Abstract
Achyranthes bidentata (A. bidentata) Blume is a medicinal herb with the property of strengthening bones and muscles and ensuring proper downward flow of blood in terms of the therapeutic theory of traditional medicine. In the present study, the effect of A. bidentata root extract (AE) on osteoblast function was investigated in osteoblastic MC3T3-E1 cells. AE caused a significant elevation of alkaline phosphatase activity, collagen synthesis, osteocalcin production, and mineralization in the cells (P < 0.05). AE also decreased the production of TNF-α, IL-6, and RANKL induced by antimycin A, mitochondrial electron transport inhibitor. Exposure of MC3T3-E1 cells to antimycin A caused significant reduction of cell viability and mineralization. However, pretreatment with AE prior to antimycin A exposure significantly reduced antimycin A-induced cell damage by preventing mitochondrial membrane potential dissipation, ATP loss, ROS release, and nitrotyrosine increase, suggesting that AE may be useful for protecting mitochondria against a burst of oxidative stress. Moreover, AE increased the phosphorylation of cAMP-response element-binding protein inhibited by antimycin A. Our study demonstrates that A. bidentata could significantly prevent osteoblast damage in aged patients.
Keywords: Achyranthes bidentata, Differentiation, Mitochondrial dysfunction, MC3T3-E1 cells, Oxidative stress
Introduction
Bone is a permanently regenerating organ which is continually renewed in a complex process of formation and resorption. Bone reorganization begins with the recruitment of osteoclasts. These cells resorb a defined bone volume in a ‘basic multicellular unit’ resulting in a ‘resorption cavity’. By means of a coupling mechanism, osteoblasts are later attracted to fill the resorption lacuna with unmineralized matrix. Primary and secondary mineralization completes the remodelling process (Teitelbaum 2007). Aging is a multi-faceted process associated with several functional and structural deficits as well as major defects of the skeleton itself. The health and activity of the mitochondria are central in the aging process. Mitochondrial free radical generation was lower in long-lived than in short-lived species and oxygen radicals of mitochondrial origin oxidatively damaged mitochondrial DNA in a way related to the rate of aging of each species (Barja and Herrero 2000). Mitochondria energy metabolism is extremely sensitive to impairment by free radicals and mitochondrial oxidative stress limits metabolic recovery (Fiskum et al. 2004). When electrons pass through complexes I–IV of the electron transport chain (ETC), 2–5 % of electrons leak out of the ETC and interact with oxygen to form superoxide (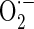 ) in mitochondria, which accounts for about 85 % of total intracellular
) in mitochondria, which accounts for about 85 % of total intracellular 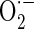 (Droge 2002). Inhibiting complex III of the mitochondrial respiratory chain with antimycin A has been shown to stimulate mitochondrial
(Droge 2002). Inhibiting complex III of the mitochondrial respiratory chain with antimycin A has been shown to stimulate mitochondrial 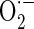 and consequential H2O2 production (Aon et al. 2003), affording means for augmenting mitochondrial reactive oxygen species (ROS).
and consequential H2O2 production (Aon et al. 2003), affording means for augmenting mitochondrial reactive oxygen species (ROS).
Many plant extracts derived from medicinal herbs have proven to be important in modulating physiological and pathological processes in human body, including signal transduction, regulation of gene expression and protein synthesis (Yang et al. 2004). A. bidentata Blume is a medicinal herb with the property of strengthening bones and muscles and ensuring proper downward flow of blood in terms of the therapeutic theory of traditional medicine. It is commonly prescribed for arthritis treatment or immnopotentiation (Xiang and Li 1993). A. bidentata root extract (AE) is rich in active phytochemicals compounds such as saponins (Li et al. 2005), ketosteroids (Zheng et al. 2008) and flavonoids (Stefan et al. 1996). The study of Li et al. (2005) demonstrated that five new oleanolic acid glycosides from A. bidentata could inhibit the formation of osteoclast. Gao et al. demonstrated that ecdysterone from A. bidentata increased osteoblastic activity (Gao et al. 2000). Wattel et al. demonstrated that flavonoid quercetin decreased osteoclastic differentiation (Wattel et al. 2004). According to ancient records, roasted A. bidentata is recommended to reinforce the muscles and bones, improve the tone of the liver and kidneys, promoting blood flow and removing blood stasis, and increase longevity (Meng and Li 2001). Recently, Zhang et al. (2012) reported that 16 weeks of AE treatment improve bone biomechanical quality through modifications of bone mineral density, and trabecular microarchitecture without hyperplastic effect on uterus in ovariectomy-induced osteoporosis in rats. Moreover, it was reported that the polysaccharides from this medicinal plant have oxidative stress protective effect (Xue et al. 2009), anti-HIV activity (Peng et al. 2008) and inhibit non-enzymatic glycation in a d-galactose induced mouse aging model (Deng et al. 2003). In the present study, we investigated the effects of AE on osteoblastic functions and antimycin A-induced cell damage using osteoblastic cell line MC3T3-E1, which is widely used in studies on various aspects of osteoblast differentiation since it expresses osteoblast markers and forms a mineralized extracellular matrix.
