Abstract
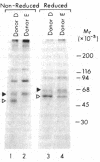
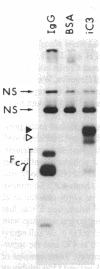
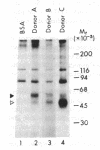
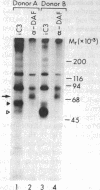
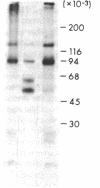
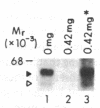
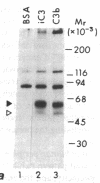
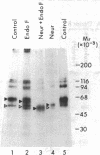
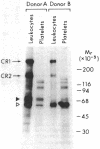
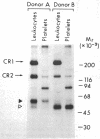
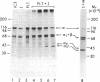
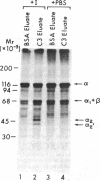
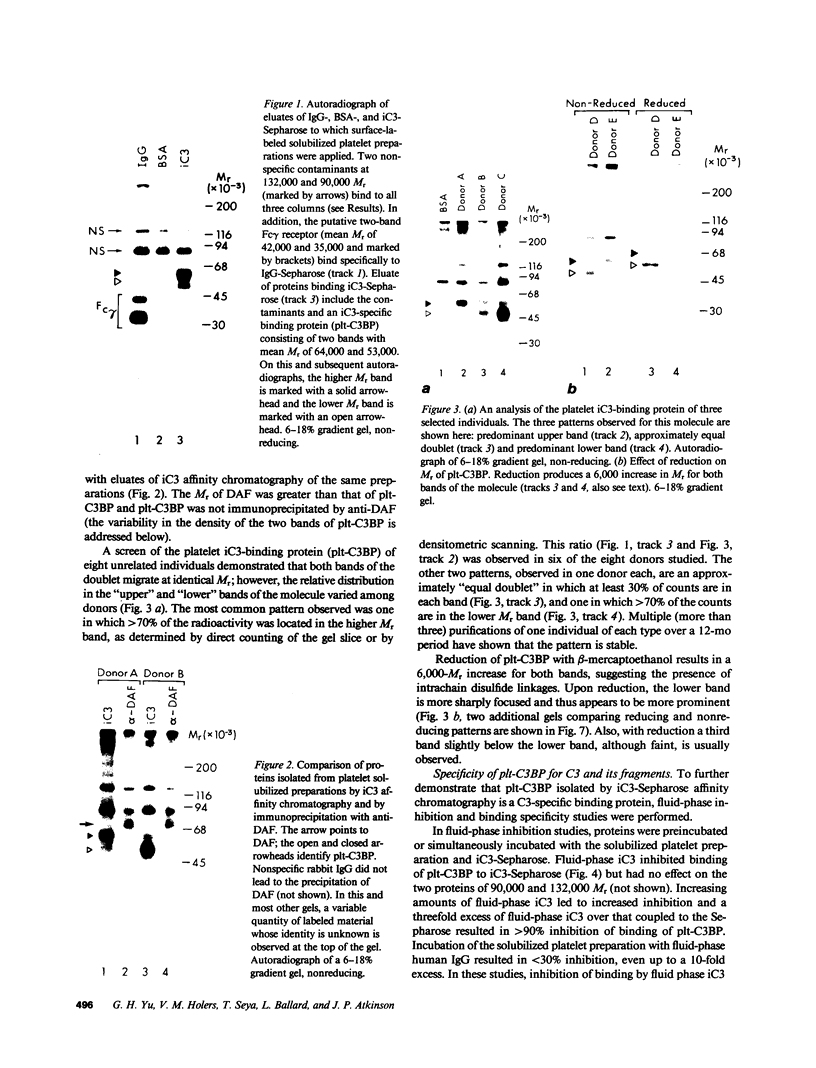
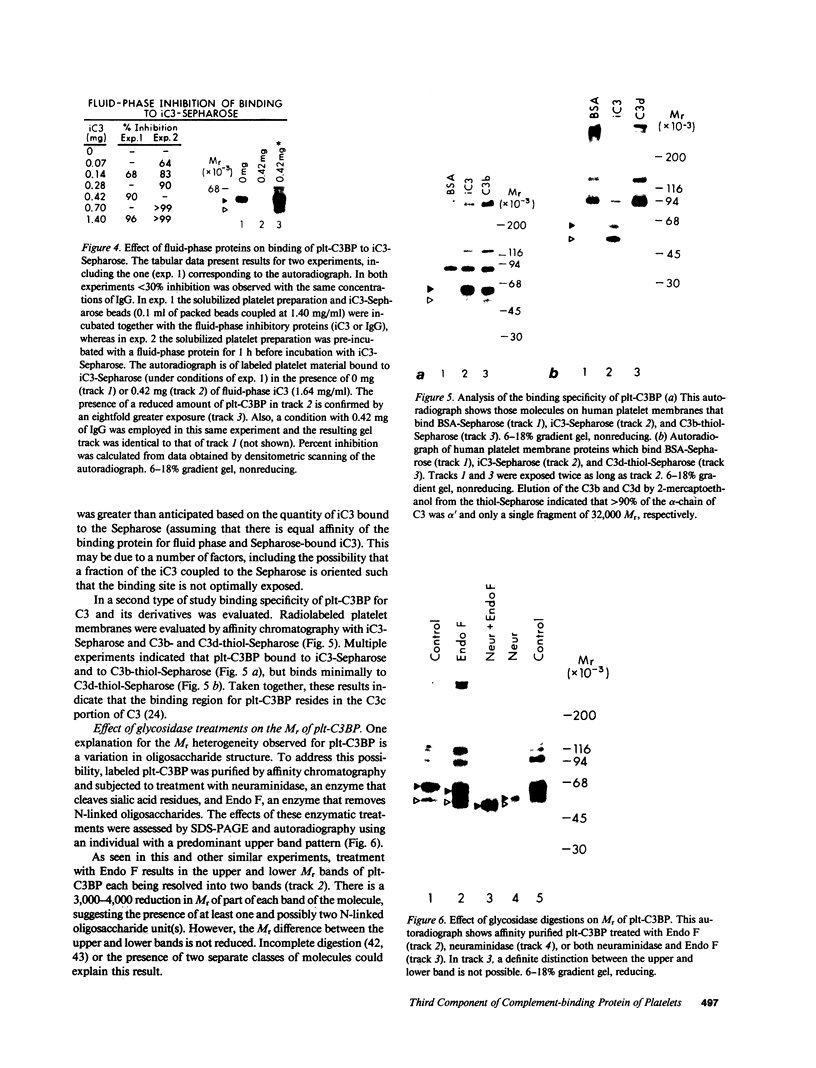
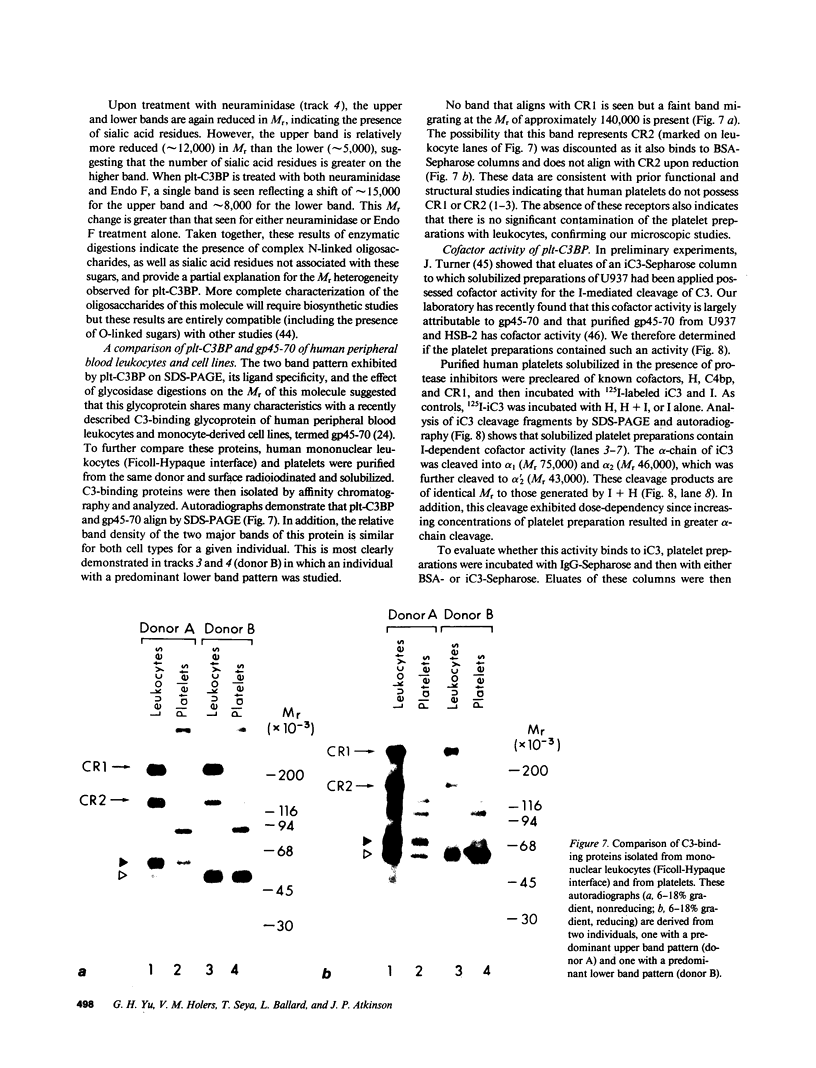
Utilizing affinity chromatography, a C3-specific binding protein was isolated from 125I surface-labeled human platelets. Analysis by sodium dodecyl sulfate-polyacrylamide gel electrophoresis demonstrated two bands with mean Mr of 64,000 and 53,000, characteristic variability in the relative density of the two bands in a given individual, and the presence of N-linked complex oligosaccharides as well as sialic acid residues not associated with N-linked sugars. These characteristics are similar to those of a human leukocyte iC3- and C3b-binding glycoprotein, termed gp45-70. Further analysis showed that leukocyte gp45-70 and the platelet C3-binding glycoprotein have identical Mr and other similar structural features. Functional characterization of solubilized platelet preparations indicated that gp45-70 has cofactor activity. This membrane glycoprotein is structurally and antigenically distinct from decay accelerating factor (DAF), a complement regulatory protein previously identified on human platelet membranes. DAF and gp45-70 have complementary activity profiles inasmuch as DAF can prevent assembly of and dissociate the C3 convertases but has no cofactor activity, whereas gp45-70 has cofactor activity but no decay accelerating activity. We suggest that these two proteins function conjointly to prevent autologous complement activation.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnaout M. A., Todd R. F., 3rd, Dana N., Melamed J., Schlossman S. F., Colten H. R. Inhibition of phagocytosis of complement C3- or immunoglobulin G-coated particles and of C3bi binding by monoclonal antibodies to a monocyte-granulocyte membrane glycoprotein (Mol). J Clin Invest. 1983 Jul;72(1):171–179. doi: 10.1172/JCI110955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atkinson J. P., Jones E. A. Biosynthesis of the human C3b/C4b receptor during differentiation of the HL-60 cell line. Identification and characterization of a precursor molecule. J Clin Invest. 1984 Nov;74(5):1649–1657. doi: 10.1172/JCI111581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ault K. A., Springer T. A. Cross-reaction of a rat-anti-mouse phagocyte-specific monoclonal antibody (anti-Mac-1) with human monocytes and natural killer cells. J Immunol. 1981 Jan;126(1):359–364. [PubMed] [Google Scholar]
