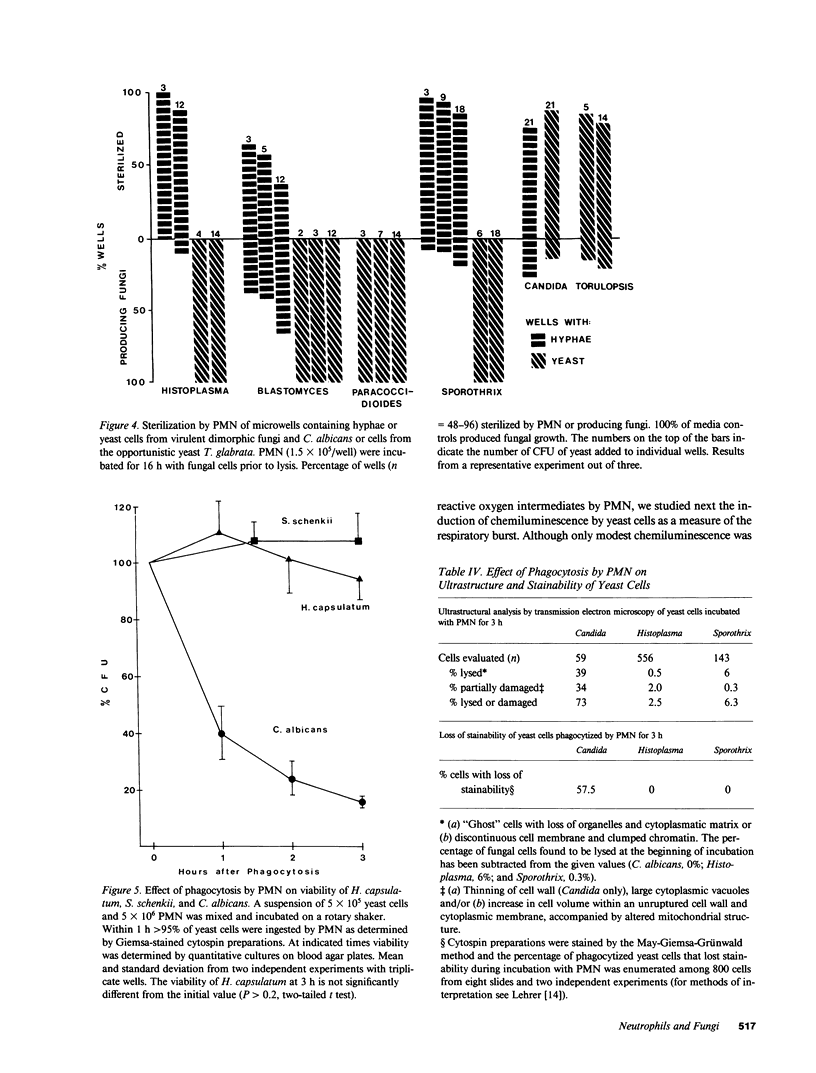
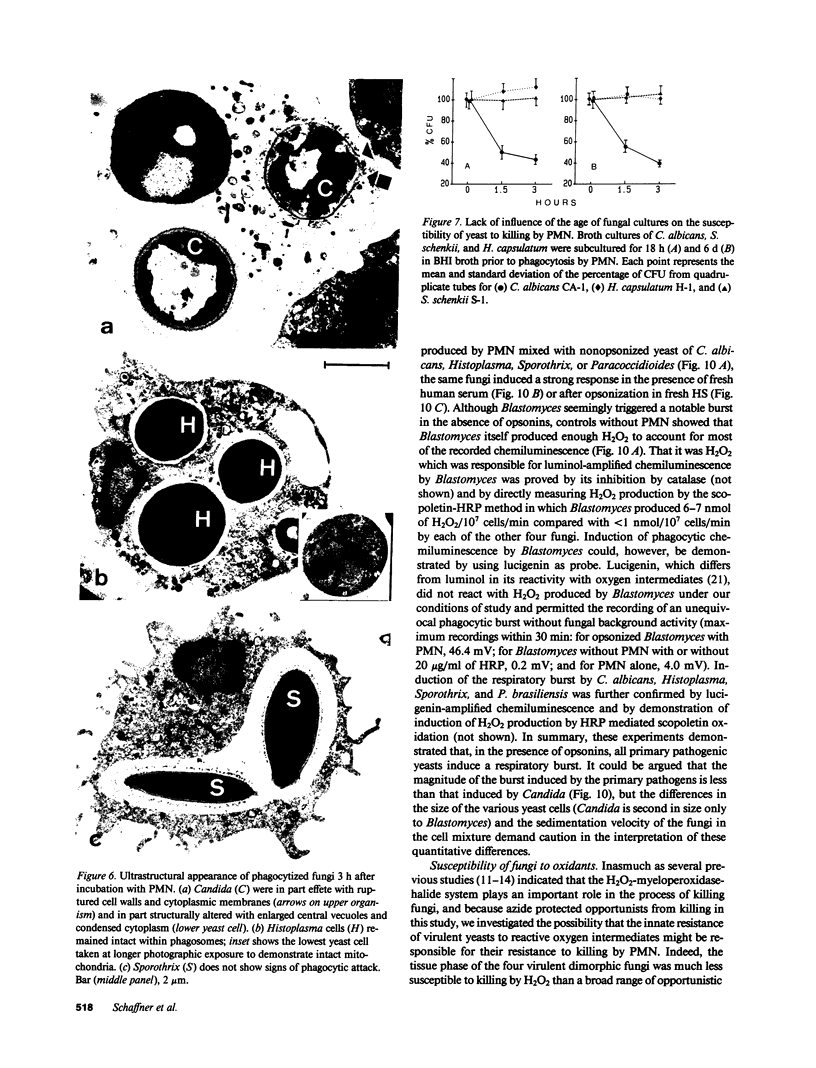
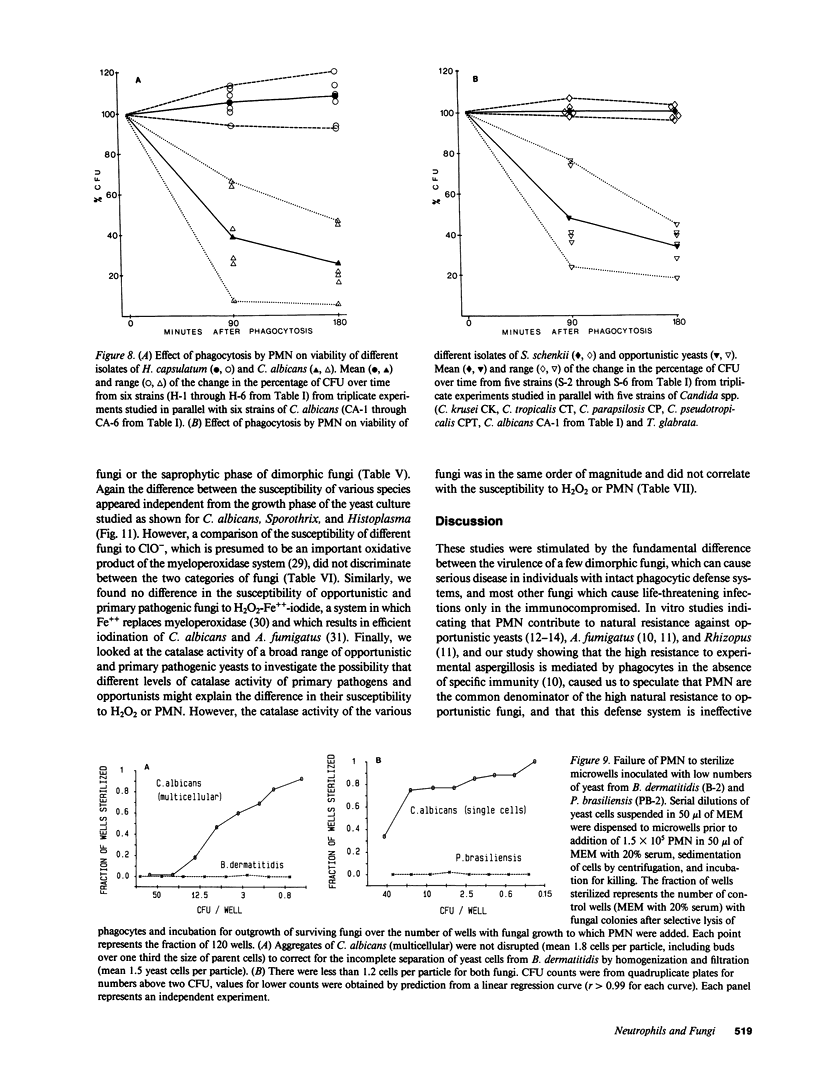
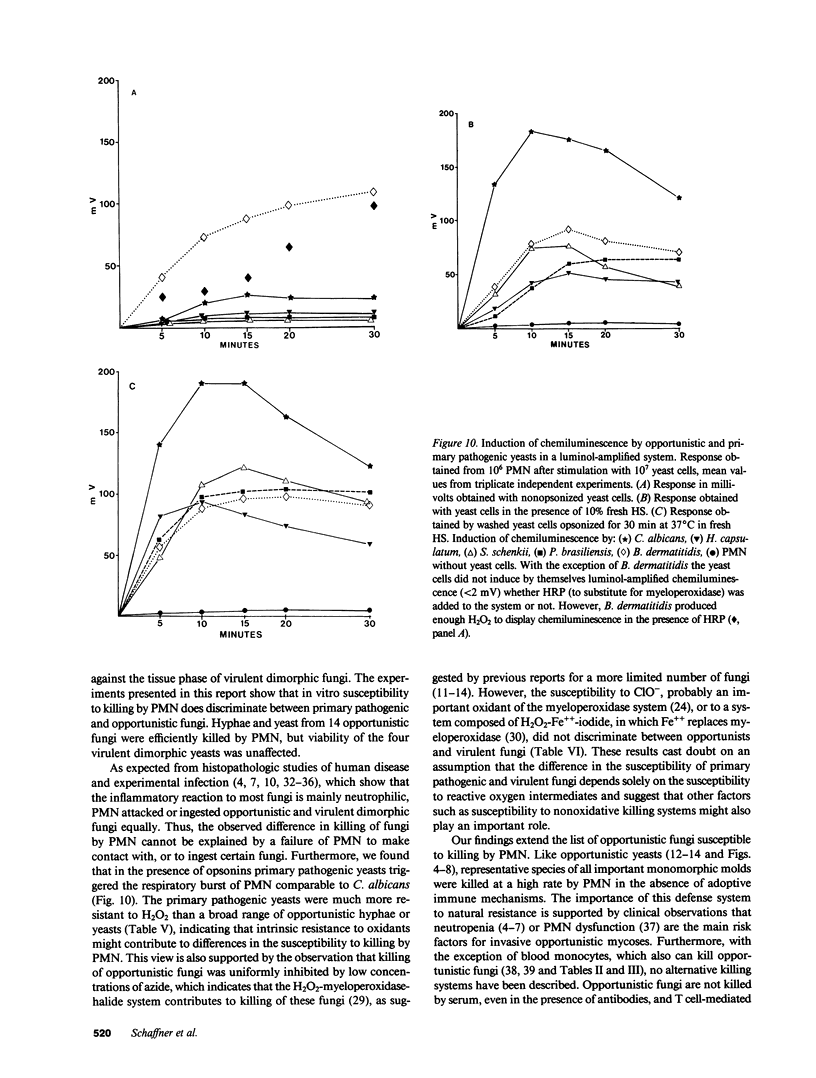
Abstract
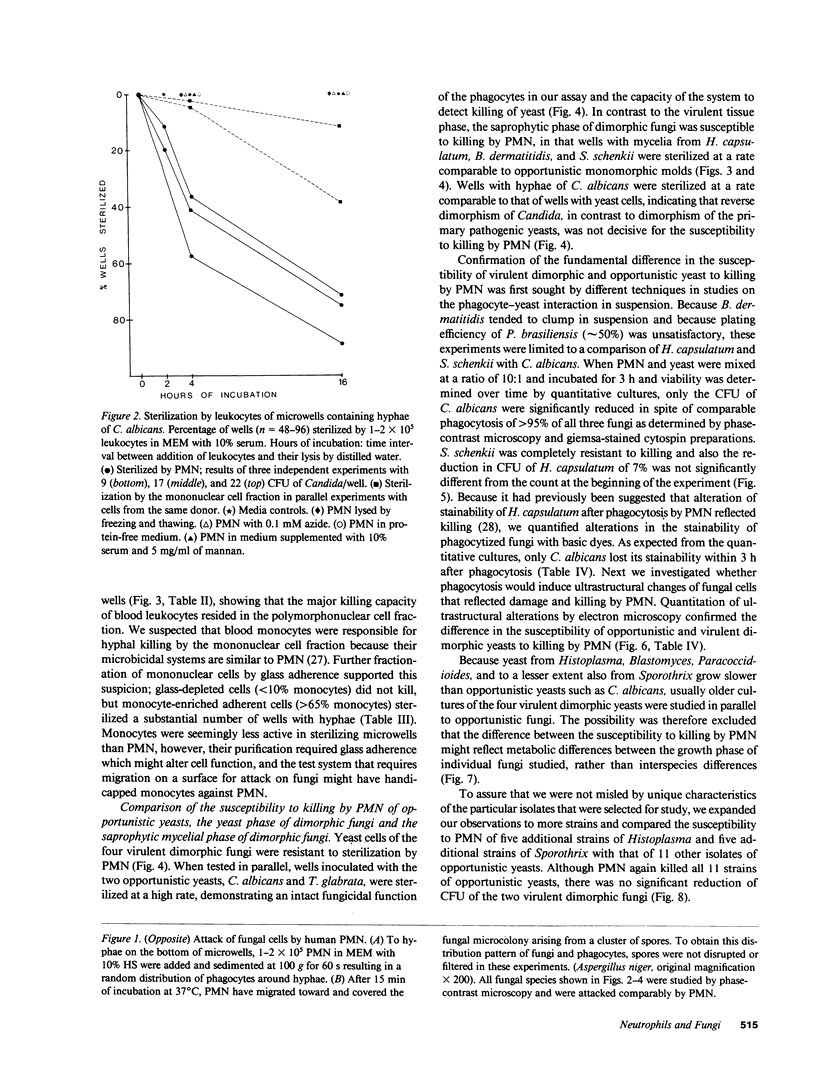
Pathogenic fungi, according to their propensity to cause infection of apparently normal individuals, can be grouped into either primary pathogens (e.g., Coccidioides, Histoplasma, Paracoccidioides, Blastomyces, and Sporothrix) or opportunists (e.g., Candida, Mucoraceae, Aspergillus spp., Petriellidium, and Trichosporon). There is, however, no unifying concept explaining the difference between the virulence of the two fungal categories. Previously we have speculated that neutrophils are the common denominator of the high natural resistance to opportunistic fungi. Accordingly, we then compared the susceptibility to killing by neutrophil granulocytes of Histoplasma, Blastomyces, Paracoccidioides, and Sporothrix with that of 14 opportunistic fungi. We found the four virulent dimorphic yeasts, in contrast to opportunistic fungi, to be resistant to killing by neutrophils. Virulent dimorphic yeasts were ingested by neutrophils, and triggered a respiratory burst comparably to opportunists but were less susceptible to hydrogen peroxide, suggesting that differences in the susceptibility to microbicidal products of leukocytes may explain the difference in virulence.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Armstrong D., Gold J. W., Dryjanski J., Whimbey E., Polsky B., Hawkins C., Brown A. E., Bernard E., Kiehn T. E. Treatment of infections in patients with the acquired immunodeficiency syndrome. Ann Intern Med. 1985 Nov;103(5):738–743. doi: 10.7326/0003-4819-103-5-738. [DOI] [PubMed] [Google Scholar]
