Abstract
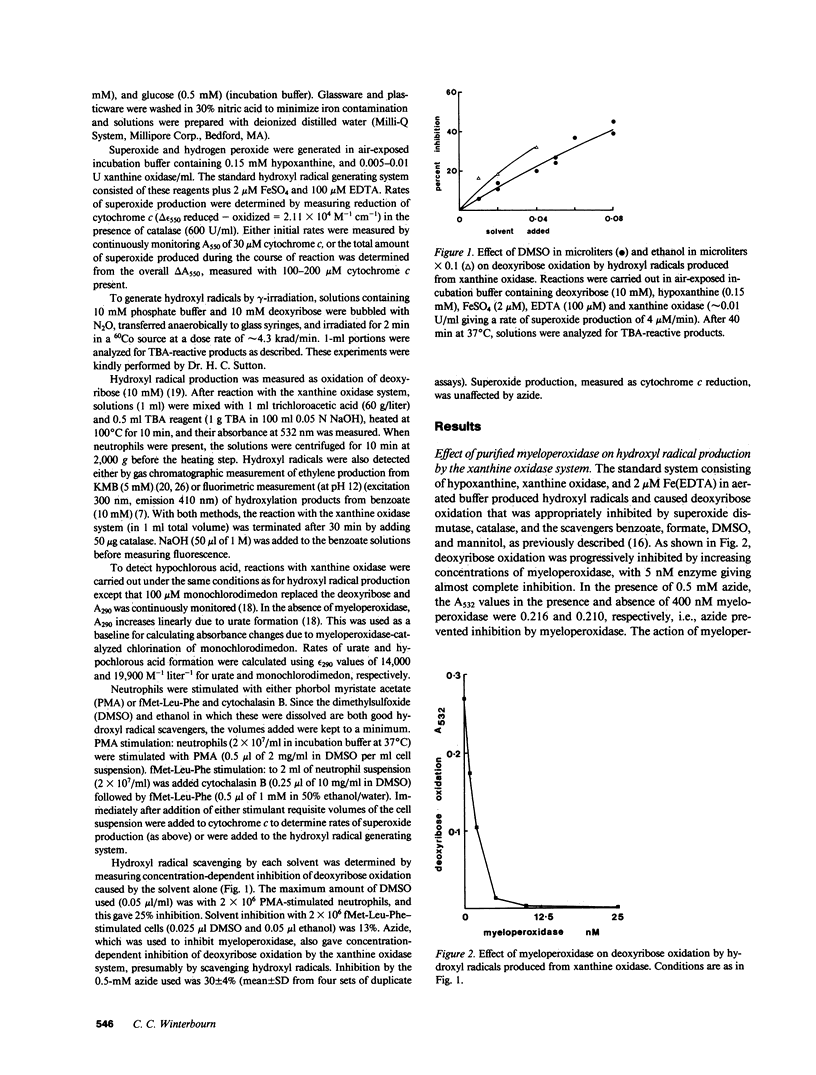
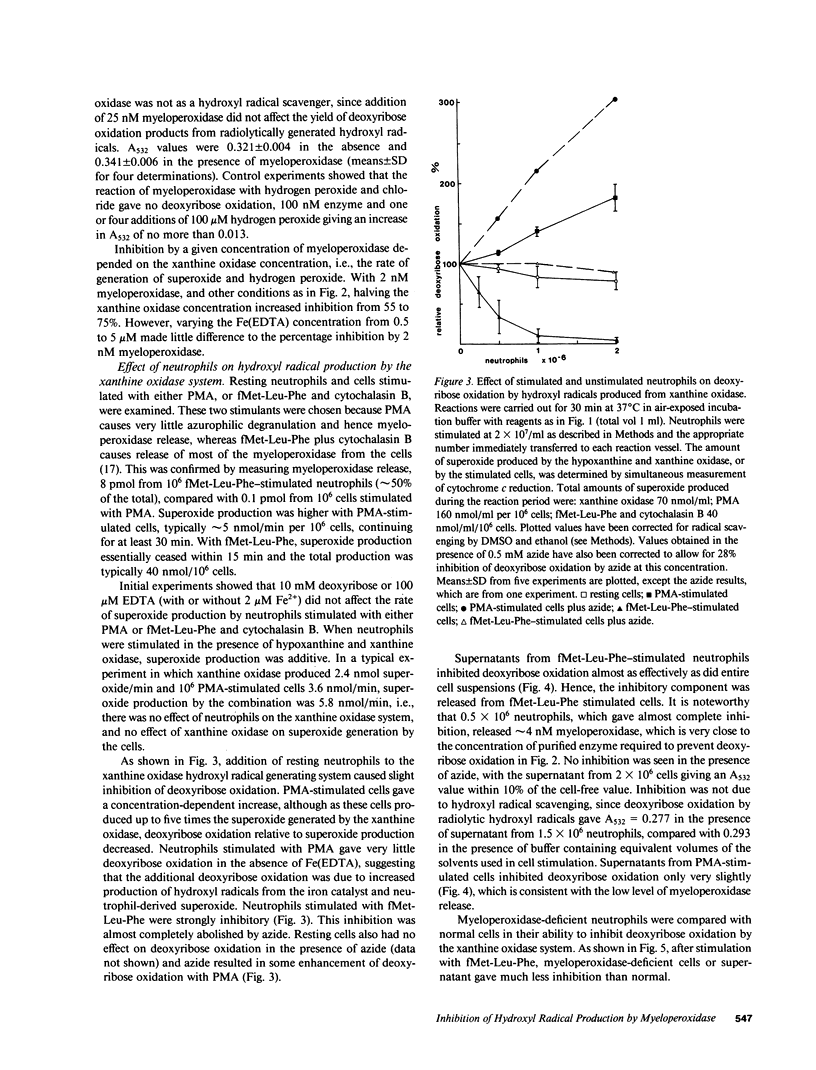
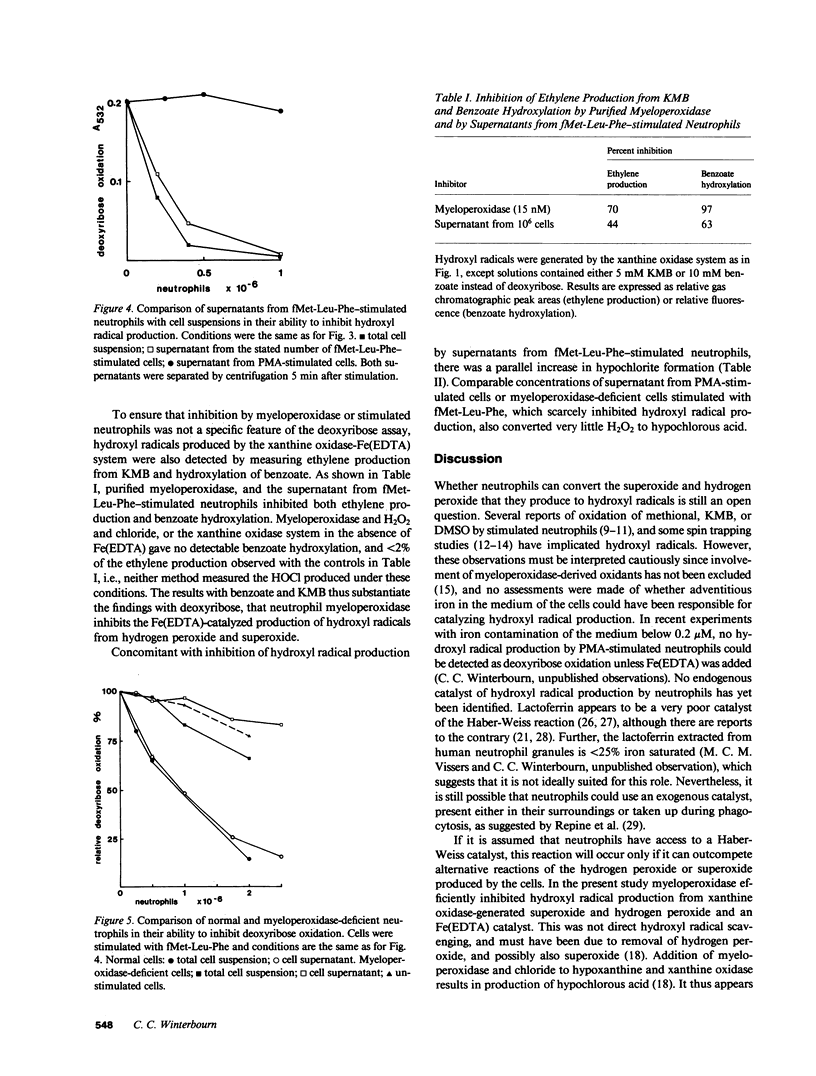
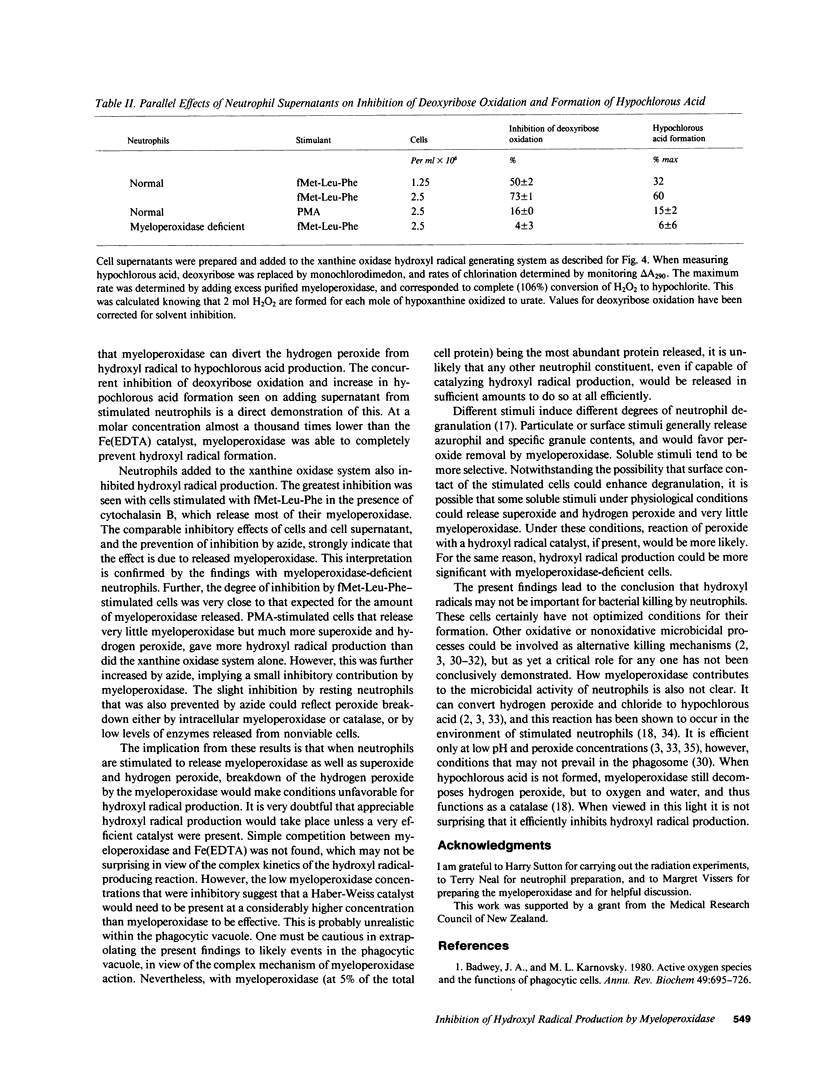
Hydroxyl radicals have been generated from hydrogen peroxide and superoxide (produced with xanthine oxidase), and an iron (EDTA) catalyst, and detected with deoxyribose, or in some cases with benzoate or alpha-keto-gamma-methiolbutyric acid. Purified myeloperoxidase, and neutrophils stimulated with fMet-Leu-Phe and cytochalasin B, strongly inhibited this hydroxyl radical production in a concentration-dependent manner. Supernatants from stimulated cells also inhibited, and inhibition by cells or supernatant was prevented by azide. There was much less inhibition by myeloperoxidase-deficient neutrophils. Inhibition thus was due to myeloperoxidase released by the cells. With neutrophils stimulated with phorbol myristate acetate, which release very little myeloperoxidase, hydroxyl radical production was enhanced due to the additional superoxide produced by the cells. It is concluded that under conditions where neutrophils release myeloperoxidase as well as superoxide and hydrogen peroxide, breakdown of hydrogen peroxide by myeloperoxidase would make conditions unfavorable for hydroxyl radical production.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ambruso D. R., Johnston R. B., Jr Lactoferrin enhances hydroxyl radical production by human neutrophils, neutrophil particulate fractions, and an enzymatic generating system. J Clin Invest. 1981 Feb;67(2):352–360. doi: 10.1172/JCI110042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews P. C., Krinsky N. I. A kinetic analysis of the interaction of human myeloperoxidase with hydrogen peroxide, chloride ions, and protons. J Biol Chem. 1982 Nov 25;257(22):13240–13245. [PubMed] [Google Scholar]
