Abstract
Ganges River dolphin, Platanista gangetica gangetica, is one of the three obligatory freshwater dolphins in the world and is distributed in the Ganges–Brahmaputra–Meghna and Sangu–Karnaphuli River systems in India, Nepal, and Bangladesh. This species is facing considerable threats to its survival, and its population has dwindled from 4000 to 5000 in the early 1980s to 3500 in 2014 in the distribution range. This article reviews current status of the sub-species, habitat use, and the potential threats that the dolphins face for their survival (details of taxonomic status and genetics, evolutionary adaptations and anatomical peculiarities, physical adaptation, primitive characteristics, biology, behavior, surfacing behavior and dive times, mating and birth, and life span/age have been placed as Electronic Supplementary Materials). Recommendations have been made for the protection and developing strategies for the conservation of this Endangered and endemic sub-species.
Electronic supplementary material
The online version of this article (doi:10.1007/s13280-014-0534-7) contains supplementary material, which is available to authorized users.
Keywords: Ganges River dolphin, Conservation, Population, Endangered species
Introduction
Ganges River dolphins, commonly known as susu, Platanista gangetica gangetica, are distributed throughout the Ganges–Brahmaputra–Meghna and Karnaphuli–Sangu river systems of Nepal, India, Bangladesh, and potentially Bhutan (Mohan et al. 1997; Sinha et al. 2000; Smith et al. 2001). There is no credible estimate of the range-wide numbers, but the subspecies was listed as “endangered” on the 1996 International Union for Conservation of Nature (IUCN) Red List, due to a reduction in its historical distribution range and projected declines in population size due to increasing threats (IUCN 1996).
Although the Ganges dolphin is mentioned in mythological and historical literature, its occurrence in the Hooghly River, the tidal zone of the Ganges, was first documented in 1801 by William Roxburgh, Superintendent of the Botanical Garden, Calcutta (Roxburgh 1801). Anderson (1879) provided the first description of the distribution range, morphology, and anatomy of the dolphin, although he did not discuss the dolphin’s population status or ecology. Approximately 100 years later, however, a few papers reported some details on the population status of the Ganges dolphin as of the 1980s (Jones 1982; Mohan 1989). Nevertheless, these reports were not based on continuous or systematic surveys, and the population status was most likely a rough estimate. Overall, information on ecology and conservation status of river dolphins in India is spatially and temporally patchy.
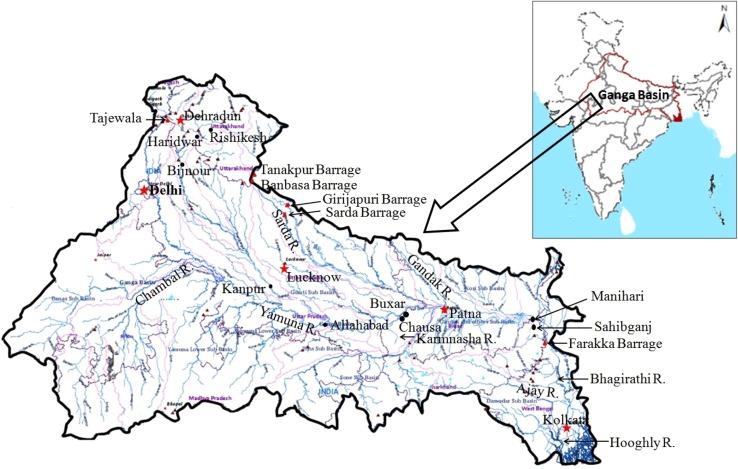
Our research team at Patna University (Patna) has conducted several surveys in discrete segments of the Ganges River from 1991 to 1996, under the Dolphin Conservation Project sponsored by the Ganga Project Directorate, Ministry of Environment and Forests, Government of India (Sinha 1996, 1997; Sinha et al. 2000; Sinha and Sharma 2003a, b). During 1990–1994, other researchers have conducted dolphin surveys at several sections of the Ganges River and its tributaries (Behera 1995; Smith et al. 1994; Behera and Rao 1999). Continuous surveys were conducted in the Ganges River in a stretch of over 1900 km from Haridwar, at the foothills of the Himalayas, to Farakka near the India–Bangladesh border in 1996–1998 (Sinha 1999; Sinha et al. 2000) with support of Biodiversity Support Program (BSP), a consortium of World Wildlife Fund, the Nature Conservancy, and the World Resources Institute. From the results of surveys conducted in 1982–1985, Singh and Sharma (1985) estimated that 45 dolphins were present in approximately 305 km segment of the River Chambal between Batesura and the confluence of the Yamuna River. With support from the National River Conservation Directorate (earlier the Ganga Project Directorate under the Ministry of Environment and Forests, Government of India), Patna University undertook intensive studies from 2001 to 2007 in a 500-km stretch of the Ganges in the state of Bihar, where over 50 % of the total population of the Ganges River dolphin in India (currently over 3,000) survive (Sinha et al. 2010a). During the same period, other researchers conducted continuous surveys in the Brahmaputra River, a large river of the Ganges river system in India, in the state of Assam (Biswas and Boruah 2000; Wakid 2005, 2009). A map of the Ganges basin and major locations mentioned in this article is presented in Fig. 1. A summary of the various survey efforts to study the distribution and status of the Ganges River dolphin in various sections of the Ganges River is listed in Tables 1, 2, and 3.
Fig. 1.
The Ganges River basin in India
Table 1.
Population status and distribution of Ganges river dolphin in the main stem of the Ganges River in India
| River | Segment | No. of Dolphin | Year | Reference | Remarks |
|---|---|---|---|---|---|
| Ganga | Between Haridwar and Middle Ganga Barrage at Bijnor (approx. 100 km) | 0 | December 1996 | Sinha et al. (2000) | The current upstream limit of the range of Ganges dolphins in the Ganges main stem appears to be below the Bijnor Barrage |
| Ganga | Between Bijnor and Narora Barrages (approx. 166 km) | 22/56 | 1993-95/2010 | Behera (1995)/Pers. comm. S. Behera | The isolated dolphin population between the two barrages appears to be increasing |
| Ganga | Between Narora and Kanpur (358 km) | 0 | 1997 | Sinha (1999) | Very low water in this stretch |
| Ganga | Between Kanpur and Allahabad (approx.252 km) | 98 | 2012 | Pers. comm. S. Behera | |
| Ganga | Allahabad to Buxar (approx. 425 km) | 204 (Downstream Survey) | 1997 | Sinha (1999) | |
| Ganga | Buxar to Maniharighat (500 km) | 808 (Upstream Survey) | 2006 | Sinha et al. (2010a) | The river has more water in this stretch as all the four major tributaries from Nepal discharge into the Ganges and create more hydro-geomorphological complexities |
| Ganga | Maniharighat to Farakka (approx. 70 km) | 115 (Downstream Survey) | 1998 | Sinha (1999) | |
| Ganga | Farakka Feeder Canal (38.2 km) | 21 (Downstream Survey) | 1996 | Sinha et al. (2000) | Farakka Barrage diverts regulated Ganges water to the River Bhagirathi through this canal |
| Bhagirathi | Jangipur to Triveni Ghat (320 km) | 119 (Downstream Survey) | 1995 | Sinha (1997) | Dolphin population is relatively low as the river receives regulated water with low silt resulting in low hydrogeological complexities |
| Hoogly | Triveni to Sagar Island (190 km) | 97 (Downstream Survey) | 2008 | Pers. comm. Gopal Sharma) | This tidal zone has high river traffic and large vessels |
Table 2.
Population status and distribution of Ganges river dolphin in various tributaries of the Ganges River in India
| River | Segment | No. of Dolphin | Year | Reference | Remarks |
|---|---|---|---|---|---|
| Yamuna | From confluence of Chambal river to Yamuna-Ganga confluence at Allahabad (250 km) | 31 | 2012 | Pers. comm. S. Behera | |
| Girwa | India/Nepal border to Girijapuri Barrage (approx. 20 km) | 39 | 2012 | Pers. comm. S. Behera | This is a protected area, Katarniaghat Gharial Sanctuary |
| Ghaghara | Girijapuri Barrage to Deorighat (505 km) | 295 | 2006 | WWF-Nepal (2006) | |
| Rapti | 15–20 km | 8 | 2012 | Pers. comm. S. Behera | |
| Saryu | 22 km | 16 | 2012 | Pers. comm. S. Behera | |
| Chambal | Rajghat to Panchnada (approx. 550 km) | 85 | 2012 | Pers. comm. S. Behera | |
| Sone | From Uttar Pradesh/Bihar border to its confluence with Ganges about 35 km upstream Patna in the state of Bihar (approx. 300 km) | 0 | 2001 | Sinha and Sharma (2003b) | Not enough water to sustain dolphin in this stretch |
| Sone | Between Bicchi in Madhya Pradesh to Banjari (130 km) | 10 | 1998 | Sinha et al. (2000) | These dolphins were sighted in some deep pools in Madhya Pradesh |
| Sarda | Sarda Barage to Palia (approx. 100 km) | 0 | 1994 | Sinha and Sharma (2003a) | Not enough water to sustain dolphin in this stretch |
| Kosi | Between Kosi Barrage to Kursela (approx. 200 km) | 85 | 2001 | Sinha and Sharma (2003b) | |
| Gandak | Gandak Barrage to Gandak-Ganges confluence at Patna (approx. 320 km) | 257 | 2010 | Choudhary et al. (2012) | |
| Ken | Confluence with Yamuna to Sindhan Kalan village (30 km) | 8 | 1998 | Sinha et al. (2000) | |
| Betwa | Confluence with Yamuna to Orai (84 km) | 6 | 1998 | Sinha et al. 2000 | |
| Sind | Confluence with Yamuna to 110 km upstream | 5 | 1998 | Sinha et al. 2000 | |
| Rupnarayan | Gadiara to Mankur (42 km), West Bengal | 18 | 2006 | WWF-Nepal (2006) |
Table 3.