Materials and methods
Extraction
Achyranthes bidentata Blume root, purchased from a local Korean medicine grocery (Kyungdong Pharmaceuticals, Hwaseong, Korea) was authenticated by Dr. S.J. Koo at the Kyunghee University. A. bidentata root powder were extracted three times with 70 % ethanol at room temperatures for 3 days each and the combined extracts (AE) were concentrated in vacuo and then freeze dried.
Cell culture
MC3T3-E1 Subclone 4 line was obtained from the ATCC (Manassas, VA, USA). MC3T3-E1 cells were cultured at 37 °C in 5 % CO2 atmosphere in α-modified minimal essential medium (α-MEM; GIBCO, Grand Island, NY, USA). Unless otherwise specified, the medium contained 10 % heat-inactivated fetal bovine serum (FBS), 100 U/ml penicillin and 100 μg/ml streptomycin (all: Sigma Aldrich, St. Louis, MO, USA).
Collagen content
The cells were treated, at confluence, with culture medium containing 5 mM β-glycerophosphate and 50 μg/ml ascorbic acid (all: Sigma Aldrich, St. Louis, MO, USA) to initiate differentiation. After 6 days, the cells were incubated with AE for 48 h. Collagen content was quantified by Sirius Red-based colorimetric assay. Cultured osteoblasts were washed with PBS, followed by fixation with Bouin’s fluid (Sigma Aldrich, St. Louis, MO, USA) for 1 h. After fixation, the fixation fluid was removed and the culture dishes were washed by immersion in running tap water for 15 min. The culture dishes were air dried and stained by Sirius Red dye reagent (Sigma Aldrich, St. Louis, MO, USA) for 1 h under mild shaking on a shaker. Thereafter, the solution was removed and the cultures were washed with 0.01 N HCl to remove non-bound dye. The stained material was dissolved in 0.1 N NaOH and absorbance was measured at 550 nm.
Alkaline phosphatase (ALP) activity
The cells were treated, at confluence, with culture medium containing 5 mM β-glycerophosphate and 50 μg/ml ascorbic acid to initiate differentiation. After 6 days, the cells were incubated with AE for 48 h. Then the cells were lysed with 0.2 % Triton X-100, with the lysate centrifuged at 14,000×g for 5 min. The clear supernatant was used to measure the ALP activity, which was determined using an ALP activity assay kit (Asan Co., Seoul, Korea). Protein concentrations were determined using the BioRad protein assay reagent (Hercules, CA, USA).
Measurement of osteocalcin
The cells were treated, at confluence, with differentiation medium. After 14 days, the cells were cultured with medium containing AE for 2 days. Osteocalcin content in cytosol was measured using a sandwich ELISA assay kit (Biomedical Technologies Inc., Stoughton, MA, USA). Two mouse osteocalcin antibodies are employed, each directed toward an end of the (C or N terminal) osteocalcin molecule. The N-terminal antibody is bound to the well, which binds the mouse osteocalcin standard or sample. The biotin labeled C-terminal mouse osteocalcin antibody completes the sandwich. This sandwich ELISA kit is specific for intact mouse osteocalcin only. Both carboxylated and decarboxylated mouse osteocalcin are recognized.
Calcium deposition assay
The cells were treated, at confluence, with culture medium containing 5 mM β-glycerophosphate and 50 μg/ml ascorbic acid. After 14 days, the cells were cultured with medium containing AE for 2 days. On harvesting, the cells were fixed with 70 % ethanol for 1 h, and then stained with 40 mM Alizarin Red S for 10 min with gentle shaking. To quantify the bound dye, the stain was solubilized with 10 % cetylpyridinum chloride (Sigma Aldrich) by shaking for 15 min. The absorbance of the solubilized stain was measured at 561 nm.
Cell viability
The cells were treated, at confluence, with culture medium containing 5 mM β-glycerophosphate and 50 μg/ml ascorbic acid to initiate differentiation. After 6 days, the cells were treated with AE and after 1 h antimycin A was added. After 48 h, surviving cells were counted by the MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) method. MTT 20 μl in 7.2 mM phosphate buffer solution, pH 6.5 (5 mg/ml), was added to each well, and the plates were incubated for an additional 2 h. After the removal of the solution in well, dimethyl sulfoxide was added to dissolve formazan products, and the plates were shaken for 5 min. The absorbance of each well was recorded on a microplate spectrophotometer at 570 nm.
