Abstract
Sentinel Lymph Node (SLN) biopsy using a combination of radioisotopes and blue dyes have a good accuracy rate in predicting subclinical neck nodal metastases in head and neck cancers. However, the limited availability of lymphoscintigraphy facilities in India requires exploration of alternative methods of SLN detection. We evaluated the feasibility of using methylene blue dye alone in detecting SLN in cN0 early oral cancers. 32 patients with cN0 early (T1, T2) oral squamous cell cancers underwent SLN biopsy using peri tumoural methylene blue dye injection. Blue dye stained (SLN) nodes were sent for frozen section analyses. Patients who had microscopic metastases in SLN underwent modified radical neck dissections and the rest underwent selective neck dissections. Paraffin sections and IHC studies were done on all nodes. SLN was identified in 29 patients (Identification rate = 90.6 %) of which SLN was positive for metastases on frozen section in 5 patients. The sensitivity, specificity and NPV of SLN with frozen section were 80 %, 95.8 % and 95.8 % respectively. IHC with cytokeratins increased the sensitivity (100 %) and NPV (100 %) at the loss of specificity (87.5 %). Methylene blue dye alone can be successfully used for SLN identification in early oral cancers with a good accuracy and sensitivity. This method will be of use especially in resource limited countries and centres where nuclear medicine facilities are not widely available. However, it has to be validated by larger randomised multi institutional trials for wider applicability. Immunohistochemistry increases the sensitivity and negative predictive value of SLN but its applicability in real time decision making is limited.
Keywords: Oral cancer, Sentinel lymph node biopsy
Introduction
Oral cancers are one of the most common cancers and a leading cause of cancer related mortality in India [1, 2]. The primary lymphatic drainage of oral cancers is to the neck nodes. The incidence of neck nodal metastases depends on various factors like stage, sub site, depth of infiltration, differentiation, etc. and varies between less than ten percent to up to fifty percent [3]. The treatment of neck in clinically node negative (cN0) early oral cancers remains controversial and the choice of treatment depends mostly on the treatment of the primary, physician preference, facilities available, etc. [3–6]. The problem in these patients with cN0 disease is to know whether the nodes are truly negative or harbour occult metastases. The chance of occult or subclinical nodal metastases in cN0 early oral cancers can be up to 3% [3, 7]. Often such a patient is either undertreated or over treated for the neck.
Oral cancers have sequential lymphatic spread which makes them a good candidate for sentinel node studies. In patients with cN0 treated with surgery, it would be ideal to do a neck dissection only in SLN positive patients and spare its morbidity in the rest. Most SLN studies in oral cancers use lymphoscintigraphy with radio labelled particles and gamma probe localisation combined with a blue dye injection [8–19]. However lymphoscintigraphy and nuclear medicine facilities are not widely available in India [20]. Therefore alternate techniques for SLN Biopsy need evaluation.
Methylene blue is a low cost, less allergic and effective dye that has shown promising results in SLN studies in breast cancer when used alone or in combination with lymphoscintigraphy [21–24]. In this study, we evaluated the feasibility of using methylene blue dye alone in identifying sentinel nodes in early oral cancers.
Patients and Methods
We conducted a prospective study of SLN Biopsy with methylene blue dye injection in 32 clinically node negative (cN0) early oral cancer (T1, T2 squamous cell carcinoma) patients treated with surgery in our hospital between 2010 and 2012. Exclusion criteria included oral cancer patients with T3&T4 cancers, clinical node positivity, lesions in or crossing midline, with previous surgery/scar in the neck, with previous history of neck irradiation and medically unfit patients. Institute ethics committee approval was obtained and informed consent taken from all patients. Evaluation included history, clinical examination, ultrasound and CT scans of the neck, chest X ray and blood analysis as appropriate. Clinical N0 was confirmed by independent neck examinations by two consultants and ultrasound of the neck. The patients underwent surgery after pre anaesthetic evaluation.
