Abstract
Background
Non-surgical approaches to treatment of lateral epicondylitis are numerous. The aim of this systematic review is to examine randomized, controlled trials of these treatments.
Methods
Numerous databases were systematically searched from earliest records to February 2013. Search terms included “lateral epicondylitis,” “lateral elbow pain,” “tennis elbow,” “lateral epicondylalgia,” and “elbow tendinopathy” combined with “randomized controlled trial.” Two reviewers examined the literature for eligibility via article abstract and full text.
Results
Fifty-eight articles met eligibility criteria: (1) a target population of patients with symptoms of lateral epicondylitis; (2) evaluation of treatment of lateral epicondylitis with the following non-surgical techniques: corticosteroid injection, injection technique, iontophoresis, botulinum toxin A injection, prolotherapy, platelet-rich plasma or autologous blood injection, bracing, physical therapy, shockwave therapy, or laser therapy; and (3) a randomized controlled trial design. Lateral epicondylitis is a condition that is usually self-limited. There may be a short-term pain relief advantage found with the application of corticosteroids, but no demonstrable long-term pain relief. Injection of botulinum toxin A and prolotherapy are superior to placebo but not to corticosteroids, and botulinum toxin A is likely to produce concomitant extensor weakness. Platelet-rich plasma or autologous blood injections have been found to be both more and less effective than corticosteroid injections. Non-invasive treatment methods such as bracing, physical therapy, and extracorporeal shockwave therapy do not appear to provide definitive benefit regarding pain relief. Some studies of low-level laser therapy show superiority to placebo whereas others do not.
Conclusions
There are multiple randomized controlled trials for non-surgical management of lateral epicondylitis, but the existing literature does not provide conclusive evidence that there is one preferred method of non-surgical treatment for this condition. Lateral epicondylitis is a condition that is usually self-limited, resolving over a 12- to 18-month period without treatment.
Level of Evidence
Therapeutic Level II. See Instructions to Authors for a complete description of level of evidence.
Keywords: Elbow tendinopathy, Extensor tendinopathy, Lateral elbow pain, Lateral epicondylalgia, Lateral epicondylitis, Tennis elbow
Introduction
Lateral epicondylitis is a common source of lateral elbow pain. Population studies have shown a prevalence of 1.3 % among those between 30 and 64 years of age, peaking between 45 and 54. It typically affects the dominant upper extremity and is associated with repetitive and forceful activity [53]. Pain is often most pronounced with wrist extension.
Lateral epicondylitis is believed to be a degenerative process, which stems from repetitive microtrauma. Typically, samples from the affected tissue demonstrate angiofibroblastic hyperplasia at the extensor origin of the forearm [60]. Activities requiring repeated contraction of the wrist extensors are implicated, with the extensor carpi radialis brevis (ECRB) tendon most commonly involved. Studies comparing cadaveric and surgical specimens indicate that lateral epicondylitis evolves through several stages, beginning with degenerative angiogenesis and ending with fibrosis and calcification [38, 49, 60].
The majority of patients diagnosed with lateral epicondylitis can be effectively managed without surgery, as it is usually a self-limited process from which up to 90 % of patients will recover by 1 year without surgical intervention [5, 12, 60]. Non-surgical approaches to treatment are numerous. The aim of this review is to examine randomized controlled trials (RCTs) of the non-surgical treatment of lateral epicondylitis.
Materials and Methods
Study Identification
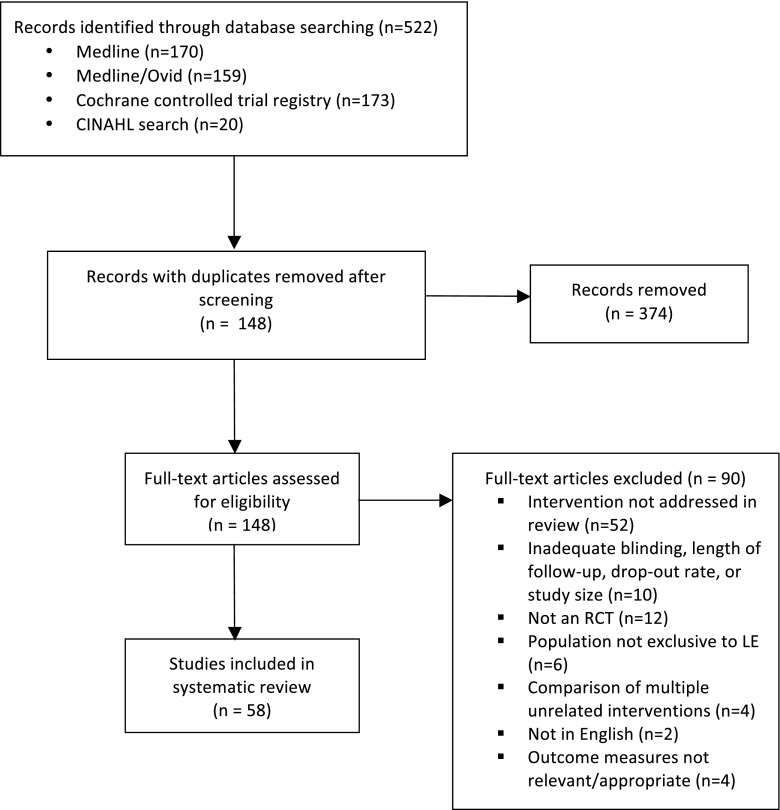
Articles from Medline/Ovid, Cochrane Database of Systematic Reviews, and CINAHL were systematically searched from earliest records to February 2013 (Fig. 1). Search terms included “lateral epicondylitis,” “lateral elbow pain,” “tennis elbow,” “lateral epicondylalgia,” and “elbow tendinopathy” combined with “randomized controlled trial.” The abstracts of the resulting articles were reviewed by two of the authors for the following eligibility criteria: (1) a target population of patients with symptoms of lateral epicondylitis; (2) evaluation of treatment of lateral epicondylitis with one of the following non-operative techniques: corticosteroid injection, injection technique, iontophoresis, botulinum toxin A injection, prolotherapy, platelet-rich plasma (PRP) or autologous blood (ABI) injection, bracing, physical therapy (PT), shockwave therapy, or laser therapy; and (3) a RCT design. Articles meeting the above criteria based on the abstract were considered for inclusion, and full text was retrieved. The quality of each article was assessed according to randomization, blinding, outcome measures, and proportion of patients lost to follow-up. Only articles representing level I or II evidence for a particular intervention were included. This represents double-blind RCTs or single-blind studies for interventions in which blinding was either impossible or had little potential effect on outcome measures. Loss to follow-up was limited to 20 %, with few exceptions, which are noted in the text. Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines were followed wherever applicable. A summary of all articles included in this review can be found in Table 1.
Fig. 1.
Flow diagram representing process of study identification. RCT randomized controlled trial, LE lateral epicondylitis
Table 1.
Characteristics of included studies
| Author, year | Participants | Experimental groups | Blinding | Outcome measures | Follow‐up periods | Lost to follow‐up | Results | Side effects (number of patients affected) | Conflict of interest statement and/or source of funding |
|---|---|---|---|---|---|---|---|---|---|
| Corticosteroid injection | |||||||||
| Hay, 1999 | 164 patients with new episode of LE | (1) Injection of 20 mg methylprednisolone plus lignocaine | Patient and doctors aware of treatment but assessor was blinded | Primary: participant global assessment of improvement (5-point scale) | 4 weeks, 6 months, 12 months; patients not restricted from receiving additional intervention | 1/164 | At 4 weeks, 92 % of injection group, 57 % naproxen group, and 50 % placebo group were completely better or improved; the difference between injection and other groups was statistically significant. Results at 12 months not statistically significant | Injection: local skin atrophy at the lateral epicondyle (3); naproxen: GI side effects (4), allergic reaction, edema (1) | Funded by Arthritis Research Campaign; methyprednisolone provided by UpJohn and naproxen by Syntex |
| (2) Naproxen 500 mg twice daily for 2 weeks | Secondary: pain, function, and main complaint on Likert scale | ||||||||
| (3) Placebo tablets | |||||||||
| Verhaar, 1996 | 106 patients with LE not previously treated with Cyriax physiotherapy | (1) Injection of 1 mL, 1 % triamcinolone with 1 mL, 1 % lidocaine +/− 2nd and 3rd injections at 2 and 4 weeks; injections given at extensor origin | Patient blinding not possible, no mention of assessor blinding | Primary: Verhaar criteria | 6 weeks, 52 weeks; patients not restricted from receiving additional interventions after 6 weeks | 3/106 patients not assessed at 6 weeks | At 6 weeks, 69 % success rate in injection group vs 27 % of physiotherapy group, this difference was statistically significant. No difference between groups at 52 weeks | None | No benefits received from a commercial party related directly or indirectly to subject of this article |
| (2) Cyriax physiotherapy, 12 treatments over 4 weeks | Secondary: grip strength | ||||||||
| Tonks, 2007 | 48 patients with LE not treated in prior 6 months | (1) Single injection 10 mg triamcinolone plus 2 % lignocaine | Patient blinding not possible, no mention of assessor blinding | Primary: pain free grip strength | 7 weeks | 11/48; 41 % drop-out rate from physiotherapy groups | Patients receiving injection statistically significantly better for all outcome measures, no significant effect of physiotherapy for any outcome measure, no significant interaction found between physiotherapy and injection | Injection: skin depigmentation and atrophy and injection site (1) | None reported |
| (2) Physiotherapy | Secondary: extensor strength, PRFEQ, complications of treatment | ||||||||
| (3) Injection plus physiotherapy | |||||||||
| (4) No treatment; injections given at point of maximum tenderness | |||||||||
| Smidt, 2002 | 185 patients with LE for at least 6 weeks, no physiotherapy or injections in prior 6 months | (1) Injection of 10 mg triamcinolone with 1 mL, 2 % lidocaine +/− 2nd and 3rd injection | Patient blinding not possible, research physiotherapist doing assessment blinded | Primary: general improvement 6‐point Likert scale; severity of main complaint, pain during the day, and inconvenience on 11‐point scale, modified pain‐free function questionnaire (PFFQ), researcher assessed overall severity | 3, 6, 12, 26, and 52 weeks | 2/178 | At 6 weeks, injection group significantly better in all outcome measures (92 % success vs 47 % for physiotherapy and 32 % for wait‐and‐see). At 52 weeks, injection group significantly worse than other intervention groups but no significant difference between physiotherapy and wait-and‐see | Injection: facial flush (2), skin irritation (3), red swollen elbow (2), skin color change over elbow (7) | No conflict of interest; funded by Health Insurance Council and Netherlands Organization for Scientific Research |
| (2) 9 physiotherapy treatments over 6 weeks | Secondary: PFG, MGS, pain-pressure threshold | Physiotherapy: skin irritation (3), red swollen elbow (3), skin color change over elbow (3) | |||||||
| (3) Wait-and‐see | |||||||||
| Bisset, 2006 | 198 patients with LE for at least 6 weeks, no treatment for 6 months | (1) Injection of 10 mg triamcinolone and 1 mL, 1 % lidocaine +/− 2nd injection at 2 weeks | Patient blinding not possible, assessor and study personnel blinded | Primary: global improvement on 6‐point Likert scale (completely recovered or much improved considered success), pain-free grip force, assessor's rating of severity, recurrence rates beyond 6 weeks | 3, 6, 12, 26, and 52 weeks | 8/198 | At 6 weeks, injection group had significantly better outcomes with 78 % success vs 27 % for wait‐and‐see and 65 % for physiotherapy. Injection group had significantly more recurrences than other treatment groups. At 52 weeks, physiotherapy had significantly better outcomes than injection but no significant difference in any primary outcome compared to wait-and‐see | Injection: loss of skin pigment (2), atrophy of subcutaneous tissue (1) | Funded by University of Queensland and the National Health and Medical Research Council, Primary Health Care Project Grant |
| (2) 8 physiotherapy treatments over 6 weeks | Secondary: VAS, PFFQ | ||||||||
| (3) Wait-and‐see | |||||||||
| Bisset, 2007 | Patient data extracted from 2 RCTs: Bisset (2006) and Smidt (2002) outlined above | Treatment groups of respective studies, analysis of the following subgroups performed: baseline pain on VAS (<60, >60), duration of symptoms (<14 weeks, >14 weeks), previous history of symptoms, employment status (manual, non-manual, no work) | See individual studies | Global improvement on 6-point Likert scale | Patient data from 6- to 52-week follow-up used | See individual studies | Subgroups of patients determined by baseline patient characteristics do have significantly different outcomes over time | See individual studies | See individual studies |
| Coombes, 2013 | 165 patients with unilateral LE for at least 6 weeks | (1) CSI of 10 mg/mL of triamcinolone acetonide in a 1 mL injection plus 1 mL of 1 % lignocaine | Assessors blinded, patients were blinded to injection type but not to PT | 6-point Likert scale, VAS, PRTEE, EuroQuol EQ‐5D, use of non-allocated treatments, adverse events | 4, 8, 12, 26, and 52 weeks | 2/165 patients lost to follow-up | 4 weeks: Treatment with CSI or PT alone showed improvement 26; 52 weeks: CSI with lower complete recovery or improvement, no difference in groups with or without physical therapy | Pain after CSI/PT, transient depigmentation or subcutaneous atrophy, hand numbness, vomiting, swelling, skin irritation | 2 authors received payment for lectures and a book chapter |
| (2) Placebo injection 0.5 mL of 0.9 % isotonic saline | |||||||||
| (3) CSI and 8 weekly PT sessions | |||||||||
| (4) Placebo and 8 weekly PT sessions | |||||||||