- Baenziger N. L., Majerus P. W. Isolation of human platelets and platelet surface membranes. Methods Enzymol. 1974;31:149–155. doi: 10.1016/0076-6879(74)31015-4. [DOI] [PubMed] [Google Scholar]
- Barel M., Charriaut C., Frade R. Isolation and characterization of a C3b receptor-like molecule from membranes of a human B lymphoblastoid cell line (Raji). FEBS Lett. 1981 Dec 21;136(1):111–114. doi: 10.1016/0014-5793(81)81225-2. [DOI] [PubMed] [Google Scholar]
- Beller D. I., Springer T. A., Schreiber R. D. Anti-Mac-1 selectively inhibits the mouse and human type three complement receptor. J Exp Med. 1982 Oct 1;156(4):1000–1009. doi: 10.1084/jem.156.4.1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger M., Gaither I. A., Frank M. M. Complement receptors. Clin Immunol Rev. 1981;1(4):471–545. [PubMed] [Google Scholar]
- Breard J., Reinherz E. L., Kung P. C., Goldstein G., Schlossman S. F. A monoclonal antibody reactive with human peripheral blood monocytes. J Immunol. 1980 Apr;124(4):1943–1948. [PubMed] [Google Scholar]
- Böyum A. Isolation of mononuclear cells and granulocytes from human blood. Isolation of monuclear cells by one centrifugation, and of granulocytes by combining centrifugation and sedimentation at 1 g. Scand J Clin Lab Invest Suppl. 1968;97:77–89. [PubMed] [Google Scholar]
- Cheng C. M., Hawiger J. Affinity isolation and characterization of immunoglobulin G Fc fragment-binding glycoprotein from human blood platelets. J Biol Chem. 1979 Apr 10;254(7):2165–2167. [PubMed] [Google Scholar]
- Cole J. L., Housley G. A., Jr, Dykman T. R., MacDermott R. P., Atkinson J. P. Identification of an additional class of C3-binding membrane proteins of human peripheral blood leukocytes and cell lines. Proc Natl Acad Sci U S A. 1985 Feb;82(3):859–863. doi: 10.1073/pnas.82.3.859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahms N. M., Hart G. W. Lymphocyte function-associated antigen 1 (LFA-1) contains sulfated N-linked oligosaccharides. J Immunol. 1985 Jun;134(6):3978–3986. [PubMed] [Google Scholar]
- Dixit R., Schneider R., Law S. K., Kulczycki A., Jr, Atkinson J. P. Ligand binding specificity of a rabbit alveolar macrophage receptor for C3b. J Biol Chem. 1982 Feb 25;257(4):1595–1597. [PubMed] [Google Scholar]