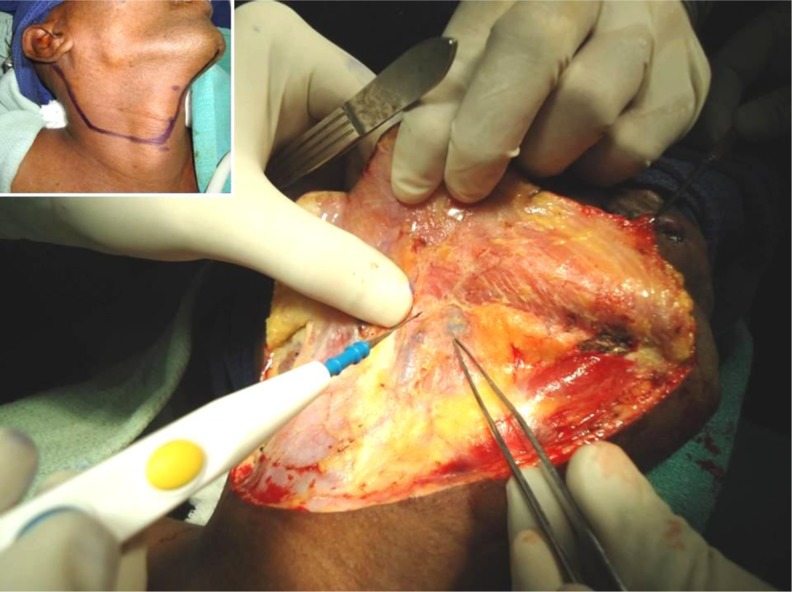
Under general anaesthesia, methylene blue dye was injected peritumourally (0.5 ml each in 3, 6, 9 &12 O’clock positions). Surgery for the neck was done first. A modified curvilinear neck incision was made from mentum to mastoid curved along the upper border of hyoid and midpoint of sternocleidomastoid (Fig. 1 inset). Flaps were raised using electrocautery. Systematic evaluation of levels IA, IB, IIA, IIB, III & IV nodes was done. Sentinel node (s) were identified by the blue dye staining within 12–20 min. SLN were dissected and sent for frozen section (FS) (Fig. 1).
Fig. 1.
Identification of SLN
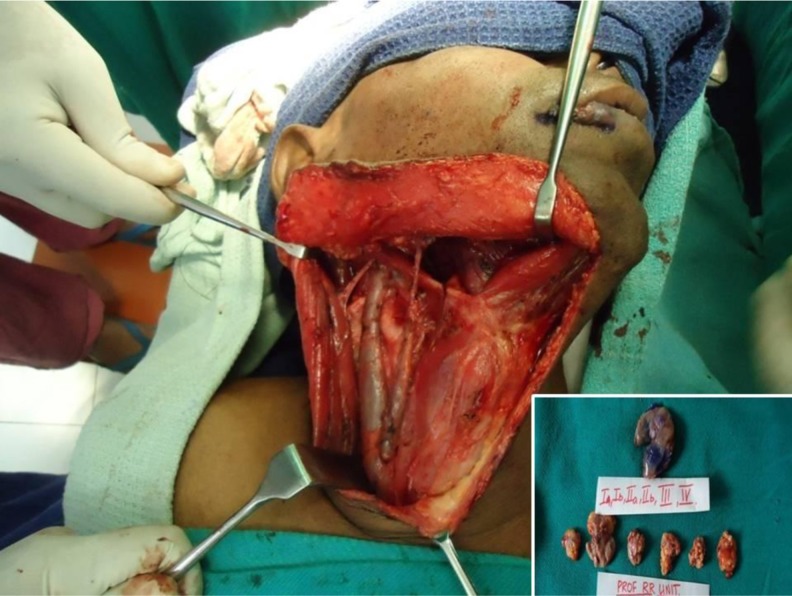
If SLN was negative on Frozen Section (FS) the patient underwent selective neck dissection (SND- Extended Supra Omohyoid Neck Dissection). If SLN was positive on Frozen section (FS), the patient underwent a type I Modified Radical Neck Dissection (MRND-I preserving the spinal accessory nerve) by converting the incision to a modified Crile’s incision by the addition of a vertical limb. Surgery for the primary was then completed. All nodes in Levels I, IIa, IIb, III, IV (and V in MRND-1) including SLN (blue dye stained nodes) were dissected, marked separately (Fig. 2) and sent for histopathological examination (HPE) with serial step sectioning of paraffin fixed specimens and H&E (IHC) for cytokeratins.
Fig. 2.
Neck dissection
The primary aim of the study was to analyse identification rates, sensitivity, specificity and negative predictive value (NPV) of SLN using methylene blue dye and frozen section. Statistical analysis was done using SPSS 16 (IBM Inc, USA) and EXCEL 2007 (Microsoft, USA).