- BAKER O., BRAUDE A. I. A study of stimuli leading to the production of spherules in coccidioidomycosis. J Lab Clin Med. 1956 Feb;47(2):169–181. [PubMed] [Google Scholar]
- BAUER H., SHELDON W. H. Leukopenia with granulocytopenia in experimental mucormycosis (Rhizopus oryzae infection). J Exp Med. 1957 Oct 1;106(4):501–508. doi: 10.1084/jem.106.4.501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker R. D. The phycomycoses. Ann N Y Acad Sci. 1970 Oct 30;174(2):592–605. doi: 10.1111/j.1749-6632.1970.tb45585.x. [DOI] [PubMed] [Google Scholar]
- Beaman L., Holmberg C. A. Interaction of nonhuman primate peripheral blood leukocytes and Coccidioides immitis in vitro. Infect Immun. 1980 Sep;29(3):1200–1201. doi: 10.1128/iai.29.3.1200-1201.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaman L., Pappagianis D., Benjamini E. Significance of T cells in resistance to experimental murine coccidioidomycosis. Infect Immun. 1977 Sep;17(3):580–585. doi: 10.1128/iai.17.3.580-585.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brummer E., Stevens D. A. Opposite effects of human monocytes, macrophages, and polymorphonuclear neutrophils on replication of Blastomyces dermatitidis in vitro. Infect Immun. 1982 Apr;36(1):297–303. doi: 10.1128/iai.36.1.297-303.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brummer E., Sugar A. M., Stevens D. A. Immunological activation of polymorphonuclear neutrophils for fungal killing: studies with murine cells and blastomyces dermatitidis in vitro. J Leukoc Biol. 1984 Oct;36(4):505–520. doi: 10.1002/jlb.36.4.505. [DOI] [PubMed] [Google Scholar]
