the control group received the intervention and weight control activity participation was measured across all 3 groups. No one in the control group was reported to have participated in the interventions (Fig 1/ Pg 1568) Usual care participants had access only to weight management services generally available to members of HealthPartners. (Pg 1567/ Col 1/ Para 2) Participation measures: Weight control activity participation was assessed in two ways using the tracking systems that are part of the CHP delivery platform. These records document the dates and types of all contacts between CHP staff and members, both for the WTB program and other CHP programs. Analysis variables for mail and phone group participants included enrollment status (yes/no) and number of WTB course sessions completed (0-10). Additionally, the total number of weight-related encounters outside of the WTB protocols were examined. The operational definition of an ‘encounter’ was an educational interaction that focused on the topics of weight, diet, and/or physical activity between CHP staff and a participant. This information was available for all three study conditions. (Pg 1567/ Col 2/ Para 2) |
Locations of supporting text in published study indicated by (Page number/ Column number/ Paragraph number) e.g. (Pg 150/ Col 1/ Para 4)
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Ashley 2001 | Lack of standard care arm. Participants not recruited in the context of a healthcare setting |
| Atkinson 1977 | No standard care arm. The intervention was not led solely by qualified healthcare professionals |
| Balch 1976 | No standard care arm. Recruitment of overweight participants did not occur in a healthcare setting |
| Ben Noun 1988 | The comparator was not normal care. |
| Boltri 2007 | Not all of the patients were overweight or obese (Table 1). It did not report weight or weight loss at the end of sudy (i.e. no objective outcome measure was reported) |
| Counterweight Prog 2004 | No outcome data for the control arm available at present. (Personal communication with the authors.) |
| De Mello 2004 | Study used participants who were children. The participants were not recruited in the context of a healthcare setting, nor was the intervention led by a qualified healthcare professional |
| Donnelly 2007 | No standard care arm. Unclear if subjects were recruited in the context of a healthcare setting. There was also concern that the “experienced health educators” used in this study were not qualified health professionals |
| Dunstan 2006 | No standard care arm. Not all participants were recruited in the context of a healthcare setting. Overweight and obese participants were not supervised by health professionals in the gym (but by YMCA staff) |
| Ferstl 1975 | No standard care arm, not all participants were recruited in the context of a healthcare setting, nor did the intervention take place in healthcare setting; the whole intervention was devised for this study and delivered at a non-profit institute for therapy research |
| Finnish DPS Group 1999 | The study participants were not recruited in the context of a healthcare setting. “The study subjects were recruited through various methods, e. g. from epidemiological surveys and by opportunistic population screenings with special emphasis on the high-risk groups such as obese subjects and first-degree relatives of Type II diabetic patients. Subjects were also recruited through advertising in local newspapers.” (Page 794/ col 2 / para 5 of Eriksson 1999) |
| Hagen 1974 | Recruitment of overweight participants did not occur in a healthcare setting. Not all participants were over 18 |
| Hakala 1994 | RCT organisation: inpatient vs outpatient. Too much variation in content between the two groups. |
| Jeffery 1979 | No standard care arm. It only compared the frequency of therapist contact. The intervention has been designed for the study at Stanford University and it’s unclear if any sort of care program was in place for overweight undergraduates at the University |
| Jeffery 1982 | No standard care arm. Participants were not recruited in the context of a healthcare setting. The intervention was not led by qualified healthcare professionals |
| Kromann 1985 | This study appears to be a CBA with a convenience sample of patients. Also the standard care arm physicians received additional training and is described by the authors as not representative of other GP practices |
| Levitz 1974 | Participants were not recruited in the context of a healthcare setting. Weight loss intervention led by non-health professionals |
| Lindstrom 1976 | Patients not recruited in the context of a healthcare setting. The intervention was not led by a qualified healthcare professional |
| McDonald 1984 | No objective patient outcome data reported i.e. it did not report patient weight or weight loss at the end of the study |
| Meyers 1996 | No standard care arm, the face to face group is not “standard care” and also the intervention was designed solely for the study. The participants were not recruited in the context of a healthcare setting |
| Ogden 1997 | No objective outcome measures i.e. it did not report weight or weight loss at the end of study |
| Perri 1987 | Patients not recruited in context of a healthcare setting. No standard care arm |
| Richman 1996 | Not an RCT (controlled before and after study). |
| Simkin-Silverman 1997 | No objective outcome measures i.e. it did not report weight or weight change at the end of study |
| Vinicor 1987 | Not all of the participants were overweight or obese (Table 1, pg 350) |
| Willaing 2004 | Not all of the participants were obese or overweight. |
DATA AND ANALYSES
Comparison 1.
Professional or organisational interventions versus standard care
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size | |
|---|---|---|---|---|---|
| 1 Results | Other data | No numeric data | |||
| 1.1 Professional interventions vs. standard care | Other data | No numeric data | |||
| 1.2 Organisational interventions vs. standard care | Other data | No numeric data |
Comparison 2.
Educational intervention versus standard care
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Weight (kg) at longest follow up | 3 | Mean Difference (Random, 95% CI) | −1.24 [−2.84, 0.37] | |
| 1.1 Change in weight between baseline and end of study | 2 | Mean Difference (Random, 95% CI) | −1.77 [−2.80, −0.74] | |
| 1.2 Weight at end of study | 1 | Mean Difference (Random, 95% CI) | 1.3 [−1.86, 4.46] | |
| 2 Weight (kg) at 1 year follow up (or closest timepoint available) | 3 | Mean Difference (Random, 95% CI) | −1.29 [−2.77, 0.20] | |
| 2.1 Change in weight between baseline and end of study | 2 | Mean Difference (Random, 95% CI) | −1.77 [−2.80, −0.74] | |
| 2.2 Weight at end of study | 1 | Mean Difference (Random, 95% CI) | 1.0 [−1.96, 3.96] |
Comparison 3.
Reminders versus standard care
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Weight (kg) at longest follow up | 1 | 108 | Mean Difference (IV, Fixed, 95% CI) | −3.75 [−8.46, 0.96] |
| 1.1 Amount overweight at end of study (Men) | 1 | 20 | Mean Difference (IV, Fixed, 95% CI) | −11.2 [−20.66, −1.74] |
| 1.2 Amount overweight at end of study (Women) | 1 | 88 | Mean Difference (IV, Fixed, 95% CI) | −1.30 [−6.73, 4.13] |
Comparison 4.
Organisational intervention versus standard care
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Weight (kg) at longest follow up | 2 | Mean Difference (Random, 95% CI) | Totals not selected | |
| 1.1 Doctor/dietitian vs. standard care | 1 | Mean Difference (Random, 95% CI) | Not estimable | |
| 1.2 Dietician vs. standard care | 1 | Mean Difference (Random, 95% CI) | Not estimable | |
| 1.3 Mail intervention vs. standard care | 1 | Mean Difference (Random, 95% CI) | Not estimable | |
| 1.4 Telephone intervention vs. standard care | 1 | Mean Difference (Random, 95% CI) | Not estimable | |
| 2 Weight (kg) at 1 yr follow up (or closest timepoint available) | 2 | Mean Difference (Random, 95% CI) | Totals not selected | |
| 2.1 Doctor/dietitian vs. standard care | 1 | Mean Difference (Random, 95% CI) | Not estimable | |
| 2.2 Dietician vs. standard care | 1 | Mean Difference (Random, 95% CI) | Not estimable | |
| 2.3 Mail intervention vs. standard care | 1 | Mean Difference (Random, 95% CI) | Not estimable | |
| 2.4 Telephone intervention vs. standard care | 1 | Mean Difference (Random, 95% CI) | Not estimable |
Analysis 1.1. Comparison 1 Professional or organisational interventions versus standard care, Outcome 1 Results
Results
| Study | Comparisons | Main process effect | Main patient outcome |
|---|---|---|---|
| Professional interventions vs. standard care | |||
| Cohen 1991 | I: One educational teaching session C: Usual care. |
Not done, except for recording how many patients the 18 GPs I: N=10; C: N=8) recruited each | See Table 2. Weight change from baseline (SD) (kg) 0-6 months I: (n=15) −1.8 (3.4) C: (n=15) 0.56 (2.5) I-C = −2.36 (favours I) 6-12 months I: (n=15) 0.94 (3.3) C: (n=15) 0.73 (2.2) I-C = 0.21 (favours C) 0-12 months I: (n=15) −0.88 (4.0) C: (n=15) 1.3 (3.0) I-C = −2.18 (favours I) |
| Martin 2006 | I: GP targeted intervention: remote educational teaching session and interventions tailored to the character of the overweight and obese patients by a multidisciplinary team delivered over 6 months C: Standard care |
No post intervention assessment of GP practice. | See Table 2 (ITT analysis) and page 1417/Col. 1/end of para 2 Weight change from baseline (SD) (kg) 0-6 months I: (n=69) −1.44 (3.30) C: (n=69) 0.25 (3.30) I-C: −1.69 (favours I) |
| Moore 2003 | I: Three 90 minutes educational sessions over 4 weeks targeted at GPs and their teams C: Usual care |
Values are numbers responding “yes” at 12 months Evidence that weight discussed in consultation. (n = 650) I: 186 C: 129 Odds ratio 2.0 (95%CI:1.3 to 3.2) P=0.003 Weight recorded. (n=650) I: 197 C: 137 Odds ratio 2.0 (95% CI:1.3 to 3.3) P = 0.004 Target weight recorded (n=643) I: 46 C: 9 Odds ratio 13.6 (95% CI: 4.2 to 44. 3) P <0.001 Dietary targets recorded (n=648) I: 48 C: 14 Odds ratio 4.5 (95% CI:1.2 to 16.7) P=0.02 Exercise targets recorded (n=648) I: 46 C: 25 Odds ratio 1.9 (95%:0.7 to 5.0) P=0.2 |
See Figure 2 and Table 2. Difference in weight, I- C (SE) 3 months I: n=331 C: n=333 I-C: 0.6(1.38) 12 months I: n=279 C: n=286 I-C: 1.0(1.51) (Favours C) 18 months I: n=256 C: n=275 I-C: 1.3(1.61) (Favours C) Difference in BMI (kg/m2): I- C (SE) 3 months I-C:-0.2 (0.52) (Favours I) 12 months 0 (0.52) 18 months I-C: 0.1 (0.55) (Favours C) |
| Rogers 1982 | I:Computerised reminders C: Usual care |
Number of diets given or reviewed:
Reminders vs control: Year 1: 2 (4.8%) Year 2: 4 (9.1%) Done both years: 7 (13.5%) Not done: 24 (27.5%) p = 0.007 ’for all obese patients combined for sex’ (but not clear which of the above figures this is for). (No SD/SEs so not possible to calculate CIs.) |
See Table 5, page 71. Follow-up: 147 patients classified as obese, 23 dropped out, but data collected for: Mean kg. overweight : 10-15 months Men: I: (n = 15) 20.4 (7.5) C: (n = 11) 25.7 (12.6) I-C= −5.3, SE=4.35 (favours I) Women: I: (n = 42) 23.4 (13.3) C: (n = 46) 24.8 (11.5) I-C= −1.4, SE=2.76 (favours I) 22-24 months Men: I: (n = 11) 15.8 (6.6) C: (n = 9) 27.0 (13.2) (favours I) Women: I: (n = 42) 23.6 (14.7) C: (n = 46) 24.9 (10.8) (favours I) |
| Organisational interventions vs. standard care | |||
| Pritchard 1999 | I1: Doctor/ dietitian I2: Dietician C: Usual care |
None measured | Page 314: sections on “Weight outcomes”, “Blood pressure outcomes”; n from Table 2 I1: n= 92 I2: n=88 C: n = 90 Weight change relative to control 12 months I1-C: −6.7 (0.42) I2-C: −5.6 (0.39) I1 - I2: −1.1 (0.92) Change in blood pressure relative to control (mmHg) 12 months I1-C: −12 (1.56) I2-C: −7 (1.56) I1 - I2: −5 (1.56) Total cost per group: I1-C: (n=93) $8240.30 I2-C: (n=89) $5715.06 C: (n=91) $2103.53 Additional cost per kg lost: I1-C: (n=93) $9.76 I2-C: (n=89) $7.30 |
| Sherwood 2006 | I1: Mail delivered intervention I2: Phone delivered intervention C: Usual care |
None measured | See Table 2 and Table 5. Weight change (kg), mean (SD): 18 months I1: (n=600) −2.27 (5.9) I2: (n=601) −2.35 (5.9) C: (n=600) −1.91 (5.9) 24 months I1: (n=600) −0.73 (5.4) I2: (n=601) −0.93 (5.4) C: (n=600) −0.59 (5.4) I1-C: −0.14 I2-C: −0.34 (Favours I1, I2) Total costs/participant: I1: (n=600) $50.45 I2: (n=601) $127.39 C: (n=600) $42.18 Cost/weight loss of 1kg: I1: (n=600) $72.08 I2: (n=601) $132.70 C: (n=600) $71.50 |
Analysis 2.1. Comparison 2 Educational intervention versus standard care, Outcome 1 Weight (kg) at longest follow up
Review: Interventions to change the behaviour of health professionals and the organisation of care to promote weight reduction in overweight and obese adults
Comparison: 2 Educational intervention versus standard care
Outcome: 1 Weight (kg) at longest follow up
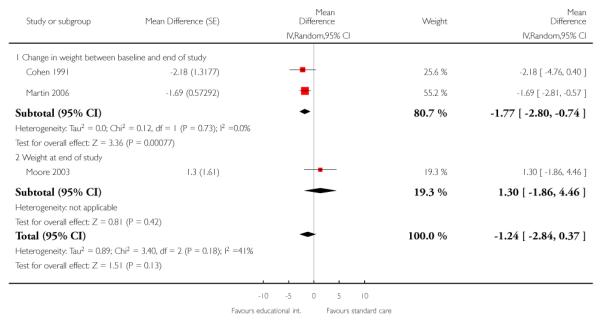
|
Analysis 2.2. Comparison 2 Educational intervention versus standard care, Outcome 2 Weight (kg) at 1 year follow up (or closest timepoint available)
Review: Interventions to change the behaviour of health professionals and the organisation of care to promote weight reduction in overweight and obese adults
Comparison: 2 Educational intervention versus standard care
Outcome: 2 Weight (kg) at 1 year follow up (or closest timepoint available)
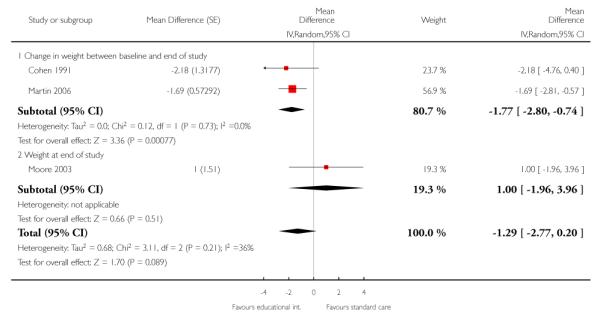
|
Analysis 3.1. Comparison 3 Reminders versus standard care, Outcome 1 Weight (kg) at longest follow up
Review: Interventions to change the behaviour of health professionals and the organisation of care to promote weight reduction in overweight and obese adults
Comparison: 3 Reminders versus standard care
Outcome: 1 Weight (kg) at longest follow up
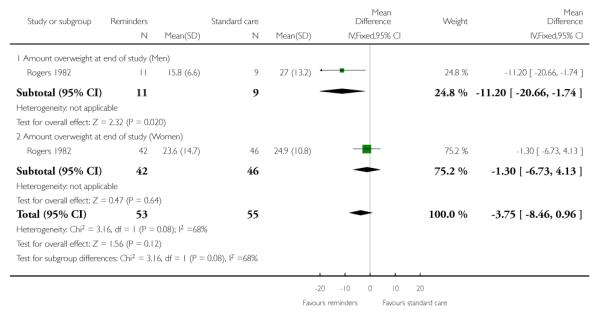
|
Analysis 4.1. Comparison 4 Organisational intervention versus standard care, Outcome 1 Weight (kg) at longest follow up
Review: Interventions to change the behaviour of health professionals and the organisation of care to promote weight reduction in overweight and obese adults
Comparison: 4 Organisational intervention versus standard care
Outcome: 1 Weight (kg) at longest follow up
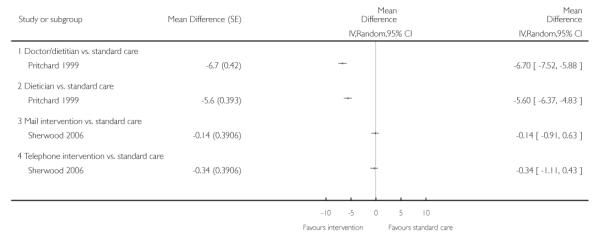
|
Analysis 4.2. Comparison 4 Organisational intervention versus standard care, Outcome 2 Weight (kg) at 1 yr follow up (or closest timepoint available)
Review: Interventions to change the behaviour of health professionals and the organisation of care to promote weight reduction in overweight and obese adults
Comparison: 4 Organisational intervention versus standard care
Outcome: 2 Weight (kg) at 1 yr follow up (or closest timepoint available)
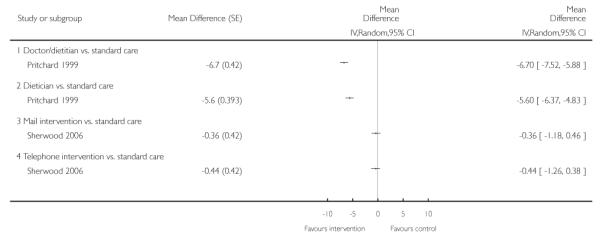
|
Appendix 1. EPOC Taxonomy
INTERVENTIONS
EPOC reviews include professional, financial, organisational or regulatory interventions.