Population status and distribution of Ganges river dolphin in the Brahmaputra River and its tributaries
| River | Segment | No. of Dolphin | Year | Reference |
|---|---|---|---|---|
| Brahmaputra | Arunachal Pradesh/Assam to India/Bangladesh border (856 km) | 583 | 2012 | Pers. comm. Wakid |
| Subhansiri | Katai Sapori to its confluence with the Brahmaputra at Jamuguri (94 km) | 35 | 2012 | Pers. comm. Wakid |
| Kulsi | From Gharamara to its confluence with the Brahmaputra at Nagarbera (76 km) | 17 | 2012 | Pers. comm. Wakid |
We also documented various threats to which the Ganges dolphins were exposed, including directed and incidental killings by the fishermen to extract oil from their blubber for use as bait in the oil fishery in the dolphins’ distribution range (Sinha 2002). The dolphin oil is used as an attractant to catch two economically important fish—Clupisoma garua and Eutropiichthyes vacha (Sinha 2002). In addition, we collected dolphin carcasses from the Ganges and its tributaries in the 1980s and 1990s, tissues of which were analyzed for toxic pollutants, including heavy metals; organochlorines, including polychlorinated biphenyls (PCBs) and pesticides (e.g., DDT and HCH); organotin compounds; and perfluorinated chemicals (PFCs) (Kannan et al. 1993, 1994, 1997; Senthilkumar et al. 1999; Yeung et al. 2009). One of the projects (Project No. 23), recommended by the IUCN/SSC Cetacean Specialist Group Action Plan, was to develop an alternative to dolphin oil for use as a fish lure (Perrin 1988). As a part of this investigation, oil from fish scraps was developed as an alternative to dolphin oil (Sinha 2002). Other threats including the effects of dams and barrages on Ganges dolphins were studied by Sinha (2000) and Smith et al. (2000). Similar to that in India, status and threats that the Ganges dolphins face in Nepal and Bangladesh have been reported earlier (Kasuya 1972; Kasuya and Haque 1972; Smith 1993; Smith et al. 1998, 2001, 2006, 2010).
Our constant efforts to study the susu led to declaration of the Ganges dolphin as the “National Aquatic Animal of India” on October 5, 2009. Despite this designation, the species is facing severe threats of extinction throughout its distributional range, and there are many aspects of the animal’s biology and ecology that remain obscure. Under these circumstances, renewed efforts are needed to generate consistent information about the species’ ecological requirements throughout its distribution range, and its response to anthropogenic and natural disturbances, as the basis for the design and implementation of relevant conservation strategies. This review is based on the results from several of the surveys and studies listed above, including some unpublished data from the lead author and information gathered from local people and organizations in India. We have made an attempt to describe the current state of knowledge on the Ganges dolphin in India, and narrated the future scope of work to address the challenges ahead in the conservation of this species.
Distribution
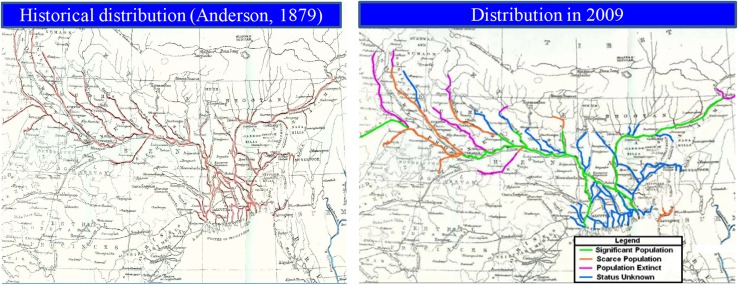
The range of distribution of Platanista in the Ganges River was, between longitudes 77°E and 89°E, from mouth of the river in Bay of Bengal to as far up as the river was navigable near the foothill of Himalayas (Anderson 1879) (Fig. 2). Anderson (1879) stated that, in the Brahmaputra River, Platanista was present “throughout all the main rivers, as far eastwards as longitude 95°E by latitude 27°30′N, frequenting all its larger tributaries.” Outside the Ganges–Brahmaputra–Meghna river systems, susus were present in the Karnaphuli River (Anderson 1879) and possibly the Sangu River in eastern Bangladesh (Haque 1976).
Fig. 2.
Distribution map of Platanista gangetica in the 1870s (Anderson 1879) and 2009; red highlight on the left panel indicates the historical distribution range
Currently, the Ganges dolphin (Platanista gangetica gangetica) is an endangered sub-species of the South Asian river dolphin (Platanista gangetica), which is distributed in the Ganges–Brahmaputra–Meghna river systems in India, Nepal, and Bangladesh and the Sangu–Karnaphuli Rivers in Bangladesh from the deltas upstream to where they were blocked by rocky barriers, shallow waters, dams, and barrages. The river dolphins prefer areas that create eddy countercurrents, such as small islands, sand bars, river bends, and convergent tributaries. In the monsoon season, Ganges dolphins migrate locally to tributaries and then return to larger river channels in the dry, winter season (Smith 1993; Sinha et al. 2000; Sinha and Sharma 2003a, b) The dolphins have been reported to move along the coast of the Bay of Bengal when monsoons flush freshwater out along the southeastern coast of India (Moreno 2003).
Kasuya and Haque (1972) recorded susus as far as Dioghat on the Narayani River, Nepal, 250 m above sea level and approximately 100 km farther upstream than Anderson recorded in 1879. Shreshtha (1989) reported dolphins in the four main river systems of Nepal: the Mahakali, Karnali, Narayani, and Kosi Rivers. Susus ascend the Meghna river systems in Bangladesh at least to Sunamganj (Jones 1982). Nine susus were also sighted in the Barak River in 2006 at Silchar in Assam (pers. comm. Pawlen Singha. Email: thpawlensingha@gmail.com) in India (Barak River is called Meghna River in Bangladesh). Jones (1982) stated that the broad plume of freshwater created by the Ganges outflow in the Bay of Bengal may facilitate the dispersal of susus to rivers outside the Ganges–Brahmaputra–Meghna systems. In 2006, one susu entered the Burhabalang River in the state of Orissa, which discharges into the Bay of Bengal almost 300 km southwest of the mouth of the Ganges (Pers. comm. S. K. Behera). This river has never been connected with the Ganges system.
In the recent years, the dolphin’s range in substantial portions of the Ganges system, especially in upstream areas, has diminished. For example, a continuous survey in low-water season in December 1996 showed that no dolphins were sighted in the 100-km stretch of the Ganges River between Bhimgoda Barrage at Haridwar and Middle Ganga Barrage at Bijnor, at the upstream limit of their historical range in the river (Sinha et al. 2000). Since December 1996, dolphin sighting was not reported in the River Ganges upstream Middle Ganga Barrage at Bijnor. After 1967, dolphins have not been reported in the Yamuna River above the Chambal River confluence near Etawa to Tajewala near the foothills of the Himalayas (736 km) during the dry season (October–June) (Sinha et al. 2000). Historically, dolphins were found year-round in the Yamuna River at Delhi (Anderson 1879), 512-km upstream of the Chambal confluence. Ganges dolphins apparently have been extirpated from a 163-km stretch of the Sarda River (also called the Mahakali River in Nepal) between Lower Sarda Barrage at Sardanagar in Uttar Pradesh state and Upper Sarda Barrage (also called Banbasa Barrage) at Tanakpur along the India-Nepal border in Uttarakhand state during the dry season (Sinha and Sharma 2003a) (Fig. 1); in a 300 linear-km segment of the Sone River, above and below the Indrapuri Barrage (during the dry season, October–June); and upstream of the Ganges confluence (Sinha and Sharma 2003b). We did not find any dolphins crossing the Lower Sarda Barrage both during the flood season (July–September) as well as lean season in the month of March–April when the gates of the barrage were opened. Additionally, no dolphins were observed in the section of the Mahakali River (called River Sarda in India) that flows through Nepal (Smith et al. 1994).
It is challenging to assess whether the extirpations were due to population fragmentation or habitat degradation caused by construction of dams and barrages. Physiographic and hydrologic complexities play an important role in making rivers suitable for inhabitation of dolphins, whereas dams and barrages degrade dolphin habitats, as they reduce physiographic and hydrologic complexity (Reeves and Leatherwood 1994).
Abundance
Statistically robust and standardized density and population estimates are necessary to determine the conservation status and to monitor trends of the river dolphin population worldwide (Reeves and Leatherwood 1994; IWC 2000; Smith and Reeves 2000). In the absence of a robust method, direct counts in discrete sections of rivers generally have been conducted (Smith and Reeves 2000). Capture-recapture analysis of photo-identified animals is commonly used to estimate the abundance of cetaceans (Hammond 2009). This method relies upon capturing images of uniquely marked animals; the proportion of identified individuals recaptured during subsequent sampling events is then used to estimate the population abundance (Borchers et al. 2002). This method has substantial limitations for the survey of Platanista spp.; however, because (1) these animals are extremely difficult to photograph, as they surface alone, unpredictably, for about one second or less, and they seldom approach boats or vessels; and (2) they lack a prominent dorsal fin, and the individuals rarely possess any readily identifying marks or features (Braulik et al. 2012a). In an earlier survey, not a single individual could be identified from 1,200 photographs of Ganges dolphins taken during that time (Smith and Reeves 2000). During 2012 surveys in the Ganges River, we took about one thousand photographs of susu, of which three individual dolphins had identifying features: The upper jaw and lower jaw of two individuals were broken, and there was a deep cut in the dorsal fin of the third. Tropical rivers, such as the Ganges, are often turbid, as they carry heavy loads of silt, and, therefore, underwater photography is almost impossible.
The primary challenge to the application of line or strip transect methods of population survey in the Indus, Ganges, and Brahmaputra Rivers is that the rivers are very shallow, and survey vessels are restricted to traveling up or down the thalweg (the line that follows the deepest part of the river) along a single curving transect that periodically approaches alternate banks as the river meanders. A thalweg transect survey unavoidably samples unrepresentative habitats as it passes through areas with higher densities; in addition, the animals are unlikely to be uniformly distributed in the surveyed strips (Braulik et al. 2012b). Transects that run from bank to bank, perpendicular to the flow, are used for line transect surveys of cetaceans in the Amazon River (Vidal et al. 1997; Martin and da Silva 2004), but in the comparatively shallow, sand-bedded, South Asian rivers, navigational constraints preclude this approach. A single transect parallel to and a standard distance from the river banks also has been used for strip transect surveys in the Amazon (Vidal et al. 1997; Martin and da Silva 2004) and for adapted line transect surveys on the Yangtze River (Zhao et al. 2008), but this is not possible on South Asian rivers, as the channel width changes rapidly and vessels cannot maintain a standard distance from the banks due to shallow depths (Braulik et al. 2012b). In the Ganges and tributaries, we followed the direct count method suggested by Smith and Reeves (2000).
A total of approximately 3,526 dolphins were sighted in their distribution range in India, Nepal, and Bangladesh during the recent surveys, details of which are shown in Table 4. However, various researchers have not followed consistent and robust methods. Many tributaries north of the Ganges River, such as Mahananda, Mechi, Bagmati, Kamala, Balan, Burhi Gandak, are yet to be surveyed, of which Mahananda and Bagmati are large rivers.
Table 4.