- Babior B. M., Kipnes R. S., Curnutte J. T. Biological defense mechanisms. The production by leukocytes of superoxide, a potential bactericidal agent. J Clin Invest. 1973 Mar;52(3):741–744. doi: 10.1172/JCI107236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babior B. M. Oxidants from phagocytes: agents of defense and destruction. Blood. 1984 Nov;64(5):959–966. [PubMed] [Google Scholar]
- Badwey J. A., Karnovsky M. L. Active oxygen species and the functions of phagocytic leukocytes. Annu Rev Biochem. 1980;49:695–726. doi: 10.1146/annurev.bi.49.070180.003403. [DOI] [PubMed] [Google Scholar]
- Baggiolini M., Hirsch J. G., De Duve C. Resolution of granules from rabbit heterophil leukocytes into distinct populations by zonal sedimentation. J Cell Biol. 1969 Feb;40(2):529–541. doi: 10.1083/jcb.40.2.529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker M. S., Gebicki J. M. The effect of pH on the conversion of superoxide to hydroxyl free radicals. Arch Biochem Biophys. 1984 Oct;234(1):258–264. doi: 10.1016/0003-9861(84)90348-5. [DOI] [PubMed] [Google Scholar]
- Bannister J. V., Bannister W. H., Hill H. A., Thornalley P. J. Enhanced production of hydroxyl radicals by the xanthine-xanthine oxidase reaction in the presence of lactoferrin. Biochim Biophys Acta. 1982 Mar 15;715(1):116–120. doi: 10.1016/0304-4165(82)90056-3. [DOI] [PubMed] [Google Scholar]
- Bannister J. V., Bellavite P., Davoli A., Thornalley P. J., Rossi F. The generation of hydroxyl radicals following superoxide production by neutrophil NADPH oxidase. FEBS Lett. 1982 Dec 27;150(2):300–302. doi: 10.1016/0014-5793(82)80755-2. [DOI] [PubMed] [Google Scholar]