State all interventions for each comparison/study group. (The categories are not mutually exclusive.)
Type of intervention
1) Professional interventions
a) Distribution of educational materials (Distribution of published or printed recommendations for clinical care, including clinical practice guidelines, audio-visual materials and electronic publications. The materials may have been delivered personally or through mass mailings.)
b) Educational meetings (Health care providers who have participated in conferences, lectures, workshops or traineeships.)
c) Local consensus processes (Inclusion of participating providers in discussion to ensure that they agreed that the chosen clinical problem was important and the approach to managing the problem was appropriate.)
d) Educational outreach visits (Use of a trained person who met with providers in their practice settings to give information with the intent of changing the provider’s practice. The information given may have included feedback on the performance of the provider(s).)
e) Local opinion leaders (Use of providers nominated by their colleagues as ‘educationally influential’. The investigators must have explicitly stated that their colleagues identified the opinion leaders.)
f) Patient mediated interventions (New clinical information (not previously available) collected directly from patients and given to the provider e.g. depression scores from an instrument.)
g) Audit and feedback (Any summary of clinical performance of healthcare over a specified period of time. The summary may also have included recommendations for clinical action. The information may have been obtained from medical records, computerised databases, or observations from patients.)
i) The following interventions are excluded
(1) Provision of new clinical information not directly reflecting provider performance which was collected from patients e.g. scores on a depression instrument, abnormal test results. These interventions should be described as patient mediated.
(2) Feedback of individual patients’ health record information in an alternate format (e.g. computerised). These interventions should be described as organisational.
h) Reminders (Patient or encounter specific information, provided verbally, on paper or on a computer screen, which is designed or intended to prompt a health professional to recall information. This would usually be encountered through their general education; in the medical records or through interactions with peers, and so remind them to perform or avoid some action to aid individual patient care. Computer aided decision support and drugs dosage are included.)
i) Marketing (Use of personal interviewing, group discussion (‘focus groups’), or a survey of targeted providers to identify barriers to change and subsequent design of an intervention that addresses identified barriers.)
j) Mass media
i) Varied use of communication that reached great numbers of people including television, radio, newspapers, posters, leaflets, and booklets, alone or in conjunction with other interventions.
ii) Targeted at the population level.
k) Other (Other categories to be agreed in consultation with the EPOC editorial team.)
2) Financial interventions
i) Provider interventions
(1) Fee-for-service (provider has been paid for number and type of service delivered)
(2) Prepaid (no other description)
(3) Capitation (provider was paid a set amount per patient for providing specific care)
(4) Provider salaried service (provider received basic salary for providing specific care)
(5) Prospective payment (provider was paid a fixed amount for healthcare in advance)
(6) Provider incentives (provider received direct or indirect financial reward or benefit for doing specific action)
(7) Institution incentives (institution or group of providers received direct or indirect financial rewards or benefits for doing specific action)
(8) Provider grant/allowance (provider received direct or indirect financial reward or benefit not tied to specific action)
(9) Institution grant/allowance (institution or group of providers received direct or indirect financial reward or benefit not tied to specific action)
(10) Provider penalty (provider received direct or indirect financial penalty for inappropriate behaviour)
(11) Institution penalty (institution or group of providers received direct or indirect financial penalty for inappropriate behaviour)
(12) Formulary (added or removed from reimbursable available products)
(13) Other (other categories to be agreed in consultation with the EPOC editorial team)
ii) Patient interventions
(1) Premium (Patient payment for health insurance. It is important to determine if the patient paid the entire premium, or if the patient’s employer paid some of it. This includes different types of insurance plans.)
(2) Co-payment (Patient payment at the time of healthcare delivery in addition to health insurance e.g. in many insurance plans that cover prescription medications the patient may pay 5 dollars per prescription, with the rest covered by insurance.)
(3) User-fee (Patient payment at the time of healthcare delivery.)
(4) Patient incentives (Patient received direct or indirect financial reward or benefit for doing or encouraging them to do specific action.)
(5) Patient grant/allowance (Patient received direct or indirect financial reward or benefit not tied to specific action.)
(6) Patient penalty (Patient received direct or indirect financial penalty for specified behaviour e.g. reimbursement limits on prescriptions.)
(7) Other (other categories to be agreed in consultation with the EPOC editorial team)
3) Organisational interventions
a) Provider orientated interventions
i) Revision of professional roles (Also known as ‘professional substitution’, ‘boundary encroachment’ and includes the shifting of roles among health professionals. For example, nurse midwives providing obstetrical care; pharmacists providing drug counselling that was formerly provided by nurses and physicians; nutritionists providing nursing care; physical therapists providing nursing care. Also includes expansion of role to include new tasks.)
ii) Clinical multidisciplinary teams (Creation of a new team of health professionals of different disciplines or additions of new members to the team who work together to care for patients.)
iii) Formal integration of services (Bringing together of services across sectors or teams or the organisation of services to bring all services together at one time also sometimes called ‘seamless care’.)
iv) Skill mix changes (Changes in numbers, types or qualifications of staff.)
v) Continuity of care (including one or many episodes of care for inpatients or outpatients).
vi) Arrangements for follow up.
vii) Case management (including co-ordination of assessment, treatment and arrangement for referrals).
viii) Satisfaction of providers with the conditions of work and the material and psychic rewards (e.g. interventions to ‘boost morale’).
ix) Communication and case discussion between distant health professionals (e.g. telephone links; telemedicine; there is a television/video link between specialist and remote nurse practitioners).
x) Other (other categories to be agreed in consultation with the EPOC editorial team).
b) Patient orientated interventions
i) Mail order pharmacies (e.g. compared to traditional pharmacies).
ii) Presence and functioning of adequate mechanisms for dealing with patients’ suggestions and complaints.
iii) Consumer participation in governance of healthcare organisation.
iv) Other (other categories to be agreed in consultation with the EPOC editorial team).
4) Structural interventions
a) Changes to the setting/site of service delivery (e.g. moving a family planning service from a hospital to a school).
b) Changes in physical structure, facilities and equipment (e.g change of location of nursing stations, inclusion of equipment where technology in question is used in a wide range of problems and is not disease specific, for example an MRI scanner).
c) Changes in medical records systems (e.g. changing from paper to computerised records, patient tracking systems).
d) Changes in scope and nature of benefits and services.
e) Presence and organisation of quality monitoring mechanisms.
f) Ownership, accreditation, and affiliation status of hospitals and other facilities.
g) Staff organisation.
h) Other (other categories to be agreed in consultation with the EPOC editorial team).
5) Regulatory interventions
a) Any intervention that aims to change health services delivery or costs by regulation or law. (These interventions may overlap with organisational and financial interventions.)
b) Changes in medical liability.
c) Management of patient complaints.
d) Peer review.
e) Licensure.
f) Other (other categories to be agreed in consultation with the EPOC editorial team).
Appendix 2. MEDLINE search strategy
MEDLINE (OVID), 1950 to May 2009
Syntax Guide
/ - index term (MeSH heading)
exp - explode: includes narrower terms to the index term being exploded
.tw. - text word In title or abstract fields
$ - truncation/ wild card: adds no or more characters
? - truncation/ wild card: adds no or one character
# - truncation/ wild card: retrieves alternative single character
adjx - adjacency: required words are adjacent to each other, or within × words of each other
.pt. - Publication type
Description of search strategy
Condition: line 4
Study design: line 137
Interventions: line 138
exp Obesity/
(obes$ or overweight$).tw.
weight loss/
or/1-3
exp *education, continuing/
(education$ adj2 (program$ or intervention? or meeting? or session? or strateg$ or workshop? or visit?)).tw.
(behavio?r$ adj2 intervention?).tw.
*pamphlets/
(leaflet? or booklet? or poster or posters).tw.
((written or printed or oral) adj information).tw.
(information$ adj2 campaign).tw.
(education$ adj1 (method? or material?)).tw.
outreach.tw.
((opinion or education$ or influential) adj1 leader?).tw.
facilitator?.tw.
academic detailing.tw.
consensus conference?.tw.
Practice Guidelines as Topic/
*guideline adherence/
practice guideline?.tw.
(guideline? adj2 (introduc$ or issu$ or impact or effect? or disseminat$ or distribut$)).tw.
((effect? or impact or evaluat$ or introduc$ or compar$) adj2 training program$).tw.
*reminder systems/
reminder?.tw.
(recall adj2 system$).tw.
(prompter? or prompting).tw.
algorithm?.tw.
*feedback/ or feedback.tw.
(feedback adj1 (loop? or control? or regula$ or mechanism? or inhib$ or system? or circuit? or sensory or visual or audio$ or auditory)).tw.
28 or 29
27 not 30
chart review$.tw.
((effect? or impact or records or chart?) adj2 audit).tw.
compliance.tw.
marketing.tw.
or/5-26,31-35
exp *reimbursement mechanism/
fee for service.tw.
*capitation fee/
*“deductibles and coinsurance”/
cost shar$.tw.
(copayment? or co payment?).tw.
(prepay$ or prepaid or prospective payment?).tw.
*hospital charges/
formular$.tw.
fundhold$.tw.
*medicaid/
*medicare/
blue cross.tw.
or/37-49
exp *Health Personnel/
clinical pharmacist?.tw.
paramedic?.tw.
nutritionist?.tw.
dieti#ian?.tw.
or/51-55
*patient care team/
exp *patient care planning/
(team? adj2 (care or treatment or assessment or consultation)).tw.
(integrat$ adj2 (care or service?)).tw.
(care adj2 (coordinat$ or program$ or continuity)).tw.
(case adj1 management).tw.
*ambulatory care/
*home care services/
*hospices/
*office visits/
*house calls/
*day care/
*aftercare/
*community health nursing/
(chang$ adj1 location?).tw.