Estimated population of the Ganges River dolphins in the early 2000s
| Location | Number of Dolphins | Source |
|---|---|---|
| Ganges River Main Stem and tributaries | 2381 | Sinha (1997), Sinha et al. (2000, 2010a, b), Sinha (1999), Sinha and Sharma (2003a, b); Behera, S. K. pers. comm. January 2014 |
| Brahmaputra and tributaries | 635 | Wakid, A. pers. comm. January 2014 |
| Ganges River system and Sundarbans area in Bangladesh | 460 | Smith et al. (2006, 2009); Alam and Sarker (2012) |
| Karnali River and tributaries—River Mohana and Pathariya; and River Koshi in Nepal |
>50 | Smith et al. (1994), pers. comm. Bhojraj Shreshtha; and pers. comm. Kevin Denlay Dec. 2013 |
| Total | 3526 |
In the recent past, a local activist, Mr. Bhoj Raj Shreshtha, founded a Dolphin Conservation Center in the Kailali District of western Nepal. Mr. Shreshtha has successfully motivated villagers in the area to keep records of dolphin sightings, especially during the flood period of July to September, when a good number of dolphins ascend into the smaller tributaries of the Karnali River. The number of dolphins sighted in the river was claimed to be over fifty, but the sighting records are not based on a “scientific method.” Nevertheless, these data have been collected from an area that is not traditionally monitored, and further efforts, which include the help of local organizations, are needed to comprehensively assess the status of dolphins.
An estimate of the abundance of Ganges dolphins was generated in 2004, 2005, 2006, and 2012 by both upstream and downstream vessel-driven direct counts by the same team of experts in the same stretch (500–525 km) of the Ganges River in the middle segment of the river in the state of Bihar (Sinha et al. 2010a; Sinha 2013). This segment of the Ganges supports maximum density of the dolphins, as all of the four major rivers of Nepal discharge into the Ganges in this stretch, which results in more water and the creation of suitable habitats for the dolphins. The surveys were conducted between Buxar and Maniharighat (500 km) in 2004–2006. In 2012, a survey was conducted in a 525-km stretch in the Ganges between Chausa (15 km upstream of Buxar, where the Karmanasa River joins the Ganges from south and forms the political boundary of Uttar Pradesh and Bihar states) and Sahibganj, located about 10 km downstream of Maniharighat on the opposite bank of the Ganges. A summary of the findings with confidence intervals and Standard Error is presented in Table 5. The population growth rate is an important measure for the assessment of the health and survival of dolphins, but such information is not currently available. Thus, further studies should focus on assessing the growth rate of the Ganges dolphin population.
Table 5.
Summary of Ganges dolphin sightings in the Ganges River (upstream survey) between Maniharighat and Buxar during 2004–2006, and between Sahibganj and Chausa (15 km upstream Buxar) in November–December 2012
| Parameter | Distance traveled (km) | Average vessel speed (km/h) | Total no. of sightings | Best estimate of dolphins | Confidence intervals | Average group size of dolphins | Std. Error of group size | Range of group size | Encounter rate (dolphin/km) |
|---|---|---|---|---|---|---|---|---|---|
| Period of survey | |||||||||
| March 2004 (u/s) | 505.7 | 6.8 | 358 | 777 | 765–816 | 2.2 | 0.088 | 1–11 | 1.5 |
| March 2004 (d/s) | 499.4 | 10.9 | 314 | 696 | 687–730 | 2.2 | 0.079 | 1–10 | 1.4 |
| November 2005(u/s) | 507.4 | 6.3 | 425 | 664 | 578–750 | 1.6 | 0.039 | 1–7 | 1.3 |
| November 2005(d/s) | 494.8 | 11.2 | 349 | 517 | 465–576 | 1.5 | 0.037 | 1–4 | 1.0 |
| December 2006 (u/s) | 517.1 | 6.3 | 557 | 808 | 729–931 | 1.5 | 0.030 | 1–4 | 1.6 |
| December 2006 (d/s) | 501.0 | 10.2 | 405 | 559 | 486–668 | 1.4 | 0.032 | 1–7 | 1.1 |
| October–November 2012 (u/s) | 526.7 | 6.5 | 813 | 813 | 736–838 | 2.1 | 0.077 | 1–15 | 1.6 |
| October–November 2012 (d/s) | 519.3 | 12.0 | 439 | 439 | 415–476 | 2.2 | 0.100 | 1–11 | 0.9 |
u/s Upstream Survey (against the river current)
d/s Downstream Survey (along the river current)
Population Density
The frequency of dolphin sightings remains high in the middle and lower reaches of the main stem of the Ganges, as the river has more hydro-physiographic complexity and greater hydraulic refuge as induced by minor geomorphic features. The river is productive due to the seasonal flood pulse that brings adequate nutrients and has reduced velocity due to its low gradient (1300:1) (Sinha and Prasad 2012). A mean encounter rate of 1.8 dolphins/linear km was reported for the Vikramshila Gangetic Dolphin sanctuary (Choudhary et al. 2006) in the middle of the Ganges River. In November 2012, our team recorded an average density of 2.3 dolphins/linear km in the sanctuary and 1.6 dolphins/linear km in the 525-km stretch of the middle Ganges between Sahibganj and Chausa.
The encounter rates of dolphins reported in other surveys are shown in Table 5. It should be noted that the encounter rate is dependent on the speed of the vessel. In the upstream survey, the encounter rate was 1.3–1.6 dolphins/linear km, and the vessel speed was 6.3–6.8 km/h. In the downstream survey, the encounter rate was 0.9–1.4 dolphins/linear km, with a vessel speed of 10.2–12 km/h. Thus, the speed of the vessel has a direct bearing on the encounter rate of the dolphin. In 2001, the encounter rate recorded for the Guddu–Sukkur subpopulation was almost five times greater than that of any other river dolphin subpopulation (Braulik 2006). This encounter rate (averaging 3.60 dolphin/km, peaking at 5.05 dolphin/km) was several times greater than that recorded for the Ganges dolphin in the rivers of India and Bangladesh. The high density of the Guddu–Sukkur subpopulation is probably due to a ban on hunting since the 1970s (Braulik 2006). More dolphins are sighted in the main channel as compared to the larger secondary channels or braids (Braulik 2006; Basir et al. 2010).
The spatial and temporal habitat selection of dolphins is a complex and dynamic function of requirements for food, mates, avoidance of predators and competitors, and the ability to move between habitat patches (Davis et al. 2002; Schofield 2003). Fluvial habitat within river networks is often described as a mosaic of habitat patches of different sizes that are formed principally by hydro-geomorphic forces (Crook et al. 2001; Thorp et al. 2006). Consequently, fluvial aquatic species are variably distributed, and variations in hydrology and geomorphology play a critical role in determining species distribution (Stazner and Higler 1986; Poff and Allan 1995; Power et al. 1995; Poff et al. 1997). The distribution of prey is likely to be one of the most important factors that influences the distribution of river dolphins; however, habitat selection is frequently assessed in terms of physical habitat characteristics, as these are the primary determinants of prey distribution and are more easily measured (Gregr and Trites 2001; Caňadas et al. 2002; Davis et al. 2002; Bearzi et al. 2008). Most riverine fish prefer specific types of habitat, and water depth is widely considered the most important variable that drives their distribution (Baird and Beaseley 2005; Sarkar and Bain 2007). For example, small or young fish often prefer shallow and slow water, whereas larger or older fish prefer deeper areas, often with faster flows (Sarkar and Bain 2007).
Dispersal
Research has indicated that dolphins move downstream in the winter season when river discharge is low and that, as the flood waters rise in the monsoon season, dolphins move into upstream waters that comprise smaller tributaries (Anderson 1879; Kasuya and Haque 1972; Shreshtha 1989; Sinha and Sharma 2003a; Kelkar et al. 2010). Given the large variation in river discharge and velocity, a seasonal movement is probable. During the flood season, many dolphins enter into the smaller tributaries, and most return to the main channel of the large rivers after the flood. However, some individuals stay back in pools of the tributaries during the dry season (Pelletier and Pelletier 1980), which makes them vulnerable and subject to being killed by local fishermen. On two occasions in 2001 and in 2013, such dolphins were successfully rescued and translocated to the nearby large rivers in West Bengal and Bihar, respectively, by our team in Patna. Between 2002 and 2012, no dolphin was found or reported stayed back in small tributaries which required to be rescued and translocated. Efforts to rescue such individuals are important to conserve the dolphin population, and resources/infrastructure should be made available for this purpose. Susus have been reported to have lived for several years in a lake near Kaziranga, Assam (Pilleri 1970).
Habitat Use
Several researchers have noted extremely patchy distributions of river dolphins in rivers of South Asia, with a preference for confluences (Jerdon 1874; Kasuya and Haque 1972; Haque et al. 1997; Sinha 1997; Sinha et al. 2000; Basir et al. 2010). Nearly all reports, however, are qualitative. A few studies reported that preferred habitats in rivers include downstream of shallow and narrow areas (Kasuya and Haque 1972), in narrow and deep sections of rivers (Pilleri 1970), in deep locations where the current is weak (Pilleri and Zbinden 1973–74; Bairagi et al. 1997), in deep water pools off the mouths of irrigation canals (Basir et al. 2010), near villages and ferry crossings (Pilleri and Bhatti 1982; Sinha 1997), downstream of bridge pilings (Sinha 1997; Smith et al. 2001; Choudhary et al. 2006), downstream of sand bars and sharp meanders, near bathing ghats, cremation ghats (Sinha 1997), and in channels with muddy and rocky substrates (Kelkar et al. 2010). The river dolphins preferentially congregate in such locations that are preferred by local fishermen, and the sites with dolphins had a higher biomass of small fish than did areas in which their presence was not recorded (Kelkar et al. 2010). We understand that, in areas of human activities such as bathing and washing ghats, ferry ghats, and cremation ghats, people tend to throw into the water some edible items that could attract fish and, ultimately, dolphins.
It is clear that South Asian river dolphins are patchily distributed according to characteristics of their habitat, but there have been few studies that have statistically tested which types of habitat are preferred in different seasons or locations. The three most comprehensive studies are as follows: (1) Smith (1993) conducted a study in the Karnali River in Nepal, which is the extreme upstream limit of the Ganges dolphin distribution. Primary and marginal habitats were identified, and it was concluded that dolphins consistently used the same areas characterized by high prey availability and low water flow velocity. River dolphins were assumed to exploit the “hydraulic refuge” provided by counter-current eddies in deep pools. (2) A study by Smith et al. (2009), in the extreme downstream, limits in the Sundarbans delta mangrove forest, river dolphins showed a consistent preference of water of approximately 12-m deep, from a possible range of 0–40 m, irrespective of seasons. Generalized additive models showed that the dolphin distribution was dependent on water with low salinity, high turbidity, and moderate depth during both low and high flows, with a preference for wide sinuous channels with at least two small confluences or one large confluence in the tidal zone in the Sundarbans. (3) A study by Braulik et al. (2012a) found that dolphins selected locations in the Indus River with significantly greater mean depth, cross-sectional area, and hydraulic radius, and significantly narrower river width and a lower degree of braiding. Dolphins with higher frequency at river constrictions and river confluences were also recorded. Channel cross-sectional area was the most important factor that affected dolphin presence and abundance. The greatest influence on presence and abundance of dolphin is exerted by area of water depth below one meter. Indus dolphins avoided channels with a small cross-sectional area (<700 m2).