- Bøyum A. Isolation of lymphocytes, granulocytes and macrophages. Scand J Immunol. 1976 Jun;Suppl 5:9–15. [PubMed] [Google Scholar]
- CHITALE A. R., BHENDE Y. M. THE INCIDENCE OF CANDIDA ALBICANS IN THROAT AND FECES OF HEALTHY PERSONS AND PATIENTS ON ANTIBIOTIC THERAPY. J Postgrad Med. 1965 Jan;11:30–33. [PubMed] [Google Scholar]
- Cauley L. K., Murphy J. W. Response of congenitally athymic (nude) and phenotypically normal mice to Cryptococcus neoformans infection. Infect Immun. 1979 Mar;23(3):644–651. doi: 10.1128/iai.23.3.644-651.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen M. S., Isturiz R. E., Malech H. L., Root R. K., Wilfert C. M., Gutman L., Buckley R. H. Fungal infection in chronic granulomatous disease. The importance of the phagocyte in defense against fungi. Am J Med. 1981 Jul;71(1):59–66. doi: 10.1016/0002-9343(81)90259-x. [DOI] [PubMed] [Google Scholar]
- Corbel M. J., Eades S. M. Experimental mucormycosis in congenitally athymic (nude) mice. Mycopathologia. 1977 Dec 16;62(2):117–120. doi: 10.1007/BF01259402. [DOI] [PubMed] [Google Scholar]
- Corbel M. J., Eades S. M. The relative susceptibility of New Zealand black and CBA mice to infection with opportunistic fungal pathogens. Sabouraudia. 1976 Mar;14(1):17–32. doi: 10.1080/00362177685190051. [DOI] [PubMed] [Google Scholar]
- Cozad G. C., Chang C. T. Cell-mediated immunoprotection in blastomycosis. Infect Immun. 1980 May;28(2):398–403. doi: 10.1128/iai.28.2.398-403.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham K. M., Bulmer G. S., Rhoades E. R. Phagocytosis and intracellular fate of Sporothrix schenckii. J Infect Dis. 1979 Nov;140(5):815–817. doi: 10.1093/infdis/140.5.815. [DOI] [PubMed] [Google Scholar]