(domicillary or domiciliary).tw.
(home adj1 treat$).tw.
day surgery.tw.
*health facilities/ or *academic medical centers/ or exp *ambulatory care facilities/ or *birthing centers/ or *health facilities, proprietary/ or *hospital units/ or exp hospitals/ or *pharmacies/ or *physicians’ offices/ or *rehabilitation centers/ or exp *residential facilities/
*group practice/ or *institutional practice/ or *nursing faculty practice/ or *partnership practice/ or *private practice/
or/57-76
*medical records/
*medical records systems, computerized/
(information adj2 (management or system?)).tw.
*peer review/ or *peer review, healthcare/
*utilization review/
exp *health services misuse/
*physician’s practice patterns/
*Quality Assurance, Health Care/
quality assurance.tw.
exp “Outcome and Process Assessment (Health Care)”/
Total Quality Management/
Quality of Health Care/
*program evaluation/
*length of stay/
(early adj1 discharg$).tw.
discharge planning.tw.
offset.tw.
triage.tw.
exp *referral/ and consultation/
*drug therapy, computer assisted/
near patient testing.tw.
*medical history taking/
*telephone/
(physician patient adj (interaction? or relationship?)).tw.
*health maintenance organizations/
managed care.tw.
(hospital? adj1 merg$).tw.
or/78-104
((standard or usual or routine or regular or traditional or conventional or pattern) adj2 care).tw.
(program$ adj2 (reduc$ or increas$ or decreas$ or chang$ or improv$ or modify$ or monitor$ or care)).tw.
((effect? or impact or evaluat$ or introduc$ or compar$) adj2 treatment program$).tw.
((effect? or impact or evaluat$ or introduc$ or compar$) adj2 care program$).tw.
((effect? or impact or evaluat$ or introduc$ or compar$) adj2 screening program$).tw.
((effect? or impact or evaluat$ or introduc$ or compar$) adj2 prevent$ program$).tw.
(computer$ adj2 (dosage or dosing or diagnosis or therapy or decision?)).tw.
((introduc$ or impact or effect? or implement$ or computer$) adj protocol?).tw.
((effect? or impact or introduc$) adj2 (legislation or regulations or policy)).tw.
or/106-114
randomized controlled trial.pt.
random allocation/
double blind method/
single blind method/
or/116-119
clinical trial.pt.
exp Clinical Trial/
(clin$ adj25 trial$).tw.
((singl$ or doubl$ or trebl$ or tripl$) adj25 (blind$ or mask$)).tw.
placebo/
placebo$.tw.
random$.tw.
research design/
Or/121-129
comparative study/
exp evaluation studies/
follow up studies/
prospective studies/
(control$ or prospective$ or volunteer$).ti,ab.
intervention$.ti,ab
Or/130-135
120 or 129 or 136
36 or 50 or 56 or 77 or 105 or 115
4 and 137 and 138
animal/
human/
140 not (140 and 141)
139 not 142
Appendix 3. EMBASE search strategy
EMBASE (OVID), 1980 to May 2009
Syntax Guide
/ - index term (EMTREE heading)
exp - explode: includes narrower terms to the index term being exploded
.tw. - text word In title or abstract fields
$ - truncation/ wild card: adds no or more characters
? - truncation/ wild card: adds no or one character
# - truncation/ wild card: retrieves alternative single character
adjx - adjacency: required words are adjacent to each other, or within × words of each other
.pt. - Publication type
Description of search strategy
Condition: line 4
Study design: line 152
Interventions: line 153
exp Obesity/
(obes$ or overweight$).tw.
weight reduction/
or/1-3
exp medical education/
exp paramedical education/
(education$ adj2 (program$ or intervention? or meeting? or session? or strateg$ or workshop? or visit?)).tw.
(behavio?r$ adj2 intervention?).tw.
publications/
medical information/
information dissemination/
information service/
(leaflet? or booklet? or poster or posters).tw.
((written or printed or oral) adj information).tw.
(information$ adj2 campaign).tw.
(education$ adj1 (method? or material?)).tw.
outreach.tw.
((opinion or education$ or influential) adj1 leader?).tw.
facilitator?.tw.
academic detailing.tw.
consensus conference?.tw.
exp Practice Guideline/
practice guideline?.tw.
(guideline? adj2 (introduc$ or issu$ or impact or effect? or disseminat$ or distribut$)).tw.
((effect? or impact or evaluat$ or introduc$ or compar$) adj2 training program$).tw.
reminder system/
reminder?.tw.
decision support system/
(recall adj2 system$).tw.
(prompter? or prompting).tw.
algorithm?.tw.
*feedback/ or feedback.tw.
(feedback adj1 (loop? or control? or regula$ or mechanism? or inhib$ or system? or circuit? or sensory or visual or audio$ or auditory)).tw.
32 or 33
31 not 34
chart review$.tw.
((effect? or impact or records or chart?) adj2 audit).tw.
compliance.tw.
marketing.tw.
or/5-30,35-39
exp reimbursement/
fee for service.tw.
capitation fee/
cost shar$.tw.
(copayment? or co payment?).tw.
(prepay$ or prepaid or prospective payment?).tw.
hospital charge/
formular$.tw.
fundhold$.tw.
*medicaid/
*medicare/
blue cross.tw.
or/41-52
exp Health Care Personnel/
clinical pharmacist?.tw.
nutritionist?.tw.
or/54-56
patient care/
patient care planning/
(team? adj2 (care or treatment or assessment or consultation)).tw.
(integrat$ adj2 (care or service?)).tw.
(care adj2 (coordinat$ or program$ or continuity)).tw.
(case adj1 management).tw.
case management/
rehabilitation care/
exp primary healthcare/
*ambulatory care/
home care/
*hospice/
office visit$.tw.
house call$.tw.
*day care/
*aftercare/
*community health nursing/
(chang$ adj1 location?).tw.
(domicillary or domiciliary).tw.
(home adj1 treat$).tw.
day surgery.tw.
exp hospital/
residential home/
nursing home/
rehabilitation center/
health center/
mental health center/
cancer center/
community health center/
healthcare facility/
assisted living facility/
*group practice/ or *faculty practice/ or *private practice/
general practice/
healthcare practice/
medical practice/
or/58-92
*medical record/
(computeri#ed adj2 “medical records system$”).tw.
(information adj2 (management or system?)).tw.
“peer review”/ or “peer review, health care”/
“utilization review”/
((abuse$ or misuse$ or overutili#ation) adj2 (health or service?)).tw
clinical practice/
quality assurance.tw.
Outcome Assessment/
Total Quality Management/
Health Care Quality/
“program evaluation”.tw.
“length of stay”.tw.
(early adj1 discharg$).tw.
discharge planning.tw.
offset.tw.
triage.tw.
patient referral/
computer assisted drug therapy/
near patient testing.tw.
anamnesis/
*telephone/
(physician patient adj (interaction? or relationship?)).tw.
*health maintenance organizations/
managed care.tw.
(hospital? adj1 merg$).tw.
or/94-119
((standard or usual or routine or regular or traditional or conventional or pattern) adj2 care).tw.
(program$ adj2 (reduc$ or increas$ or decreas$ or chang$ or improv$ or modify$ or monitor$ or care)).tw.
((effect? or impact or evaluat$ or introduc$ or compar$) adj2 treatment program$).tw.
((effect? or impact or evaluat$ or introduc$ or compar$) adj2 care program$).tw.
((effect? or impact or evaluat$ or introduc$ or compar$) adj2 screening program$).tw.
((effect? or impact or evaluat$ or introduc$ or compar$) adj2 prevent$ program$).tw.
(computer$ adj2 (dosage or dosing or diagnosis or therapy or decision?)).tw.
((introduc$ or impact or effect? or implement$ or computer$) adj protocol?).tw.
((effect? or impact or introduc$) adj2 (legislation or regulations or policy)).tw.
or/121-129
randomized controlled trial/
controlled clinical trial/
double blind procedure/
single blind procedure/
Or/131-134
exp Clinical Trial/
(clin$ adj25 trial$).tw.
((singl$ or doubl$ or trebl$ or tripl$) adj25 (blind$ or mask$)).tw.
placebo/
placebo$.tw.
random$.tw.
methodology/
Or/136-142
comparative study/
exp evaluation/
follow up/
prospective studies/
(control$ or prospective$ or volunteer$).tw.
intervention$.tw.
control$.tw.
Or/144-150
135 or 143 or 151
40 or 53 or 57 or 93 or 120 or 130
4 and 152 and 153
nonhuman/
154 not 155
Appendix 4. CINAHL search strategy
CINAHL (EBSCO), 1982 to May 2009
Syntax Guide
MH - CINAHL subject heading
MM - CINAHL major subject heading
+ - explode: includes narrower terms to the index term being exploded
TI - word in the title field
AB - word in the abstract field
* - truncation/ wild card: adds no or more characters
Nx - adjacency: required words are adjacent to each other, or within × words of each other
PT - Publication type
Description of search strategy
Condition: line 3
Study design: line 67
Interventions: line 59
(MH “Obesity+”) or (MM “Weight Loss”)
TI (obes* or overweight* ) or AB (obes* or overweight* )
1 or 2
(MH “Education, Continuing+”) or (MM “Pamphlets”) or (MM “Practice Guidelines”) or (MM “Professional Compliance”) or (MM “Reminder Systems”)
TI (education* N2 program*) or TI (education* N2 intervention*) or TI (education* N2 meeting*) or TI (education* N2 session*) or TI (education* N2 strateg*) or TI (education* N2 workshop*) or TI (education* N2 visit*) or AB (education* N2 program*) or AB (education* N2 intervention*) or AB (education* N2 meeting*) or AB (education* N2 session*) or AB (education* N2 strateg*) or AB (education* N2 workshop*) or AB (education* N2 visit*)
TI (behavior* N2 intervention*) or TI (behaviour* N2 intervention*) or AB (behavior* N2 intervention*) or AB (behaviour* N2 intervention*)
TI (leaflet* or booklet* or poster or posters) or AB (leaflet* or booklet* or poster or posters)
TI (written information) or TI (printed information) or TI (oral information) or AB (written information) or AB (printed information) or AB (oral information)
TI (information* N2 campaign) or AB (information* N2 campaign)
TI (education* N1 method*) or TI (education* N1 material*) or AB (education* N1 method*) or AB (education* N1 material*)
TI (outreach) or AB (outreach) or TI (facilitator*) or AB (facilitator*) or TI (academic detailing) or AB (academic detailing) or TI (consensus conference*) or AB (consensus conference*)
TI (opinion N1 leader*) or TI (education* N1 leader*) or TI (influential N1 leader) or AB (opinion N1 leader*) or AB (education* N1 leader*) or AB (influential N1 leader)
TI (practice guideline*) or AB (practice guideline*)
TI (guideline* N2 introduc*) or TI (guideline* N2 issu*) or TI (guideline* N2 impact) or TI (guideline* N2 effect*) or TI (guideline* N2 disseminat*) or TI (guideline* N2 distribut*) or AB (guideline* N2 introduc*) or AB (guideline* N2 issu*) or AB (guideline* N2 impact) or AB (guideline* N2 effect*) or AB (guideline* N2 disseminat*) or AB (guideline* N2 distribut*)
TI (effect* N2 training program*) or TI (impact N2 training program*) or TI (evaluat* N2 training program*) or TI (introduc* N2 training program*) or TI (compar* N2 training program*) or AB (effect* N2 training program*) or AB (impact N2 training program*) or AB (evaluat* N2 training program*) or AB (introduc* N2 training program*) or AB (compar* N2 training program*)
TI (reminder*) or AB (reminder*) or TI (recall N2 system*) or AB (recall N2 system*) or TI (prompter*) or AB (prompter*) or TI (prompting) or AB (prompting)
TI (algorithm*) or AB (algorithm*)
(MM “Feedback”)
TI (feedback) or AB (feedback)
TI (feedback N1 loop*) or TI (feedback N1 control*) or TI (feedback N1 regula*) or TI (feedback N1 mechanism*) or TI (feedback N1 inhib*) or TI (feedback N1 system*) or TI (feedback N1 circuit*) or TI (feedback N1 sensory) or TI (feedback N1 visual) or TI (feedback N1 audio*) or TI (feedback N1 auditory) or AB (feedback N1 loop*) or AB (feedback N1 control*) or AB (feedback N1 regula*) or AB (feedback N1 mechanism*) or AB (feedback N1 inhib*) or AB (feedback N1 system*) or AB (feedback N1 circuit*) or AB (feedback N1 sensory) or AB (feedback N1 visual) or AB (feedback N1 audio*) or AB (feedback N1 auditory)TI (feedback N1 loop*) or TI (feedback N1 control*) or TI (feedback N1 regula*) or TI (feedback N1 mechanism*) or TI (feedback N1 inhib*) or TI (feedback N1 system*) or TI (feedback N1 circuit*) or TI (feedback N1 sensory) or TI (feedback N1 visual) or TI (feedback N1 audio*) or TI (feedback N1 auditory) or AB (feedback N1 loop*) or AB (feedback N1 control*) or AB (feedback N1 regula*) or AB (feedback N1 mechanism*) or AB (feedback N1 inhib*) or AB (feedback N1 system*) or AB (feedback N1 circuit*) or AB (feedback N1 sensory) or AB (feedback N1 visual) or AB (feedback N1 audio*) or AB (feedback N1 auditory)