There is no quantitative information on which aspects of the riverine habitat that are important to the Ganges dolphin in India, where the river has vast floodplains and many confluences and meanders, and is highly braided, with many deep pools, hydraulic refuges, and hydro-geomorphic complexities. River dolphins are expected to be most vulnerable during the low-water season, when the habitat is limited, and it is, therefore, important to determine which habitats are preferentially used at this time, so that conservation efforts can be focused in those locations.
Ganges Dolphin as a Bioindicator Species
Rivers are at risk from multiple stressors, including changes in water quantity and quality, habitat modification, over-exploitation of resources, climate change, pollution, and invasive species. The current impacts of these stressors on rivers are dramatically increasing (Alcamo et al. 2005; Foley et al. 2005). Currently, 65 % of global river discharge is considered to be under moderate to high threat, and the water security of 80 % of the human population is at high risk (Vorosmarty et al. 2010). In addition, biodiversity in freshwater ecosystems is in rapid decline, and this reduction in freshwater system is considered even more threatened than are marine ecosystems (Revenga et al. 2000; Vorosmarty et al. 2010).
Degradation of the freshwater ecosystem is sometimes measured using a suite of ecological indicators, such as macro-invertebrates, fishes, and macrophytes. Carefully selected indicators can provide warning signals of cryptic but significant changes to ecosystems (Karr 1999; Noss 1999). Top predators, such as mammalian carnivores, sea birds, and raptors, are among the widely used indicator species (Furness and Camphuysen 1997; Sergio et al. 2005, 2006, 2008; Piatt et al. 2007). Top predators tend to be concentrated in important biodiversity hotspots (Worm et al. 2003; Sergio et al. 2005, 2006). The reduction or disappearance of top predators is related to significant ecosystem transformations, including impacts on several trophic levels and changes in energy flows, over-exploitation of resources, and changes in the behavior of prey or food chain structure (Soule et al. 2005; Heithans et al. 2008; Braum and Worm 2009). Moreover, their presence or absence can indicate the extent of the footprint of human pressures.
River dolphins are top predators that inhabit some of the largest tropical river basins in Asia and South America and may be ideal candidates for ecological indicators. Gomez-Salazar et al. (2012) investigated the relationships between measures of ecosystem degradation and river dolphins as potential ecological indicators. They tested three ecological indicators of freshwater ecosystem degradation using river dolphins: (i) density of river dolphins, (ii) mean group size of dolphins, and (iii) dolphin sighting rates. A strong negative relationship between measures of habitat degradation and river dolphin density estimates was found in selected locations of the Amazon and Orinoco Rivers. It was suggested that river dolphins are flagship and sentinel species for monitoring the conservation status of large tropical rivers in South America. The contents of micro-pollutants, such as organochlorines, organotin compounds, and perfluorinated chemicals in the Ganges dolphin tissues (Kannan et al. 1993, 1994, 1997; Senthilkumar et al. 1999; Yeung et al. 2009), which were otherwise below detectable levels in the river water or in other invertebrates and fishes, suggest that dolphins are sentinels of toxic chemical pollution in the river. Low dolphin populations in the river’s upstream dams and barrages on the India–Nepal border and in other areas indicate ecosystem degradation. Thus, Ganges dolphins’ low population in some locations can be related to environmental degradation in the Ganges basin.
Threats
Several cetacean subpopulations are under siege from various stressors, such as climate change; chemical, pathogen, and noise pollution; ship traffic; and fishery bycatches; and the Ganges dolphin population is no exception. Freshwater cetaceans have declined dramatically in numbers and range, especially in Asia (Reeves et al. 2000; Smith and Jefferson 2002). The Yangtze River dolphin is already extinct (Turvey et al. 2007). The threats are diverse, longstanding, and very difficult to assess or manage.
All of the existing river dolphins are endangered, mainly due to human activities and multiple threats, including direct or incidental catch; hydroelectric power plants; construction of dams, barrages, and embankments; strikes by vessels; chemical pollution from the discharge of domestic effluents, from the agriculture, industry, mining, and health sector; noise pollution due to underwater explosions and vessels; and deforestation, which lead to heavy siltation and competing demands of freshwater for irrigation, especially in the Indian subcontinent. All freshwater cetaceans require adequate water flow and water quality within their range; these are the basic elements of a suitable habitat and are needed by the animals to support their physical health, mobility, and ability to forage efficiently and to find prey. River dolphins face intense competition with humans for resources such as fish and freshwater. The Ganges dolphins share their lowland riverine habitat with hundreds of millions of people, which results in high mortality rates from hunting, entanglement in fishing gear or entrapment in irrigation canals, population fragmentation by dams and barrages, and severe habitat depletion by water extraction, and degradation by pollution and altered flow regimes (Sinha et al. 2000, 2010b; Sinha 2002).
The future of the South Asian river dolphins is intimately tied to the region’s water security. South Asia has approximately 25 % of the world’s human population but only 4.5 % of its renewable water resources (Babel and Wahid 2008). As per Indian Census 2011, the average population density in the Ganga Basin in India is 581/km2, and, in Bihar state (located in the Ganges basin), it is 1102/km2, compared to the world’s average population density of 13.3/km2. Thus, the Ganges basin is one of the most densely populated regions in the world, and the loss of freshwater biodiversity is inevitable and the prospects for the South Asian river dolphins uncertain.
Effects of Dams and Barrages
Construction of at least 50 dams and barrages within the known or suspected historical range of the Ganges dolphin (Smith et al. 2000) has dramatically affected its habitat, abundance, and population structure during the last 45–50 years. Dams and barrages (low-gated diversion dams) restrict the movement of dolphins, rendering them isolated into separate sub-populations. A subpopulation is defined by the IUCN as “geographically or otherwise distinct groups in the population between which there is little demographic or genetic exchange (typically one successful migrant individual or gamete per year or less)” (IUCN 2001).
The Farakka Barrage (24.7891°N, 87.8878°E; located on the Ganges River 400 km downstream of Patna and 400 km upstream of Calcutta near the India–Bangladesh border; Fig. 3) has affected the dolphin population in the Ganges, as the barrage has not only created a physical barrier for movement of the dolphin but also the reach of the river has been changed from a lotic to a lentic ecosystem (Sinha 2000). Due to the increased sedimentation rate, more than 75 % of the “head pond” has been filled, and a huge sand bar (3 km × 0.3 km) was formed in 2004 (Sinha 2013). Sediments are trapped behind dams and barrages and reduce the volume of suspended matter transported downstream, lessening the potential for bars and sand islands to form in the lower reaches of the river. Barrages reduce or eliminate the “freshet effect,” which, in many wild rivers, renew the floodplains and contributes to meandering (Reeves and Leatherwood 1994).
Fig. 3.
A sand bar (3 km × 0.3 km) in the head pond of Farakka Barrage along the Ganges River in December 2004
The Bhagirathi River receives regulated flow with a low sediment load from the Farakka Barrage through a 38.2-km long feeder canal. The water with the low sediment load has reduced the physiographic and hydrologic complexity in the Bhagirathi River up to Katwa (155 km). The reduced complexity has led to a very low dolphin population (0.3 dolphin/linear km) compared to the other, lower segments of the Ganges and Bhagirathi Rivers, which have about 0.5–1 dolphin/linear km (Sinha 1997). A small tributary, Ajay River, with heavy loads of silt from the highlands of Jharkhand, discharges into the Bhagirathi River from the west at Katwa. The Bhagirathi River has more hydro-physiographic complexity downstream of Katwa, which is evident from the presence of more dolphins and avian fauna (Sinha 1997). Dams and barrages have a number of potential problems, including downstream effects on prey caused by changes in flow rate and sediment transport (Reeves and Leatherwood 1994).
Embankments cause sediment deposits in the riverbed instead of in floodplains, thereby eliminating or reducing the extent of the eddy-counter currents, where dolphins are generally found (Smith et al. 1998). The embankments also restrict access of riverine fishes to the floodplain habitat critical to their reproduction and growth (Boyce 1990). Approximately 3,500 km of embankments have been constructed along the Ganges main stem and the Gandak, Burhi Gandak, Bagmati, Kamala, Yamuna, Punpun, and Sone tributaries (Mishra 1999). Dolphins were apparently extirpated from at least 35 km of the Punpun tributary of the Ganges after embankments were constructed in 1975 (Sinha et al. 2000). Other sources of habitat degradation in the distribution range include heavy siltation in river beds, due to loss of green cover in the catchments area, and change in land use pattern (e.g., crop farming in floodplains); water abstraction from surface pumps, especially in the Ganges system, where the mean dry-season water depth has declined dramatically in recent years; dredging; and removal of stones (Shreshtha 1989), sand (Mohan et al. 1998), and wood debris (Smith 1993).
A large number of completed and ongoing hydroelectric projects on the Ganges and in tributaries in the Himalayas are further expected to aggravate the problem of flow decline in the middle and lower reaches of the Ganges, where dolphins survive (Sinha et al. 2010b). The cumulative effects of these activities compromise the ecological integrity of the riverine ecosystems, especially the small tributaries where the suitable habitat is limited and disproportionately vulnerable to local disturbance. Declining flows in the rivers have received little attention for a long time. The newly established National Ganga River Basin Authority by the Indian government in 2009, an apex body under the chairmanship of the Prime Minister of India, has the mandate of “Aviral Dhara Nirmal Dhara” (uninterrupted quality flow). Such efforts may help restore the riverine environment.
Chemical Pollution
The riverine ecosystem is in close proximity to human activities and, therefore, is an ultimate sink for the discharge of sewage and industrial wastewater that emanates from human activities. The Ganges River basin is the most densely populated basin in the world and is heavily polluted by fertilizers, pesticides, industrial chemicals, and domestic effluents. Exposure of dolphins to toxic chemicals can affect their reproduction and survival. In the Ganges River food chain, the dolphins, as an apex predator, have been shown to accumulate high levels of persistent and toxic chemicals in their tissues. Several studies conducted by our research group have reported elevated levels of DDT in the blubber of Ganges dolphins (Kannan et al. 1994; Senthilkumar et al. 1999) (Table 6). Notable levels of immunotoxic chemicals, such as butyltins and perfluorinated chemicals, have been found in the tissues of Ganges dolphins (Kannan et al. 1997, 2005; Yeung et al. 2009). Heavy metals, including cadmium and lead, have been measured in the livers of Ganges dolphins (Kannan et al. 1993).