| Newcomer, 2001 | 39 patients with LE for less than 4 weeks and no prior treatment | (1) Injection of 6 mg betamethasone and 4 mL, 0.25 % bupivacaine | Subjects and physician performing injections were blinded to medication used | VAS, functional pain questionnaire, MGS | 4 and 8 weeks, telephone follow-up at 6 months (excluding grip strength) | 4/39 discontinued to pursue alternative treatment | Both groups improved in a statistically significant manner over time, there were no significant differences in outcome between groups with the exception of an improvement in VAS in the injection group from 8 weeks to 6 months | None reported | None reported |
| (2) Injection of 5 mL, 0.25 % bupivacaine; patients not allowed to undergo any other prescribed treatment during 6-month study period | |||||||||
| Lindenhovius, 2008 | 64 patients with LE for less than 6 months, no prior treatment with CSI or iontophoresis | (1) Injection of 4 mg dexamethasone and 1 mL, 1 % lidocaine | Patient and treating surgeon blinded to treatment | DASH, VAS, MGS | 1 and 6 months | 6 patients dropped from study, 6 patients missed 1-month follow‐up, 9 patients missed 6-month follow-up; included in analysis until dropout | Both groups improved over time, no statistically significant difference between groups in any outcome measure at 1 or 6 months | Slight discoloration of skin around injection site (2 placebo, 1 dexamethasone) | None reported |
| (2) Injection of 2 mL, 1 % lidocaine; patients free to pursue other forms of treatment during study | |||||||||
| Altay, 2002 | 120 patients with LE, no prior CSI | (1) Injection of 2 mL lidocaine | Patient and assessor blinded to injection type | Verhaar criteria | 2, 6, and 12 months | 0/120 | Both groups improved over time, no statistically significant difference between groups at 2 months, results were not changed at 6 and 12 months | No complications observed | None reported |
| (2) Injection of 1 mL lidocaine and 1 mL triamcinolone; peppering technique used for both groups, option for 2nd injection at 2 weeks | |||||||||
| Dogramaci, 2009 | 75 patients with LE, no prior CSI | (1) Injection of 1 mL triamcinolone and 1 mL lidocaine | Patient and assessor blinded to injection type | VAS, Verhaar criteria (excellent or good outcomes considered successful) | 3 weeks and 6 months | Not discussed | No statistically significant differences between groups at 3 weeks; at 6 months, successful outcomes statistically higher and VAS statistically lower in peppered CS injection group than other treatment groups | No complications observed | None reported |
| (2) Peppered injection of 1 mL lidocaine | |||||||||
| (3) Peppered injection of 1 mL triamcinolone and 1 mL lidocaine; all groups +/− 2nd injection at 3 weeks | |||||||||
| Okcu, 2012 | 80 patients with LE, no prior CSI | (1) Injection of 1 mL betamethasone and 1 mL prilocaine | Patient could not be blinded to peppering, assessor blinded to injection technique | Turkish DASH | 3, 6, 12, 18 months, and final follow-up (average 21.6 months) | 31/80, not included in analysis | A statistically significant difference in favor of the peppered injection group was found for DASH scores at 12 and 18 months and at final follow-up | None reported | Authors declare no conflict of interest |
| (2) Peppered injection of 1 mL betamethasone and 1 mL prilocaine; injections given at point of maximum tenderness | |||||||||
| Iontophoresis | |||||||||
| Demirtas, 1998 | 40 patients with LE | (1) Dermal iontophoresis of sodium diclofenac followed by infrared treatment | Not discussed | Pain with pressure to LE, with resisted wrist extension, during functional activity, and at rest | 18 days | None | Statistically significant improvement in both groups | None | None reported |
| (2) Dermal iontophoresis of sodium salicylate followed by infrared treatment | |||||||||
| Labelle, 1997 | 129 patients with LE, no steroid intake in prior 6 weeks, no NSAID use in prior 3 weeks | (1) Long-arm cast immobilization and daily slow release diclofenac 150 mg | Patients and assessor blinded to treatment | Primary: MPFGS | 14, 21, and 28 days | 1/129 | At 28 days, all outcomes improved for both groups in a statistically significant manner. The difference between groups was only statistically significant for VAS (pain) and MGS ratio, in favor of the NSAID group | Headache (4 placebo, 2 NSAID), abdominal pain (6 placebo, 19 NSAID), diarrhea (13 placebo, 25 NSAID) | Supported by grant form National Health and Welfare Canada. Ciba‐Geigy Canada provided both NSAID and placebo |
| (2) Long-arm cast immobilization and daily placebo; cast was removed at 14 days, medication given daily for 28 days; no other medication for LE allowed during study period | Secondary: MPFGS ratio of affected to normal side, MGS, MGS ratio, VAS (pain and function), pain-free function index | ||||||||
| Nirschl, 2003 | 199 patients with medial or lateral epicondylitis for less than 3 months, no CSI in prior year | (1) Dermal iontophoresis of dexamethasone | Patients and investigator blinded to treatment | Primary: investigator's global evaluation of improvement (−1, worsened, to 4, no symptoms), patient's global evaluation of improvement on same scale, VAS Secondary: patient's assessment of symptoms (0, none, to 4, very severe), investigator's tenderness evaluation on same scale | 2 days (in person) and 1 month (by phone) post-treatment | 9/199 | Dexamethasone produced a statistically significant improvement in VAS (23 vs 14 mm for placebo) and more scores of moderate or better on the investigator's global improvement scale (52 vs 33 %) at 2 days post-treatment, but outcomes were not significantly different between groups at 1 month | Dexamethasone: application site vesicles (2), blister (2), localized skin reaction (2), burning sensation (1), atopic dermatitis (1), erythema (1), hypersensitivity (1), pruritus (1), skin irritation (1) | Supported by a grant from Iomed, Inc, Salt Lake City, Utah, who also provided solution |
| (2) Dermal iontophoresis of sodium chloride; 6 treatments within 15 days, patients were not to begin other treatments until after 2 day follow-up | Placebo: pruritus (3), erythema (2), arthralgia (2), application site pain (1), application site pruritus (1), burning sensation (1), elbow pain (1) | ||||||||
| Stefanou, 2012 | 101 patients with LE less than 2 years, no steroid medication within 6 months | (1) Self-contained iontophoresis patch in place for 2 days with 10 mg dexamethasone in reservoir, +/− second patch replaced 2–10 days after removal of first | Patients could not be blinded to treatment, physician who determined work status not blinded to treatment | Primary: grip strength, PRTEE | Completion of 8-week protocol, 6 months after completion of 8-week protocol | 19/101 left the study due to non-compliance, treatment failure, or loss to follow-up and were excluded from analysis | Iontophoresis group had statistically significant increase in grip strength at both follow-ups while the other 2 groups only achieved this by 6 months; all 3 groups had statistically significant improvement in PRTEE (pain and function) at 6-month follow-up; improvement in work status at the first follow-up was only statistically significantly for the iontophoresis group | Injection: post-injection pain (<10 % patients) | None reported |
| (2) Injection of 10 mg dexamethasone | Secondary: work status (no restrictions, work with restrictions, no work | Iontophoresis: skin irritation (1) | |||||||
| (3) Injection of 10 mg triamcinolone; treatment for all groups included an 8-week protocol of rest, mobility, and strengthening | |||||||||
| Botulinum toxin | |||||||||
| Hayton, 2005 | 40 patients with LE for more than 6 months; all patients received at least 1 CSI and full course of PT | (1) Injection of 50 U of botulinum toxin type A in 2 mL normal saline | Patient, physician administering injection, and assessor blinded to injection type | MGS, VAS, QOL measured with SF-12 health status questionnaire | 3 months | 2 patients had an operation (1 from each experimental group), 1 patient lost to follow-up | At 3 months, there were no significant differences between treatment groups for any outcome measure | Transient extensor lag (12/18 patients receiving botulinum toxin injection) | Authors did not receive benefits in support of their research or preparation of this manuscript. 1 or more authors received benefits from a commercial entity (botulinum toxin supplied by Allergan) |
| (2) Injection of 2 mL normal saline; injections administered 5 cm distal to area of maximal tenderness in line with the middle of the wrist | |||||||||
| Wong, 2005 | 60 patients with LE for more than 3 months; no prior injection | (1) Injection of 60 U of Dysport botulinum toxin | Patient and physician blinded to injection type | Primary: VAS | 4 and 12 weeks | No loss to follow-up | At 4 weeks, the botulinum group showed a statistically significant improvement in VAS over the placebo group, which was maintained at 12 weeks; no significant difference in grip strength was found between groups at any time | Botulinum: post-injection pain (2), weakness in finger extension (10), paresis of digits (4) | Funded by a donation from New World Development Ltd. Sponsor had no part in design of study, data collection or interpretation, or final manuscript preparation |
| (2) Injection of an equivalent volume of normal saline; injections administered 1 cm from the lateral epicondyle and were aimed toward the tender spot | Secondary: MGS | Placebo: post-injection pain (1), post-injection nausea (1), weakness in finger extension (6) | |||||||
| Placzek, 2007 | 132 patients with LE for more than 4 months, 3 or more conservative therapies tried without success | (1) Injection of 60 U botulinum toxin A in 0.6 mL normal saline | Patient and assessor blinded to injection type | Clinical pain score, VAS (continuous pain past 48 h, max pain past 48 h), MGS, global assessment of improvement by patient and physician (4, substantially better, to 0, substantially worse) | 2, 6, 12, and 18 weeks | 2/132 | Improvement was more statistically significant for the botulinum group than the placebo group in the clinical pain score at every follow‐up and in VAS for continuous pain and global assessments of improvement from the 6th week onward | Strength of extension of the third finger was decreased significantly in the botulinum group at 2 weeks, this weakness resolved by week 18 | 1 or more authors received outside funding or grants in excess of $10,000 from Ipsen Pharma in support of their research for or preparation of this work |
| (2) Injection of 0.6 mL normal saline; injections administered 3–4 cm distal to tender epicondyle | |||||||||
| Espandar, 2010 | 48 patients with LE for at least 6 months and a previous course of PT and/or CSI | (1) Injection of 60 U botulinum toxin A in 1 mL of normal saline | Patient and physician blinded to injection type | Primary: VAS | 4, 8, and 16 weeks | No loss to follow‐up | Intensity of pain at rest and during max pinch decreased more in the botulinum group than the placebo group, this difference was statistically significant | Botulinum: tingling sensation around injection site (5) subjective feeling of muscle spasm around injection site (8), pain at injection site (7) | Study funded by Vice Chancellor of Research at Tehran University of Medical Sciences. No competing interest declared |
| (2) Injection of 1 mL of normal saline; injection given at 1/3 the length of the forearm from the tip of the lateral epicondyle on the course of the posterior interosseus nerve | Secondary: pain during max grip and max pinch, grip strength | Placebo: pain at injection site (2) | |||||||
| Lin, 2010 | 16 patients (19 elbows), no CSI in prior 3 months | (1) Injection of 50 U botulinum toxin type A in 1 mL normal saline | Patient, and assessor blinded to injection type | VAS pain, pain‐free grip strength, WHO QOL‐BREF questionnaire to assess quality of life | 4, 8, and 12 weeks | 1 patient did not receive post‐treatment evaluations, excluded from analysis | At 4 weeks, both groups reported decreased pain with a significantly greater reduction reported by the steroid group, this pain remained reduced at 8 and 12 weeks but difference between groups were not significantly significant. No statistically significant differences in QOL were observed between groups at any time | All patients in botulinum toxin A group experienced mild weakness in wrist and middle digit extension that resolved by 12 weeks | Study funded by Council of Labor Affairs of Taiwan government. Allergan Inc. provided Botox. Authors report no conflict of interest |
| (2) Injection of 1 mL, 40 mg triamcinolone; injections into the ECRB muscle near common extensor origin | |||||||||
| Prolotherapy | |||||||||