18 or 19 or 20
17 not 21
TI (chart review*) or AB (chart review*)
TI (effect* N2 audit) or TI (impact N2 audit) or TI (records N2 audit) or TI (chart* N2 audit) or AB (effect* N2 audit) or AB (impact N2 audit) or AB (records N2 audit) or AB (chart* N2 audit)
TI (compliance) or AB (compliance) or TI (marketing) or AB (marketing)
4 or 5 or 6 or 7 or 8 or 9 or 10 or 11 or 12 or 13 or 14 or 15 or 16 or 22 or 23 or 24 or 25
(MH “Reimbursement Mechanisms+”) or (MM “Fee for Service Plans”) or (MM “Capitation Fee”) or (MM “Health Facility Charges”) or (MM “Medicaid”) or (MM “Medicare”)
TI (fee for service) or AB (fee for service) or TI (“deductibles and coinsurance”) or AB (“deductibles and coinsurance”) or TI (cost shar*) or AB (cost shar*) or TI (copayment*) or TI (“co payment*”) or TI (“co-payment*”) or AB (copayment*) or AB (“co payment*”) or AB (“co-payment*”)
TI (prepay*) or TI (prepaid) or TI (“prospective payment*”) or AB (prepay*) or AB (prepaid) or AB (“prospective payment*”)
TI (formular*) or AB (formular*) or TI (fundhold*) or AB (fundhold*)
TI (blue cross) or AB (blue cross)
27 or 28 or 29 or 30 or 31
(MH “Health Personnel+”)
TI (clinical pharmacist* or paramedic* or nutritionist*) or AB (clinical pharmacist* or paramedic* or nutritionist*)
33 or 34
(MH “Multidisciplinary Care Team+”) or (MM “Nursing Care Plans”) or (MM “Ambulatory Care”) or (MM “Home Health Care”) or (MM “Hospices”) or (MM “Office Visits”) or (MM “Home Visits”) or (MM “Day Care”) or (MM “After Care”) or (MM “Community Health Nursing”) or (MM “Health Facilities”) or (MM “Academic Medical Centers”) or (MH “Ambulatory Care Facilities+”) or (MM “Alternative Birth Centers”) or (MM “Hospital Units”) or (MH “Hospitals+”) or (MM “Pharmacy, Retail”) or (MM “Practitioner’s Office”) or (MM “Rehabilitation Centers”) or (MH “Residential Facilities+”) or (MH “Health Facility Departments+”) or (MM “Group Practice”) or (MM “Faculty Practice”) or (MM “Private Practice”)(MH “Multidisciplinary Care Team+”) or (MM “Nursing Care Plans”) or (MM “Ambulatory Care”) or (MM “Home Health Care”) or (MM “Hospices”) or (MM “Office Visits”) or (MM “Home Visits”) or (MM “Day Care”) or (MM “After Care”) or (MM “Community Health Nursing”) or (MM “Health Facilities”) or (MM “Academic Medical Centers”) or (MH “Ambulatory Care Facilities+”) or (MM “Alternative Birth Centers”) or (MM “Hospital Units”) or (MH “Hospitals+”) or (MM “Pharmacy, Retail”) or (MM “Practitioner’s Office”) or (MM “Rehabilitation Centers”) or (MH “Residential Facilities+”) or (MH “Health Facility Departments+”) or (MM “Group Practice”) or (MM “Faculty Practice”) or (MM “Private Practice”)
TI (“patient care planning” or “case management” or domicillary or domiciliary or “day surgery” or “institutional practice” or “partnership practice”) or AB (“patient care planning” or “case management” or domicillary or domiciliary or “day surgery” or “institutional practice” or “partnership practice”)
TI (team* N2 care) or TI (team* N2 treatment) or TI (team* N2 assessment) or TI (team* N2 consultation) or AB (team* N2 care) or AB (team* N2 treatment) or AB (team* N2 assessment) or AB (team* N2 consultation)
TI (integrat* N2 care) or TI (integrat* N2 service*) or AB (integrat* N2 care) or AB (integrat* N2 service*)
TI (care N2 coordinat*) or TI (care N2 program*) or TI (care N2 continuity) or AB (care N2 coordinat*) or AB (care N2 program*) or AB (care N2 continuity)
TI (chang* N2 location*) or AB (chang* N2 location*) or TI (home N2 treat*) or AB (home N2 treat*)
36 or 37 or 38 or 39 or 40 or 41
(MH “Medical Records+”) or (MM “Peer Review”) or (MM “Utilization Review”) or (MH “Health Services Misuse+”) or (MM “Quality Assurance”) or (MM “Process Assessment (Health Care)”) or (MM “Outcome Assessment”) or (MM “Quality Improvement”) or (MM “Quality of Health Care”) or (MM “Program Evaluation”) or (MM “Length of Stay”) or (MH “Referral and Consultation+”) or (MM “Drug Therapy, Computer Assisted”) or (MM “Patient History Taking”) or (MM “Telephone”) or (MM “Health Maintenance Organizations”)
TI (information N2 management) or TI (information N2 system*) or AB (information N2 management) or AB (information N2 system*)
TI (“physician practice patterns”) or AB (“physician practice patterns”) or TI (“quality assurance”) or AB (“quality assurance”) or TI (early N1 discharg*) or AB (early N1 discharg*)
TI (“discharge planning” or “offset” or “triage” or “near patient testing” or “managed care”) or AB (“discharge planning” or “offset” or “triage” or “near patient testing” or “managed care”)
TI (“physician patient interaction*”) or TI (“physician patient relationship*) or AB (“physician patient interaction*”) or AB (“physician patient relationship*) or TI (hospital* N1 merg*) or AB (hospital* N1 merg*)
43 or 44 or 45 or 46 or 47
TI (standard N2 care) or TI (usual N2 care) or TI (routine N2 care) or TI (regular N2 care) or TI (traditional N2 care) or TI (conventional N2 care) or TI (pattern N2 care) or AB (standard N2 care) or AB (usual N2 care) or AB (routine N2 care) or AB (regular N2 care) or AB (traditional N2 care) or AB (conventional N2 care) or AB (pattern N2 care)
TI (program* N2 reduc*) or TI (program* N2 increas*) or TI (program* N2 decreas*) or TI (program* N2 chang*) or TI (program* N2 improv*) or TI (program* N2 modif*) or TI (program* N2 monitor*) or TI (program* N2 care) or AB (program* N2 reduc*) or AB (program* N2 increas*) or AB (program* N2 decreas*) or AB (program* N2 chang*) or AB (program* N2 improv*) or AB (program* N2 modif*) or AB (program* N2 monitor*) or AB (program* N2 care)
TI (effect* N2 “treatment program*”) or TI (impact N2 “treatment program*”) or TI (evaluat* N2 “treatment program*”) or TI (introduc* N2 “treatment program*”) or TI (compar* N2 “treatment program*”) or AB (effect* N2 “treatment program*”) or AB (impact N2 “treatment program*”) or AB (evaluat* N2 “treatment program*”) or AB (introduc* N2 “treatment program*”) or AB (compar* N2 “treatment program*”)
TI (effect* N2 “care program*”) or TI (impact N2 “care program*”) or TI (evaluat* N2 “care program*”) or TI (introduc* N2 “care program*”) or TI (compar* N2 “care program*”) or AB (effect* N2 “care program*”) or AB (impact N2 “care program*”) or AB (evaluat* N2 “care program*”) or AB (introduc* N2 “care program*”) or AB (compar* N2 “care program*”)
TI (effect* N2 “screening program*”) or TI (impact N2 “screening program*”) or TI (evaluat* N2 “screening program*”) or TI (introduc* N2 “screening program*”) or TI (compar* N2 “screening program*”) or AB (effect* N2 “screening program*”) or AB (impact N2 “screening program*”) or AB (evaluat* N2 “screening program*”) or AB (introduc* N2 “screening program*”) or AB (compar* N2 “screening program*”)
TI (effect* N2 “prevention program*”) or TI (impact N2 “prevention program*”) or TI (evaluat* N2 “prevention program*”) or TI (introduc* N2 “prevention program*”) or TI (compar* N2 “prevention program*”) or AB (effect* N2 “prevention program*”) or AB (impact N2 “prevention program*”) or AB (evaluat* N2 “prevention program*”) or AB (introduc* N2 “prevention program*”) or AB (compar* N2 “prevention program*”)
TI (computer* N2 dosage) or TI (computer* N2 dosing) or TI (computer* N2 diagnosis) or TI (computer* N2 therapy) or TI (computer* N2 decision*) or AB (computer* N2 dosage) or AB (computer* N2 dosing) or AB (computer* N2 diagnosis) or AB (computer* N2 therapy) or AB (computer* N2 decision*)
TI (introduc* N2 protocol*) or TI (impact N2 protocol*) or TI (effect* N2 protocol*) or TI (implement* N2 protocol*) or TI (computer* N2 protocol*) or AB (introduc* N2 protocol*) or AB (impact N2 protocol*) or AB (effect* N2 protocol*) or AB (implement* N2 protocol*) or AB (computer* N2 protocol*)
TI (effect* N2 legislation) or TI (effect* N2 regulations) or TI (effect* N2 policy) or TI (impact* N2 legislation) or TI (impact* N2 regulations) or TI (impact* N2 policy) or TI (introduc* N2 legislation) or TI (introduc* N2 regulations) or TI (introduc* N2 policy) or AB (effect* N2 legislation) or AB (effect* N2 regulations) or AB (effect* N2 policy) or AB (impact* N2 legislation) or AB (impact* N2 regulations) or AB (impact* N2 policy) or AB (introduc* N2 legislation) or AB (introduc* N2 regulations) or AB (introduc* N2 policy)
49 or 50 or 51 or 52 or 53 or 54 or 55 or 56 or 57
26 or 32 or 35 or 42 or 48 or 58
(MH “Clinical Trials+”) or (MM “Random Assignment”) or (MM “Placebos”) or (MM “Quantitative Studies”)
PT “Clinical trial”
TI (Clinical* trial*) or AB (Clinical* trial*)
TI (singl* N1 blind*) or TI (doubl* N1 blind*) or TI (trebl* N1 blind*) or TI (tripl* N1 blind*) or TI (singl* N1 mask*) or TI (doubl* N1 mask*) or TI (trebl* N1 mask*) or TI (tripl* N1 mask*) or AB (singl* N1 blind*) or AB (doubl* N1 blind*) or AB (trebl* N1 blind*) or AB (tripl* N1 blind*) or AB (singl* N1 mask*) or AB (doubl* N1 mask*) or AB (trebl* N1 mask*) or AB (tripl* N1 mask*)
TI (Randomised control* trial*) or TI (Randomized control* trial*) or AB (Randomised control* trial*) or AB (Randomized control* trial*)
(Random* N2 allocat*) or AB (Random* N2 allocat*)
TI (placebo*) or AB (placebo*)
60 or 61 or 62 or 63 or 64 or 65 or 66
3 and 59 and 67
Appendix 5. PsycINFO search strategy
PsycINFO (OVID), 1806 to May 2009
Syntax Guide
/ - index term (APA thesaurus)
exp - explode: includes narrower terms to the index term being exploded
.tw. - text word In title or abstract fields
$ - truncation/ wild card: adds no or more characters
? - truncation/ wild card: adds no or one character
# - truncation/ wild card: retrieves alternative single character
adjx - adjacency: required words are adjacent to each other, or within × words of each other
.pt. - Publication type
Description of search strategy
Condition: line 4
Study design: line 108
Interventions: line 103
exp Obesity/
(obes$ or overweight$).tw.
weight loss/
or/1-3
exp Continuing education/
(education$ adj2 (program$ or intervention? or meeting? or session? or strateg$ or workshop? or visit?)).tw.
(behavio?r$ adj2 intervention?).tw.
*Written communication/
(leaflet? or booklet? or poster or posters).tw.
((written or printed or oral) adj information).tw.
(information$ adj2 campaign).tw.
(education$ adj1 (method? or material?)).tw.
outreach.tw.
((opinion or education$ or influential) adj1 leader?).tw.
facilitator?.tw.
academic detailing.tw.
consensus conference?.tw.
Treatment Guidelines/
(guideline$ adj3 adher$).tw.
practice guideline?.tw.
(guideline? adj2 (introduc$ or issu$ or impact or effect? or disseminat$ or distribut$)).tw.
((effect? or impact or evaluat$ or introduc$ or compar$) adj2 training program$).tw.
reminder$.tw.
(recall adj2 system$).tw.
(prompter? or prompting).tw.
algorithm?.tw.
*feedback/ or feedback.tw.
(feedback adj1 (loop? or control? or regula$ or mechanism? or inhib$ or system? or circuit? or sensory or visual or audio$ or auditory)).tw.
27 or 28
26 not 29
chart review$.tw.
((effect? or impact or records or chart?) adj2 audit).tw.
compliance.tw.
marketing.tw.
or/5-25,30-34
(reimburs$ adj3 mechanism$).tw.
fee for service.tw.
(capitation adj3 (fee or fees)).tw.
cost shar$.tw.
(copayment? or co payment? or co-payment?).tw.
(prepay$ or prepaid or prospective payment?).tw.
*health care costs/
formular?.tw.
fundhold$.tw.
(medicaid or medicare).tw.
blue cross.tw.
or/36-46
exp *Health Personnel/
clinical pharmacist?.tw.
paramedic?.tw.
nutritionist?.tw.
dietician?.tw.
or/48-52
exp *patient care planning/
(team? adj2 (care or treatment or assessment or consultation)).tw.
(integrat$ adj2 (care or service?)).tw.
(care adj2 (coordinat$ or program$ or continuity)).tw.
(case adj1 management).tw.
*ambulatory care/
*home care/
*hospice/
*home visiting programs/
*aftercare/
(community adj 3 nurs*).tw.
(chang$ adj1 location?).tw.
(domiciliary or domicillary).tw.
(home adj1 treat$).tw.
day surgery.tw.
exp Health care services/ or exp Hospitals/ or exp Residential care institutions/ or exp Rehabilitation centers/
*Practice/
or/54-70
*medical records/
(information adj2 (management or system?)).tw.