Table 6.
Reported concentrations (ng/g wet wt) of DDT, HCH, polychlorinated biphenyls (PCBs) in the blubber and butyltins and perfluorooctanesulfonate (PFOS) in the liver of Ganges river dolphins from India
| Date of collection | Sex | Length (cm) | Tissue | Lipid (%) | PCBs | DDTs | HCHs | Butyltins* | PFOS* |
|---|---|---|---|---|---|---|---|---|---|
| 24 January 1988 | M | 70.4 | Blubber | 34 | 360 | 4700 | 190 | 2000 | |
| 6 October 1991 | M | 104 | Blubber | 31 | 410 | 9100 | 470 | 380 | |
| 21 July 1991 | F | 115 | Blubber | 41 | 620 | 12000 | 430 | 250 | |
| 27 March 1992 | F | 233 | Blubber | 74 | 420 | 13000 | 610 | 61 | |
| 11 February 1993 | F | 250 | Blubber | 51 | 1500 | 31000 | 860 | NA | |
| 27 June 1994 | M | 84 | Blubber | 53 | 13000 | 64000 | 1100 | NA | |
| 3 November 1994 | M | 123 | Blubber | 77 | 2560 | 63000 | 1100 | NA | |
| 29 November 1994 | M | 117 | Blubber | 70 | 2100 | 30000 | 1900 | NA | |
| 5 November 1996 | F | 133 | Blubber | 71 | 1100 | 21000 | 1900 | NA | |
| 1993-2007 (n = 15) | 10 M&5F | 68-248 | Liver | NA | NA | NA | NA | NA | 28 (0.74–74)** |
Although levels of the some of the toxicants were relatively low, based on the analysis of the metabolic index (see details in Kannan et al. 1994), it was found that Ganges dolphins have a low capacity to metabolize some toxic pollutants. The proximity to intense pollution sources and low capacity to metabolize pollutants make the Ganges dolphins vulnerable to the effects of chemical pollution. Several studies have shown that some freshwater aquatic mammals, such as mink and river otter, are very sensitive to the effects of chemical pollution (Kannan et al. 2000). Thus, studies are needed to assess the impact of pollutants on the health of river dolphins. In addition to the contaminants studied thus far, other emerging contaminants that arise from sewage pollution and diseases in river dolphins should be examined in future studies. Our study on mercury pollution in water, sediment, benthic macro-invertebrates, and fishes of the Ganges River at Varanasi found higher levels of mercury (0.0–91.7 ppm) in fishes than those of fishes collected from the western coast at Mumbai (0.03–0.82 ppm) (Sinha et al. 2007). Recent studies have reported elevated levels of arsenic in the Ganges river basin (Nickson et al. 2007; Kumar et al. 2010). Mercury and other industrial pollutants that arise from the discharges of wastes from several industries need to be studied in different segments of the Ganges.
Directed and Incidental Catches
Deliberate killing of the Ganges dolphins is believed to have declined in most areas but still occurs at least occasionally in the Ganges near Patna (Sinha 2002) and in the upper reaches of the Brahamaputra River in Assam (Mohan et al. 1997) for their meat and oil, which is used as a fish attractant. Mortality from fishing gears, especially monofilament nylon gillnets, is a severe problem for the Ganges dolphins throughout their range (Sinha 2002). Dolphins are particularly vulnerable, because their preferred habitat is often in the same location as the fishing grounds. A specific problem is that, because dolphin oil is highly valued as a fish attractant, fishermen have a strong incentive to kill any dolphin found alive in their nets and even to set their nets strategically in the hope of capturing dolphins, which is termed “assisted incidental capture” (Sinha 2002). Meaningful quantitative data on the magnitude of catches, either deliberate or incidental, are unavailable and unlikely to become available in the absence of organized fishing in the river system.
Although the Ganges dolphin was given legal protection in India under the Wildlife (Protection) Act of 1972, the law was not effective until the end of the 20th century. The efficacy of the Act became noticeable after the proceedings of the Patna High Court (CWJC No. 5628 of 2001). Field trials have shown that fish scrap oil is an efficient substitute for dolphin oil as a fish attractant (Sinha 2002). We conducted several extension programs, with the help of Wildlife Trust of India (Sinha 2004), a non-governmental organization, to popularize the use of fish scrap oil as an alternative to dolphin oil. Many groups of fishermen from Assam visited our laboratory at Patna University during the last 10 years, and the most recent was in 2012, to get training on how to obtain oil from fish scraps and its use in the oil fishery. Some fishermen, however, continue to use dolphin oil. In November 2012, we encountered a couple of fishermen who were using dolphin meat and oil at Sultanganj near the Vikramshila Gangetic Dolphin Sanctuary in Bihar and another fisherman at Barh in the Patna District. After these observations, we organized an interactive meeting on January 25, 2013, with fisherman who use dolphin meat and oil in their village near Sultanganj to create awareness, educate, and motivate the fishermen to save the dolphin. State government officials also participated in the meeting. Such meetings and the extension program to popularize the fish scrap oil will help to save the Ganges dolphin.
Conservation and Challenges Ahead
India has been a pioneer in conserving wildlife. The world’s first recorded wildlife conservation measures were enacted in India during the third century BC. One of the greatest Indian emperors, Ashoka the Great, who reigned from 274 to 232 BC, stressed the sanctity of an animal’s life. Some of his decrees engraved in stone have survived until today in the Pillar Edict V. The Ganges dolphin (called Ganga-puputaka in ancient days) was included in the list of animals declared inviolable by the emperor (Sinha 1996).
The Government of India provides legal protection to the Ganges River dolphin (Fig. 4) by including it in Schedule I of the Wildlife (Protection) Act, 1972 since the Act was enacted in 1972. Killing and poaching of any animal included in Schedule I of the Act are cognizable offenses, and the offender may be fined up to $500 US and/or receive a 7-year imprisonment. The efficacy of this act, however, was not evident until the 1990s, when we started intensive and extensive awareness campaigns among the general public. Help rendered by the mass media, both print and electronic, was valuable in educating people of different social strata.
Fig. 4.
Platanista gangetica gangetica surfacing in the River Ganges (Photo by Fernando Trujillo)
The IUCN categorized the Ganges dolphin as endangered in 1996 (IUCN 1996). The species was included in Appendix I of the Convention on the International Trade on Endangered Species of Flora and Fauna (CITES) and in Appendix II of the Convention on Migratory Species. The government of India declared the Ganges dolphin the National Aquatic Animal of India on October 5, 2009, and formal notification was issued on May 10, 2010. Thus, India became the first country to adopt the river dolphin as its National Aquatic Animal.
A separate Conservation Action Plan (CAP) for the Ganges river dolphin has been prepared for the Government of India (Sinha et al. 2010b). The CAP includes protection and restoration of habitats, community participation, capacity building, conducting of periodic status surveys and monitoring, establishing protected areas, providing education awareness, minimizing incidental catches, rescue and rehabilitation, and initiating researches on identified thrust areas besides, identifying agencies for implementation of the Action Plan. A National Dolphin Research Center is to be established at Patna shortly as an institutional support for the long-term conservation of the dolphin. With the help of activists, NGOs, university researchers, government departments/officials, and other stakeholders, especially fishermen, various action plans are being implemented. The State Government of Bihar designated October 5 as “Dolphin Day” and is celebrating the “Dolphin Day” every year on October 5 since 2012, as a means to help create awareness among the general public in addition to annual monitoring of government activities to save and conserve the dolphins.
One of the important tasks for researchers is the development of a robust scientific method for population estimation to provide a basis for determining which areas should be given the highest conservation priority. Further, age-wise habitat use during different seasons must be studied as a means to help prioritize the conservation efforts.
Recommendations for Conservation
The baiji’s extinction clearly demonstrates that, without appropriate and timely actions, the future of the remaining freshwater cetaceans is precarious. All freshwater cetaceans require adequate water flow and water quality within their range; these are the basic elements of a suitable habitat and are needed by the animals to support their physical health, mobility, and ability to forage efficiently.
The long-term viability of freshwater cetacean populations requires management of entire ecosystems and watersheds, i.e., an ecosystem approach for the conservation and management of rivers and river dolphins. Watershed management, especially in upstream sections, is required to reduce sedimentation from agriculture, forestry, and land conversion; to limit water removal and dramatic changes in flow regimes by dams and barrages; to ensure adequate water and sustain essential geomorphic features in cetacean habitat; and to reduce toxic effluents and chemical pollution from agriculture, industry, industrial transport, and human settlements.
Organochlorine and butyltin concentrations in samples from the tissues of Ganges dolphins were high enough to cause concern about their effects. Further, several unstudied pollutants that arise from the disposal of sewage are expected to compromise the health of dolphins. Pollutant loads can be expected to increase with industrialization, and the spread of intensive agricultural practices is facilitated by water diversion. River dolphins may be particularly vulnerable to industrial pollution, because their habitats in counter-current pools downstream of confluences and sharp meanders often place them in proximity to point sources in major urban areas in India. Further, the capacity of rivers to dilute pollutants has been drastically reduced in many areas because of upstream water abstraction, diversion, and impoundment. This problem is destined to worsen as more development takes place along the river. It is of utmost importance to maintain pollution-free, uninterrupted flow in the rivers to address these issues. Particularly in river systems where there is great demand for fresh water for human use, critical minimum flow and the maintenance of natural flow variability are of overarching importance.
It is important to determine which habitats are preferentially used by dolphins during the low-water season so that conservation efforts can be focused in these locations. In the dry season, channel constrictions, confluences, and channels with high cross-sectional areas are all high-use dolphin habitats that could benefit from management as discrete dolphin conservation zones. The monitoring of river dolphin populations and habitats on a regular basis, as has been performed for tigers and elephants, is very much required. Involvement of fishermen in dolphin conservation efforts will encourage them to have a sense of “ownership.” Further, a study is needed on the implications of climate change on freshwater cetaceans that include consideration of habitat resilience.
It is important to collect as much scientific information as possible on behaviors and other ecological requirements of the dolphin in the Ganges River. We recommend building a microcosm in the Ganges River at Patna for captive breeding and rescue efforts. For this, a big enclosure (2–3 km × 100 m × 5 m) could be created, using smooth metal poles and wire mesh (30 cm × 30 cm) along the left bank of the river at Patna, where enough water flow is available year-round. This will ensure availability of enough water flow and prey through the enclosure. A couple of male and female dolphins can be kept in the enclosure to study their behaviors and the possibilities of “breeding” in natural habitat.