- Bors W., Lengfelder E., Saran M., Fuchs C., Michel C. Reactions of oxygen radical species with methional: a pulse radiolysis study. Biochem Biophys Res Commun. 1976 May 3;70(1):81–87. doi: 10.1016/0006-291x(76)91111-6. [DOI] [PubMed] [Google Scholar]
- Böyum A. Isolation of mononuclear cells and granulocytes from human blood. Isolation of monuclear cells by one centrifugation, and of granulocytes by combining centrifugation and sedimentation at 1 g. Scand J Clin Lab Invest Suppl. 1968;97:77–89. [PubMed] [Google Scholar]
- Diguiseppi J., Fridovich I. Ethylene from 2-keto-4-thiomethyl butyric acid: the Haber-Weiss reaction. Arch Biochem Biophys. 1980 Dec;205(2):323–329. doi: 10.1016/0003-9861(80)90114-9. [DOI] [PubMed] [Google Scholar]
- Green M. R., Hill H. A., Okolow-Zubkowska M. J., Segal A. W. The production of hydroxyl and superoxide radicals by stimulated human neutrophils- measurements by EPR spectroscopy. FEBS Lett. 1979 Apr 1;100(1):23–26. doi: 10.1016/0014-5793(79)81123-0. [DOI] [PubMed] [Google Scholar]
- Gutteridge J. M., Paterson S. K., Segal A. W., Halliwell B. Inhibition of lipid peroxidation by the iron-binding protein lactoferrin. Biochem J. 1981 Oct 1;199(1):259–261. doi: 10.1042/bj1990259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halliwell B., Gutteridge J. M. Formation of thiobarbituric-acid-reactive substance from deoxyribose in the presence of iron salts: the role of superoxide and hydroxyl radicals. FEBS Lett. 1981 Jun 15;128(2):347–352. doi: 10.1016/0014-5793(81)80114-7. [DOI] [PubMed] [Google Scholar]
- Halliwell B., Gutteridge J. M. Oxygen toxicity, oxygen radicals, transition metals and disease. Biochem J. 1984 Apr 1;219(1):1–14. doi: 10.1042/bj2190001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison J. E., Schultz J. Studies on the chlorinating activity of myeloperoxidase. J Biol Chem. 1976 Mar 10;251(5):1371–1374. [PubMed] [Google Scholar]