- Cutler J. E. Acute systemic candidiasis in normal and congenitally thymic-deficient (nude) mice. J Reticuloendothel Soc. 1976 Feb;19(2):121–124. [PubMed] [Google Scholar]
- Diamond R. D., Clark R. A., Haudenschild C. C. Damage to Candida albicans hyphae and pseudohyphae by the myeloperoxidase system and oxidative products of neutrophil metabolism in vitro. J Clin Invest. 1980 Nov;66(5):908–917. doi: 10.1172/JCI109958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond R. D., Haudenschild C. C., Erickson N. F., 3rd Monocyte-mediated damage to Rhizopus oryzae hyphae in vitro. Infect Immun. 1982 Oct;38(1):292–297. doi: 10.1128/iai.38.1.292-297.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond R. D., Krzesicki R., Epstein B., Jao W. Damage to hyphal forms of fungi by human leukocytes in vitro. A possible host defense mechanism in aspergillosis and mucormycosis. Am J Pathol. 1978 May;91(2):313–328. [PMC free article] [PubMed] [Google Scholar]
- Diamond R. D., Oppenheim F., Nakagawa Y., Krzesicki R., Haudenschild C. C. Properties of a product of Candida albicans hyphae and pseudohyphae that inhibits contact between the fungi and human neutrophils in vitro. J Immunol. 1980 Dec;125(6):2797–2804. [PubMed] [Google Scholar]
- Drutz D. J., Frey C. L. Intracellular and extracellular defenses of human phagocytes against Blastomyces dermatitidis conidia and yeasts. J Lab Clin Med. 1985 Jun;105(6):737–750. [PubMed] [Google Scholar]