Having declared the river dolphin, Platanista gangetica gangetica, the National Aquatic Animal, the Indian government should complement this commendable action by setting up a national network of protected/conserved areas for river dolphins and associated aquatic fauna and considering initiating a National River Dolphin Project along the lines of Project Tiger, Project Elephant, Project Snow Leopard, and Project Rhino. In doing so, the project should identify the dolphins’ present pattern of distribution and status in the context of their historical distribution throughout the Ganges and Brahmaputra systems, Indus tributaries, and coastal waters of India (including Sundarbans). Given that fishery interactions are the primary cause of river dolphin mortality, the Inland Fisheries Act needs to be reviewed and amended so that rules and regulations are in place, making fisheries sustainable, and reducing risks to dolphins and other aquatic wildlife.
We need to consider the development of community-based river dolphin conservation areas, where sustainable fisheries and dolphin conservation measures are promoted in an integrated manner, with possible model planning, design, and implementation of ecotourism projects focused on dolphin watching, with appropriate safeguards against disturbance (harassment). Such projects should incorporate education and awareness efforts and should be promoted as a preferable alternative to dolphinariums. We also need to design and implement a national awareness campaign on river dolphins through innovative media programs and the establishment of interpretation and information centers in dolphin conservation/protected areas.
Electronic supplementary material
Biographies
Ravindra Kumar Sinha
PhD, is a Professor of Zoology at Patna University, Patna, India. His research interests include Conservation of the Ganges River dolphin, River Ecology, Pollution Biology, Bio-monitoring, and Limnology. He has conducted several field surveys on river dolphins in India and Nepal for over 25 years.
Kurunthachalam Kannan
PhD, is a Research Scientist at Wadsworth Center, New York State Department of Health and a Professor of Department of Environmental Health Sciences, State University of New York at Albany, New York, USA. His research interests include understanding of environmental distribution, fate, and toxic effects of organic pollutants. His research has focused on the effects of toxic environmental chemicals on marine mammals and other aquatic wildlife.
Contributor Information
Ravindra K. Sinha, Email: rksinha.pu@gmail.com
Kurunthachalam Kannan, Phone: 1-518-474-0015, Email: kkannan@wadsworth.org.
References
- Alam SMI, Sarker NJ. Status and distribution of the Gangetic dolphin, Platanista gangetica gangetica (Roxburgh, 1801) in River Burhiganga during 2003–2004 and its conservation. Bangladesh Journal of Zoology. 2012;40:21–31. doi: 10.3329/bjz.v40i1.12890. [DOI] [Google Scholar]
- Alcamo J, van Vuuren D, Cramer W. Anonymous Scenarios. Washington, DC: Millenium Ecosystem Assessment; 2005. Changes in ecosystem services and their drivers across the scenarios; pp. 297–373. [Google Scholar]
- Anderson J. Anatomical and Zoological researches: Comprising an account of zoological results of the two expeditions to western Yunnan in 1868 and 1875; and a monograph of the two cetacean genera Platanista and Orcella. London, UK: Bernard Quaritich; 1879. [Google Scholar]
- Babel, M.S., and S.W. Wahid. 2008. Freshwater under threat: South Asia. United Nations Environment Program, Nairobi, Kenya.
- Basir T, Khan A, Gautam P, Behra SK. Abundance and prey availability assessment of Ganges river dolphin (Platanista gangetica gangetica) in a stretch of Upper Ganges River, India. Aquatic Mammals. 2010;36:19–26. doi: 10.1578/AM.36.1.2010.19. [DOI] [Google Scholar]
- Bairagi SP, Dey SC, Mohan RSL. The status of a resident population of Ganges River dolphin (Platanista gangetica) in Kulsi River of north east India. Tiger Paper. 1997;24(2):11–13. [Google Scholar]
- Baird IG, Beaseley I. Irrawaddy dolphin Orcaella brevirostris in the Cambodian Mekong River: An initial survey. Oryx. 2005;39:301–310. doi: 10.1017/S003060530500089X. [DOI] [Google Scholar]
- Bearzi G, Azzellino A, Politi E, Costa M, Bastianini M. Influence of seasonal forcing on habitat use by bottlenose dolphins Tursiops truncates in the Northern Adriatic Sea. Ocean Science Journal. 2008;43:175–182. doi: 10.1007/BF03029922. [DOI] [Google Scholar]
- Behera, S.K.1995. Studies on Population Dynamics, Habitat Utilization and Conservation Aspects of Gangetic Dolphins (Platanista gangetica) in a Stretch of Ganga River from Rishikesh to Kanpur. PhD Thesis, Gwalior: School of Studies in Zoology, Jiwaji University.
- Behera SK, Rao RJ. Observations on the behaviour of Gangetic dolphin Platanista gangetica in the Upper Ganga River. Journal of the Bombay Natural History Society. 1999;96:42–48. [Google Scholar]
- Biswas SP, Boruah S. Ecology of river dolphin (Platanista gangetica) in the Upper Brahmaputra. Hydrobiology. 2000;430:97–111. doi: 10.1023/A:1004077215276. [DOI] [Google Scholar]
- Borchers DL, Buckland ST, Zucchini W. Estimating animal abundance: Closed populations. London: Springer; 2002. [Google Scholar]
- Braulik G. Status assessment of Indus River dolphin, Platanista gangetica minor, March–April 2001. Biological Conservation. 2006;129:579–590. doi: 10.1016/j.biocon.2005.11.026. [DOI] [Google Scholar]
- Braulik G, Bhatti ZI, Ehsan T, Hussain B, Khan AR, Khan A, Khan U, Kundli KU, Rajput R, Reichert AP, Northridge SP, Bhagat HB, Garstang R. Robust abundance estimate for endangered river dolphin subspecies in South Asia. Endangered Species Research. 2012;17:201–215. doi: 10.3354/esr00425. [DOI] [Google Scholar]
- Braulik G, Reichert AP, Ehsan T, Khan S, Northridge SP, Alexander JS, Garstang R. Habitat use by a freshwater dolphin in the low-water season. Aquatic Conservation: Marine and Freshwater Ecosystem. 2012;22:533–546. doi: 10.1002/aqc.2246. [DOI] [Google Scholar]
- Braum JL, Worm B. Cascading top-down effects of changing oceanic predator abundances. Journal of Animal Ecology. 2009;78:699–714. doi: 10.1111/j.1365-2656.2009.01531.x. [DOI] [PubMed] [Google Scholar]
- Boyce JK. Birth of a megaproject: political economy of flood control in Bangladesh. Environmental Management. 1990;14:158–165. doi: 10.1007/BF02394131. [DOI] [Google Scholar]
- Caňadas A, Sagarminaga R, Garcia-Tiscar S. Cetacean distribution related with depth and slope in the Mediterranean waters off southern Spain. Deep Sea Research. 2002;49:2053–2073. doi: 10.1016/S0967-0637(02)00123-1. [DOI] [Google Scholar]
- Choudhary SK, Smith BD, Dey S, Dey S, Prakash S. Conservation and biomonitoring in the Vikramshila Gangetic dolphin sanctuary, Bihar, India. Oryx. 2006;40:189–197. doi: 10.1017/S0030605306000664. [DOI] [Google Scholar]
- Choudhary S, Dey S, Dey S, Sagar V, Nair G, Kelkar N. River dolphin distribution in regulated river system; implications for dry season flow regimes in the Gangetic basin. Aquatic Conservation: Marine and Freshwater Ecosystems. 2012;22(1):11–25. doi: 10.1002/aqc.1240. [DOI] [Google Scholar]
- Crook DA, Robertson AI, King AJ, Humphries P. The influence of spatial scale and habitat arrangement on diel patterns of habitat use of two low land river fishes. Oecologia. 2001;129:525–533. doi: 10.1007/s004420100750. [DOI] [PubMed] [Google Scholar]
- Davis RW, Ortega-Ortiz JG, Ribic CA, Evans WE, Biggs DC, Ressler PH, Cady RB, Leben RR, Mullin KD, Wursig B. Cetacean habitat in the northern oceanic Gulf of Mexico. Deep Sea Research. 2002;49:212–242. [Google Scholar]
- Foley JA, Defries R, Asner GP, Barford C, Bonan G, Carpenter SR, Chapin FS, Col MT, Daily GC, Gibbs HK. Global consequences of land use. Science. 2005;309:570. doi: 10.1126/science.1111772. [DOI] [PubMed] [Google Scholar]
- Furness RW, Camphuysen KCJ. Seabirds as monitors of the marine environment. ICES Journal of Marine Science. 1997;54:726. doi: 10.1006/jmsc.1997.0243. [DOI] [Google Scholar]
- Gomez-Salazar C, Coll M, Whitehead H. River dolphins as indicators of ecosystem degradation in large tropical rivers. Ecological Indicators. 2012;23:19–26. doi: 10.1016/j.ecolind.2012.02.034. [DOI] [Google Scholar]
- Gregr EJ, Trites AW. Predictions of critical habitat for five whale species in the waters of coastal British Columbia. Canadian Journal of Fisheries and Aquatic Sciences. 2001;58:1265–1285. doi: 10.1139/f01-078. [DOI] [Google Scholar]
- Hammond PS. Mark-recapture. In: Perrin WF, Wursig B, Thewissen JGM, editors. Encyclopedia of marine mammals. 2. San Diego, CA: Academic Press; 2009. pp. 705–709. [Google Scholar]
- Haque, A. K. M. A. 1976. Comments on the abundance and distribution of the Ganges susu Platanista gangetica and the effects of the Farakka barrage on its population. FAO, ACMRR, Scientific Consultation on Marine Mammals, ACMRR/MM/SC/132.
- Haque AKMA, Nishiwaki M, Kasuya T, Tobayama T. Observations on the behaviour and other biological aspects of the Ganges susu, Platanista gangetica. The Scientific Report of Whales Research Institute. 1997;29:87–94. [Google Scholar]
- Heithans MR, Frid A, Wirsing AJ, Worm B. Predicting ecological consequences of marine top predator declines. Trends in Ecology & Evolution. 2008;23:202–210. doi: 10.1016/j.tree.2008.01.003. [DOI] [PubMed] [Google Scholar]
- IUCN. 1996. 1996 IUCN Red List of threatened animals. IUCN, Gland, Switzerland and Cambridge, UK. 448 pp.
- IUCN. 2001. IUCN Red List categories and criteria: version 3.1. IUCN Species Survival Commission. IUCN Gland, Switzerland and Cambridge, UK.