- Klebanoff S. J., Rosen H. Ethylene formation by polymorphonuclear leukocytes. Role of myeloperoxidase. J Exp Med. 1978 Aug 1;148(2):490–506. doi: 10.1084/jem.148.2.490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matheson N. R., Wong P. S., Travis J. Isolation and properties of human neutrophil myeloperoxidase. Biochemistry. 1981 Jan 20;20(2):325–330. doi: 10.1021/bi00505a015. [DOI] [PubMed] [Google Scholar]
- Repine J. E., Eaton J. W., Anders M. W., Hoidal J. R., Fox R. B. Generation of hydroxyl radical by enzymes, chemicals, and human phagocytes in vitro. Detection with the anti-inflammatory agent, dimethyl sulfoxide. J Clin Invest. 1979 Dec;64(6):1642–1651. doi: 10.1172/JCI109626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Repine J. E., Fox R. B., Berger E. M. Hydrogen peroxide kills Staphylococcus aureus by reacting with staphylococcal iron to form hydroxyl radical. J Biol Chem. 1981 Jul 25;256(14):7094–7096. [PubMed] [Google Scholar]
- Rosen H., Klebanoff S. J. Hydroxyl radical generation by polymorphonuclear leukocytes measured by electron spin resonance spectroscopy. J Clin Invest. 1979 Dec;64(6):1725–1729. doi: 10.1172/JCI109637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segal A. W. How do phagocytic cells kill bacteria? Med Biol. 1984;62(2):81–84. [PubMed] [Google Scholar]
- Selsted M. E., Szklarek D., Lehrer R. I. Purification and antibacterial activity of antimicrobial peptides of rabbit granulocytes. Infect Immun. 1984 Jul;45(1):150–154. doi: 10.1128/iai.45.1.150-154.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutton H. C. Efficiency of chelated iron compounds as catalysts for the Haber-Weiss reaction. J Free Radic Biol Med. 1985;1(3):195–202. doi: 10.1016/0748-5514(85)90118-7. [DOI] [PubMed] [Google Scholar]