- Edwards J. E., Jr, Lehrer R. I., Stiehm E. R., Fischer T. J., Young L. S. Severe candidal infections: clinical perspective, immune defense mechanisms, and current concepts of therapy. Ann Intern Med. 1978 Jul;89(1):91–106. doi: 10.7326/0003-4819-89-1-91. [DOI] [PubMed] [Google Scholar]
- Galgiani J. N., Hayden R., Payne C. M. Leukocyte effects on the dimorphism of Coccidioides immitis. J Infect Dis. 1982 Jul;146(1):56–63. doi: 10.1093/infdis/146.1.56. [DOI] [PubMed] [Google Scholar]
- Harvey R. P., Schmid E. S., Carrington C. C., Stevens D. A. Mouse model of pulmonary blastomycosis: utility, simplicity, and quantitative parameters. Am Rev Respir Dis. 1978 Apr;117(4):695–703. doi: 10.1164/arrd.1978.117.4.695. [DOI] [PubMed] [Google Scholar]
- Howard D. H. Fate of Histoplasma capsulatum in guinea pig polymorphonuclear leukocytes. Infect Immun. 1973 Sep;8(3):412–419. doi: 10.1128/iai.8.3.412-419.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard D. H., Otto V. Experiments on lymphocyte-mediated cellular immunity in murine histoplasmosis. Infect Immun. 1977 Apr;16(1):226–231. doi: 10.1128/iai.16.1.226-231.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurtrel B., Langrange P. H., Michel J. C. Absence of correlation between delayed-type hypersensitivity and protection in experimental systemic candidiasis in immunized mice. Infect Immun. 1981 Jan;31(1):95–101. doi: 10.1128/iai.31.1.95-101.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerkering T. M., Espinel-Ingroff A., Shadomy S. Detection of candida antigenemia by counterimmunoelectrophoresis in patients with invasive candidiasis. J Infect Dis. 1979 Nov;140(5):659–664. doi: 10.1093/infdis/140.5.659. [DOI] [PubMed] [Google Scholar]