Results
The mean age of patients was 43 years (range: 26–70 years). General patient characteristics are shown in table 1. The most common sub sites were tongue (56 %) and buccal mucosa (22 %). Sixty percent (n = 19) of the patients were tobacco/betel users and 31% (n = 10) were alcoholics.
Table 1.
General Patient Characteristics
| Characteristics | N (%) | |
|---|---|---|
| Total Patients | Male | 24 (75 %) |
| Female | 8 (25 %) | |
| Total | 32 | |
| T Stage | cT1 | 16 |
| cT2 | 16 | |
| Sub site | Alveolus | 2 (7 %) |
| Buccal mucosa | 7 (22 %) | |
| Floor of mouth | 1 (3 %) | |
| Hard palate | 2 (6 %) | |
| Retro molar trigone (RMT) | 2 (6 %) | |
| Tongue | 18 (56 %) | |
| Grade | Grade 1 | 19 |
| Grade 2 | 12 | |
| Grade 3 | 1 | |
SLN Identification
SLN was identified in 29 patients (SLN identification rate: 29/32 = 90.6 %). A total of 707 nodes were dissected in 32 patients, of which 50 nodes (in 29 patients) were blue dye stained (SLN). In 21 patients (72.4 %) more than one SLN was identified. Mean SLN yield was 1.56 (median = 2) with the highest yield in RMT cancers (mean =2.25).
SLN was positive in 5 patients (5/32 = 15.6 %) and 8 nodes (8/50 = 16 %) on frozen section (Table 2). These patients underwent MRND-1. Three out of 5 patients had SLN as the only site of metastases (Table 3). No blue bye stained nodes (SLN) were identified in Level IIB and IV. None of the non SLN nodes in Level IIB and IV had metastases on HPE and IHC. There were no skip metastases, extra nodal disease or overflow phenomenon.
Table 2.
Distribution of harvested Lymph Nodes
| Level | Total No. of nodes (mean) | Total No. of SLN | No. of Positive SLN |
|---|---|---|---|
| Ia | 89 (2.8) | 7 | 0 |
| IB | 104 (3.3) | 18 | 3 |
| IIa | 145 (4.5) | 17 | 3 |
| IIB | 98 (3.1) | 0 | 0 |
| III | 149 (4.7) | 8 | 2 |
| IV | 114 (3.6) | 0 | 0 |
| V | 22 (4.4)* | 0 | 0 |
| Total | 707 (22.1) | 50 Nodes/29 Patients | 8 Nodes/5 patients |
*In 5 patients who underwent MRND
Table 3.
Details of SLN Positive Patients
| S.No | Patient Age in years/Sex | Primary Sub site | Surgery | Total no. of Nodes dissected | No. of SLN identified | No. of Positive SLN | LN Levels | Final HPE | Follow-up |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 40/F | Tongue | Hemiglossectomy | 21 | 3 | 3 | IB, IIA, IIA | pT2N2a (4/21) | 25 months, Alive NED |
| 2 | 55/M | Tongue | Hemiglossectomy | 29 | 1 | 1 | IIA | pT1N1 (1/29) | 24 months, Alive NED |
| 3 | 40/M | Tongue | Hemiglossectomy | 33 | 2 | 2 | IB, IIA | pT2N2a (3/33) | 17 months, Alive, NED |
| 4 | 43/F | Buccal Mucosa | Wide Excision | 21 | 1 | 1 | IIA | pT1N1 (1/21) | 14 months, Alive, NED |
| 5 | 55/M | FOM | Wide Excision + Marginal Mandibulectomy | 31 | 1 | 0 | IB | pT1N1 (1/31) (False Negative) | 18 months Alive, NED |
| 6 | 32/M | Tongue | Wide Excision | 38 | 2 | 1 | IIA*, III | pT1N0 (0/38) (False Positive) | 22 months, Alive, NED |
*Only Level IIA node was positive on frozen section, NED—No evidence of disease
SLN Accuracy
Frozen section (FS) had one false positive and one false negative result on final HPE. The patient (patient 5, table 3) with false negative SLN had the SLN as the only nodal site of metastasis. IHC for cytokeratins showed three more patients (including the above patient) with micro metastases only in SLN nodes. Since these patients had micro metastases only in the SLN, it was decided that they do not undergo MRND or receive adjuvant radiotherapy and be in close follow up. The patient (patient 6, table no. 3) with false positive SLN (both IHC and HPE negative, pT1N0) did not receive any adjuvant therapy.