- Tauber A. I., Babior B. M. Evidence for hydroxyl radical production by human neutrophils. J Clin Invest. 1977 Aug;60(2):374–379. doi: 10.1172/JCI108786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss S. J., Klein R., Slivka A., Wei M. Chlorination of taurine by human neutrophils. Evidence for hypochlorous acid generation. J Clin Invest. 1982 Sep;70(3):598–607. doi: 10.1172/JCI110652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss S. J., Rustagi P. K., LoBuglio A. F. Human granulocyte generation of hydroxyl radical. J Exp Med. 1978 Feb 1;147(2):316–323. doi: 10.1084/jem.147.2.316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winterbourn C. C. Comparison of superoxide with other reducing agents in the biological production of hydroxyl radicals. Biochem J. 1979 Aug 15;182(2):625–628. doi: 10.1042/bj1820625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winterbourn C. C., Garcia R. C., Segal A. W. Production of the superoxide adduct of myeloperoxidase (compound III) by stimulated human neutrophils and its reactivity with hydrogen peroxide and chloride. Biochem J. 1985 Jun 15;228(3):583–592. doi: 10.1042/bj2280583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winterbourn C. C. Lactoferrin-catalysed hydroxyl radical production. Additional requirement for a chelating agent. Biochem J. 1983 Jan 15;210(1):15–19. doi: 10.1042/bj2100015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winterbourn C. C., Sutton H. C. Iron and xanthine oxidase catalyze formation of an oxidant species distinguishable from OH.: comparison with the Haber-Weiss reaction. Arch Biochem Biophys. 1986 Jan;244(1):27–34. doi: 10.1016/0003-9861(86)90090-1. [DOI] [PubMed] [Google Scholar]