- Kim M. H., Rodey G. E., Good R. A., Chilgren R. A., Quie P. G. Defective candidacidal capacity of polymorphonuclear leukocytes in chronic granulomatous disease of childhood. J Pediatr. 1969 Aug;75(2):300–303. doi: 10.1016/s0022-3476(69)80403-8. [DOI] [PubMed] [Google Scholar]
- Klebanoff S. J. Myeloperoxidase-halide-hydrogen peroxide antibacterial system. J Bacteriol. 1968 Jun;95(6):2131–2138. doi: 10.1128/jb.95.6.2131-2138.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klebanoff S. J. The iron-H2O2-iodide cytotoxic system. J Exp Med. 1982 Oct 1;156(4):1262–1267. doi: 10.1084/jem.156.4.1262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozel T. R., Gotschlich E. C. The capsule of cryptococcus neoformans passively inhibits phagocytosis of the yeast by macrophages. J Immunol. 1982 Oct;129(4):1675–1680. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- LURIE H. I. Histopathology of sporotrichosis. Notes on the nature of the asteroid body. Arch Pathol. 1963 Apr;75:421–437. [PubMed] [Google Scholar]
- Lehrer R. I., Cline M. J. Interaction of Candida albicans with human leukocytes and serum. J Bacteriol. 1969 Jun;98(3):996–1004. doi: 10.1128/jb.98.3.996-1004.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehrer R. I. Measurement of candidacidal activity of specific leukocyte types in mixed cell populations I. Normal, myeloperoxidase-deficient, and chronic granulomatous disease neutrophils. Infect Immun. 1970 Jul;2(1):42–47. doi: 10.1128/iai.2.1.42-47.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehrer R. I. The fungicidal mechanisms of human monocytes. I. Evidence for myeloperoxidase-linked and myeloperoxidase-independent candidacidal mechanisms. J Clin Invest. 