Considering HPE on paraffin fixed blocks as gold standard, FS had a sensitivity, specificity and NPV of 80 %, 95.8 % and 95.8 % respectively. IHC with cytokeratins increased the sensitivity (100 %) and NPV (100 %) of histopathology at the loss of specificity (87.5 %) and PPV (62.5 %) (Table 4).
Table 4.
Sensitivity, specificity of SLN
| Frozen Section | HPE | IHC | |
|---|---|---|---|
| Total Positive | 5 | 5 | 8 |
| Total Negative | 24 | 24 | 21 |
| False Positive | 1 | Gold Standard | 3 |
| False Negative | 1 | 0 | |
| Sensitivity | 80 % | 100 % | |
| Specificity | 95.8 % | 87.5 % | |
| Positive Predictive Value (PPV) | 80 % | 62.5 % | |
| Negative Predictive Value (NPV) | 95.8 % | 100 % |
Patient Follow up
All SLN positive patients are alive with no disease on follow-up (mean: 18 months, range 14–30). Two other patients, both with tongue cancers (one with isolated tumour cells on IHC) developed contra lateral nodal recurrence (4 months and 8 months respectively). Both patients had no SLN/non SLN positive nodes initially. They underwent contra lateral MRND and neck irradiation at the time of diagnosis of recurrence. One patient, also with tongue cancer had a local recurrence post Hemiglossectomy (rcT2N1M0). She underwent wide excision with MRND-1 with neck irradiation.
Discussion
Sentinel Lymph Node Biopsy (SLNB) is not the standard of care in oral cavity cancers [19]. However, multiple single institution studies [8–16] and two multi institution studies [17, 18] have successfully demonstrated its feasibility in oral cancers with high detection rates (around 95 %) and negative predictive values (88–100 %). Three meta analyses by Paleri et al. [20], Govers et al.[21] and Thompson et al.[22] based on pooled data samples have also confirmed its use in the staging and treatment of early stage head and neck cancers.
SLNB is a technically demanding procedure, has a longer learning curve, requires expertise and facilities like lymphoscintigraphy, gamma camera, etc. The advantages of SLNB include less morbidity and better cosmetic outcome with comparable local failure and 2- year survival rates [23].
The current NCCN guidelines [24] for oral cavity cancers recommend offering SLN in T1/T2 N0 oral cancers as an alternative to elective neck dissection in centres with adequate expertise and facilities. NCCN guidelines recommend completion of neck dissection in patients who have metastatic disease in the sentinel node (pSLN+) and in patients in whom SLN was not detected. Sentinel node negative patients (pSLN0) may be observed with the caveat that there are no adverse features like extra capsular spread, perineural invasion, lymphovascular space invasion, positive margins, etc. Further, it advises caution when using the procedure in sub sites like floor of mouth, palate and gingiva.
Most SLN studies in oral cancers use lymphoscintigraphy with radio labelled particles with gamma probe localisation and blue dye injection [8–18]. However lymphoscintigraphy and nuclear medicine facilities are not widely available in India [25]. SLN identification in breast cancer using blue dye alone has been widely reported with identification rates slightly inferior or equal to combined techniques [26–29]. Methylene blue has been shown to be an effective and safer alternative to isosulphan blue dye in breast cancer SLN studies [28, 29]. There are very few studies that assess the feasibility of using blue dye alone in detecting SLN in oral cancers [30]. We used methylene blue dye for the study because of its lower cost, easier availability, good safety profile and comparable efficacy.
In our study, the SLN identification rate using methylene blue alone was 90.6% (n = 29/32), which is comparable to the identifications rates in other SLN studies in oral cancers (92–100 %) [8–18]. In 72% (n = 23) of patients, more than one SLN were identified in the same level or in more than one level. Age (P = 0.11), sex (P = 0.56), tumour subside (P = 0.9), T stage (P = 0.7), tumour grade (P = 0.9), Tobacco/Betel use (P = 0.5) or alcohol use (P = 0.6) did not affect SLN identification rate.