- IWC (International Whaling Commission) Report of the standing sub-committee on small cetaceans. Journal of Cetacean Research and Management. 2000;1(Supplement):211–225. [Google Scholar]
- Jerdon TC. The mammals of India; a natural history of all animals known to inhabit continental India. London: J. Wheldon; 1874. p. 335. [Google Scholar]
- Jones S. The present status of the Gangetic susu, Platanista gangetica (Roxburgh), with comments on the Indus susu, Platanista minor Owen. FAO Advisory Committee on Marine Resources Research Working Party on Marine Mammals. FAO Fish. Ser. (5) 1982;4:97–115. [Google Scholar]
- Kannan K, Sinha RK, Tanabe S, Ichihashi H, Tatsukawa R. Heavy metals and organochlorine residues in Ganges river dolphin from India. Marine Pollution Bulletin. 1993;26:159–162. doi: 10.1016/0025-326X(93)90128-7. [DOI] [Google Scholar]
- Kannan K, Tanabe S, Tatsukawa R, Sinha RK. Biodegradation capacity and residue pattern of organochlorines in Ganges river dolphins from India. Toxicological and Environmental Chemistry. 1994;42:249–261. doi: 10.1080/02772249409358010. [DOI] [Google Scholar]
- Kannan K, Senthilkumar K, Sinha RK. Sources and accumulation of butyltin compounds in Ganges river dolphin, Platanista gangetica. Applied Organometallic Chemistry. 1997;11:223–230. doi: 10.1002/(SICI)1099-0739(199703)11:3<223::AID-AOC543>3.0.CO;2-U. [DOI] [Google Scholar]
- Kannan K, Blankenship AL, Jones PD, Giesy JP. Toxicity reference values for the toxic effects of polychlorinated biphenyls in aquatic mammals. Human and Ecological Risk Assessment. 2000;6:181–201. doi: 10.1080/10807030091124491. [DOI] [Google Scholar]
- Kannan K, Ramu K, Kajiwara N, Sinha RK, Tanabe S. Organochlorine pesticides, polychlorinated biphenyls and polybrominated diphenyl ethers in Irrawaddy dolphins from India. Archives of Environmental Contamination and Toxicology. 2005;49:415–420. doi: 10.1007/s00244-005-7078-6. [DOI] [PubMed] [Google Scholar]
- Karr JR. Defining and measuring river health. Freshwater Biology. 1999;41:221–234. doi: 10.1046/j.1365-2427.1999.00427.x. [DOI] [Google Scholar]
- Kasuya T. Some information on the growth of the Ganges dolphin with a comment on the Indus dolphin. The Scientific Reports of the Whales Research Institute. 1972;24:87–108. [Google Scholar]
- Kasuya T, Haque AKMA. Some informations on distribution and seasonal movement of the Ganges dolphin. The Scientific Reports of the Whales Institute. 1972;24:109–115. [Google Scholar]
- Kelkar N, Krishnaswamy J, Choudhary S, Sutaria D. Coexistence of fisheries with river dolphin conservation. Conservation Biology. 2010;24:1130–1140. doi: 10.1111/j.1523-1739.2010.01467.x. [DOI] [PubMed] [Google Scholar]
- Kumar P, Kumar M, Ramanathan AL, Tsujimura M. Tracing the factors responsible for arsenic enrichment in groundwater of the middle Gangetic Plain, India: A source identification perspective. Environmental Geochemistry and Health. 2010;32:129–146. doi: 10.1007/s10653-009-9270-5. [DOI] [PubMed] [Google Scholar]
- Martin AR, da Silva VMF. River dolphins and flooded forests; seasonal habitat use and sexual segregation of boto, Inia geoffrensis in an extreme cetacean environment. Journal of the Zoological Society of London. 2004;263:295–305. doi: 10.1017/S095283690400528X. [DOI] [Google Scholar]
- Mishra DK. Above the danger mark. Himal. 1999;12:12–17. [Google Scholar]
- Mohan, R.S.L. 1989. Conservation and management of the Ganges River dolphin, Platanista gangetica, in India. 64–69 pp, In Biology and conservation of the river dolphins, Occasional papers of the IUCN/SSC, ed, by W.F. Perrin, R.L. Brownell, Jr., Z. Kaiya, and L. Jiankang, Vol. 3. Gland, Switzerland: IUCN.
- Mohan RSL, Dey SC, Bairagi SP, Roy S. On a survey of the Ganges River dolphin Platanista gangetica of Brahmaputra River, Assam. The Journal of the Bombay Natural History Society. 1997;94:483–495. [Google Scholar]
- Mohan RSL, Dey SC, Bairagi SP. On a resident population of the Ganges River dolphin, Platanista gangetica in the Kulsi River (Assam), a tributary of Brahmaputra. The Journal of the Bombay Natural History Society. 1998;95:1–7. [Google Scholar]
- Moreno, P. 2003. Ganges and Indus Dolphins. 13–17 pp. In Grzimek’s animal life encyclopedia, ed. by M. Hutchins, D. Kleinman, V. Geist, J. Murphy, D. Thoney, Vol. 15, 2nd Edition. Farmington Hills: Gale Group.
- Nickson R, Sengupta C, Mitra P, Dave SN, Banerjee AK, Bhattacharya A, Basu S, Kakoti N, Moorthy NS, Wasuja M, Kumar M, Mishra DS, Ghosh A, Vaish DP, Srivastava AK, Tripathi RM, Singh SN, Prasad R, Bhattacharya S, Deverill P. Current knowledge on the distribution of arsenic in groundwater in five states of India. Journal of Environmental Science and Health—Part A. 2007;42:1707–1718. doi: 10.1080/10934520701564194. [DOI] [PubMed] [Google Scholar]
- Noss RF. Assessing and monitoring forest biodiversity a suggested framework and indicators. Forest Ecology and Management. 1999;115:135–146. doi: 10.1016/S0378-1127(98)00394-6. [DOI] [Google Scholar]
- Pelletier C, Pelletier FX. Report sur I’expedition delphinisia (Septembre 1977–Septembre 1978) Annales de la societe des sciences naturelle de la charaente maritime. 1980;6:647–679. [Google Scholar]
- Perrin, W.F. 1988. Dolphins, porpoises and whales. An action plan for conservation of biological diversity: 1988–1992. Gland, Switzerland: IUCN.
- Piatt JF, Harding AMA, Shultz M, Speckman SG, Van Pelt TI, Drew GC, Kettle AB. Seabirds as indicators of marine food supplies; Cairns revisited. Seabirds as Indicators of Marine Ecosystems. 2007;352:221–234. [Google Scholar]
- Pilleri G. Observations on the behaviour of Platanista gangetica in the Indus and Brahmaputra rivers. Investigations on Cetacea. 1970;2:27–59. [Google Scholar]
- Pilleri G, Bhatti MU. Status of the Indus dolphin population (Platanista indi, Blyth, 1859) between Sukkur and Taunsa barrages. Investigations on Cetacea. 1982;13:245–252. [Google Scholar]
- Pilleri G, Zbinden K. Size and ecology of the dolphin population (Platanista indi) between Sukkur and Guddu Barrages, Indus River. Investigations on Cetacea. 1973–74;5:59–70. [Google Scholar]
- Poff NL, Allan JD. Functional organization of stream fish assemblages in relation to hydrological variability. Ecology. 1995;76:606–627. doi: 10.2307/1941217. [DOI] [Google Scholar]
- Poff NL, Allan JD, Bain MB, Karr JR, Prestegaard KL, Richter BD, Sparks RE, Stromberg JC. The natural flow regime: A paradigm for river conservation and restoration. BioScience. 1997;47:769–784. doi: 10.2307/1313099. [DOI] [Google Scholar]
- Power ME, Sun A, Parker G, Dietrich WE, Wooton JT. Hydraulic food chain models—An approach to the study of food-web dynamics in large rivers. BioScience. 1995;45:159–167. doi: 10.2307/1312555. [DOI] [Google Scholar]
- Reeves RR, Leatherwood S. Dams and River Dolphins: Can They Coexist? AMBIO. 1994;23:172–175. [Google Scholar]
- Reeves RR, Smith BD, Kasuya T, editors. Biology and conservation of freshwater cetaceans in Asia. Gland, Switzerland: IUCN; 2000. [Google Scholar]
- Revenga C, Brunner J, Henniger N, Kassem K, Payner R. Pilot analysis of global ecosystems, freswater systems. Washington, DC: World Resources Institute; 2000. [Google Scholar]
- Roxburgh W. An account of a new species of Dolphinus, an inhabitant of the Ganges. Asiatic Research. 1801;7:170–174. [Google Scholar]
- Sarkar UK, Bain MB. Priority habitats for the conservation of large river fish in the Ganges River basin. Aquatic Conservation: Marine and Freshwater Ecosystems. 2007;17:349–359. doi: 10.1002/aqc.782. [DOI] [Google Scholar]
- Schofield PJ. Habitat selection of two gobies (Microgobius gulosus, Gobiosoma robustum) influence of structural complexities, competitive interactions and presence of a predator. Journal of Experimental Marine Biology and Ecology. 2003;288:125–137. doi: 10.1016/S0022-0981(03)00004-2. [DOI] [Google Scholar]
- Sergio F, Newton I, Marchesi L. Conservation: Top predators and biodiversity. Nature. 2005;436:192. doi: 10.1038/436192a. [DOI] [PubMed] [Google Scholar]
- Sergio F, Newton I, Marchesi L, Pedrini P. Ecologically justified charisma: Preservation of top predators delivers biodiversity conservation. Journal of Applied Ecology. 2006;43:1049–1055. doi: 10.1111/j.1365-2664.2006.01218.x. [DOI] [Google Scholar]
- Sergio F, Caro T, Brown D, Clucas B, Hunter J, Ketchum J, McHugh K, Hiraldo F. Top predators as conservation tools: Ecological rationale, assumptions, and efficacy. Annual Review of Ecology Evolution and Systematics. 2008;39:1–19. doi: 10.1146/annurev.ecolsys.39.110707.173545. [DOI] [Google Scholar]
- Senthilkumar K, Kannan K, Sinha RK, Tanabe S, Giesy JP. Bioaccumulation profiles of polychlorinated biphenyl congeners and organochlorine pesticides in Ganges River dolphins. Environmental Toxicology and Chemistry. 1999;18:1511–1520. doi: 10.1002/etc.5620180725. [DOI] [Google Scholar]
- Shreshtha, T.K. 1989. Biology, status and conservation of the Ganges River dolphin, Platanista gangetica, in Nepal. In Biology and conservation of the river dolphins. Occasional Paper of the IUCN Species Survival Commission, ed. by W.F. Perrin, R.L. Brownell, Jr., K. Zhou, J. Liu, Vol. 3, 70–76 pp. Gland, Switzerland: IUCN.
- Singh LAK, Sharma RK. Gangetic dolphin, Platanista gangetica: Observations on the habits and distribution pattern in National Chambal Sanctuary. Journal of the Bombay Natural History Society. 1985;82:648–653. [Google Scholar]
- Sinha, R. K. 1996. Final Technical Report on Dolphin Conservation Project, Patna University, Patna. Submitted to the Ganga Project Directorate, Ministry of Environment and Forests, Govt of India.