- Dykman T. R., Cole J. L., Iida K., Atkinson J. P. Polymorphism of human erythrocyte C3b/C4b receptor. Proc Natl Acad Sci U S A. 1983 Mar;80(6):1698–1702. doi: 10.1073/pnas.80.6.1698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dykman T. R., Cole J. L., Iida K., Atkinson J. P. Structural heterogeneity of the C3b/C4b receptor (Cr 1) on human peripheral blood cells. J Exp Med. 1983 Jun 1;157(6):2160–2165. doi: 10.1084/jem.157.6.2160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dykman T. R., Hatch J. A., Aqua M. S., Atkinson J. P. Polymorphism of the C3b/C4b receptor (CR1): characterization of a fourth allele. J Immunol. 1985 Mar;134(3):1787–1789. [PubMed] [Google Scholar]
- Dykman T. R., Hatch J. A., Atkinson J. P. Polymorphism of the human C3b/C4b receptor. Identification of a third allele and analysis of receptor phenotypes in families and patients with systemic lupus erythematosus. J Exp Med. 1984 Mar 1;159(3):691–703. doi: 10.1084/jem.159.3.691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elder J. H., Alexander S. endo-beta-N-acetylglucosaminidase F: endoglycosidase from Flavobacterium meningosepticum that cleaves both high-mannose and complex glycoproteins. Proc Natl Acad Sci U S A. 1982 Aug;79(15):4540–4544. doi: 10.1073/pnas.79.15.4540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fearon D. T. Identification of the membrane glycoprotein that is the C3b receptor of the human erythrocyte, polymorphonuclear leukocyte, B lymphocyte, and monocyte. J Exp Med. 1980 Jul 1;152(1):20–30. doi: 10.1084/jem.152.1.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fearon D. T. Regulation of the amplification C3 convertase of human complement by an inhibitory protein isolated from human erythrocyte membrane. Proc Natl Acad Sci U S A. 1979 Nov;76(11):5867–5871. doi: 10.1073/pnas.76.11.5867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fearon D. T. The human C3b receptor. Springer Semin Immunopathol. 1983;6(2-3):159–172. doi: 10.1007/BF00205871. [DOI] [PubMed] [Google Scholar]
- Fingeroth J. D., Weis J. J., Tedder T. F., Strominger J. L., Biro P. A., Fearon D. T. Epstein-Barr virus receptor of human B lymphocytes is the C3d receptor CR2. Proc Natl Acad Sci U S A. 1984 Jul;81(14):4510–4514. doi: 10.1073/pnas.81.14.4510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frade R., Barel M., Ehlin-Henriksson B., Klein G. gp140, the C3d receptor of human B lymphocytes, is also the Epstein-Barr virus receptor. Proc Natl Acad Sci U S A. 1985 Mar;82(5):1490–1493. doi: 