*peer review/ or *peer review, health care/
*utilization review/
((physician or doctor) adj2 practice).tw.
*Quality of Care/
quality assurance.tw.
*program evaluation/
*length of stay/
(early adj1 discharg$).tw.
discharge planning.tw.
offset.tw.
triage.tw.
Professional Referral/ or Professional Consultation/
near patient testing.tw.
*Patient history/
(physician patient adj (interaction? or relationship?)).tw.
*health maintenance organizations/
managed care.tw.
(hospital? adj1 merg$).tw.
or/72-91
((standard or usual or routine or regular or traditional or conventional or pattern) adj2 care).tw.
(program$ adj2 (reduc$ or increas$ or decreas$ or chang$ or improv$ or modify$ or monitor$ or care)).tw.
((effect? or impact or evaluat$ or introduc$ or compar$) adj2 treatment program$).tw.
((effect? or impact or evaluat$ or introduc$ or compar$) adj2 care program$).tw.
((effect? or impact or evaluat$ or introduc$ or compar$) adj2 screening program$).tw.
((effect? or impact or evaluat$ or introduc$ or compar$) adj2 prevent$ program$).tw.
(computer$ adj2 (dosage or dosing or diagnosis or therapy or decision?)).tw.
((introduc$ or impact or effect? or implement$ or computer$) adj2 protocol?).tw.
((effect or impact or introduc$) adj2 (legislation or regulations or policy)).tw.
or/93-101
35 or 47 or 53 or 71 or 92 or 102
103 and 4
((Clinical adj3 trial*) or (controlled adj3 trial*) or (randomi* adj3 trial*) or (random* adj3 allocat*) or placebo*).tw.
((singl* or doubl* or treb* or trip*) adj25 (mask* or blind*)).tw.
((comparative adj3 stud*) or (evaluat* adj3 stud*) or (follow adj up adj3 stud*) or prospective stud*).tw.
or/105-107
104 and 108
Appendix 6. Data extraction form
THE DATA COLLECTION CHECKLIST
July 2008
DATA COLLECTION
For brevity, obese and overweight participants in the trials are referred to as patients in this checklist, although it is recognised that they might not be symptomatic at the time of the study.
Once potentially relevant studies have been identified for a review, the following data should be extracted independently by two reviewers.
Please record your name and the Study ID (first author and year of publication) in the header of this document.
For most items reviewers should mark an X against the appropriate response in each case in the column labelled Relevant supporting text and location. In addition it will be helpful if you cut and paste relevant supporting text and state its original location in the paper (page/column/paragraph). This facilitates later comparisons of extracted data. Any other comments can also be recorded in this column. The column will expand to fit the amount of text you insert. Where appropriate add additional rows.
Data which is missing or UNCLEAR in a published report should be marked clearly on the data collection form (usually in the far right hand column). KD will contact the study authors for any necessary clarification or additional information.
Items in the data extraction sheet which are clearly not applicable to the study in question should be marked accordingly (i.e. N/A).
1. INCLUSION CRITERIA
1.1. Reviews scope
|
1.1 Reviews scope:
Any intervention that aims to improve the way health professionals work to reduce the weight of overweight or obese people. That is the effect(s) of a behavioural/ educational, financial, organisational or regulatory intervention (s) is evaluated |
RELEVANT SUPPORTING TEXT AND LOCATION (page/column/paragraph) | |
| YES | The effect of intervention(s) that aims to improve the way health professionals work to reduce the weight of overweight or obese people is evaluated. NB the population must be overweight or obese OR the overweight or obese population’s results are segregated for at least one of our significant outcomes (weight loss or objective measure of health professional’s behaviour change) | |
| NO | ||
| UNCLEAR | The intervention does not appear to be clearly described. Discuss the paper with KD before beginning data extraction |
If you scored NO for item 1.1, the study should not be included in the review.
COLLECT NO FURTHER DATA
1.1. Study design
| 1.2 Randomised controlled trial (RCT) | RELEVANT SUPPORTING TEXT AND LOCATION (page/column/paragraph) | |
| YES | Statement of random allocation of health professionals, patients, episodes of care, locations of care, etc given by authors | |
| NO | No statement of random allocation of health professionals, patients, episodes of care, locations of care, etc | |
| UNCLEAR | Discuss the paper with KD before beginning data extraction |
If you scored NO for the above criteria in item 1.2, the study should not be included in the review .
COLLECT NO FURTHER DATA.
1.2. Methodological inclusion criteria
| 1.3.1 Paper reports objective measurement of provider performance/behaviour or patient outcome(s) | RELEVANT SUPPORTING TEXT AND LOCATION (page/column/paragraph) | |
| YES | E.g. Primary outcome : Patient weight loss, OR Secondary Patient outcomes: psychological outcomes (depression, dietary restraint); morbidity (measures of disease status, sick leave); fat or BMI measures; effects on risk factors (differences in cholesterol levels, blood pressure); patient behaviour (attendance levels at weight management or physical exercise programmes); and number of withdrawals from treatment OR Secondary Health professional outcomes: measures of health practitioners behaviour, knowledge. |
|
| NO | E.g. self-report data, measures of attitudes or beliefs or perceptions or satisfaction. Studies reporting only knowledge or attitudes of health professionals or patient satisfaction with no objective measure of professional performance or patient outcomes are to be excluded | |
| UNCLEAR | Discuss the paper with KD before beginning data extraction |
| 1.3.2 Relevant and interpretable data presented or obtainable (e.g. by reading points off a graph) | |
| YES | Data is presented or obtainable |
| NO | Relevant data is not presented and is clearly unobtainable |
| UNCLEAR | Discuss the paper with KD before beginning data extraction |
If you scored NO for either of the above criteria in item 1.3, the study should not be included in the review.
COLLECT NO FURTHER DATA.
A study must meet the minimum criteria for scope, design, and methodology for inclusion in the reviews. If it does not, COLLECT NO FURTHER DATA. If you are unclear whether a paper meets any of the inclusion criteria please contact Katherine Deane.
2.0 METHODS
2.1 Units of allocation and analysis
|
2.1.1 Unit of allocation
(i.e. who or what was allocated to study groups, and was it cluster or individual randomisation) |
Relevant supporting text and location. (page/column/paragraph) |
| Patient | |
| Episode of care | |
| Clinic Day | |
| Provider | |
| Firm | |
| Practice | |
| Institution | |
| Community | |
| Other: (Please specify) | |
| UNCLEAR |
|
2.1.2 Unit of analysis
(e.g. results analysed as events per practice) |
Relevant supporting text and location. (page/column/paragraph) |
| Patient | |
| Episode of care | |
| Clinic Day | |
| Provider | |
| Firm | |
| Practice | |
| Institution | |
| Community | |
| Other: (Please specify) | |
| UNCLEAR |
2.2 Power calculation
| 2.2 Power calculation: | Relevant supporting text and location. (page/column/paragraph) | |
| YES | Study has sufficient statistical power to detect clinically important effects as statistically significant | |
| Number expected to be recruited / number actually recruited | ||
| NO | No power calculation | |
| UNCLEAR |
2.3 Risk of Bias Assessment
|
2.3.1 SEQUENCE GENERATION
Was the allocation sequence adequately generated? Describe the method used to generate the allocation sequence in sufficient detail to allow an assessment of whether it should produce comparable groups |
Relevant supporting text and location. (page/column/paragraph) |
The unit of allocation was health professional, patient or episode of care and the investigators describe a random component in the sequence generation process such as:
*Minimization may be implemented without a random element, and this is considered to be equivalent to being random. |
|
The unit of allocation was health professional, patient or episode of care and the investigators describe a quasi-random component in the sequence generation process such as:
|
|
The investigators describe a non-random component in the sequence generation process. E.g
|
|
| Insufficient information about the sequence generation |
|
2.3.2 ALLOCATION CONCEALMENT
Was allocation adequately concealed? Describe the method used to conceal the allocation sequence in sufficient detail to determine whether intervention allocations could have been foreseen in advance of, or during, enrolment |
Relevant supporting text and location. (page/column/paragraph) | |
| YES | The unit of allocation was health professional, patient or episode of care and participants and investigators enrolling participants could not foresee assignment because one of the following, or an equivalent method, was used to conceal allocation:
|
|
| NO | Participants or investigators enrolling participants could possibly foresee assignments and thus introduce selection bias, such as allocation based on:
Again for cluster randomisation the judgement is whether a study where allocation was performed by the study statistician is regarded as biased |
|
| UNCLEAR | Insufficient information to permit judgement of Yes or No. This is usually the case if the method of concealment is not described or not described in sufficient detail to allow a definite judgement? E.g. if the use of assignment envelopes is described, but it remains unclear whether envelopes were sequentially numbered, opaque and sealed |
|
2.3.3.1 BLINDING OF OUTCOME ASSESSORS:
Was knowledge of the allocated interventions adequately prevented during the study? Describe all measures used, if any, to blind the outcome assessors from knowledge of which intervention a participant received. Provide any information relating to whether the intended blinding was effective |
|||
| YES | Any one of the following:
|
||
| NO | Any one of the following:
|
||
| UNCLEAR | Any one of the following:
|
||
|
Reported Outcome(s)
(Add rows as necessary) |
Low Risk of Bias: YES/NO/UNCLEAR | ||
|
2.3.4.1 INCOMPLETE OUTCOME DATA:
Were incomplete outcome data adequately addressed? Describe the completeness of outcome data for each main outcome, including attrition and exclusions from the analysis. State whether attrition and exclusions were reported, the numbers in each intervention group (compared with total randomized participants), reasons for attrition/ exclusions where reported, and any re-inclusions in analyses performed by the review authors |
|||
| YES | Any one of the following:
|
||
| NO | Any one of the following:
|
||
| UNCLEAR | Any one of the following:
|
||
|
Reported Outcome(s)
(Add rows as necessary) |
Low Risk of Bias: YES/NO/UNCLEAR | ||
|
2.3.5 SELECTIVE OUTCOME REPORTING
Are reports of the study free of suggestion of selective outcome reporting? State how the possibility of selective outcome reporting was examined by the review authors, and what was found NB. KD will try to find study protocols if not present in your paper, you dont need to do this |
Relevant supporting text and location. (page/column/paragraph) | |
| YES | Any of the following:
|
|
| NO | Any one of the following:
|
|
| UNCLEAR | Insufficient information to permit judgement of Yes or No. It is likely that the majority of studies will fall into this category |
NB. We do not expect data extractors to go find the study protocols, the Newcastle base will try to find these down along with any other queries for the study authors that arise from the data extraction.