| Scarpone, 2008 | 24 patients with LE for at least 6 months and failure of conservative treatments, no CSI in prior 6 weeks | (1) 0.5 mL prolotherapy injections (50 % dextrose, 5 % sodium morrhuate, 4 % lidocaine, 0.5 % sensorcaine) | Patient and physician blinded to injection type | Primary: resting elbow pain on 11‐point Likert scale | 8 and 16 weeks, telephone interview at 52 weeks | 4/24 patients dropped out of study, excluded from analysis | The prolotherapy group, but not the control group, showed a statistically significant improvement in pain scores at 8 and 16 weeks. Isometric strength for the prolotherapy group improved significantly at 8 and 16 weeks, the difference between groups were statistically significant at 16 weeks | Prolotherapy: local erythema, irritation, and discomfort 1 day after injection (2), all patients experienced self‐limited post‐injection pain | Authors have no financial interest in the products mentioned in this article |
| (2) Injections of 0.5 mL normal saline; 3 injections administered into supracondylar ridge, lateral epicondyle, and annular ligament for total of 1.5 mL at baseline, 4, and 8 weeks | Secondary: resting grip strength, isometric resistance strength | ||||||||
| Carayannopoulos, 2001 | 24 patients with LE from 3 months to 2 years, no CSI in prior 6 months | (1) Injection of 1 mL procaine, 0.9 mL of P2G (phenol 1.2 %, glycerine 12.5 %, dextrose 12.5 % in sterile water), and 0.1 mL sodium morrhuate | Patient and physician blinded to injection type | Primary: VAS | 1 and 3 months, telephone follow‐up at 6 months | 7/24, lost to follow‐up, excluded from analysis | The prolotherapy group experienced a statistically significant improvement in VAS, QVAS, and DASH at 3 and 6 months, while the steroid group only saw this improvement in QVAS at 6 months and in DASH at 3 and 6 months. No significant differences between groups were observed at any time | 1 patient from prolotherapy group withdrew from study because of excessive pain, no other adverse reaction reported by patients | Peer reviewers and all others who control content have no relevant financial relationships to disclose |
| (2) Injection of 1 mL procaine and 1 mL methylprednisolone; above solutions split between 3 injection sites: annular ligament, origin of common extensor tendon, radial collateral ligament at tubercle of radius; repeat injections at 1 month | Secondary: quadruple VAS (QVAS), DASH, grip strength, pain free grip strength | ||||||||
| Zeisig, 2008 | 32 patients (36 elbows) with LE for more than 3 months, no interventions in prior 3 months | (1) Injection of 0.5 mL polidocanol | Patient and physician blinded to injection type | Primary: patient satisfaction with treatment (yes/no), VAS (elbow pain during grip activities of daily life) | 3 months; patients in group 2 offered injection of polidocanol after initial 3-month follow-up, all patients followed up 12 months after 1st injection | 2/36 withdrew from trial before first follow‐up, not included in analysis | There were no statistically significant differences between treatment groups for any outcome measures at any time. Patients in both groups had statistically significant decreases in VAS at both follow‐ups | Patients in group 2 noted increased elbow pain and stiffness during first week after injection, no other adverse effects | Authors declare no conflict of interest |
| (2) Injection of 0.5 mL lidocaine plus epinephrine; injections delivered to target vessels inside extensor origin under ultrasound guidance | Secondary: MGS | ||||||||
| Platelet-rich plasma/autologous blood injection | |||||||||
| Peerbooms, 2010 | 100 patients with LE for more than 6 months, failed conservative therapy | (1) PRP injection (PRP collected with Recover system by Biomet Biologics) | Patient and assessor blinded to injection type | VAS, DASH; successful treatment defined as more than 25 % reduction in VAS or DASH without a reintervention at 1 year | 4, 8, 12, 26, and 52 weeks | 8/100 lost to follow-up, included in analysis with last data point carried forward | Success rates were 49 and 73 % according to VAS and 51 and 73 % according to DASH for the steroid group and PRP group respectively. These differences were statistically significant in favor of the PRP group | Apart from local inflammation causing increased pain 3–4 weeks after PRP injection, no systemic or other local reactions were seen | Sponsored by Biomet, the funding source had no involvement in study design; collection, analysis, and interpretation of data; writing of report; or decision to submit for publication |
| (2) Injection of triamcinolone 40 mg/mL with epinephrine; for both groups 1 mL was injected directly into area of maximum tenderness, remaining solution was injected into common extensor tendon using peppering technique; use of NSAIDs prohibited | |||||||||
| Gosens, 2011 | Continuation of Peerbooms (2010) | According to Peerbooms (2010) | Patient and assessor blinded to injection type | VAS, DASH; successful treatment defined as more than 25 % reduction in VAS or DASH without a reintervention at 2 years | 2 years | 8/100 lost to follow-up, included in analysis with last data point carried forward | Differences in success rates according to VAS and DASH were statistically significant in favor of the PRP group. Compared with baseline VAS and DASH scores, a number of patients deteriorated at 2 years, with more patients being from the steroid group. This difference was statistically significant for both VAS and DASH scores | Apart from local inflammation causing increased pain 3–4 weeks after PRP injection, no systemic or other local reactions were seen | Biomet supplied the Recover system used at a discounted rate but did not have any influence on the collection and analysis of data |
| Kazemi, 2010 | 60 patients with a new episode of LE, no CSI in prior 3 months | (1) Injection of AB with 2 % lidocaine | Patients not blinded because blood taken for ABI but assessors blinded to injection type | Primary: VAS (within last 24 h) | 4 and 8 weeks | No loss to follow‐up | Differences in outcome measures between groups was statistically significant in favor of ABI for VAS, pain in grip, pressure pain threshold, and Quick DASH (not significant for other outcomes) at 4 weeks and in all outcome measures at 8 weeks | There were no noticeable or reported side effects of treatment in either group | Authors report no conflict of interest |
| (2) Injection of 20 mg methylprednisolone with 2 % lidocaine; injections given proximal to lateral epicondyle; no other therapy allowed for duration of study | Secondary: pain‐free function questionnaire, pain with max grip, quick DASH, modified Nirschl score, MGS, pressure pain threshold | ||||||||
| Krogh, 2013 | 60 patients with LE for at least 3 months, tendinopathy on US | (1) Injection of 3–3.5 mL PRP (prepared with Biomet GPS II); injections delivered deep to extensor origin with a peppering technique | Assessors and patients blinded | PRTEE | 4, 12, 26, and 52 weeks | 0/60 at 3 months, 34/60 at 6 months, 44/60 at 12 months | CSI superior at 1 month, PRP or CSI not superior to placebo at 3 months with regard to pain reduction. CSI reduces tendon thickness on US more than PRP or placebo | Rash, post-injection pain, skin atrophy, loss of pigmentation | None reported |
| (2) CSI of 1 mL triamcinolon 40 mg/mL and 2 mL lidocaine 10 mg/mL | |||||||||
| (3) Placebo injection 3 mL | |||||||||
| Wolf, 2011 | 34 patients with LE, no CSI in prior 6 months | (1) Injection of saline with lidocaine | Patient but not physician giving injection blinded to injection type | DASH, PRFEQ, VAS | 2 weeks, 2 and 6 months | 6/34 patients lost to follow‐up, not included in analysis | There were no statistically significant differences between groups for any outcome measure at any time | None reported | Funded by Clinical Research Grant from the American Society for Surgery of the Hand. No benefits related to the article received |
| (2) Injection of CS with lidocaine | |||||||||
| (3) Injection of AB with lidocaine; injections given with multiple passes of needle in fanlike fashion under the extensor origin | |||||||||
| Creaney, 2011 | 150 patients with LE for at least 6 months failing conservative measures, no previous injection | (1) Injection of PRP (isolated from AB spun in a centrifuge with siphoning of buffy coat layer) | Patient and assessor but not investigator blinded to injection type | PRTEE; success defined as an improvement in PRTEE of 25 points at final analysis | 1, 3, and 6 months | 20/150 lost to follow-up, not included in analysis | At 6 months, both groups experienced a reduction in PRTEE pain scores. The difference in success rate between the 2 groups was not statistically significant. 12/17 patients in the AB group compared to 7/24 patients in the PRP group elected to proceed to surgery | None reported | No competing interests |
| (2) Injection of AB; tendons first bathed with bupivacaine before PRP/AB ultrasound guided injection into extensor origin under minimal pressure, second injection given at 1 month | |||||||||
| Thanasas, 2011 | 28 patients with LE for at least 3 months | (1) Injection of 3 mL AB | Assessors blinded, patients aware of treatment as blood was drawn from ABI group | VAS, Liverpool elbow score | 6 weeks, 3 and 6 months | 1/28 patients lost to follow‐up | Improvement in VAS was larger in PRP group at every follow‐up but only the difference at 6 weeks was statistically significant. No significant difference in Liverpool elbow score was noted at any time | With the exception of local pain and discomfort post-injection, no other complications noted | Authors report no conflict of interest |
| (2) Injection of 3 mL PRP (prepared with Biomet GPS III); injections delivered deep to extensor origin with a peppering technique | |||||||||
| Bracing | |||||||||
| Van De Streek, 2004 | 43 patients with LE for at least 3 weeks, no CSI in prior month | (1) Thamert Epi‐med elbow band | Patient blinding not possible, further blinding not discussed | MGS with pain score (1–10), PRFEQ | 6 weeks | 3 patients dropped out of study | All outcome measures improved in a statistically significant manner for both groups, there were no statistically significant differences between groups | None | None reported |
| (2) Forearm/hand splint (combination of Thamert Epi-med band, orthoflex brace, and aluminum bar from elbow to palm with 30° dorsiflexion at wrist; both groups instructed to wear as much as possible for 6 weeks during all daily activities | |||||||||
| Garg, 2010 | 70 patients (74 elbows) with LE, no treatments in prior 6 months | (1) Hely & Weber Velcro wrist extension splint | Patient blinding not possible, further blinding not discussed | ASES Elbow Assessment Form derived score, MEP | 6 weeks | 25 patients lost to follow‐up, 3 did not complete outcome measurement forms; 42 patients (44 elbows) included in final analysis | The improvement in the pain component of ASES score was statistically more significant for the wrist extension group at 6 weeks, both groups improved over time but there were no other significant differences in outcome measures | None | No benefits from any commercial entity related to the subject of this article received |
| (2) Hely & Weber forearm counterforce strap brace; patients instructed to wear brace during all daytime hours for 6 weeks and given standard home icing and stretching exercise | |||||||||
| Altan, 2008 | 50 patients with LE for less than 12 weeks, no prior treatment with CSI or physical therapy during that period | (1) Lateral epicondyle bandage | Patient blinding not possible, researcher unaware of brace type | VAS pain during rest and with movement, pain with pressure (0–3), grip strength, response to treatment (1, bad, to 4, excellent) | 2 and 6 weeks | 1/50 | Both groups improved in a statistically significant manner over time in all parameters, improvement in resting pain was statistically more significant in the wrist extension splint group at 2 weeks compared to, there was no difference for other parameters between groups at either evaluation | None | This study was not funded by any institution and there are no conflicts of interest |
| (2) Wrist extension splint; patients instructed to wear braces continuously for 6 weeks, removal for no more than 1 h at a time | |||||||||
| Struijs, 2004 | 180 patients with LE for at least 6 weeks, no treatment in prior 6 months | (1) 9 physiotherapy (PT) treatments over 6 weeks (pulsed ultrasound, friction massage, strengthening exercises) | Patient blinding not possible, assessors blinded | Primary: global improvement (1, completely recovered, to 6, much worse), severity of patient's complaints (11‐point scale), pain intensity (11‐point scale), modified PFFQ | 6 weeks, 1 year | 10/180, included in analysis | No significant differences between groups at 1 year. At 6 weeks, statistically significant benefits of PT over brace were found for pain, disability, and satisfaction. Brace superior only for inconvenience during daily activities. No significant benefit to combination therapy compared to PT, but compared to brace, severity of complaints and satisfaction were statistically significant in favor of combination | None | None reported |
| (2) Epipoint elbow band worn during the day for 6 weeks | Secondary: inconvenience during daily activities (11‐point scale), pain‐free grip strength, MGS, pressure pain threshold, satisfaction of patient (11‐point scale) | ||||||||