It is prudent to mention here that we did have a learning curve in dye injection, SLN identification and dissection. All three patients in whom no SLN were identified were during the first half of the study. The time taken to identify SLN also varied between 12 and 20 min from dye injection. We especially experienced difficulty in dye injection in hard palate, RMT and alveolar lesions.
There has been considerable debate regarding the method of pathological evaluation of SLN. Imprint Cytology, H&E staining in paraffin embedded blocks, Serial step sectioning of the paraffin blocks and Immunohistochemistry have been compared in their ability to detect occult metastases. Imprint Cytology and Frozen section are usually inferior to serial step section and IHC studies [30, 31]. We used frozen section to evaluate metastases in SLN and compared it with HPE based on serial step sectioning and IHC for cytokeratins. The sensitivity, specificity and NPV of 80 %, 95.8 % and 95.8 % respectively of FS with methylene blue alone are also comparable to SLN studies using both radio colloid and blue dye [8–16].
IHC improves SLN accuracy and NPV. However, the importance of micro metastases/isolated tumour cells detected by IHC only is debatable [30] with some authors like Broglie et al.[11] favouring completion of neck dissection as further treatment and others like Chone et al.[16] against it. Also, it is not feasible to apply IHC studies for “on table” decision making during SLNB as done with FS. In our study, IHC for cytokeratins revealed micro metastases in SLN in 3 additional patients which were not detected by FS or HPE. This increased the sensitivity (100 %) and NPV (100 %) at the cost of specificity (87.5 %) and PPV (62.5 %). These three patients with IHC only micro metastases did not receive any adjuvant treatment and one patient developed contra lateral nodal recurrence during follow up. Thus the role of IHC detected micrometastases/isolated tumour cells in staging and their optimal treatment needs further evaluation.
The seemingly high false negativity of 20 % (n = 1) is probably due to the small study population. However relapse/recurrence in the neck (2/29 = 6.9 %) is comparable and acceptable.
One important finding in our study was the absence of SLN/non SLN positive nodes in Level IIB in any patient in spite of 98 nodes (mean = 3.1) being dissected. Similar low incidences of level IIB nodal metastases in early oral cancers have been reported [32, 33]. Level IIB node dissection leads to an increased incidence of spinal accessory nerve injury [34]25. Similarly, we did not have any SLN positive nodes in Level IV, even in tongue cancers. Though the number of patients in our study is small to show significant differences, these issues definitely have to be revisited in a larger trial setting.
We would like to emphasize the limitations of using methylene blue dye alone for SLNB. While using lymphoscintigraphy, the size of the neck incision can be minimised over hot spots before extending it for formal neck dissections. However, the mandatory elevation of the neck flaps before SLN identification while using methylene blue alone probably negates the idea of less morbidity associated with SLNB. The other drawbacks are the inability to evaluate the contra lateral neck and the chance of missing some deeper nodes. In our study, two patients developed contra lateral recurrence on follow-up.
Larger multi institutional trials will help us to better understand and address the drawbacks of this technique for its widespread applicability in decision making especially in countries like India where lymphoscintigraphy is not widely available.
Conclusion
Methylene blue dye alone can be successfully used for SLN identification in early oral cancers with a good accuracy and sensitivity. This method will be of use especially in resource limited countries and centres where nuclear medicine facilities are not widely available. However, it has to be validated by larger randomised multi institutional trials for wider applicability. Immunohistochemistry increases the sensitivity and negative predictive value of SLN but its applicability in real time decision making is limited.