2.3.6 Other sources of bias
| 2.3.6 BASELINE MEASUREMENT | Relevant supporting text and location. (page/column/paragraph) |
| Performance or patient outcomes measured prior to the intervention, and no substantial differences present across study groups in main outcome measures and also in possible confounding variables (e.g. sex, age) | |
| Differences at baseline in main outcome measures or confounding variables (e.g. sex, age) likely to undermine the post intervention differences, e.g. differences between groups before the intervention similar to those found post intervention or had extreme baseline imbalance | |
| Baseline measures not reported, or unclear whether baseline measures are different across study groups |
| 2.3.7 RELIABLE PRIMARY OUTCOME MEASURE(S) | |||
| YES | Two or more raters with agreement ≥ 90% or kappa ≥ 0.8 OR outcome assessment is objective, e. g. length of hospital stay, drug levels assessed by a standardised test | ||
| NO | Two or more raters with agreement < 90% or kappa < 0.8. | ||
| UNCLEAR | Reliability not reported for outcome measures obtained by chart extraction or collected by an individual | ||
|
Reported Outcome(s)
(Add rows as necessary) |
Low Risk of Bias: YES/NO/UNCLEAR | ||
| 2.3.8 PROTECTION AGAINST CONTAMINATION | Relevant supporting text and location. (page/column/paragraph) | |
| YES | Allocation by community, institution or practice and unlikely that control group received the intervention | |
| NO | Likely that control group received the intervention, e.g. cross-over trials or if patients rather than professionals were randomised | |
| UNCLEAR | Professionals allocated within a clinic or practice and possible that communication between experimental and control group professionals could have occurred |
2 PARTICIPANTS
2.1 Characteristics of participating healthcare providers
|
2.1.1 Profession (mark all appropriate): Please state the numbers of each profession involved. Also please note if the numbers come from baseline, the remaining population at the endpoint, or other time period (e.g. sequential accrual) |
Relevant supporting text and location. (page/column/paragraph) |
| Physicians | |
| Nurses | |
| Pharmacists | |
| Physiotherapists | |
| Dietitianss/Nutritionists | |
| Psychologists | |
| Other: (Please specify) | |
| UNCLEAR |
| 3.1.2 Level of training: | Relevant supporting text and location. (page/column/paragraph) |
| In post-graduate training (House Officer/Intern, Registrar/Resident) | |
| Fully trained (Consultant/Attending) | |
| Mixed | |
| Other (Specify i.e. copy all information available in paper) | |
| UNCLEAR (information not available) |
| 3.1.3 Age of health professional: | Relevant supporting text and location. (page/column/paragraph) |
| Mean age | |
| UNCLEAR (information not available) |
| 3.1.4 Years since graduation or in practice: | Relevant supporting text and location. (page/column/paragraph) |
| Mean | |
| UNCLEAR (information not available) |
| 3.1.5 Proportion of eligible providers (or allocation units) who participated in the evaluation: | Relevant supporting text and location. (page/column/paragraph) |
| Report the numbers or the percentage of providers in target population who were allocated to study groups | |
| UNCLEAR (information not available) |
3.2 Characteristics of the participating patients
|
3.2.1 Clinical problem(s) of participating patients:
Please give information on the authors definitions of the conditions e.g. over 5lbs over the recommended maximum weight for their height. Please also note the numbers with each condition and if they come from baseline, the remaining population at the endpoint, or other time period (e.g. sequential accrual) |
Relevant supporting text and location. (page/column/paragraph) |
| 3.2.1.1 Overweight (BMI over 25 but less than 30) | |
| UNCLEAR (information not available) | |
| 3.2.1.2 Obese (BMI 30 or over) | |
| UNCLEAR (information not available) | |
| 3.2.1.3 Diabetes | |
| UNCLEAR (information not available) | |
| 3.2.1.4 Ischemic heart disease | |
| UNCLEAR (information not available) |
|
3.2.2 Other characteristics of participating patients:
Please note if the numbers come from baseline, the remaining population at the endpoint, or other time period (e.g. sequential accrual) |
Relevant supporting text and location. (page/column/paragraph) |
| 3.2.2.1 Age: | |
| Mean | |
| Range | |
| UNCLEAR (information not available) | |
| 3.2.2.2 Gender | |
| UNCLEAR (information not available) | |
| 3.2.2.3 Ethnicity | |
| UNCLEAR (information not available) | |
| 3.2.2.4 Other (Please specify) | |
| UNCLEAR (information not available) |
|
3.2.3 The number randomised into the trial
(i.e. all those who actually entered the study) |
Relevant supporting text and location. (page/column/paragraph) |
| 3.2.3.1 Episodes of care: | |
| UNCLEAR (information not available) | |
| 3.2.3.2 Patients | |
| UNCLEAR (information not available) | |
| 3.2.3.3 Providers | |
| UNCLEAR (information not available) | |
| 3.2.3.4 Practices | |
| UNCLEAR (information not available) | |
| 3.2.3.5 Hospitals | |
| UNCLEAR (information not available) | |
| 3.2.3.6 Communities or regions | |
| UNCLEAR (information not available) |
3.3 SETTING
3.3.1 Reimbursement system
| 3.3.1 Reimbursement system: | Relevant supporting text and location. (page/column/paragraph) |
| Fee for service (provider paid for number and type of services delivered) | |
| Capitation (provider paid set amount per patient for providing specific care) | |
| Prospective payment | |
| Global budget | |
| Mixed | |
| UNCLEAR |
3.4 Setting of care
| 3.4 Setting of care: | Relevant supporting text and location. (page/column/paragraph) |
| Inpatient | |
| Outpatient (e.g. ambulatory care provided by hospitals, specialists etc.) | |
| General practice or community-based | |
| Mixed | |
| UNCLEAR |
3.5 Academic status
| 3.5 Academic status of the setting of care: | Relevant supporting text and location. (page/column/paragraph) |
| University (teaching) hospital | |
| Non-teaching or university affiliated | |
| Mixed | |
| Other (please specify) | |
| UNCLEAR |
3.6 Country
| 3.6 Country: | Relevant supporting text and location. (page/column/paragraph) |
| USA | |
| Canada | |
| UK | |
| Australia | |
| Netherlands | |
| Other (Please specify) | |
| UNCLEAR (information not available) |
4.0 CHARACTERISTICS OF THE INTERVENTIONS
4.1 Professional interventions
|
4.1 Professional interventions:
Record the intervention(s) aimed at the health professionals for each study group or period. If there is more than one form of intervention add rows |
Location of text (page/column/ paragraph) | |
|
Describe intervention
(Report this in the words of the paper) |
||
|
Describe intervention
(Report this in the words of the paper) |
||
4.2 Timing of intervention
|
4.2 Timing:
For each intervention aimed at the health professionals, state the following (for each score UNCLEAR if information not available) |
Relevant supporting text and location. (page/column/paragraph) | |
| Proximity to clinical decision-making (this item may be particularly relevant to audit and feedback and reminder interventions) | Describe. | |
| UNCLEAR | ||
| Frequency/number of intervention events | Describe | |
| UNCLEAR | ||
| Duration of intervention | Describe | |
| UNCLEAR |
4.3 Recipient
|
4.3 Healthcare professional recipient:
State whether each intervention was delivered to an individual, a group or was not stated (UNCLEAR) |
Relevant supporting text and location. (page/column/paragraph) | ||
| Intervention Group |
Describe whether delivered to individual, group, or UNCLEAR
(Report this in the words of the paper) |
||
| Control Group |
Describe whether delivered to individual, group, or UNCLEAR
(Report this in the words of the paper) |
|
4.4 Intervention deliverer:
State who (or what) delivered the intervention (if not stated code as UNCLEAR) e.g. local expert, computer system |
Relevant supporting text and location. (page/column/ paragraph) | ||
| Intervention Group |
Describe who (or what) delivered the intervention
(Report this in the words of the paper) |
||
| Control Group |
Describe who (or what) delivered the intervention
(Report this in the words of the paper) |
||
4.5 Types of targeted behaviour of the health professionals
|
4.5 Type(s) of targeted behaviour of the health professionals
e.g. increased rates of referral. Report this in the words of the paper |
Location of text in paper. (page/column/paragraph) |
4.6 Development of the intervention
|
4.6.1 Consultation with professional recipients:
Was the intervention aimed at the health professional developed through consultation with the professional recipient(s)? |
Relevant supporting text and location. (page/column/ paragraph) | |
| YES | Specified in the paper that recipients were involved in development of intervention. Describe the method of involvement e.g. formal consensus process | |
| NO | Specified in the paper that recipients were not involved in development of intervention | |
| UNCLEAR | Not specified |
|
4.6.2 Evidence base of intervention:
Was the intervention based on good evidence? |
Relevant supporting text and location. (page/column/paragraph) | |
| YES | Intervention based on good evidence e.g. clear reference to a systematic review or RCT. Describe | |
| NO | Explicitly not evidence-based. | |
| UNCLEAR | Not specified |
4.7 Consumer Involvement
|
4.7 Consumer Involvement:
Were consumers (i.e. potential patients) involved at any point of the design, conduct or interpretation of the study? (E.g., consumers involved in clinical practice guideline development, or their views collected.) |
Relevant supporting text and location. (page/column/paragraph) | ||
| YES | Specified in the paper that consumers were involved in the design, conduct or interpretation of the study. Describe | ||
| NO | Specified in the paper that consumers were not involved in the design, conduct or interpretation of the study | ||
| UNCLEAR | Not specified | ||
4.8 Barriers to change
|
4.8 Barriers to change:
Did the investigators prospectively identify specific barriers to change in the target population, which were addressed by the intervention |
Relevant supporting text and location. (page/column/paragraph) | |
| Describe. | ||
| Not done | ||
| Not clear | ||
4.9 Source of funding for study
| 4.9 Source of funding for study | Relevant supporting text and location. (page/column/paragraph) | |
| Describe. | ||
| Not clear | ||
4.10 Ethical Approval
| 4.10 Ethical Approval | Relevant supporting text and location. (page/column/paragraph) | |
| YES | Ethical approval sought and obtained for study | |
| UNCLEAR | Not reported |
5.0 CHARACTERISTICS OF OUTCOMES
| 5.1 Economic variables | Relevant supporting text and location. (page/column/paragraph) | |
| Were costs of the intervention reported? | YES (describe costs) | |
| NO (not reported) | ||
| Were changes in direct healthcare costs as a result of the intervention reported (e.g. drugs, hospital stays, etc.)? | YES (describe costs) | |
| NO (not reported) | ||
| Were changes in non-healthcare costs as a result of the intervention reported (e.g. patient travel or time off work for hospital visits)? | YES (describe costs) | |
| NO (not reported) | ||
| Were costs associated with the intervention linked with provider or patient outcomes in an economic evaluation (e.g. net cost per unit change in rate of prescribing, or cost per life year saved)? | YES (describe ratio) | |
| NO (no economic evaluation reported) | ||
| UNCLEAR (not adequately described in the paper) |
|
5.2 For how long were outcomes measured after initiation of the intervention?
(State all time points relevant) |
Relevant supporting text and location. (page/column/paragraph) |
|
5.3 Losses to follow-up:
NB please give all information provided (add rows as needed) e.g. numbers of practices and numbers of patients |
Relevant supporting text and location. (page/column/paragraph)
CONTROL GROUP |
Relevant supporting text and location. (page/column/paragraph)
INTERVENTION GROUP |
| Number randomised | ||
| Number completing follow-up (note when) | ||
| Reasons for loss to follow-up |
|
5.4 Has a possible ceiling effect been identified?
(e.g. there was little room for improvement in provider performance, because it was adequate without the intervention, based on baseline measurements or control group performance) |
Relevant supporting text and location. (page/column/paragraph) | |
| YES | ||
| NO | ||
| UNCLEAR | ||
| YES | ||
| NO | ||
| UNCLEAR | ||
6.0 RESULTS
Record results. Use extra forms for additional outcomes and/or comparisons. State the results as they will be entered in the review, and describe how calculated (e.g. relative percentage differences attributable to the intervention).
a) State the main results of the main outcome(s), for each study group, in natural units
b) For each available comparison, report the baseline and post intervention differences between study and control groups, in natural units. Include statistical significance if reported. Indicate whether the units of allocation and analysis were different and, if so, whether appropriate adjustment was made (e.g. the intra-practice correlation coefficient indicates the independence of the event analysed).
In all cases, report a more favourable provider/patient outcome in the more active intervention group as a positive (+) finding (i.e., where differences in the groups are in the intended direction).
Finally if the results are presented in the paper in a different format to that provided by us, please just cut and paste their whole results table(s) into this section.
6.0 Results
Comparison no. ......
Groups compared (use same labelling as intervention and effect modifiers table):
Describe comparison (e.g. intervention [specify type] vs. no intervention):
Outcome no. ....... Type of outcome: Process / Patient / Cost
Describe outcome measure: ............................................
Was the outcome adjusted for baseline covariates?
Was the data extracted from a graph (i.e. measured with a ruler). YES/NO/not applicable
NB If YES please enlarge the graph in order to maximise accuracy of measurements.
EVENT DATA Results in natural units (report intervention group first):
| Baseline period | Post-intervention period | Location (page/column/paragraph or table) | |||
| No. with event | Total observed | No. with event | Total observed | Intervention | |
| Control |
Total observed: no. of cases in group who were completely monitored for that outcome.
No. with event: no. of cases in group in which specified outcome occurred.
NB. if process data e.g. number of referrals within intervention period, only complete post-intervention period data block.
CONTINOUS DATA Results in natural units (report intervention group first):
| Baseline period | Post-intervention period | Location (page/column/paragraph or table) | No. | ||||
| Mean | SD | No. | Mean | SD | Authors report of which average and variance used (e.g. mean and SD) | ||
| Intervention | |||||||
| Control | |||||||
Statistical significance: ......
Statistical test used: ..................... Comments (e.g. one / two-tailed test)
Unit of analysis error: Yes / No
If No, was appropriate adjustment made (e.g. measure of intra-cluster correlation): Yes / No
Further comments:
FEEDBACK
Suggested change in title, 10 November 2010
Summary
Given the exclusion criteria for this review exclude trials of interventions targetting health professionals who are working solely with children, should the title for this review maybe refer to ‘adults’ instead of ‘people’? I think you found very few, if any studies that were excluded solely on the basis of the age of participating patients, but do you think there might be scope for a similar review that focuses on similar interventions aimed ultimately on improving care for children and young people in particular? Maybe you are planning one? Submitter has modified conflict of interest statement:
I am currently conducting a review of the views of young people in the UK about obesity, body size shape and weight and have published another on the same topic but including studies of children aged 4-11. I work for a University Social Sciences Research Department that has received funding to conduct a programme of research work in the area of obesity.
I have no other potential conflicts of interest
Reply
Thank you for your suggestion. We have changed the title as suggested to more clearly indicate the scope of this review.
Contributors
Rebecca Rees
Martin Eccles
Alain Mayhew
HISTORY
Protocol first published: Issue 2, 1998
Review first published: Issue 1, 1999
| Date | Event | Description |
|---|---|---|
| 17 March 2010 | Amended | Minor edits |
| 16 February 2010 | New search has been performed | New search up to June 2009. Revised inclusion criteria and new team of authors |
| 16 February 2010 | New citation required but conclusions have not changed | The searches were updated, and the criteria was changed to only include RCTs. There are now 6 studies in the review and it is very difficult to make any conclusions about the effectiveness of the interventions due to methodological weaknesses or heterogeneity |
| 25 July 2008 | Amended | Converted to new review format. |
| 13 January 2001 | New citation required and conclusions have changed | Substantive amendment |
WHAT’S NEW
Last assessed as up-to-date: 14 June 2009.
| Date | Event | Description |
|---|---|---|
| 10 November 2010 | Amended | Title changed. |
| 10 November 2010 | Feedback has been incorporated | See comment in feedback section; title changed. |
Footnotes
DECLARATIONS OF INTEREST Carolyn Summerbell and Emma Harvey were advisors to the BiO Project (Moore 2003) - a study mentioned in this review. Jim Brown is a shareholder in Glaxo Smithkline and Sanofi Aventis.
NOTES Fifteen of the previously included studies were excluded due to changed inclusion criteria. Three new studies have been included in this review update. The conclusions have been changed in issues of detail, but the overall message of the review has not changed.