| (3) Combination of above 2 treatments | |||||||||
| Luginbuhl, 2008 | 35 patients (36 elbows) with LE, no prior treatment with forearm band or strengthening, no more than 3 CSI in prior 6 months | (1) Forearm support band worn during the day for at least 3 months | Patient blinding not possible, no mention assessor blinding | ROM, MGS, Nirschl–Pettrone score | 6 weeks, 3 months, and 1 year | 6/36 elbows lost to follow‐up, not included in analysis; 16 of remaining patients did not have follow‐up at 1 year but were included in other time points | All groups had a statistically significant improvement in Nirschl–Pettrone score at 6 weeks and latest follow-up, no significant differences between groups were found at any time point | None reported | None reported |
| (2) Strengthening exercises for 3 months | |||||||||
| (3) Combination of above 2 treatments; all patients initially treated with injection of 2–3 mL Scandicain and 40 mg triamcinolone | |||||||||
| Physical therapy | |||||||||
| Peterson, 2011 | 81 patients with LE for more than 3 months | (1) Daily exercise program for 3 months | Patient blinding not possible, data collection not blinded | Primary: VAS (during MVC, MME) | 1, 2, and 3 months | 2/81, included in analysis | The exercise group had greater and faster regression of pain during MVC and MME than wait‐and‐see, this difference was statistically significant | Not discussed | Funding sources had no involvement in study. Authors declare no conflict of interest |
| (2) Wait‐and‐see | Secondary: extensor strength, DASH, Gothenburg QOL Instrument | ||||||||
| Park, 2009 | 31 patients with LE for at least 6 weeks, no CSI in prior 3 months | (1) Immediate physical therapy | Patient blinding not possible | VAS, modified Nirschl–Pettrone score, MEPS | 1, 3, 6, and 12 months | 5/31 | At 1 month, the difference between groups for the modified Nirschl–Pettrone score and VAS was statistically significant in favor of the immediate therapy group. No other significant differences were found during the study period | Not discussed | Authors declare no conflict of interest |
| (2) Physical therapy started after 4 weeks oral NSAIDS | |||||||||
| Viswas, 2012 | 20 patients with LE for 8–10 weeks, no previous therapy | (1) Supervised exercise program | Patient blinding not possible, assessor blinded | VAS, TEFS | 4 weeks | No loss to follow‐up | Both groups improved over time, but the group undergoing the supervised exercise program improved more in both outcomes than the Cyriax group, this difference was statistically significant | Not discussed | Authors declare no conflict of interest |
| (2) Cyriax Physiotherapy; 3 sessions per week for 4 weeks | |||||||||
| Tyler, 2010 | 21 patients with LE for more than 6 weeks, no CSI in prior 6 weeks | (1) Standard physical therapy | Patient blinding not possible, assessor blinded | DASH, VAS, wrist and middle finger extension strength, pain with pressure | At completion of treatment | No loss to follow‐up | Improvements in all outcomes were greater for the eccentric group, this difference was statistically significant | None | Hygenic Corporation donated flexbars to study. Authors did not receive benefits from any commercial entity related to the article |
| (2) Standard physical therapy plus isolated eccentric wrist extensor strengthening (Thera‐Band FlexBar); treatments continued until symptoms resolved or patients were referred back to their physician with continued symptoms | |||||||||
| Martinez, 2005 | 94 patients with LE for more than 3 months | (1) Stretching | Patient blinding not possible | PFG, PRFEQ, DASH, VAS, pain and function assessment with SF‐36 patient's subjective assessment of results | 6 weeks | 13/94, included in intent‐to‐treat analysis | At 6 weeks, all groups improved in a statistically significant manner for PFG, VAS, DASH, and SF‐36. No significant difference was found between groups | Not discussed | Not reported |
| (2) Stretching plus concentric strengthening program | |||||||||
| (3) Stretching plus eccentric strengthening program | |||||||||
| Extra‐corporeal shock wave therapy | |||||||||
| Rompe, 1996 | 115 patients with LE for more than 12 months, unsuccessful conservative therapy in prior 6 months | (1) ESWT 1000 pulses per treatment | Patient and assessor blinded to treatment allocation | VAS, MGS, patient assessed overall outcome (1, excellent, to 4, poor) | 3, 6, and 24 weeks after last treatment | 15 patients discontinued treatment during first 6 weeks, not included in analysis | In the active treatment group there was a statistically significant decrease in VAS compared to placebo group at each follow‐up. There were 24 good or excellent results in the active group compared with only 3 in the placebo group | No complications observed | No benefits received from a commercial party related to the subject of this article |
| (2) Subtherapeutic ESWT; 3 treatments at weekly intervals; no other therapy allowed during course of treatment | |||||||||
| Rompe, 2004 | 78 recreational tennis players with LE for at least 1 year unresponsive to conservative treatments including CSI, no therapy in prior 2 months | (1) ESWT 2,000 pulses per treatment | Patient and assessor blinded to treatment allocation, but 29 of 38 active treatment patients guessed allocation correctly | Primary: VAS during resisted wrist extension | 3 and 12 months after last treatment | 4 patients withdrew to pursue additional therapy, 2 patients refused follow‐up, 2 patients only had phone follow‐up; included in intention to treat analysis | At 3 months, the difference in results between treatment groups was statistically significant for all outcome measures, in favor of the active treatment group. This significant difference was not maintained at 12 months | Temporary reddening after treatment and pain during treatment were common in both groups. 8 patients from the active group and 1 patient from placebo group experienced nausea during treatment | No author or related institution received financial benefit from research study |
| (2) Placebo ESWT with shock waves deflected; 3 treatments at weekly intervals; no other therapy allowed during 3 month follow‐up | Secondary: 50 % reduction in primary outcome measure, Roles and Maudsley score (1, excellent, to 4, poor), Upper Extremity Function Scale, grip strength, overall patient satisfaction | ||||||||
| Pettrone, 2005 | 114 patients with LE for at least 6 months unresponsive to 2 conventional therapy; no CSI in prior 6 weeks, PT in prior 4 weeks, or NSAIDS in prior week | (1) ESWT 2000 pulses per treatment | Patients and evaluating physicians blinded to treatment allocation | Primary: 50 % reduction of VAS during resisted wrist extension | 1, 4, 8, 12 weeks and 6 and 12 months after last treatment; patients not responding to treatment could be unblinded and placebo patients offered active treatment | 6 patients discontinued study prior to 12 weeks, 34 crossover patients considered lost to placebo group, 13 patients discontinued study prior to 12 months; patients included in intention to treat analysis | At 12 weeks, a statistically significant difference between groups was observed for reduction in pain, improvement in upper extremity function, and overall impression of disease state in favor of the active treatment group. Most of the placebo group was lost to crossover at 12 weeks and not available for comparison past this point | Active ESWT: transient treatment‐related pain (28), nausea during treatment (10) | 1 or more authors received outside funding from Siemens Medical in support of their research |
| (2) Placebo ESWT with shock waves deflected; 3 treatments at weekly intervals targeting point of max tenderness | Secondary: Upper Extremity Functional Scale, grip strength, patient overall impression of disease state | Placebo ESWT: transient treatment‐related pain (13) | |||||||
| Haake, 2002 | 272 patients with LE, at least 6 months unsuccessful conservative therapy and no treatment prior 2 weeks | (1) ESWT 2,000 pulses per treatment | Patients and assessor blinded to treatment allocation | Primary: success rate after 12 weeks (defined as score of 1 or 2 on Roles and Maudsley score and no additional treatment during study period) | 6 and 12 weeks after last treatment | 26/272, included in intention to treat analysis | There were no significant differences between groups in any outcome measure at any time | No severe adverse events noted. Most common side effects were reddening of skin (42 ESWT, 11 placebo) and pain (15 ESWT, 6 placebo) | Dornier Medizintechnik provided shock wave equipment. Benefits were directed to an institution with which 1 or more authors are associated |
| (2) Placebo ESWT with shock waves deflected; 3 treatments received, injection of 3 mL 1 % mepivacaine administered before each treatment | Secondary: Roles and Maudsley score, VAS, MGS | ||||||||
| Speed, 2002 | 75 patients with LE for at least 3 months, no treatment in prior 6 weeks | (1) ESWT 1,500 pulses per treatment with Sonocur Plus Unit (Siemens) | Patients and assessor blinded to treatment allocation | VAS | 1 month after last treatment | 4 patients withdrew from study | Both groups had statistically significant improvement over time, no significant differences existed between groups at any time | 2 patients in active treatment group withdrew due to worsening symptoms, no other adverse effects reported | Funded by Cambridge Arthritis Research Endeavour |
| (2) Placebo ESWT, subtherapeutic doses without skin contact; 3 treatments at monthly intervals directed to site of maximum pain; no other treatments permitted during study | |||||||||
| Melikyan, 2003 | 86 patients with LE who had received extensive conservative treatment and were awaiting surgery | (1) ESWT starting at a low energy and gradually increased, total of 1,000 mJ/mm2 energy delivered | Patient and assessor blinded to treatment allocation | DASH, MGS, analgesic requirement | 1, 3, and 12 months after last treatment | 12/86, not included in analysis | None of the outcome measures showed a statistically significant difference between groups at any time. All patients improved significantly over time | None reported | Supported by BUPA research fund. Benefits received from a commercial party related article are directed to a research fund or other non‐profit institution with which 1 or more author is associated |
| (2) Placebo ESWT with shock waves deflected; 3 treatments targeting common extensor tendon under ultrasound guidance | |||||||||
| Chung, 2005 | 60 patients with untreated LE for between 3 weeks and 1 year | (1) ESWT 2,000 pulses, 0.03–0.17 mJ/mm2 energy delivered | Patient and assessor blinded to treatment allocation | VAS (overall pain, resting pain, pain during sleep, pain during activity, pain at its worst, pain at its least), QOL on EuroQol 5D instrument (EQ5D), max PFGS, pain medication used (success defined as 50 % reduction in overall elbow pain, max overall elbow pain of 4 cm, and no use of pain medication for 2 weeks prior to 8‐week evaluation) | 4 and 8 weeks after last treatment | 1 patient dropped out of study, 3 were lost to follow‐up, included in intention to treat analysis | At 8 weeks, there was no statistically significant difference in success rate between treatment groups | No severe or moderate adverse events were observed | Funded by Sport Science Association of Alberta and Alberta Provincial Canadian Institutes of Health. No author received financial benefit from research in this study |
| (2) Placebo ESWT with shock waves deflected; 3 treatments at weekly intervals targeting point of maximum pain, all patients provided a stretching protocol | |||||||||
| Staples, 2008 | 68 patients with LE for at least 6 weeks, no oral/topical NSAID in prior 2 weeks, CSI in prior month, or oral CS in prior 6 weeks | (1) ESWT 240 pulses/min, 2,000 pulses per treatment | Patient and assessor blinded to treatment allocation | VAS (pain, function), discomfort in daily activities, DASH, QOL using Medical Outcome Study SF-36, max PFG, Problem Elicitation Technique | 6 weeks, 3 months, and 6 months after completion of 3-week treatment | 2 patients withdrew after treatment, 3 patients did not attend 6‐week follow-up, 3, did not attend 3‐month follow-up, and 5 did not attend 6‐month follow-up; all patients included in intention to treat analysis | There were no significant differences between groups in any outcome measures at 6‐week and 6‐month follow‐up visits. At 3 months, there was a statistically significant difference between groups in favor of the active group for DASH (work and sport), but neither section was completed by all patients | Active ESWT: bruising after 1st treatment (1), pain/tenderness in arm (4), burning sensation (1) Placebo ESWT: pain and/or lump in treated area (2) | Supported by Australian National Health and Medical Research Council Practitioner Fellowship. |