1975 Feb;55(2):338–346. doi: 10.1172/JCI107937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levitz S. M., Diamond R. D. Killing of Aspergillus fumigatus spores and Candida albicans yeast phase by the iron-hydrogen peroxide-iodide cytotoxic system: comparison with the myeloperoxidase-hydrogen peroxide-halide system. Infect Immun. 1984 Mar;43(3):1100–1102. doi: 10.1128/iai.43.3.1100-1102.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Löfgren S., Tärnvik A., Thore M., Carlsson J. A wild and an attenuated strain of Francisella tularensis differ in susceptibility to hypochlorous acid: a possible explanation of their different handling by polymorphonuclear leukocytes. Infect Immun. 1984 Feb;43(2):730–734. doi: 10.1128/iai.43.2.730-734.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montoro M., Collea J. V., Frasier S. D., Mestman J. H. Successful outcome of pregnancy in women with hypothyroidism. Ann Intern Med. 1981 Jan;94(1):31–34. doi: 10.7326/0003-4819-94-1-31. [DOI] [PubMed] [Google Scholar]
- Nelson R. D., Quie P. G., Simmons R. L. Chemotaxis under agarose: a new and simple method for measuring chemotaxis and spontaneous migration of human polymorphonuclear leukocytes and monocytes. J Immunol. 1975 Dec;115(6):1650–1656. [PubMed] [Google Scholar]
- Restrepo A., Vélez H. Efectos de la fagocitosis in vitro sobre el Paracoccidioides brasiliensis. Sabouraudia. 1975 Mar;13(Pt 1):10–21. [PubMed] [Google Scholar]