References to studies included in this review
* Indicates the major publication for the study
- Cohen 1991 {published data only} .*; Cohen MD, D’Amico FJ, Merenstein JH. Weight reduction in obese hypertensive patients. Family Medicine. 1991;23(1):25–8. [PubMed] [Google Scholar]
- Martin 2006 {published data only} .Davis Martin P, Rhode PC, Dutton GR, Redmann SM, Ryan DH, Brantley PJ. A primary care weight management intervention for low-income African-American women. Obesity. 2006;14:1412–20. doi: 10.1038/oby.2006.160. [DOI] [PubMed] [Google Scholar]
- Moore 2003 {published data only} .*; Moore H, Summerbell CD, Greenwood DC, Tovey P, Griffiths J, Henderson M, et al. Improving management of obesity in primary care: cluster randomised trial. BMJ. 2003;327:1085–8. doi: 10.1136/bmj.327.7423.1085. [DOI] [PMC free article] [PubMed] [Google Scholar]; Moore H, Summerbell CD, Vail A, Greenwood DC, Adamson AJ. The design features and practicalities of conducting a pragmatic cluster randomized trial of obesity management in primary care. Statistics in Medicine. 2001;20(3):331–40. doi: 10.1002/1097-0258(20010215)20:3<331::aid-sim795>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- Pritchard 1999 {published data only} .*; Pritchard DA, Hyndman J, Taba F. Nutritional counselling in general practice: a cost effective analysis. Journal of Epidemiology and Community Health. 1999;53(5):311–6. doi: 10.1136/jech.53.5.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers 1982 {published data only} .Haring OM. Report prepared for the National Centre for Health Services Research. Hyattsville; Maryland, USA: May 31. 1976. Improving patient care by automated record summaries. issue Report Number PB–267 486. [Google Scholar]; *; Rogers JL, Haring OM, Wortman PM, Watson RA, Goetz JP. Medical information systems: assessing impact in the areas of hypertension, obesity and renal disease. Medical Care. 1982;20(1):63–74. doi: 10.1097/00005650-198201000-00005. [DOI] [PubMed] [Google Scholar]
- Sherwood 2006 {published data only} .Jeffery RW, McGuire MT, Brelje KL, Pronk NP, Boyle RG, Hase KA, et al. Recruitment to mail and telephone interventions for obesity in a managed care environment: the Weigh-To-Be project. American Journal of Managed Care. 2004;10:378–82. [PubMed] [Google Scholar]; *; Sherwood NE, Jeffery RW, Pronk NP, Boucher JL, Hanson A, Boyle R, et al. Mail and phone interventions for weight loss in a managed-care setting: weigh-to-be 2-year outcomes. International Journal of Obesity. 2006;30:1565–73. doi: 10.1038/sj.ijo.0803295. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
- Ashley 2001 {published data only} .Ashley JM, Jeor ST, Schrage JP, Perumean-Chaney SE, Gilbertson MC, McCall NL, et al. Weight control in the physician’s office. Archives of Internal Medicine. 2001;161(13):1599–604. doi: 10.1001/archinte.161.13.1599. [DOI] [PubMed] [Google Scholar]
- Atkinson 1977 {published data only} .Atkinson RL, Greenway FL, Bray GA, Dahms WT, Molitch ME, Hamilton K, et al. Treatment of obesity: comparison of physician and non-physician therapists using placebo and anorectic drugs in a double-blind trial. International Journal of Obesity. 1977;1(2):113–20. [PubMed] [Google Scholar]
- Balch 1976 {published data only} .Balch P, Balch K. Establishing a campus-wide behavioral weight reduction program through a university student health service: the use and training of health service personnel as behavioral weight therapists. Journal of the American College Health Association. 1976;25(2):148–52. [PubMed] [Google Scholar]
- Ben Noun 1988 {published data only} .Ben-Noun L. Comparison of physician-led and dietitian-led weight reducing programs [Hebrew] Harefuah. 1988;114(10):488–90. [PubMed] [Google Scholar]
- Boltri 2007 {published data only} .Boltri JM, Okosun I, vis-Smith YM, Seale JP, Roman P, Tobin BW. A simple nurse-based prompt increases screening and prevention counseling for diabetes. Diabetes Research and Clinical Practice. 2007;1:81–7. doi: 10.1016/j.diabres.2006.05.002. [DOI] [PubMed] [Google Scholar]
- Counterweight Prog 2004 {published data only} .The Counterweight Project Team A new evidence-based model for weight management in primary care: the Counterweight Programme. Journal of Human Nutrition and Dietetics. 2004;17:191–208. doi: 10.1111/j.1365-277X.2004.00517.x. [DOI] [PubMed] [Google Scholar]; The Counterweight Project Team Current approaches to obesity management in UK primary care: the Counterweight Programme. Journal of Human Nutrition and Dietetics. 2004;17:191–208. doi: 10.1111/j.1365-277X.2004.00528.x. [DOI] [PubMed] [Google Scholar]; The Counterweight Project Team Empowering primary care to tackle the obesity epidemic: The Counterweight Programme. European Journal of Clinical Nutrition. 2005;59(Suppl 1):S93–101. doi: 10.1038/sj.ejcn.1602180. [DOI] [PubMed] [Google Scholar]; The Counterweight Project Team Evaluation of the Counterweight Programme for obesity management in primary care: a starting point for continuous improvement. British Journal of General Practice. 2008;58(553):548–54. doi: 10.3399/bjgp08X319710. [DOI] [PMC free article] [PubMed] [Google Scholar]; The Counterweight Project Team Impact of obesity on drug prescribing in primary care. British Journal of General Practice. 2005;55:743–9. [PMC free article] [PubMed] [Google Scholar]; The Counterweight Project Team Influence of body mass index on prescribing cost savings of a weight management programme in primary care. Journal of Health Services Research & Policy. 2008;13(3):158–66. doi: 10.1258/jhsrp.2008.007140. [DOI] [PubMed] [Google Scholar]; The Counterweight Project Team Obesity impacts on general practice appointments. Obesity Research. 2005;13(8):1442–9. doi: 10.1038/oby.2005.174. [DOI] [PubMed] [Google Scholar]; The Counterweight Project Team Prevalence of CVD risk factors by body mass index and the impact of 10% weight change. Obesity Research & Clinical Practice. 2008;2:15–27. doi: 10.1016/j.orcp.2008.01.002. [DOI] [PubMed] [Google Scholar]
- De Mello 2004 {published data only} .De Mello ED, Luft VC, Meyer F. Individual outpatient care versus group education programs. Which leads to greater change in dietary and physical activity habits for obese children? Jornal de Pediatria. 2004;80(6):468–74. doi: 10.2223/1260. [DOI] [PubMed] [Google Scholar]
- Donnelly 2007 {published data only} .Donnelly JE, Smith BK, Dunn L, Mayo MM, Jacobsen DJ, Stewart EE, et al. Comparison of a phone vs clinic approach to achieve 10% weight loss. International Journal of Obesity. 2007;31(8):1270–6. doi: 10.1038/sj.ijo.0803568. [DOI] [PubMed] [Google Scholar]
- Dunstan 2006 {published data only} .Dunstan DW, Vulikh E, Owen N, Jolley D, Shaw J, Zimmet P. Community center-based resistance training for the maintenance of glycaemic control in adults with type 2 diabetes. Diabetes Care. 2006;29(12):2586–91. doi: 10.2337/dc06-1310. [DOI] [PubMed] [Google Scholar]
- Ferstl 1975 {published data only} .Ferstl von R, Jokusch U, Brengelmann JC. Behaviour therapy of overweight [Die verhaltenstherapeutische behandlung des Ubergewichts] International Journal of Health Education. 1975:119–36. [Google Scholar]
- Finnish DPS Group 1999 {published data only} .Eriksson J, Lindström J, Valle T, Aunola S, Hämäläinen H, Ilanne-Parikka P, et al. Prevention of Type II diabetes in subjects with impaired glucose tolerance: the Diabetes Prevention Study (DPS) in Finland. Study design and 1-year interim report on the feasibility of the lifestyle intervention programme. Diabetologia. 1999;42:793–801. doi: 10.1007/s001250051229. [DOI] [PubMed] [Google Scholar]; Lindstrom J, Louheranta A, Mannelin M, Rastas M, Salminen V, Eriksson J, et al. Finnish Diabetes Prevention Study. The Finnish Diabetes Prevention Study (DPS): Lifestyle intervention and 3-year results on diet and physical activity. Diabetes Care. 2003;26(12):3230–6. doi: 10.2337/diacare.26.12.3230. [DOI] [PubMed] [Google Scholar]; Tuomilehto J, Lindstrom J, Eriksson J, Valle T, Hamalainen H, Ilanne-Parikka P, et al. Prevention of type 2 diabetes mellitus by changes in lifestyle among subjects with impaired glucose tolerance. New England Journal of Medicine. 2001;344(18):1343–50. doi: 10.1056/NEJM200105033441801. [DOI] [PubMed] [Google Scholar]
- Hagen 1974 {published data only} .Hagen RL. Group therapy vs. bibliotherapy in weight reduction. Behavior Therapy. 1974;5:222–34. [Google Scholar]
- Hakala 1994 {published data only} .Hakala P. Weight reduction programmes at a rehabilitation centre and a health centre based on group counselling and individual support: short- and long-term follow-up study. International Journal of Obesity & Related Metabolic Disorders. 1994;18(7):483–9. [PubMed] [Google Scholar]
- Jeffery 1979 {published data only} .Jeffery RW, Wing RR. Frequency of therapist contact in the treatment of obesity. Behavior Therapy. 1979;10(2):186–92. [Google Scholar]
- Jeffery 1982 {published data only} .Jeffery RW, Danaher BG, Killen J, Farquhar JW, Kinnier R. Self-administered programs for health behavior change: smoking cessation and weight reduction by mail. Addictive Behaviors. 1982;7(1):57–63. doi: 10.1016/0306-4603(82)90025-9. [DOI] [PubMed] [Google Scholar]
- Kromann 1985 {published data only} .Kromann H. Clinical dietitian in general practice. The effects of dietary advice to obese patients and diabetics [Klinisk diaetist i almen praksis. Effekten af diaetvejledning af adipose og diabetikere] Ugeskr Laeger. 1985;147:20–3. [PubMed] [Google Scholar]
- Levitz 1974 {published data only} .Levitz LS, Stunkard AJ. A therapeutic coalition for obesity: behavior modification and patient self-help. American Journal of Psychiatry. 1974;131(4):423–7. [PubMed] [Google Scholar]
- Lindstrom 1976 {published data only} .Lindstrom LL, Balch P, Reese S. In person versus telephone treatment for obesity. Journal of Behavior Therapy and Experimental Psychiatry. 1976;7(4):367–9. [Google Scholar]
- McDonald 1984 {published data only} .McDonald CJ, Hui SL, Smith DM, Tierney WM, Cohen SJ, Weinberger M, et al. Reminders to physicians from an introspective computer medical record. A two-year randomized trial. Annals of Internal Medicine. 1984;100(1):130–8. doi: 10.7326/0003-4819-100-1-130. [DOI] [PubMed] [Google Scholar]
- Meyers 1996 {published data only} .Meyers AW, Graves TJ, Whelan JP, Barclay DR. An evaluation of a television-delivered behavioral weight loss program: are the ratings acceptable? Journal of Consulting & Clinical Psychology. 1996;64(1):172–8. doi: 10.1037//0022-006x.64.1.172. [DOI] [PubMed] [Google Scholar]
- Ogden 1997 {published data only} .Ogden J, Hoppe R. The relative effectiveness of two styles of educational package to change practice nurses’ management of obesity. International Journal of Obesity & Related Metabolic Disorders. 1997;21(11):963–71. doi: 10.1038/sj.ijo.0800503. [DOI] [PubMed] [Google Scholar]
- Perri 1987 {published data only} .Perri MG, McAdoo WG, McAllister DA, Lauer JB, Jordan RC, Yancey DZ, et al. Effects of peer support and therapist contact on long-term weight loss. Journal of Consulting & Clinical Psychology. 1987;55(4):615–7. doi: 10.1037/0022-006X.55.4.615. [DOI] [PubMed] [Google Scholar]
- Richman 1996 {published data only} .Richman RM, Webster P, Salgo AR, Mira M, Steinbeck KS, Caterson ID. A shared care approach in obesity management: the general practitioner and a hospital based service. International Journal of Obesity & Related Metabolic Disorders. 1996;20(5):413–9. [PubMed] [Google Scholar]
- Simkin-Silverman 1997 {published data only} .Simkin-Silverman LR, Wing RR. Management of obesity in primary care. Obesity Research. 1997;5(6):603–12. doi: 10.1002/j.1550-8528.1997.tb00582.x. [DOI] [PubMed] [Google Scholar]
- Vinicor 1987 {published data only} .Vinicor F, Cohen SJ, Mazzuca SA, Moorman N, Wheeler M, Kuebler T, et al. DIABEDS: a randomized trial of the effects of physician and/or patient education on diabetes patient outcomes. Journal of Chronic Diseases. 1987;40(4):345–56. doi: 10.1016/0021-9681(87)90050-6. [DOI] [PubMed] [Google Scholar]
- Willaing 2004 {published data only} .Willaing I, Ladelund S, Jørgensen T, Simonsen T, Nielsen LM. Nutritional counselling in primary healthcare: a randomized comparison of an intervention by general practitioner or dietitian. European Journal of Cardiovascular Prevention and Rehabilitation. 2004;11:513–20. doi: 10.1097/01.hjr.0000152244.58950.5f. [DOI] [PubMed] [Google Scholar]
Additional references
- Australian Bureau of Statistics 2008 .Australian Bureau of Statistics . National Health Survey, 2007-08. Australian Bureau of Statistics; 2008. [Google Scholar]
- Brownell and Puhl 2003 .Brownell K, Puhl R. Stigma and discrimination in weight management and obesity. The Permanente Journal. 2003;7:21–23. [Google Scholar]