| (2) Subtherapeutic ESWT; 3 treatments at weekly intervals targeting area of maximal tenderness over common extensor tendon; no other forms of therapy including NSAIDS allowed during first 6 weeks of study | |||||||||
| Laser | |||||||||
| Lundeberg, 1987 | 57 patients with LE for at least 3 months, no CSI in prior 6 months | (1) Placebo laser | Patients, therapists, and assessors blinded to laser type | VAS, pain and diminished power with resisted wrist dorsiflexion (0, no pain, to 3, severe pain and absent power), wrist extension strength, grip strength, patient and physician assessment of treatment (satisfactory/unsatisfactory) | Every other week during treatment and at least 3 months after treatment | Not discussed | The 3 treatment groups showed no significant difference in any outcome measure at any time | None reported | Work supported by grants from The Royal Swedish Academy of Sciences and Stiftelsen Clas Groschinskys minnesfond |
| (2) Ga–As pulse laser, 904 nm wavelength | |||||||||
| (3) He‐Ne continuous‐wave laser, 632.8 nm wavelength; 2 treatments per week given over 5 to 6 weeks with laser stimulating 10 acupuncture points | |||||||||
| Haker, 1990 | 49 patients with LE for at least 1 months | (1) Ga–As Mid 1500 IRRADIA laser, wavelength 904 nm | Patients, therapist, and assessors blinded to laser type | Subjective physician assessment (1, excellent, to 5, unchanged or worse), max pain free grip | After last treatment and 3 months post‐treatment, 1 year follow-up with questionnaire | 2 patients withdrew after treatment, 7 additional patients withdrew after 3 months follow‐up | At 3 months, there were no significant differences between treatment groups for any outcome measure | None reported | Work supported by grants from Foundations of Karolinska Institutet |
| (2) Placebo laser; 10 treatments, 2–3 times weekly targeting the extensor group of muscles | |||||||||
| Krasheninnikoff, 1994 | 48 patients with LE for at least 1 month, no CSI in prior 3 weeks or other simultaneous treatment | (1) Active Ga–Al–As laser (30 mW/830 nm) | Patients, therapists, and assessors blinded to laser type | Duration of pain, pain relief, symptoms, patients subjective impression of effect | Telephone interview at 10 weeks post‐treatment | 12/48; excluded from analysis | At 10 weeks, there were no significant differences between treatment groups for any outcome measure | None reported | None reported |
| (2) Placebo laser; 8 treatments 2 times per week targeting tender points on lateral epicondyle and in forearm extensors | |||||||||
| Papadopoulos, 1996 | 29 patients (31 elbows) with LE | (1) Ga–Al–As laser (50 mW/820 nm) | Patients, operator, and assessor blinded to treatment | VAS, pain threshold with exercising forearm extensors | 1st, 4th, and 6th treatment visit | No loss to follow‐up | There were no statistically significant differences between groups for any outcome measure at any time | None reported | Loan of equipment by Omega Technologies |
| (2) Placebo laser; 3 treatments per week for 2 weeks targeting most tender point | |||||||||
| Vasseljen, 1992 | 30 patients with LE, no other treatments in prior 3 weeks | (1) Ga–As laser (18 mW/904 nm) | Patients, operator, and assessor blinded to treatment | MGS, extension weight test, point of pain with wrist flexion, VAS, patient subjective assessment of improvement | Post‐treatment and 4 weeks after last treatment | 1 patient had alternative treatment during study, dropped and was replaced by another patient | Statistically significant improvement in laser compared to placebo was found for VAS and MGS 4 weeks after treatment | Not discussed | Supported by grant from Norwegian Fund for Post‐Graduate Education in Physiotherapy. Schreuder Instrumenten supplied lasers |
| (2) Placebo laser; no other treatments allowed during study; 8 treatments were given at a frequency of 3 times per week | |||||||||
| Basford, 2000 | 47 patients with LE for more than 30 days, no CS administered for any reason in prior 30 days | (1) Nd:YAG CW 1.06 μm continuous wave laser (204 mW/cm2) | Patient, therapists, and physician blinded to laser type | Primary: VAS for max pain in last 24 h, max tenderness on palpation, and patient perception of change | 1st, 6th, and 12th treatment visit and 1 months post‐treatment | 1 patient did not return for 1 months follow‐up | There were no statistically significant differences between groups for any outcome measure at any time | No significant treatment side effects were noted | Authors have not received a benefit from any commercial party having a direct financial interest in the results of the research supporting this article |
| (2) Inactive laser; 3 treatments per week for 4 weeks targeting 7 points along the symptomatic forearm | Secondary: grasp and pinch strength, pain with grasp and pinch | ||||||||
| Stergioulas, 2007 | 62 patients with LE for at least 5 weeks, no treatment in prior month | (1) Ga–As continuous wave laser (40 mW, 904 nm) plus plyometric exercises | Patients and physical therapist performing assessments blinded to laser type | VAS (pain at rest, with palpation of lateral epicondyle, during resisted extension), ROM, grip strength, and free weight test | Post‐treatment and 8 weeks after last treatment | 12/62 patients left study to pursue other treatments | Relative to the placebo group, the active treatment group had a statistically significant decrease in pain at rest, with palpation, during resisted middle finger extension, and during grip strength testing and an increase in grip strength and weight testing at both follow‐up intervals | No complications reported by patients | None reported |
| (2)Placebo laser plus plyometric exercise, 12 treatments over 8 weeks | |||||||||
| Lam, 2007 | 39 patients with LE, no previous episode of lateral elbow pain on same side or any prior treatment | (1) Ga–As laser (25 mW, 904 nm) plus exercise program | Patient and physician blinded | Mechanical pain threshold, MGS, VAS, DASH | 5th and 9th treatment session and 3 weeks post‐treatment | Not discussed | A statistically significant difference in improvement for all outcome measures were found between the laser group and the placebo group in favor of the active laser group, except in 2 subsections of DASH | None reported | None reported |
| (2) Placebo laser plus exercise program; 3 treatments per week for 3 weeks targeting all tender points | |||||||||
| Emanet, 2010 | 49 patients (50 elbows) with LE for less than 3 months, no PT or CSI in prior 2 months | (1) Ga–As laser (100 W/905 nm) with exercise program | Patients and assessor blinded to laser type | VAS, pain with pressure over lateral epicondyle, DASH, PRTEE, pain free grip strength, Nottingham Health Profile (NHP) | 3 and 12 weeks | 3/49, excluded from analysis | At 3 weeks, a statistically significant improvement in all outcomes was observed in both groups. At 12 weeks, a statistically significant improvement in favor of the active laser group was found for pain with resisted wrist extension, pain with pressure, DASH, PRTEE, and NHP (pain) | None reported | No competing financial interests exist |
| (2) Placebo laser with exercise program; 5 treatments a week for 3 weeks targeting the 2 most sensitive points around the lateral epicondyle | |||||||||
AB autologous blood, ASES American Shoulder and Elbow Society, CSI corticosteroid injection, ESWT extra-corporeal shock wave therapy, LE lateral epicondylitis, MEPS Mayo Elbow Performance Score, MGS maximum grip strength, MME maximum muscle elongation, MPFGS Maximum Pain Free Grip Score, MVC maximum voluntary contraction, PFFQ Pain Free Function Questionnaire, PFG pain free grip, PRFEQ Patient Rated Forearm Evaluation Questionnaire, PRP platelet-rich plasma, PRTEE Patient Rated Tennis Elbow Evaluation, PT physiotherapy, QVAS Quadruple Visual Analogue Scale, QOL quality of life, ROM range of motion, SF-12 Short Form 12, SF-36 Short Form 36, TEFS Tennis Elbow Function Score, VAS Visual Analogue Scale (for pain unless otherwise specified)
Results
Corticosteroid Injection
Injection of corticosteroid into the area over the origin of the ECRB or point of maximal tenderness has been a common treatment for relief of lateral elbow pain. Dexamethasone, betamethasone, and triamcinolone have been variously used mixed with a local anesthetic such as lidocaine or bupivacaine.
A study by Hay et al. [23] examined the management of lateral epicondylitis with corticosteroid injection, naproxen, or placebo tablets. At 4 weeks, injection showed clear advantage with 92 % of patients in this group reporting they were completely pain free or improved. Only 57 % of the naproxen group and 50 % of the placebo group reported the same. At 12 months, however, no differences were found between the groups.
Corticosteroid injection, physical therapy, and a wait-and-see approach were compared by Verhaar et al. [66] using a scoring system based on subjective pain relief, patient satisfaction, grip strength, and provoked pain to rate outcomes for treatment with corticosteroid injection or physical therapy. At 6 weeks, patients receiving corticosteroid injection had significantly (p < 0.05) more excellent and good results than the physical therapy group. There was no difference when the groups were compared at 1 year; however, at that point, 50 % of the patients had had other therapy or surgery. Another short-term study comparing corticosteroid injection, physical therapy, both injection and therapy, and no treatment was performed by Tonks et al. [62]. This study had similar results at 7 weeks, with the injection only group performing significantly (p < 0.045) better than other treatment groups. However, 23 % of participants were lost to follow-up in this study.
Smidt et al. [54] assessed long-term outcomes in a RCT comparing corticosteroid injection, physical therapy, and wait-and-see. Corticosteroid injections were found to be the most effective treatment at 6 weeks but were surpassed by physical therapy and no treatment by 52 weeks. Results from later studies by Bisset et al. [6, 7] found comparable results using the same treatment groups. Physical therapy and corticosteroid injection were more effective than wait-and-see before 6 weeks. At 52 weeks, however, there was no difference between physical therapy and wait-and-see, and subjects receiving an injection fared worse than those in other treatment groups. A recent study by Coombes et al. [11] found similar short-term improvement with corticosteroid injections over placebo at 4 weeks; however, these patients fared worse at 26 and 52 weeks of follow-up.
Two double-blind RCTs compared the effects of corticosteroid combined with local anesthetic injection to an injection of local anesthetic alone. Newcomer et al. [37] randomized patients to receive betamethasone/bupivacaine or bupivacaine alone. All patients also received physical therapy. The only statistically significant (p < 0.04) finding was a better visual analog scale (VAS) pain score in the corticosteroid group between 2 and 6 months. Lindenhovius et al. [31] compared injection of dexamethasone and lidocaine with lidocaine alone. No difference was found in any outcome measure at 1- or 6-month follow-ups.
Injection Technique
The peppering technique was described almost 50 years ago [48] and involves many small injections delivered by inserting a needle, injecting, withdrawing without exiting the skin, repositioning, and injecting again until the sensation of crepitus felt with these injections disappears. This technique is thought to initiate healing of the tendon by creating new channels through degenerative tissue in which bleeding occurs [3].
Altay et al. [3] compared injection of corticosteroid plus local anesthetic to local anesthetic alone using the above technique. A second injection of the same type was given at a 2-week follow-up in patients whose symptoms persisted. Results were categorized as excellent, good, fair, or poor according to modified Verhaar criteria (pain relief, patient satisfaction, grip strength, presence of provoked pain on resisted wrist extension). No difference between the two groups was found up to 1 year post-injection, concluding that injection technique is more important than the substance injected.
Dogramaci et al. [16] randomized patients to receive either a single injection of corticosteroid plus local anesthetic, a peppered injection of corticosteroid plus local anesthetic, or a peppered injection of local anesthetic alone. Patients receiving peppered injection of corticosteroid plus local anesthetic had significant (p < 0.011) improvement in VAS and a higher percentage of excellent results according to the Verhaar criteria than the other groups at 6 months. Okcu et al. [41] found similar results when they compared single and peppered injections of corticosteroid, assessing the outcome with Disabilities of the Arm, Shoulder, and Hand (DASH) scores (Turkish). Statistically significant (p < 0.017) results in favor of the peppered injection group were found at 1 year, but results of this study did not include the 38 % of patients with inadequate follow-up.