References
- 1.Consolidated Report of Hospital Based Cancer Registries (2001) 2003) National Cancer Registry Programme. Indian Council of Medical Research, 2007
- 2.Consolidated Report of Population Based Cancer Registries (2001) 2004 National Cancer Registry Programme. Indian Council of Medical Research, 2006
- 3.DeVita Jr. VT, Lawrence TS and Rosenberg SA. DeVita, (2011) Hellman and Rosenberg’s Cancer: principles and practice of oncology, 9th (ed). Lippincott Williams & Wilkins (USA) Chapter 70 (1)
- 4.Amaral TMP, da Silva Freire AR, Carvalho AL, et al. Predictive factors of occult metastasis and prognosis of clinical stages I and II squamous cell carcinoma of the tongue and floor of the mouth. Oral Oncol. 2004;40:780–786. doi: 10.1016/j.oraloncology.2003.10.009. [DOI] [PubMed] [Google Scholar]
- 5.Kowalski LP, Sanabria A. Elective neck dissection in oral carcinoma: a critical review of the evidence. Acta Otorhinolaryngol Ital. 2007;27:113–117. [PMC free article] [PubMed] [Google Scholar]
- 6.Yuen AP, Ho CM, Chow TL, et al. Prospective randomized study of selective neck dissection versus observation for N0 neck of early tongue carcinoma. Head Neck. 2009;31:765–772. doi: 10.1002/hed.21033. [DOI] [PubMed] [Google Scholar]
- 7.van den Brekel MW, van der Waal I, Meijer CJ, et al. The incidence of micro metastases in neck dissection specimens obtained from elective neck dissections. Laryngoscope. 1996;106:987–991. doi: 10.1097/00005537-199608000-00014. [DOI] [PubMed] [Google Scholar]
- 8.Höft S, Maune S, Muhle C, et al. Sentinel lymph-node biopsy in head and neck cancer. Br J Cancer. 2004;91:124–128. doi: 10.1038/sj.bjc.6601877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chone CT, Magalhes RS, Etchehebere E, et al. Predictive value of sentinel node biopsy in head and neck cancer. Acta Otolaryngol. 2008;128:920–924. doi: 10.1080/00016480701760114. [DOI] [PubMed] [Google Scholar]
- 10.Alkureishi LWT, Ross GL, Shoaib T, et al. Sentinel node biopsy in head and neck squamous cell cancer: 5- year follow-Up of a european multicenter trial. Ann Surg Oncol. 2010;17:2459–2464. doi: 10.1245/s10434-010-1111-3. [DOI] [PubMed] [Google Scholar]
- 11.Broglie MA, Haile SR, Stoeckl SJ. Long-term experience in sentinel node biopsy for early oral and oropharyngeal squamous cell carcinoma. Ann Surg Oncol. 2011;18:2732–2738. doi: 10.1245/s10434-011-1780-6. [DOI] [PubMed] [Google Scholar]
- 12.Christensen A, Bilde A, Therkildsen MH, et al. The prevalence of occult metastases in Non sentinel lymph nodes after step-serial sectioning and immuno histo chemistry in cN0 oral squamous cell carcinoma. Laryngoscope. 2011;121:294–298. doi: 10.1002/lary.21375. [DOI] [PubMed] [Google Scholar]
- 13.Pattani KM, Califano J. Long-term experience in sentinel node biopsy for early oral and oropharyngeal squamous cell carcinoma. Ann Surg Oncol. 2011;18:2709–2710. doi: 10.1245/s10434-011-1785-1. [DOI] [PubMed] [Google Scholar]
- 14.Pezier T, Nixon IJ, Gurney B, et al. Sentinel lymph node biopsy for T1/T2 oral cavity squamous cell carcinoma—a prospective case series. Ann Surg Oncol. 2012;19:3528–3533. doi: 10.1245/s10434-011-2207-0. [DOI] [PubMed] [Google Scholar]
- 15.Vorburger MS, Broglie MA, Soltermann A, et al. Validity of frozen section in sentinel lymph node biopsy for the staging in oral and oropharyngeal squamous cell carcinoma. J Surg Oncol. 2012;106:816–819. doi: 10.1002/jso.23156. [DOI] [PubMed] [Google Scholar]
- 16.Chone CT, Aniteli MB, Magalhães RS, et al. Impact of immunohistochemistry in sentinel lymph node biopsy in head and neck cancer. Eur Arch Otorhinolaryngol. 2013;270:313–317. doi: 10.1007/s00405-012-2032-5. [DOI] [PubMed] [Google Scholar]
- 17.Ross GL, Soutar DS, Gordon MacDonald D, et al. Sentinel node biopsy in head and neck cancer: preliminary results of a multicenter trial. Ann Surg Oncol. 2004;11:690–696. doi: 10.1245/ASO.2004.09.001. [DOI] [PubMed] [Google Scholar]