10.1073/pnas.82.5.1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iida K., Nadler L., Nussenzweig V. Identification of the membrane receptor for the complement fragment C3d by means of a monoclonal antibody. J Exp Med. 1983 Oct 1;158(4):1021–1033. doi: 10.1084/jem.158.4.1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinoshita T., Medof M. E., Silber R., Nussenzweig V. Distribution of decay-accelerating factor in the peripheral blood of normal individuals and patients with paroxysmal nocturnal hemoglobinuria. J Exp Med. 1985 Jul 1;162(1):75–92. doi: 10.1084/jem.162.1.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulczycki A., Jr, Krause V., Killion C. C., Atkinson J. P. Improved cell surface radioiodination of macrophages. J Immunol Methods. 1980;37(2):133–138. doi: 10.1016/0022-1759(80)90198-2. [DOI] [PubMed] [Google Scholar]
- Kulczycki A., Jr, Solanki L., Cohen L. Isolation and partial characterization of Fc gamma-binding proteins of human leukocytes. J Clin Invest. 1981 Dec;68(6):1558–1565. doi: 10.1172/JCI110410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lublin D. M., Griffith R. C., Atkinson J. P. Influence of glycosylation on allelic and cell-specific Mr variation, receptor processing, and ligand binding of the human complement C3b/C4b receptor. J Biol Chem. 1986 May 5;261(13):5736–5744. [PubMed] [Google Scholar]
- Medof M. E., Kinoshita T., Nussenzweig V. Inhibition of complement activation on the surface of cells after incorporation of decay-accelerating factor (DAF) into their membranes. J Exp Med. 1984 Nov 1;160(5):1558–1578. doi: 10.1084/jem.160.5.1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medof M. E., Kinoshita T., Silber R., Nussenzweig V. Amelioration of lytic abnormalities of paroxysmal nocturnal hemoglobinuria with decay-accelerating factor. Proc Natl Acad Sci U S A. 1985 May;82(9):2980–2984. doi: 10.1073/pnas.82.9.2980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore A., Nachman R. L. Platelet Fc receptor. Increased expression in myeloproliferative disease. J Clin Invest. 1981 Apr;67(4):1064–1071. doi: 10.1172/JCI110118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholson-Weller A., Burge J., Fearon D. T., Weller P. F., Austen K. F. Isolation of a human erythrocyte membrane glycoprotein with decay-accelerating activity for C3 convertases of the complement system. J Immunol. 1982 Jul;129(1):184–189. [PubMed] [Google Scholar]