Iontophoresis
Iontophoresis involves the use of a small electric current to drive charged molecules through the skin, allowing topical medications to penetrate deeper tissues. Demirtas and Oner [15] studied the short-term effects of iontophoresis of two well-known non-steroidal anti-inflammatory drugs (NSAIDs), sodium diclofenac and sodium salicylate. Significant (p < 0.001) decreases in pain were seen with both drugs after one treatment a day for up to 18 days. Sodium diclofenac produced a larger reduction in pain than sodium salicylate. A RCT of oral diclofenac and placebo was conducted by Labelle and Guibert [28]. Both groups were immobilized and the treatment group received 150 mg of diclofenac daily for 28 days. No difference was found between the groups with regard to function; however, there was a significant (p < 0.03) difference in pain reduction in the treatment group.
In Nirschl et al. [40], patients received six treatments of dexamethasone or placebo iontophoresis within 15 days. At 2 days post-treatment, those treated with dexamethasone reported significantly (p < 0.012) less pain on VAS than patients receiving placebo. There was no significant (p = 0.249) difference found between the groups at 1 month.
Stefanou et al. [57] tested the delivery of dexamethasone via a self-contained battery powered iontophoresis patch against dexamethasone injection and triamcinolone injection. All subjects also received therapy. Outcomes were assessed at the end of therapy and at 6 months. Outcomes were similar for all groups at 6 months, but only the iontophoresis group had significantly (p < 0.05) improved grip strength and higher rates of returning to work without restriction at the end of therapy.
Botulinum Toxin A Injection
Botulinum toxin A has been proposed as a non-surgical option for treatment of lateral epicondylitis. The mechanism for relief of pain is paralysis of extensor muscles, preventing further microtrauma to the tendon origins and allowing healing to occur.
Hayton et al. [24], Wong et al. [69], Placzek et al. [47], and Espandar et al. [18] compared 50–60 U of botulinum toxin A or saline injected 1–5 cm distal to the point of maximum tenderness or one third the distance along the forearm from the tip of the lateral epicondyle. Primary outcomes were pain score and grip strength at intervals up to 18 weeks. Hayton’s group did not find any difference between the treatment and placebo groups at any time. However, studies by Wong, Placzek, and Espandar found significant (p < 0.006, 0.003, and 0.01, respectively) differences in pain score but not in grip strength. All studies documented patients in the treatment groups who experienced transient weakness in finger extension.
Lin et al. [30] compared botulinum toxin A to corticosteroid injection and assessed VAS pain score, quality of life, and grip strength at 4, 8, and 12 weeks. The botulinum treatment was significantly (p < 0.02) less effective in reducing pain and resulted in significantly (p < 0.01) reduced grip strength when compared to the corticosteroid group at 4 weeks. The reduced grip strength continued at 8 weeks. There was no difference in quality of life at any time.
Prolotherapy
Prolotherapy consists of an injection composed of osmotics/irritants (dextrose or polidocanol) and/or chemotactics (sodium morrhuate), which promote inflammation in the target tissue. The iatrogenic local inflammation is proposed to induce fibroblastic growth and collagen synthesis, ultimately leading to stronger repair of damaged fibers at the lateral epicondyle.
A double-blind RCT was performed by Scarpone et al. [52] to assess the efficacy of prolotherapy vs. placebo injection in the treatment of refractory lateral epicondylitis. The study group received three injections of dextrose, sodium morrhuate, lidocaine, and sensorcaine at 4-week intervals, while the control group received saline injections. Significantly (p < 0.05) improved pain and grip strength were observed in the prolotherapy group at intervals up to 52 weeks.
Carayannopoulos et al. [8] compared prolotherapy and corticosteroid injections for the treatment of lateral epicondylitis in a later RCT. The prolotherapy group received two injections consisting of dextrose, glycerin, phenol, sodium morrhuate, and procaine, whereas the corticosteroid group received an injection of methylprednisolone and procaine. Both groups demonstrated significant (p < 0.04) improvement at 3 or 6 months, but results of this study did not include the 29 % of patients with inadequate follow-up. No difference could be found between the treatments. Zeisig et al. [70] studied ultrasound guided injections of polidocanol vs lidocaine/epinephrine in a double-blind RCT and reported improvement in grip strength and VAS in both groups at 3 months with no difference between groups.
Platelet-Rich Plasma and Autologous Blood Injection
Injections of PRP or ABI have been proposed to facilitate healing through release of growth factors directly at the site of interest. Specifically, PRP contains high levels of various growth factors including platelet-derived growth factor, transforming growth factor beta, and vascular endothelial growth factor [35]. PRP is created by withdrawing the patient’s own blood and centrifuging it to isolate a platelet-rich fraction before injecting into the patient at the site of interest. In the case of ABI, autologous blood is withdrawn from the patient but is not treated before reinjection.
Several studies have compared the injection of PRP or ABI to injection of corticosteroids. Gosens et al. and Peerbooms et al. [20, 44] performed double-blind RCTs demonstrating a significant (p < 0.005) difference in favor of the groups receiving PRP injections over those receiving corticosteroid injections with follow-up intervals up to 2 years. Similarly, Kazemi et al. [25] compared ABI to corticosteroid injection in a RCT where assessors were unaware of treatment. At 8 weeks, ABI was found to be more effective in all outcomes. A recent study by Krogh et al. [27] did not find PRP superior to corticosteroid injection at 1 month or to either corticosteroid injection of placebo at 3 months of follow-up; however, tendon thickness was found to be reduced on ultrasound examination in corticosteroid injection over PRP or placebo test subjects.
Wolf et al. [68] attempted to account for any placebo effect associated with injection by adding a control group to their RCT study design. Patients were randomized to receive one of three treatments including ABI, corticosteroid/lidocaine injection, and saline/lidocaine injection. Outcomes improved over time in all three groups with no differences at 2- and 6-month follow-ups.
PRP has also been compared directly to ABI. Creaney et al. [14] conducted a double-blind RCT with patients receiving injections at 1 month intervals of either autologous blood or PRP. Both preparations produced improvement over 6 months, but there were no differences between groups. Thanasas et al. [61] also compared the two in a RCT in which assessors were unaware of treatment allocation. Ultrasound guidance and peppering injection technique was used in both groups. Statistically significant (p < 0.05) improvement in pain for PRP over ABI was present only at 6 weeks.
Bracing
Two commonly used braces are the proximal forearm strap (counter force brace) and wrist extension splint. By holding the wrist in extension, the splint is thought to unload the extensor origin and relax the wrist extensors. There are several theories as to how the forearm strap reduces the force exerted on the common extensor origin, one being that its compressive force limits expansion and thereby the force generated by the extensors. Another is that the strap serves as a secondary origin for the ECRB muscle, reducing the force conducted proximally [39]. Both mechanisms would theoretically decrease stress on the damaged tendons, allowing healing.
Several authors have compared the effects of the proximal forearm strap and wrist extension splint on lateral epicondylitis symptoms. One study found no difference between the two orthotics [64], and two found a substantial difference in favor of the wrist splint in reduction of pain [2, 19]. Both studies were limited to 6 weeks of follow-up and lacked a control group; one study [19] did not include 40 % of patients with inadequate follow-up, making interpretation of the true benefit of either splint difficult to determine in these studies.
Struijs et al. [59] randomly assigned 180 patients to forearm strap, physical therapy, or combination and found no differences at 26- or 52-week follow-ups. At 6 weeks, the strap-only group was significantly (p < 0.05) better in ability to perform daily activities. Luginbuhl et al. [32] compared use of a forearm strap for at least 3 months, strengthening exercises, and a combination with the addition of a corticosteroid injection at the beginning of treatment. All groups experienced significant (p < 0.0001) improvement in pain at 6 weeks and 1 year, and there was no difference between groups at any time.
Physical Therapy
PT is often employed for non-surgical treatment of lateral epicondylitis. There are many studies comparing the efficacy of PT to other modalities of treatment that are included elsewhere in this review. There are also several RCTs which have compared PT to wait-and-see or examined different methods of PT. Peterson et al. [45] and Park et al. [43] compared PT to wait-and-see, with mixed findings. Peterson’s group found significant (p < 0.0016) improvement in pain for the treatment group while Park saw significantly (p < 0.01) better pain scores for the control group. Viswas et al. [67] evaluated a stretching/strengthening program against Cyriax friction massage, finding no difference in outcomes between the methods. Tyler et al. [63] and Martinez-Silvestrini et al. [34] examined various combinations of eccentric and concentric exercises compared to standard PT with inconsistent results; Tyler’s study found a significant difference (p < 0.011) in all measures for the eccentric group while Martinez-Silvestrini found no difference in outcomes between any group. The recent study examining corticosteroid injection and physical therapy by Coombes et al. [11] previously discussed found short-term improvement with physical therapy alone; however, this effect disappeared by 26 and 52 weeks of follow-up.
Shockwave Therapy
Extracorporeal shock wave therapy (ESWT) applies energy to the interface of two substances with differing acoustic impedance. It has been proposed that this energy promotes tissue healing and possibly reduces pain by stimulating nerve fibers to produce analgesia [56].
Of the several double-blind RCTs done to evaluate the efficacy of ESWT, only two authors have produced significant (p < 0.001) findings. Rompe et al. conducted two studies [50, 51] looking at the efficacy of ESWT in the treatment of lateral epicondylitis. Both studies were double-blind RCTs looking at patients with refractory lateral epicondylitis of at least 12 months. Patients received three weekly treatments of either active or sham ESWT. In the earlier study, significant (p < 0.001) improvement in pain and grip strength was observed in the active treatment group at 3, 6, and 24 weeks. In the later study, significant (p < 0.001) difference in favor of the active treatment group was found up to 12 months after treatment. Pettrone and McCall [46] also saw a positive effect of ESWT on pain and function 12 weeks post-treatment in patients with refractory lateral epicondylitis for more than 6 months. Other investigators, Haake et al. [21], Speed et al. [55], Melikyan et al. [36], Chung et al. [10], and Staples et al. [56] did not find any substantial difference between treatment and placebo groups with any outcome measure.
Laser Therapy
Low-level laser therapy (LLLT) is proposed to have a biostimulatory effect on tissue, reducing levels of TNF alpha [1] and reducing cell apoptosis [9] although the precise method by which this occurs is incompletely understood. The effect of LLLT on lateral epicondylitis has been studied with conflicting results. Early double-blind RCTs did not show an advantage to LLLT. Lundeberg et al. [33] studied two different types of laser (pulsed Ga–As and continuous He–Ne), with no difference between treatment and placebo groups up to 3 months after treatment. Four more RCTs studied the effect of either a Ga–As or Ga–Al–As laser vs. sham laser therapy. Varying levels of energy were delivered per point in each study, and follow-up periods ranged from 7 weeks to 1 year. Three studies did not demonstrate a difference in results between laser therapy and placebo [22, 26, 42] (although one of these studies [26] did not include 25 % of subjects lost to follow-up) whereas a fourth study did [65].
Results of more recent studies conflict with those found previously. Basford et al. [4] conducted a double-blind RCT with a Nd-YAG laser and placebo which did not demonstrate a difference in outcome at 4 weeks. However, a study by Stergioulas [58] combined plyometric exercise with Ga–As laser or placebo laser and found a significant (p < 0.05) improvement in VAS and strength at 8 and 16 weeks in the active treatment group. A similar study by Lam and Cheing [29], looking only at short-term outcomes of Ga–As laser treatment at 3 weeks found comparable results (p < 0.0125). Emanet et al. [17] also found positive results of LLLT in their double-blind RCT using a Ga–As laser and additional physical therapy for both groups with a statistically significant (p < 0.05) difference with respect to improved pain, grip strength, and functional assessment in favor of the treatment group at 12 weeks.
Discussion
Many non-surgical treatments for lateral epicondylitis have been developed and studied. Corticosteroid injection, injection technique, iontophoresis, botulinum toxin A, prolotherapy, platelet-rich plasma/autologous blood injections, bracing, physical therapy, extracorporeal shock wave therapy, and laser therapy have been subjected to RCTs. When taken in aggregate, we propose there are several conclusions to be drawn from the collection of studies reviewed herein.