- Sinha RK. Status and conservation of Ganges River dolphin in Bhagirathi—Hooghly River systems in India. International Journal of Ecology and Environmental Sciences. 1997;23:343–355. [Google Scholar]
- Sinha, R. K. 1999. The Ganges River dolphin—a tool for baseline assessment of biological diversity in River Ganges, India. Final Technical Report No. 1/99. Patna University, Patna, India.
- Sinha, R.K. 2000. Status of the Ganges River dolphin (Platanista gangetica) in the vicinity of Farakka Barrage, India. In Biology and conservation of freshwater cetaceans in Asia, ed. by R.R. Reeves, B.D. Smith, T. Kasuya, Vol. 23, 42–48 pp. Occasional Gland, Switzerland: Paper of the IUCN Species Survival Commission.
- Sinha RK. An alternative to dolphin oil as a fish attractant in the Ganges River system: Conservation of the Ganges River dolphin. Biological Conservation. 2002;107:253–257. doi: 10.1016/S0006-3207(02)00058-7. [DOI] [Google Scholar]
- Sinha, R. K. 2004. ‘Bait and Watch’: Popularization of alternatives to Dolphin oil among fishermen for the conservation of the Ganges River Dolphin (Platanista gangetica) in Bihar, 1–14 pp. New Delhi: Wildlife Trust of India.
- Sinha, R.K. 2013. The Gangetic dolphin and action plan for its conservation in Bihar, 52 pp. India: Department of Environment and Forests, Govt. of Bihar.
- Sinha, R.K., and K. Prasad. 2012. Management of water quality and biodiversity of the River Ganga. In Ecosystem & integrated water resources management in South Asia, ed. E.R.N. Gunawardane, B. Gopal, H. Kotagama, 104–132 pp. London, UK: Routeledge, Taylor and Francis Group.
- Sinha RK, Sharma G. Faunal diversity of the River Sarda, Uttar Pradesh, India. Journal of Ecophysiology Occupational Health. 2003;3:103–116. [Google Scholar]
- Sinha RK, Sharma G. Current status of Ganges dolphin, Platanista gangetica in River Son and Kosi in Bihar. The Journal of the Bombay Natural History Society. 2003;100:27–37. [Google Scholar]
- Sinha, R.K., B.D Smith, G. Sharma, K. Prasad, B.C. Choudhary, K. Sapkota, R.K. Sharma, and S.K. Behera. 2000. Status and distribution of the Ganges susu (Platanista gangetica) in Ganges River system of India and Nepal. In Biology and conservation of freshwater cetaceans in Asia, ed. R.R. Reeves, B.D. Smith, T. Kasuya, Vol. 23, 42–48 pp. Gland, Switzerland: Occasional Paper of the IUCN Species Survival Commission.
- Sinha RK, Sinha SK, Kedia DK, Kumari A, Rani N, Sharma G, Prasad K. A holistic study on mercury pollution in the Ganga River system at Varanasi, India. Current Science. 2007;92:1223–1228. [Google Scholar]
- Sinha, R.K., S.K. Verma, and L. Singh. 2010a. Population status and Conservation of the Ganges River dolphin (Platanista gangetica gangetica) in the Indian subcontinent, Chapter 22, In Biology, evolution, and conservation of river Dolphins within South America and Asia, ed. M. Ruiz-Garcia, and J. Shostell. New York, USA: Nova Science Publishers. Inc. ISBN: 978-1-60876-633-8.
- Sinha, R.K., S.K. Behera, and B.C Choudhury. 2010b. Conservation Action Plan for the Gangetic dolphins. National Ganga River Basin Authority, Ministry of Environment and Forests, Govt of India. pp 44.
- Smith BD. Status and conservation of the Ganges River dolphin Platanista gangetica in the Karnali River, Nepal. Biological Conservation. 1993;66:159–169. doi: 10.1016/0006-3207(93)90002-I. [DOI] [Google Scholar]
- Smith, B.D., and G.T. Braulik. 2009. Susu and Bhulan. Encyclopedia of Marine Mammals, 2nd ed. San Diego, CA, USA: Academic Press.
- Smith BD, Jefferson TA. Status and conservation of facultative freshwater cetaceans in Asia. The Raffles Bulletin of Zoology. 2002;10((Suppl.)):173–187. [Google Scholar]
- Smith, B.D. and Reeves, R. R. 2000. Report of the second meeting of the Asian River Dolphin Committee, Rajendrapur, Bangladesh, 22–24 February 1997. In Biology and conservation of freshwater cetaceans in Asia, ed. R.R. Reeves, B.D. Smith, T. Kasuya Occasional Paper of the IUCN Species Survival Commission, Vol. 23, 1–14 pp. Gland, Switzerland: IUCN.
- Smith BD, Sinha RK, Regmi U, Sapkota K. Status of Ganges River dolphins (Platanista gangetica) in the Karnali, Narayani and Saptakosi Rivers of Nepal and India in 1993. Marine Mammal Science. 1994;10:68–75. [Google Scholar]
- Smith BD, Haque AKMA, Hossain MS, Khan A. River dolphins in Bangladesh: Conservation and the effects of water developments. Environmental Management. 1998;22:323–335. doi: 10.1007/s002679900108. [DOI] [PubMed] [Google Scholar]
- Smith, B.D., R.K. Sinha, K. Zhou, A.A. Chaudhry, L. Renjun, D. Wang, B. Ahmed, A.K.M. Aminul Haque, K. Sapkota, and R.S.L. Mohan. 2000. Register of water development projects affecting Asian river cetaceans. In Biology and conservation of freshwater cetaceans in Asia, ed. R.R. Reeves, B.D. Smith, T. Kasuya, Vol. 23, 22–39 pp. Gland, Switzerland: Occasional Paper of the IUCN Species Survival Commission.
- Smith BD, Ahmed B, Ali ME, Braulik G. Status of the Ganges River dolphin or shushuk Platanista gangetica in Kaptai Lake and the southern rivers of Bangladesh. Oryx. 2001;35:61–72. doi: 10.1017/S0030605300031549. [DOI] [Google Scholar]
- Smith BD, Braulik G, Strindberg S, Ahmed B, Mansur R. Abundance of Irrawaddy dolphins (Orcaella brevirostris) and Ganges River dolphins (Platanista gangetica gangetica) estimated using concurrent counts made by independent teams in waterways of the Sundarbans mangrove forest in Bangladesh. Marine Mammal Science. 2006;22:527–547. doi: 10.1111/j.1748-7692.2006.00041.x. [DOI] [Google Scholar]
- Smith BD, Braulik G, Strindberg S, Mansur R, Diyan MAA, Ahmed B. Habitat selection of freshwater cetaceans and the potential effects of declining freshwater flows and sea-level rise in waterways of the Sundarbans mangrove forest, Bangladesh. Aquatic Conservation: Marine and Freshwater Ecosystems. 2009;19:209–225. doi: 10.1002/aqc.987. [DOI] [Google Scholar]
- Smith, B.D., M.A.A. Diyan, R.M. Mansur, E.F. Mansur, and B. Ahmed. 2010. Identification and channel characteristics of cetacean hotspots in waterways of the eastern Sundarbans mangrove forest, Bangladesh. Fauna and Flora International. Oryx 1–7. doi: 10.1017/S003060530990159.
- Soule ME, Estes JA, Miller B, Honnold DL. Strongly interacting species: conservation policy, management and ethics. BioScience. 2005;55:168–176. doi: 10.1641/0006-3568(2005)055[0168:SISCPM]2.0.CO;2. [DOI] [Google Scholar]
- Stazner B, Higler B. Stream hydraulics as a major determinant of benthic invertebrate zonation patterns. Freshwater Biology. 1986;16:127–139. doi: 10.1111/j.1365-2427.1986.tb00954.x. [DOI] [Google Scholar]
- Thorp JH, Thoms MC, Delong MD. The riverine ecosystem synthesis: Biocomplexity in river networks across space and time. River Research and Applications. 2006;22:123–147. doi: 10.1002/rra.901. [DOI] [Google Scholar]
- Turvey ST, Pitman RL, Taylor BL, Barlow J, Akamasu T, Barrett LA, Xiujiang Z, Reeves RR, Steward BS, Wang K, Zhou W, Zhang X, Pusser LT, Richlen M, Brandon J, Ding W. First human caused extinction of cetacean species? Biology Letters. 2007;3:537–540. doi: 10.1098/rsbl.2007.0292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vidal O, Barlow J, Hurtado LA, Torre J, Cendon P, Ozeda Z. Distribution and abundance of the Amazon River dolphin (Inia geoffrensis) and the tucuxi (Sotalia fluviatilis) in the Upper Amazon River. Marine Mammal Science. 1997;13:427–445. doi: 10.1111/j.1748-7692.1997.tb00650.x. [DOI] [Google Scholar]
- Vorosmarty CJ, Mcintyre PB, Gessner MO, Dudgeon D, Prusevich A, Green P, Glidden S, Bunn SE, Sullivan CA, Lierman CR. Global threats to human water security and river biodiversity. Nature. 2010;467:555–561. doi: 10.1038/nature09440. [DOI] [PubMed] [Google Scholar]
- Wakid A. Status and distribution of newly documented residential Gangetic Dolphin (Platanista gangetica gangetica) population in eastern Assam. The Journal of the Bombay Natural History Society. 2005;102:158–161. [Google Scholar]
- Wakid A. Status and distribution of the endangered Gangetic dolphin (Platanista gangetica gangetica) in the Brahmaputra River within India in 2005. Current Science. 2009;97:1143–1151. [Google Scholar]
- Worm B, Lotze HK, Myers RA. Predator diversity hotspots in the blue ocean. PNAS USA. 2003;1000:9884. doi: 10.1073/pnas.1333941100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WWF-Nepal. 2006. Conservation and management of river dolphins in Asia. Proceedings of the regional meeting on conservation and management of river dolphins. 26–27 May, Kathmandu, Nepal.
- Yeung LWY, Yamashita N, Taniyasu S, Lam KS, Sinha RK, Borole DV, Kannan K. A survey of perfluorinated compounds in surface water and biota including dolphins from the Ganges River and in other water bodies in India. Chemosphere. 2009;76:55–62. doi: 10.1016/j.chemosphere.2009.02.055. [DOI] [PubMed] [Google Scholar]
- Zhao X, Barlow J, Taylor BL, Pitman RL, Wang K, Wei Z, Stewart BS, Turvey ST, Akamatsu T, Reeves RR, Wang D. Abundance and conservation status of the Yangtze finless porpoise in the Yangtze River, China. Biological Conservation. 2008;141:3006–3018. doi: 10.1016/j.biocon.2008.09.005. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.