- Root R. K., Metcalf J., Oshino N., Chance B. H2O2 release from human granulocytes during phagocytosis. I. Documentation, quantitation, and some regulating factors. J Clin Invest. 1975 May;55(5):945–955. doi: 10.1172/JCI108024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose H. D., Varkey B. Deep mycotic infection in the hospitalized adult: a study of 123 patients. Medicine (Baltimore) 1975 Nov;54(6):499–507. doi: 10.1097/00005792-197511000-00004. [DOI] [PubMed] [Google Scholar]
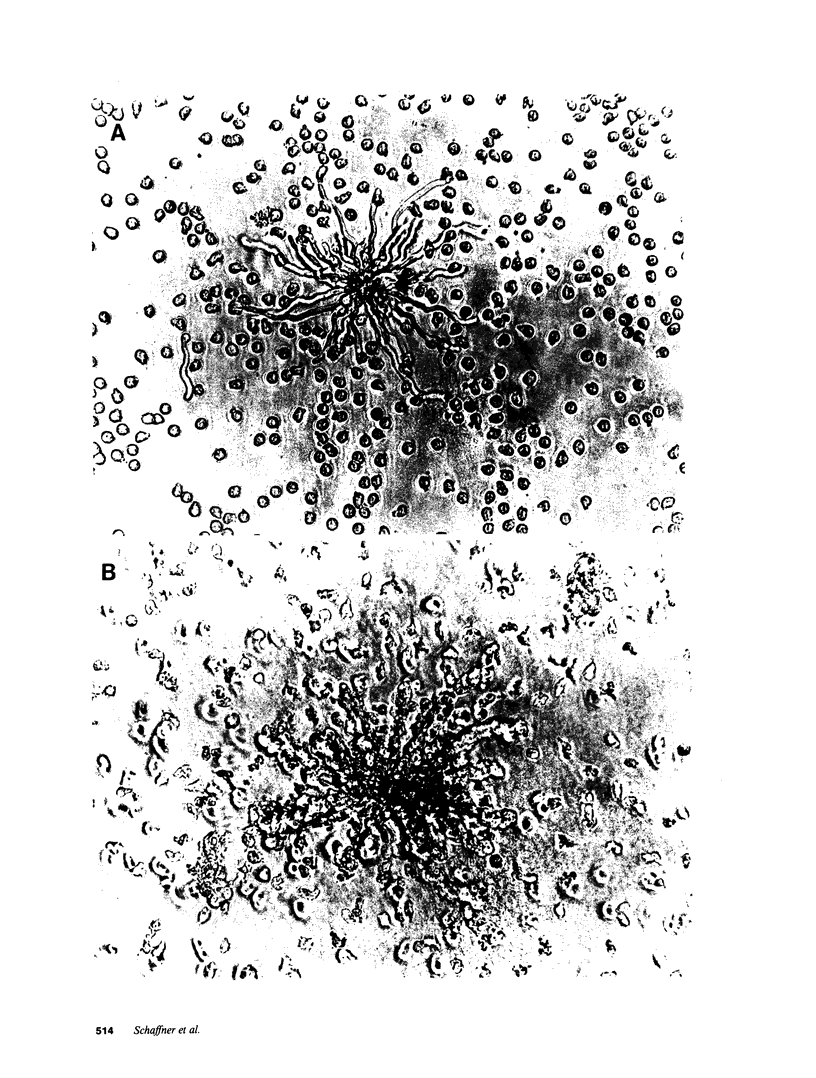
- Schaffner A., Douglas H., Braude A. Selective protection against conidia by mononuclear and against mycelia by polymorphonuclear phagocytes in resistance to Aspergillus. Observations on these two lines of defense in vivo and in vitro with human and mouse phagocytes. J Clin Invest. 1982 Mar;69(3):617–631. doi: 10.1172/JCI110489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiraishi A., Nakagaki K., Arai T. Experimental sporotrichosis in congenitally athymic (nude) mice. J Reticuloendothel Soc. 1979 Sep;26(3):333–336. [PubMed] [Google Scholar]
- Sixbey J. W., Fields B. T., Sun C. N., Clark R. A., Nolan C. M. Interactions between human granulocytes and Blastomyces dermatitidis. Infect Immun. 1979 Jan;23(1):41–44. doi: 10.1128/iai.23.1.41-44.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss S. J., LoBuglio A. F., Kessler H. B. Oxidative mechanisms of monocyte-mediated cytotoxicity. Proc Natl Acad Sci U S A. 1980 Jan;77(1):584–587. doi: 10.1073/pnas.77.1.584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young R. C., Bennett J. E., Vogel C. L., Carbone P. P., DeVita V. T. Aspergillosis. The spectrum of the disease in 98 patients. Medicine (Baltimore) 1970 Mar;49(2):147–173. doi: 10.1097/00005792-197003000-00002. [DOI] [PubMed] [Google Scholar]