Given that lateral epicondylitis is thought to result from repetitive microtrauma, rather than an inflammatory process, it is not surprising that corticosteroid injections do not provide long-term relief. Patients receiving corticosteroid injections have improved pain relief over physical therapy or a wait-and-see approach for 1 to 2 months post-treatment, although these effects do not remain when examined at 1 year. The several studies testing the effect of a peppering technique of corticosteroid injection show the technique improves results at 6 months to 1 year of follow-up. RCTs of iontophoresis have shown some benefit to this technique of corticosteroid delivery, at least on a very short-term basis of days to weeks.
Other types of injection have been studied in addition to injection of corticosteroids. Treatment with botulinum toxin A may also provide pain relief when compared to placebo, but not to corticosteroid injection, and the accompanying transient extensor weakness may not be acceptable to patients. Prolotherapy is superior to saline placebo but does not appear to be more effective than corticosteroid or lidocaine injection. PRP and ABI have been found to be both less and more effective than corticosteroid injections, and PRP has been shown to also be either more or equally effective than ABI in studies.
Bracing with a proximal forearm strap or wrist extension splint, however, seems to provide little to no additional benefit when combined with physical therapy. The studies reviewing physical therapy without bracing or other concurrent treatment did not show a clear advantage to PT.
Non-invasive techniques such as laser therapy and ESWT have been the subject of RCTs. Early studies of laser therapy did not show an effect of treatment whereas more recent investigations did show substantial improvement for patients treated with laser therapy over those who received placebo therapy. These beneficial effects were most apparent at 1 to 4 months of follow-up. The preponderance of studies of extracorporeal shockwave therapy appears to indicate that this treatment is no more effective than placebo.
A weakness of this systematic review is that which is inherent in many studies, even those which are undertaken as RCT, blinded trials: Design of the perfect trial is probably impossible, as there are always unanticipated variables [13]. Patients are lost to follow-up, have subjective responses to test criteria, or there may be unintended investigator bias. However, given a large number of investigations, valuable conclusions may be drawn.
In summary, lateral epicondylitis is a condition that is usually self-limited, resolving over a 12- to 18-month period without treatment. There are many options for non-surgical treatment for pain relief. Corticosteroids, whether delivered with a standard or peppered injection technique or via iontophoresis, may provide short-term pain relief on the order of several months; however, this effect is not sustained in the long term. Injections of botulinum toxin A, prolotherapy, PRP, or ABI may also provide varying degrees of improvement in pain, although botulinum toxin A can have unacceptable side effects. Studies of LLLT do not show a clear benefit at this time. Therapies that do not include injection, such as bracing, PT, or ESWT, do not appear to provide substantial benefit in terms of pain relief or improved function. In conclusion, there are multiple randomized controlled trials for non-surgical management of lateral epicondylitis, but the existing literature does not provide conclusive evidence that there is one preferred method of non-surgical treatment for this condition.
Acknowledgments
John C. Elfar receives funding from the National Institute of Arthritis and Musculoskeletal and Skin Diseases of the National Institutes of Health under the following: “Research reported in this publication was supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases of the National Institutes of Health under Award Number K08AR060164.”
Conflict of Interest
Susan E. G. Sims declares that she has no conflict of interest.
Katherine Miller declares that she has no conflict of interest.
John C. Elfar declares that he has no conflict of interest.
Warren C. Hammert declares that he has no conflict of interest.
Statement of Human and Animal Rights
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2008.
Statement of Informed Consent
Informed consent was obtained from all patients for being included in the study.
References
- 1.Aimbire F, Albertini R, Pacheco MT, et al. Low-level laser therapy induces dose-dependent reduction of TNFalpha levels in acute inflammation. Photomed Laser Surg. 2006;24(1):33–7. doi: 10.1089/pho.2006.24.33. [DOI] [PubMed] [Google Scholar]
- 2.Altan L, Kanat E. Conservative treatment of lateral epicondylitis: comparison of two different orthotic devices. Clin Rheumatol. 2008;27(8):1015–9. doi: 10.1007/s10067-008-0862-8. [DOI] [PubMed] [Google Scholar]
- 3.Altay T, Gunal I, Ozturk H. Local injection treatment for lateral epicondylitis. Clin Orthop Relat Res. 2002;398:127–30. doi: 10.1097/00003086-200205000-00018. [DOI] [PubMed] [Google Scholar]
- 4.Basford JR, Sheffield CG, Cieslak KR. Laser therapy: a randomized, controlled trial of the effects of low intensity Nd:YAG laser irradiation on lateral epicondylitis. Arch Phys Med Rehabil. 2000;81(11):1504–10. doi: 10.1053/apmr.2000.17812. [DOI] [PubMed] [Google Scholar]
- 5.Binder AI, Hazleman BL. Lateral humeral epicondylitis—a study of natural history and the effect of conservative therapy. Br J Rheumatol. 1983;22(2):73–6. doi: 10.1093/rheumatology/22.2.73. [DOI] [PubMed] [Google Scholar]
- 6.Bisset L, Beller E, Jull G, et al. Mobilisation with movement and exercise, corticosteroid injection, or wait and see for tennis elbow: randomised trial. BMJ. 2006;333(7575):939. doi: 10.1136/bmj.38961.584653.AE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bisset L, Smidt N, Van der Windt DA, et al. Conservative treatments for tennis elbow do subgroups of patients respond differently? Rheumatology. 2007;46(10):1601–5. doi: 10.1093/rheumatology/kem192. [DOI] [PubMed] [Google Scholar]
- 8.Carayannopoulos A, Borg-Stein J, Sokolof J, et al. Prolotherapy versus corticosteroid injections for the treatment of lateral epicondylosis: a randomized controlled trial. PM R. 2011;3(8):706–15. doi: 10.1016/j.pmrj.2011.05.011. [DOI] [PubMed] [Google Scholar]
- 9.Carnevalli CM, Soares CP, Zangaro RA, et al. Laser light prevents apoptosis in Cho K-1 cell line. J Clin Laser Med Surg. 2003;21(4):193–6. doi: 10.1089/104454703768247756. [DOI] [PubMed] [Google Scholar]
- 10.Chung B, Wiley JP, Rose MS. Long-term effectiveness of extracorporeal shockwave therapy in the treatment of previously untreated lateral epicondylitis. Clin J Sport Med. 2005;15(5):305–12. doi: 10.1097/01.jsm.0000179137.69598.7e. [DOI] [PubMed] [Google Scholar]
- 11.Coombes BK, Bisset L, Brooks P, et al. Effect of corticosteroid injection, physiotherapy, or both on clinical outcomes in patients with unilateral lateral epicondylalgia: a randomized controlled trial. JAMA. 2013;309(5):461–9. doi: 10.1001/jama.2013.129. [DOI] [PubMed] [Google Scholar]
- 12.Coonrad RW, Hooper WR. Tennis elbow: its course, natural history, conservative and surgical management. J Bone Joint Surg Am. 1973;55(6):1177–82. [PubMed] [Google Scholar]
- 13.Cowan J, Lozano-Calderon S, Ring D. Quality of prospective controlled randomized trials. Analysis of trials of treatment for lateral epicondylitis as an example. J Bone Joint Surg Am. 2007;89(8):1693–9. doi: 10.2106/JBJS.F.00858. [DOI] [PubMed] [Google Scholar]
- 14.Creaney L, Wallace A, Curtis M, et al. Growth factor-based therapies provide additional benefit beyond physical therapy in resistant elbow tendinopathy: a prospective, double-blind, randomised trial of autologous blood injections versus platelet-rich plasma injections. Br J Sports Med. 2011;45(12):966–71. doi: 10.1136/bjsm.2010.082503. [DOI] [PubMed] [Google Scholar]
- 15.Demirtas RN, Oner C. The treatment of lateral epicondylitis by iontophoresis of sodium salicylate and sodium diclofenac. Clin Rehabil. 1998;12(1):23–9. doi: 10.1191/026921598672378032. [DOI] [PubMed] [Google Scholar]
- 16.Dogramaci Y, Kalaci A, Savas N, et al. Treatment of lateral epicondilitis using three different local injection modalities: a randomized prospective clinical trial. Arch Orthop Trauma Surg. 2009;129(10):1409–14. doi: 10.1007/s00402-009-0832-x. [DOI] [PubMed] [Google Scholar]
- 17.Emanet SK, Altan LI, Yurtkuran M. Investigation of the effect of GaAs laser therapy on lateral epicondylitis. Photomed Laser Surg. 2010;28(3):397–403. doi: 10.1089/pho.2009.2555. [DOI] [PubMed] [Google Scholar]
- 18.Espandar R, Heidari P, Rasouli MR, et al. Use of anatomic measurement to guide injection of botulinum toxin for the management of chronic lateral epicondylitis: a randomized controlled trial. CMAJ. 2010;182(8):768–73. doi: 10.1503/cmaj.090906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Garg R, Adamson GJ, Dawson PA, et al. A prospective randomized study comparing a forearm strap brace versus a wrist splint for the treatment of lateral epicondylitis. J Should Elb Surg. 2010;19(4):508–12. doi: 10.1016/j.jse.2009.12.015. [DOI] [PubMed] [Google Scholar]
- 20.Gosens T, Peerbooms JC, van Laar W, et al. Ongoing positive effect of platelet-rich plasma versus corticosteroid injection in lateral epicondylitis: a double-blind randomized controlled trial with 2-year follow-up. Am J Sports Med. 2011;39(6):1200–8. doi: 10.1177/0363546510397173. [DOI] [PubMed] [Google Scholar]
- 21.Haake M, Konig IR, Decker T, et al. Extracorporeal shock wave therapy in the treatment of lateral epicondylitis: a randomized multicenter trial. J Bone Joint Surg Am. 2002;84-A(11):1982–91. doi: 10.2106/00004623-200211000-00012. [DOI] [PubMed] [Google Scholar]
- 22.Haker E, Lundeberg T. Laser treatment applied to acupuncture points in lateral humeral epicondylalgia. A double-blind study. Pain. 1990;43(2):243–7. doi: 10.1016/0304-3959(90)91078-W. [DOI] [PubMed] [Google Scholar]
- 23.Hay EM, Paterson SM, Lewis M, et al. Pragmatic randomised controlled trial of local corticosteroid injection and naproxen for treatment of lateral epicondylitis of elbow in primary care. BMJ. 1999;319(7215):964–8. doi: 10.1136/bmj.319.7215.964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hayton MJ, Santini AJ, Hughes PJ, et al. Botulinum toxin injection in the treatment of tennis elbow. A double-blind, randomized, controlled, pilot study. J Bone Joint Surg Am. 2005;87(3):503–7. doi: 10.2106/JBJS.D.01896. [DOI] [PubMed] [Google Scholar]
- 25.Kazemi M, Azma K, Tavana B, et al. Autologous blood versus corticosteroid local injection in the short-term treatment of lateral elbow tendinopathy: a randomized clinical trial of efficacy. Am J Phys Med Rehabil. 2010;89(8):660–7. doi: 10.1097/PHM.0b013e3181ddcb31. [DOI] [PubMed] [Google Scholar]