- Nicholson-Weller A., March J. P., Rosen C. E., Spicer D. B., Austen K. F. Surface membrane expression by human blood leukocytes and platelets of decay-accelerating factor, a regulatory protein of the complement system. Blood. 1985 May;65(5):1237–1244. [PubMed] [Google Scholar]
- Nicholson-Weller A., Spicer D. B., Austen K. F. Deficiency of the complement regulatory protein, "decay-accelerating factor," on membranes of granulocytes, monocytes, and platelets in paroxysmal nocturnal hemoglobinuria. N Engl J Med. 1985 Apr 25;312(17):1091–1097. doi: 10.1056/NEJM198504253121704. [DOI] [PubMed] [Google Scholar]
- Schneider R. J., Kulczycki A., Jr, Law S. K., Atkinson J. P. Isolation of a biologically active macrophage receptor for the third component of complement. Nature. 1981 Apr 30;290(5809):789–792. doi: 10.1038/290789a0. [DOI] [PubMed] [Google Scholar]
- Seya T., Holers V. M., Atkinson J. P. Purification and functional analysis of the polymorphic variants of the C3b/C4b receptor (CR1) and comparison with H, C4b-binding protein (C4bp), and decay accelerating factor (DAF). J Immunol. 1985 Oct;135(4):2661–2667. [PubMed] [Google Scholar]
- Seya T., Turner J. R., Atkinson J. P. Purification and characterization of a membrane protein (gp45-70) that is a cofactor for cleavage of C3b and C4b. J Exp Med. 1986 Apr 1;163(4):837–855. doi: 10.1084/jem.163.4.837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todd R. F., 3rd, Nadler L. M., Schlossman S. F. Antigens on human monocytes identified by monoclonal antibodies. J Immunol. 1981 Apr;126(4):1435–1442. [PubMed] [Google Scholar]
- Weis J. J., Tedder T. F., Fearon D. T. Identification of a 145,000 Mr membrane protein as the C3d receptor (CR2) of human B lymphocytes. Proc Natl Acad Sci U S A. 1984 Feb;81(3):881–885. doi: 10.1073/pnas.81.3.881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong W. W., Fearon D. T. p65: A C3b-binding protein on murine cells that shares antigenic determinants with the human C3b receptor (CR1) and is distinct from murine C3b receptor. J Immunol. 1985 Jun;134(6):4048–4056. [PubMed] [Google Scholar]
- Wong W. W., Wilson J. G., Fearon D. T. Genetic regulation of a structural polymorphism of human C3b receptor. J Clin Invest. 1983 Aug;72(2):685–693. doi: 10.1172/JCI111018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright S. D., Rao P. E., Van Voorhis W. C., Craigmyle L. S., Iida K., Talle M. A., Westberg E. F., Goldstein G., Silverstein S. C. Identification of the C3bi receptor of human monocytes and macrophages by using monoclonal antibodies. Proc Natl Acad Sci U S A. 1983 Sep;80(18):5699–5703. doi: 10.1073/pnas.80.18.5699. [DOI] [PMC free article] [PubMed] [Google Scholar]