- 18.Cilantros FJ, Kitsch RP, Schuler DE, et al. Sentinel lymph node biopsy accurately stages the regional lymph nodes for T1-T2 oral squamous cell carcinomas: results of a prospective multi-institutional trial. J Clin Oncol. 2010;28:1395–1400. doi: 10.1200/JCO.2008.20.8777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kowalski LP, Sanabria A. Elective neck dissection in oral carcinoma: a critical review of the evidence. Acta Otorhinolaryngol Ital. 2007;27:113–117. [PMC free article] [PubMed] [Google Scholar]
- 20.Paleri V, Rees G, Arullendran P, et al. Sentinel node biopsy in squamous cell cancer of the oral cavity and oral pharynx: a diagnostic meta-analysis. Head Neck. 2005;27:739–747. doi: 10.1002/hed.20228. [DOI] [PubMed] [Google Scholar]
- 21.Govers TM, Hannink G, Merkx MA, et al. Sentinel node biopsy for squamous cell carcinoma of the oral cavity andoropharynx: a diagnostic meta-analysis. Oral Oncol. 2013;49:726–732. doi: 10.1016/j.oraloncology.2013.04.006. [DOI] [PubMed] [Google Scholar]
- 22.Thompson CF, St John MA, Lawson G, et al. Diagnostic value of sentinel lymph node biopsy in head and neck cancer: a meta-analysis. Eur Arch Otorhinolaryngol. 2013;270:2115–2122. doi: 10.1007/s00405-012-2320-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Samant S. Sentinel node biopsy as an alternative to elective neck dissection for staging of early oral carcinoma. Head Neck. 2014;36:241–246. doi: 10.1002/hed.23288. [DOI] [PubMed] [Google Scholar]
- 24.NCCN Clinical Practice Guidelines in Oncology Head and Neck Cancers Version 2.2014. http://www.nccn.org/professionals/physician_gls/pdf/head-and-neck.pdf
- 25.List of nuclear medicine centres in the country. Central Bureau of Health Intelligence. Director General Health Services. Ministry of Health and Family Welfare, India. http://cbhidghs.nic.in/hii2000-01/10.283.htm
- 26.Somashekhar SP, Zaveri Shabber S, Udupa Venkatesh K, et al. Sentinel lymph node biopsy in early breast cancer using methylene blue dye and radioactive sulphur colloid - a single institution Indian experience. Indian J Surg. 2008;70:111–119. doi: 10.1007/s12262-008-0033-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mathelin C, Croce S, Brasse D, et al. Methylene blue dye an accurate dye for sentinel lymph node identification in early breast cancer. Anticancer Res. 2009;29:4119–4125. [PubMed] [Google Scholar]
- 28.Varghese P, Abdel-Rahman AT, Akberali S, et al. Methylene blue dye- a safe and effective alternative for sentinel lymph node localization. Breast J. 2008;14:61–67. doi: 10.1111/j.1524-4741.2007.00519.x. [DOI] [PubMed] [Google Scholar]
- 29.Zakaria S, Hoskin TL, Degnim AC. Safety and technical success of methylene blue dye for lymphatic mapping in breast cancer. Am J Surg. 2008;196:228–233. doi: 10.1016/j.amjsurg.2007.08.060. [DOI] [PubMed] [Google Scholar]
- 30.Suresh TN, Harendra Kumar ML, Thomas AK, et al. Study of sentinel lymph node in oral squamous cell carcinoma. J Clin Biomed Sci. 2013;3:146–149. [Google Scholar]
- 31.Trivedi NP, Ravindran HK, Sundram S, et al. Pathologic evaluation of sentinel lymph nodes in oral squamous cell carcinoma. Head Neck. 2010;32:1437–1443. doi: 10.1002/hed.21345. [DOI] [PubMed] [Google Scholar]
- 32.Pantvaidya GH, Pal P, Vaidya AD, et al. (2013) A prospective study of 583 neck dissections in oral cancers: implications for clinical practice. Head Neck Aug 30. Epub [DOI] [PubMed]
- 33.Manola M, Aversa C, Moscillo L, et al. Status of level IIb lymph nodes of the neck in squamous cell carcinoma of the oral tongue in patients who underwent modified radical neck dissection and lymph node sentinel biopsy. Acta Otorhinolaryngol Ital. 2011;31:130–134. [PMC free article] [PubMed] [Google Scholar]
- 34.Lima LP, Amar A, Lehn CN. Spinal accessory nerve neuropathy following neck dissection. Braz J Otorhinolaryngol. 2011;77((2):259–262. doi: 10.1590/S1808-86942011000200017. [DOI] [PMC free article] [PubMed] [Google Scholar]