- 26.Krasheninnikoff M, Ellitsgaard N, Rogvi-Hansen B, et al. No effect of low power laser in lateral epicondylitis. Scand J Rheumatol. 1994;23(5):260–3. doi: 10.3109/03009749409103726. [DOI] [PubMed] [Google Scholar]
- 27.Krogh TP, Fredberg U, Stengaard-Pedersen K, et al. Treatment of lateral epicondylitis with platelet-rich plasma, glucocorticoid, or saline: a randomized, double-blind, placebo-controlled trial. Am J Sports Med. 2013;41(3):625–35. doi: 10.1177/0363546512472975. [DOI] [PubMed] [Google Scholar]
- 28.Labelle H, Guibert R. Efficacy of diclofenac in lateral epicondylitis of the elbow also treated with immobilization. The University of Montreal Orthopaedic Research Group. Arch Fam Med. 1997;6(3):257–62. doi: 10.1001/archfami.6.3.257. [DOI] [PubMed] [Google Scholar]
- 29.Lam LK, Cheing GL. Effects of 904-nm low-level laser therapy in the management of lateral epicondylitis: a randomized controlled trial. Photomed Laser Surg. 2007;25(2):65–71. doi: 10.1089/pho.2006.2047. [DOI] [PubMed] [Google Scholar]
- 30.Lin YC, Tu YK, Chen SS, et al. Comparison between botulinum toxin and corticosteroid injection in the treatment of acute and subacute tennis elbow: a prospective, randomized, double-blind, active drug-controlled pilot study. Am J Phys Med Rehabil. 2010;89(8):653–9. doi: 10.1097/PHM.0b013e3181cf564d. [DOI] [PubMed] [Google Scholar]
- 31.Lindenhovius A, Henket M, Gilligan BP, et al. Injection of dexamethasone versus placebo for lateral elbow pain: a prospective, double-blind, randomized clinical trial. J Hand Surg [Am] 2008;33(6):909–19. doi: 10.1016/j.jhsa.2008.02.004. [DOI] [PubMed] [Google Scholar]
- 32.Luginbuhl R, Brunner F, Schneeberger AG. No effect of forearm band and extensor strengthening exercises for the treatment of tennis elbow: a prospective randomised study. Chir Organi Mov. 2008;91(1):35–40. doi: 10.1007/s12306-007-0006-3. [DOI] [PubMed] [Google Scholar]
- 33.Lundeberg T, Haker E, Thomas M. Effect of laser versus placebo in tennis elbow. Scand J Rehabil Med. 1987;19(3):135–8. [PubMed] [Google Scholar]
- 34.Martinez-Silvestrini JA, Newcomer KL, Gay RE, et al. Chronic lateral epicondylitis: comparative effectiveness of a home exercise program including stretching alone versus stretching supplemented with eccentric or concentric strengthening. J Hand Ther. 2005;18(4):411–9. doi: 10.1197/j.jht.2005.07.007. [DOI] [PubMed] [Google Scholar]
- 35.Marx RE. Platelet-rich plasma: evidence to support its use. J Oral Maxillofac Surg. 2004;62(4):489–96. doi: 10.1016/j.joms.2003.12.003. [DOI] [PubMed] [Google Scholar]
- 36.Melikyan EY, Shahin E, Miles J, et al. Extracorporeal shock-wave treatment for tennis elbow. A randomised double-blind study. J Bone Joint Surg Br. 2003;85(6):852–5. [PubMed] [Google Scholar]
- 37.Newcomer KL, Laskowski ER, Idank DM, et al. Corticosteroid injection in early treatment of lateral epicondylitis. Clin J Sport Med. 2001;11(4):214–22. doi: 10.1097/00042752-200110000-00002. [DOI] [PubMed] [Google Scholar]
- 38.Nirschl RP. Tennis elbow: the surgical treatment of lateral epicondylitis. J Bone Joint Surg Am. 1979;61-A(6):832. [PubMed] [Google Scholar]
- 39.Nirschl RP, Ashman ES. Elbow tendinopathy: tennis elbow. Clin Sports Med. 2003;22(4):813–36. doi: 10.1016/S0278-5919(03)00051-6. [DOI] [PubMed] [Google Scholar]
- 40.Nirschl RP, Rodin DM, Ochiai DH, et al. Iontophoretic administration of dexamethasone sodium phosphate for acute epicondylitis. A randomized, double-blinded, placebo-controlled study. Am J Sports Med. 2003;31(2):189–95. doi: 10.1177/03635465030310020601. [DOI] [PubMed] [Google Scholar]
- 41.Okcu G, Erkan S, Senturk M, et al. Evaluation of injection techniques in the treatment of lateral epicondylitis: a prospective randomized clinical trial. Acta Orthop Traumatol Turc. 2012;46(1):26–9. doi: 10.3944/AOTT.2012.2577. [DOI] [PubMed] [Google Scholar]
- 42.Papadopoulos ES, Smith RW, Cawley MID, et al. Low-level laser therapy does not aid the management of tennis elbow. Clin Rehabil. 1996;10:9–11. doi: 10.1177/026921559601000103. [DOI] [Google Scholar]
- 43.Park JY, Park HK, Choi JH, et al. Prospective evaluation of the effectiveness of a home-based program of isometric strengthening exercises: 12-month follow-up. Clin Orthop Surg. 2010;2(3):173–8. doi: 10.4055/cios.2010.2.3.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Peerbooms JC, Sluimer J, Bruijn DJ, et al. Positive effect of an autologous platelet concentrate in lateral epicondylitis in a double-blind randomized controlled trial: platelet-rich plasma versus corticosteroid injection with a 1-year follow-up. Am J Sports Med. 2010;38(2):255–62. doi: 10.1177/0363546509355445. [DOI] [PubMed] [Google Scholar]
- 45.Peterson M, Butler S, Eriksson M, et al. A randomized controlled trial of exercise versus wait-list in chronic tennis elbow (lateral epicondylosis) Ups J Med Sci. 2011;116(4):269–79. doi: 10.3109/03009734.2011.600476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pettrone FA, McCall BR. Extracorporeal shock wave therapy without local anesthesia for chronic lateral epicondylitis. J Bone Joint Surg Am. 2005;87(6):1297–304. doi: 10.2106/JBJS.C.01356. [DOI] [PubMed] [Google Scholar]
- 47.Placzek R, Drescher W, Deuretzbacher G, et al. Treatment of chronic radial epicondylitis with botulinum toxin A. A double-blind, placebo-controlled, randomized multicenter study. J Bone Joint Surg Am. 2007;89(2):255–60. doi: 10.2106/JBJS.F.00401. [DOI] [PubMed] [Google Scholar]
- 48.Pruce AM, Miller JA, Berger IR, Journal A. Anatomic landmarks in joint paracentesis. Clin Symp. 1964;16:19–30. [PubMed] [Google Scholar]
- 49.Regan W, Wold LE, Coonrad R, et al. Microscopic histopathology of chronic refractory lateral epicondylitis. Am J Sports Med. 1992;20(6):746–9. doi: 10.1177/036354659202000618. [DOI] [PubMed] [Google Scholar]
- 50.Rompe JD, Decking J, Schoellner C, et al. Repetitive low-energy shock wave treatment for chronic lateral epicondylitis in tennis players. Am J Sports Med. 2004;32(3):734–43. doi: 10.1177/0363546503261697. [DOI] [PubMed] [Google Scholar]
- 51.Rompe JD, Hope C, Kullmer K, et al. Analgesic effect of extracorporeal shock-wave therapy on chronic tennis elbow. J Bone Joint Surg (Br) 1996;78(2):233–7. [PubMed] [Google Scholar]
- 52.Scarpone M, Rabago DP, Zgierska A, et al. The efficacy of prolotherapy for lateral epicondylosis: a pilot study. Clin J Sport Med. 2008;18(3):248–54. doi: 10.1097/JSM.0b013e318170fc87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Shiri R, Viikari-Juntura E, Varonen H, et al. Prevalence and determinants of lateral and medial epicondylitis: a population study. Am J Epidemiol. 2006;164(11):1065–74. doi: 10.1093/aje/kwj325. [DOI] [PubMed] [Google Scholar]
- 54.Smidt N, van der Windt DA, Assendelft WJ, et al. Corticosteroid injections, physiotherapy, or a wait-and-see policy for lateral epicondylitis: a randomised controlled trial. Lancet. 2002;359(9307):657–62. doi: 10.1016/S0140-6736(02)07811-X. [DOI] [PubMed] [Google Scholar]
- 55.Speed CA, Nichols D, Richards C, et al. Extracorporeal shock wave therapy for lateral epicondylitis—a double blind randomised controlled trial. J Orthop Res. 2002;20(5):895–8. doi: 10.1016/S0736-0266(02)00013-X. [DOI] [PubMed] [Google Scholar]
- 56.Staples MP, Forbes A, Ptasznik R, et al. A randomized controlled trial of extracorporeal shock wave therapy for lateral epicondylitis (tennis elbow) J Rheumatol. 2008;35(10):2038–46. [PubMed] [Google Scholar]
- 57.Stefanou A, Marshall N, Holdan W, et al. A randomized study comparing corticosteroid injection to corticosteroid iontophoresis for lateral epicondylitis. J Hand Surg [Am] 2012;37(1):104–9. doi: 10.1016/j.jhsa.2011.10.005. [DOI] [PubMed] [Google Scholar]
- 58.Stergioulas A. Effects of low-level laser and plyometric exercises in the treatment of lateral epicondylitis. Photomed Laser Surg. 2007;25(3):205–13. doi: 10.1089/pho.2007.2041. [DOI] [PubMed] [Google Scholar]
- 59.Struijs PA, Kerkhoffs GM, Assendelft WJ, et al. Conservative treatment of lateral epicondylitis: brace versus physical therapy or a combination of both-a randomized clinical trial. Am J Sports Med. 2004;32(2):462–9. doi: 10.1177/0095399703258714. [DOI] [PubMed] [Google Scholar]
- 60.Taylor SA, Hannafin JA. Evaluation and management of elbow tendinopathy. Sports Health. 2012;4(5):384–93. doi: 10.1177/1941738112454651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Thanasas C, Papadimitriou G, Charalambidis C, et al. Platelet-rich plasma versus autologous whole blood for the treatment of chronic lateral elbow epicondylitis: a randomized controlled clinical trial. Am J Sports Med. 2011;39(10):2130–4. doi: 10.1177/0363546511417113. [DOI] [PubMed] [Google Scholar]
- 62.Tonks JH, Pai SK, Murali SR. Steroid injection therapy is the best conservative treatment for lateral epicondylitis: a prospective randomised controlled trial. Int J Clin Pract. 2007;61(2):240–6. doi: 10.1111/j.1742-1241.2006.01140.x. [DOI] [PubMed] [Google Scholar]
- 63.Tyler TF, Thomas GC, Nicholas SJ, et al. Addition of isolated wrist extensor eccentric exercise to standard treatment for chronic lateral epicondylosis: a prospective randomized trial. J Should Elb Surg. 2010;19(6):917–22. doi: 10.1016/j.jse.2010.04.041. [DOI] [PubMed] [Google Scholar]
- 64.Van De Streek MD, Van Der Schans CP, De Greef MH, et al. The effect of a forearm/hand splint compared with an elbow band as a treatment for lateral epicondylitis. Prosthetics Orthot Int. 2004;28(2):183–9. doi: 10.1080/03093640408726703. [DOI] [PubMed] [Google Scholar]
- 65.Vasseljen O, Hoeg N, Kjeldstad B, et al. Low level laser versus placebo in the treatment of tennis elbow. Scand J Rehabil Med. 1992;24(1):37–42. [PubMed] [Google Scholar]
- 66.Verhaar JA, Walenkamp GH, van Mameren H, et al. Local corticosteroid injection versus Cyriax-type physiotherapy for tennis elbow. J Bone Joint Surg (Br) 1996;78(1):128–32. [PubMed] [Google Scholar]
- 67.Viswas R, Ramachandran R, Korde AP. Comparison of effectiveness of supervised exercise program and Cyriax physiotherapy in patients with tennis elbow (lateral epicondylitis): a randomized clinical trial. ScientificWorldJournal. 2012;2012:939645. doi: 10.1100/2012/939645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wolf JM, Ozer K, Scott F, et al. Comparison of autologous blood, corticosteroid, and saline injection in the treatment of lateral epicondylitis: a prospective, randomized, controlled multicenter study. J Hand Surg [Am] 2011;36(8):1269–72. doi: 10.1016/j.jhsa.2011.05.014. [DOI] [PubMed] [Google Scholar]
- 69.Wong SM, Hui AC, Tong PY, et al. Treatment of lateral epicondylitis with botulinum toxin: a randomized, double-blind, placebo-controlled trial. Ann Interm Med. 2005;143(11):793–7. doi: 10.7326/0003-4819-143-11-200512060-00007. [DOI] [PubMed] [Google Scholar]
- 70.Zeisig E, Fahlstrom M, Ohberg L, et al. Pain relief after intratendinous injections in patients with tennis elbow: results of a randomised study. Br J Sports Med. 2008;42(4):267–71. doi: 10.1136/bjsm.2007.042762. [DOI] [PubMed] [Google Scholar]