- Cabana 1999 .Cabana MD, Rand CS, Powe NR, Wu AW, Wilson MH, Abboud PA, et al. Why don’t physicians follow clinical practice guidelines? A framework for improvement. JAMA. 1999;282:1458–65. doi: 10.1001/jama.282.15.1458. [DOI] [PubMed] [Google Scholar]
- Colquitt 2005 .Colquitt J, Clegg A, Loveman E, Royle P, Sidhu MK. Surgery for morbid obesity. Cochrane Database of Systematic Reviews. 2005;(Issue 4) doi: 10.1002/14651858.CD003641.pub2. [DOI: 10.1002/14651858.CD003641.pub2] [DOI] [PubMed] [Google Scholar]
- CONSORT 2001 .Moher D, Schulz KF, Altman DG, CONSORT Group The CONSORTstatement: revised recommendations for improving the quality of reports of parallel-group randomised trials. Lancet. 2001;357:1191–4. [PubMed] [Google Scholar]
- CONSORT CRCT 2004 .Campbell MK, Elbourne DR, Altman DG. CONSORT statement: extension to cluster randomised trials. BMJ. 2004;328:702–8. doi: 10.1136/bmj.328.7441.702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CRD 1997 .Centre for Reviews and Dissemination The prevention and treatment of obesity. Effective Health Care. 1997;3(2):1–12. [Google Scholar]
- Curioni 2006 .Curioni C, André C. Rimonabant for overweight or obesity. Cochrane Database of Systematic Reviews. 2006;(Issue 4) doi: 10.1002/14651858.CD006162.pub2. [DOI: 10.1002/14651858.CD006162.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deeks 2001 .Deeks JJ, Altman DG, Bradburn MJ. Statistical methods for examining heterogeneity and combining results from several studies in meta-analysis. In: Egger M, Davey Smith G, Altman DG, editors. Systematic Reviews in Health Care: Meta-Analysis in Context. 2nd Edition BMJ Publication Group; London: 2001. [Google Scholar]
- Deeks 2008 .Deeks JJ, Higgins JPT, Altman DG. Chapter 9: Analysing data and undertaking meta-analyses. In: Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews on interventions. 5.0.0 the Cochrane Collaboration; 2008. [Google Scholar]
- DerSimonian 1986 .DerSimonian R, Laird N. Meta-analysis in clinical trials. Controlled Clinical Trials. 1986;7:177–88. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- Douketis 1999 .Douketis J, Feightner J, Attia J, Feldman W. Periodic health examination, 1999 update: 1. Detection, prevention and treatment of obesity. Canadian Task Force on Preventive Health Care. Canadian Medical Association Journal. 1999;160:513–25. [PMC free article] [PubMed] [Google Scholar]
- Egger 2003 .Egger M, Juni P, Bartlett C, Holenstein F, Sterne J. How important are comprehensive literature searches and the assessment of trial quality in systematic reviews? Empirical study. Health Technol Assessment. 2003;7(1):1–76. [PubMed] [Google Scholar]
- EHCB 1997 .NHS Centre for Reviews and Dissemination The prevention and treatment of obesity. Effective Health Care. 1997;3(2):1–142. [Google Scholar]
- EPOC 2002 .The Effective Practice and Organisation of Care group [accessed 14 Jan 2010];Data collection checklist. http://www.epoc.cochrane.org/en/handsearchers.html
- EPOC 2009a .The Effective Practice and Organisation of Care group [accessed 14 Jan 2010];The Specialised Registers. http//: www.epoc.cochrane.org/en/handsearchers.html
- EPOC 2009b .Effective Practice and Organisation of Care group [accessed 14 Jan 2010];EPOC Risk of bias guideline. http://www.epoc.cochrane.org/en/index.html
- Foresight 2007 .Forsight programme Foresight- Tackling obesities: Future choices- project report. Government Office for Science. 2007:1–153. [Google Scholar]
- Frank 1993 .Frank A. Futility and avoidance. Medical professionals in the treatment of obesity. JAMA. 1993;269(16):2132–3. doi: 10.1001/jama.269.16.2132. [DOI] [PubMed] [Google Scholar]
- Galuska 1999 .Galuska DA, Till JC, Serdula MK, Ford ES. Are healthcare professionals advising obese patients to lose weight? JAMA. 1999;282:1576–88. doi: 10.1001/jama.282.16.1576. [DOI] [PubMed] [Google Scholar]
- Garrow 2000 .Garrow J, Summerbell C. Obesity. In: Stevens A, Raftery J, Mant J, editors. Health Care Needs Assessment: the epidemiologically based needs assessment reviews: 3rd series. Radcliffe Medical Press Ltd; Abingdon: 2000. Electronic version available at http://hcna.radcliffe–online.com/obframe.html. [Google Scholar]
- Glenny 1997 .Glenny AM, O’Meara S, Melville A, Sheldon TA, Wilson C. The treatment and prevention of obesity: A systematic review of the literature. International Journal of Obesity & Related Metabolic Disorders. 1997;21(9):715–37. doi: 10.1038/sj.ijo.0800495. [DOI] [PubMed] [Google Scholar]
- Goldstein 2003 .Goldstein H. Multilevel Statistical Models. 3rd Edition Arnold; London: 2003. [Google Scholar]
- Haring 1976 .Haring OM. Improving patient care by automated record summaries:Final report. Northwestern University Medical School, National Center for Health services Research; 1976. [Google Scholar]
- HEA 1995 .Health Education Authority . Obesity in primary healthcare. A literature review. HEA; London: 1995. [Google Scholar]
- Higgins 2003 .Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–60. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins 2008 .Higgins JPT, Altman DG. Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions. 5.0.0 The Cochrane Collaboration; 2008. [Google Scholar]
- Higgins 2009 .Higgins JPT, Thompson SG, Spiegelhalter DJ. A re-evaluation of random effects meta-analysis. Journal of the Royal Statistical Society A. 2009;172(part 1):137–159. doi: 10.1111/j.1467-985X.2008.00552.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopewell 2009 .Hopewell S, Loudon K, Clarke MJ, Oxman AD, Dickersin K. Publication bias in clinical trials due to statistical significance or direction of trial results. Cochrane Database of Systematic Reviews. 2009;(Issue 1) doi: 10.1002/14651858.MR000006.pub3. [DOI: 10.1002/14651858.MR000006.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeffery 2004 .Jeffery RW, McGuire MT, Brelje KL, Pronk NP, Boyle RG, Hase KA, et al. Recruitment to mail and telephone interventions for obesity in a managed care environment: the weigh-to-be project. The American Journal of Managed Care. 2004;10:378–82. [PubMed] [Google Scholar]
- Moher 1998 .Moher D, Pham B, Jones A, Cook DJ, Jadad AR, Moher M, et al. Does quality of reports of randomised trials affect estimates of intervention efficacy reported in meta-analyses? Lancet. 1998;352:609–13. doi: 10.1016/S0140-6736(98)01085-X. [DOI] [PubMed] [Google Scholar]
- Moore 2001 .Moore H, Summerbell CD, Vail A, Greenwood DC, Adamson AJ. The design features and practicalities of conducting a pragmatic cluster randomized trial of obesity management in primary care. Statistics in Medicine. 2001;20(3):331–40. doi: 10.1002/1097-0258(20010215)20:3<331::aid-sim795>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- NHLBI 1998 .The National Heart, Lung, and Blood Institute . Clinical guidelines on the identification, evaluation, and treatment of overweight and obesity in adults: The evidence report. National Institutes of Health; 1998. [PubMed] [Google Scholar]
- NICE 2006 .National Institute for Health and Clinical Excellence. National Collaborating Centre for Primary Care Obesity: the prevention, identification, assessment and management of overweight and obesity in adults and children. 2006;Vol. CG43 http://www.nice.org.uk/CG43#summary [PubMed] [Google Scholar]
- O’Meara 2000 .O’Meara S, Riemsma R, Shirran E, Mather L, ter Riet G. A systematic review of the clinical effectiveness and cost-effectiveness of orlistat in the management of obesity. Report commissioned by the NHS R&D HTA Programme on behalf of The National Institute for Clinical Excellence (NICE)
- Ogden 2006 .Ogden CL, Carroll MD, Curtin LR, McDowell MA, Tabak CJ, Flegal KM. Prevalence of overweight and obesity in the United States 1999-2004. JAMA. 2006;295(13):1549–55. doi: 10.1001/jama.295.13.1549. [DOI] [PubMed] [Google Scholar]
- Padwal 2003 .Padwal R, Rucker D, Li S, Curioni C, Lau DCW. Long-term pharmacotherapy for obesity and overweight. Cochrane Database of Systematic Reviews. 2003;(Issue 4) doi: 10.1002/14651858.CD004094. [DOI: 10.1002/14651858.CD004094.pub2] [DOI] [PubMed] [Google Scholar]
- Price 1987 .Price JH, Desmond SM, Krol RA, Snyder FF, O’Connell JK. Family practice physicians’ beliefs, attitudes, and practices regarding obesity. American Journal of Preventive Medicine. 1987;3(6):339–45. [PubMed] [Google Scholar]
- Puhl and Brownell 2001 .Puhl RM, Brownell KD. Bias, discrimination, and obesity. Obesity Research. 2001;9:788–905. doi: 10.1038/oby.2001.108. [DOI] [PubMed] [Google Scholar]
- Puhl and Heuer 2009 .Puhl RM, Heuer CA. The stigma of obesity: a review and update. Obesity. 2009;17(5):941–64. doi: 10.1038/oby.2008.636. [DOI] [PubMed] [Google Scholar]
- Raab 2001 .Raab G, Butcher I. Balance in cluster randomized trials. Statistics in Medicine. 2001;20:351–65. doi: 10.1002/1097-0258(20010215)20:3<351::aid-sim797>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- Rennie 2005 .Rennie KL, Jebb SA. Prevalence of obesity in Great Britain. Obesity Reviews. 2005;6(1):11–2. doi: 10.1111/j.1467-789X.2005.00164.x. [DOI] [PubMed] [Google Scholar]
- RevMan 2008 .The Nordic Cochrane Centre. The Cochrane Collaboration . Review Manager. 5.0 The Nordic Cochrane Centre, The Cochrane Collaboration; Copenhagen: 2008. [Google Scholar]
- Schulz 1995 .Schulz KF, Chalmers I, Hayes RJ, Altman D. Empirical evidence of bias. Dimensions of methodological quality associated with estimates of treatment effects in controlled trials. JAMA. 1995;273:408–12. doi: 10.1001/jama.273.5.408. [DOI] [PubMed] [Google Scholar]
- Senn 1994 .Senn S. Testing for base-line balance in clinical trials. Statistics in Medicine. 1994;13(17):1715–26. doi: 10.1002/sim.4780131703. [DOI] [PubMed] [Google Scholar]
- Shaw 2005 .Shaw K, O’Rourke P, Del Mar C, Kenardy J. Psychological interventions for overweight or obesity. Cochrane Database of Systematic Reviews. 2005;(Issue 2) doi: 10.1002/14651858.CD003818.pub2. [DOI: 10.1002/14651858.CD003818.pub2] [DOI] [PubMed] [Google Scholar]
- Shaw 2006 .Shaw K, Gennat H, O’Rourke P, Del Mar C. Exercise for overweight or obesity. Cochrane Database of Systematic Reviews. 2006;(Issue 4) doi: 10.1002/14651858.CD003817.pub3. [DOI: 10.1002/14651858.CD003817.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shojania 2009 .Shojania KG, Jennings A, Mayhew A, Ramsay CR, Eccles MP, Grimshaw J. The effects of on-screen, point of care computer reminders on processes and outcomes of care. Cochrane Database of Systematic Reviews. 2009;(Issue 3) doi: 10.1002/14651858.CD001096.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Summerbell 1998 .Summerbell CD. Dietary treatments for obesity. In: Stock M, Kopelman P, editors. Clinical Obesity. Blackwell Scientific Press; Oxford: 1998. [Google Scholar]
- The Counterweight Project Team 2008 .The Counterweight Project Team Engaging patients, clinicians and health funders in weight management; the counterweight programme. Family Practice. 2008;25:i79–i86. doi: 10.1093/fampra/cmn081. [DOI] [PubMed] [Google Scholar]
- Thomas 2007 .Thomas DE, Elliott EJ, Baur L. Low glycaemic index or low glycaemic load diets for overweight and obesity. Cochrane Database of Systematic Reviews. 2007;(Issue 3) doi: 10.1002/14651858.CD005105.pub2. [DOI: 10.1002/14651858.CD005105.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wadden 2000 .Wadden TA, Anderson DA, Foster GD, Bennett A, Steinberg C, Sarwer DB. Obese womens’ perceptions of their physicians’ weight management attitudes and practices. Archives of Family Medicine. 2000;9:854–60. doi: 10.1001/archfami.9.9.854. [DOI] [PubMed] [Google Scholar]
- WHO 1998 .World Health Organization (WHO) International Obesity Task Force (IOTF) Obesity: Preventing and Managing the Global Epidemic. Report of a WHO consultation on obesity, Geneva 3-5 June 1997. WHO. World Health Organization; Geneva: 1998. [PubMed] [Google Scholar]
- WHO 2004 .World Health Organization . Global strategy on diet, physical activity and health. World Health Organization; Geneva: 2004. [Google Scholar]
- WHO 2005 .WHO Expert Committee on Physical Status . The use and interpretation of anthropometry: report form a WHO Expert Committee. Vol. 854. World Health Organization; 1995. pp. 1–439. [PubMed] [Google Scholar]
References to other published versions of this review
- Harvey 1999 .Harvey EL, Glenny A-M, Kirk SFL, Summerbell C. A systematic review of interventions to improve health professionals’ management of obesity. International Journal of Obesity. 1999;23:1213–22. doi: 10.1038/sj.ijo.0800932. [DOI] [PubMed] [Google Scholar]
- Harvey 2002 .Harvey EL, Glenny AM, Kirk FL, Summerbell CD. An updated systematic review of interventions to improve health professionals’ management of obesity. Obesity reviews. 2002;3:45–55. doi: 10.1046/j.1467-789x.2002.00053.x. [DOI] [PubMed] [Google Scholar]