Measurement of phosphorylated CREB (cAMP-response element-binding protein)
Phosphorylated CREB was evaluated using Cell-Based Phospho-CREB (S133) Immunoassay (R&D Systems, Minneapolis, MN, USA). This cell-Based ELISA contains the components required to run an ELISA using fluorogenic substrates to measure phosphorylated CREB (S133) in whole cells. Following stimulation with agents, cells are fixed and permeabilized in the wells. The target protein phosphorylation is measured using a double immunoenzymatic labeling procedure. The cells are simultaneously incubated with two primary antibodies: a phospho-specific antibody and a normalization antibody that recognizes the pan-protein regardless of phosphorylation status. The primary antibodies are derived from different species. Two secondary antibodies recognizing different species are labeled with either horseradish-peroxidase (HRP) or alkaline phosphatase (ALP), and two spectrally distinct fluorogenic substrates for either HRP or AP are used for detection. The fluorescence of the phosphorylated protein is normalized to that of the pan-protein in each well for the correction of well-to-well variations.
Determination of mitochondrial membrane potential (MMP)
JC-1 mitochondrial membrane potential assay kit (Cayman Chemical Co., Ann Arbor, MI, USA) was used to demonstrate the changes in the MMP in cells. JC-1 is a lipophilic and cationic dye, which permeates plasma and mitochondrial membranes. The dye fluoresces red in healthy mitochondria with high membrane potential, whereas it fluoresces green in mitochondria with diminished membrane potential. Cells were incubated with the MMP-sensitive fluorescent dye JC-1 for 20 min at 37 °C, washed twice in PBS, and then the “red” (excitation 550 nm, emission 600 nm) and “green” (excitation 485 nm, emission 535 nm) fluorescence were measured using a Fluorescence microplate reader (Molecular Devices, Sunnyvale, CA, USA). Mitochondrial depolarization (i.e., loss of MMP) manifests itself by a decrease in the red/green fluorescence ratio.
Measurement of ATP level and mitochondrial complex IV activity
The ATP concentration was determined by luciferase reaction using BioAssay Systems’ EnzyLight™ ATP Assay Kit (BioAssay Systems, Hayward, CA, USA). This ATP assay kit provides a rapid method to measure intracellular ATP. The Microplate Assay for Complex IV Activity (MitoSciences, Eugene, OR, USA) was used to determine the activity of the cytochrome c oxidase enzyme. Protein concentrations were determined using the BioRad protein assay reagent.
Measurement of intracellular ROS
Cells were loaded with 5 μM 2′,7′-dichlorofluorescin diacetate (DCFH-DA) (Sigma Aldrich) for 1 h. Following washing with DPBS (Dulbecco's Phosphate-Buffered Saline), ROS levels were determined by measuring the fluorescent intensity at excitation wavelength 485 nm and emission wavelength 530 nm.
Measurement of mitochondrial 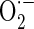
Mitochondrial 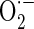 levels were detected using MitoSOX™ Red mitochondrial
levels were detected using MitoSOX™ Red mitochondrial 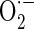 indicator (Invitrogen Molecular Probes, Carlsbad, CA, USA). MitoSOX™ Red (Ex/Em = 510 nm/580 nm) is a fluorogenic dye for highly selective detection of
indicator (Invitrogen Molecular Probes, Carlsbad, CA, USA). MitoSOX™ Red (Ex/Em = 510 nm/580 nm) is a fluorogenic dye for highly selective detection of 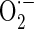 in the mitochondria of cells. Cells were incubated with 5 μM MitoSOX™ Red at 37 °C for 20 min according to the instructions of the manufacturer. After the washing of cells, the MitoSOX™ Red fluorescences were detected.
in the mitochondria of cells. Cells were incubated with 5 μM MitoSOX™ Red at 37 °C for 20 min according to the instructions of the manufacturer. After the washing of cells, the MitoSOX™ Red fluorescences were detected.
Measurement of cardiolipin peroxidation
10-N-nonyl-Acridin Orange (NAO, Molecular Probes, Inc., Eugene, OR, USA), which binds to mitochondria-specific cardiolipin, was used for the determination of cardiolipin. Decreases in the fluorescence of NAO in cells have been reported to reflect the peroxidation of intracellular cardiolipin because the fluorochrome loses its affinity for peroxidised cardiolipin. Cells were labelled with 5 μM NAO for 20 min. After washing twice, fluorescence was measured at excitation wavelength 485 nm and emission wavelength 530 nm.
Measurement of nitrotyrosine content
Cells were lysed by homogenization in lysis buffer [20 mM MOPS, 50 mM sodium fluoride, 1 mM sodium vanadate, 5 mM EGTA, 2 mM EDTA, 1 % NP-40, 1 mM DTT, 1 mM benzamidine, 1 mM PMSF, and 1 % protease inhibitor cocktail (pH 7.2) (all: Sigma Aldrich)]. After centrifugation at 10,000g for 15 min at 4 °C, supernatant was used as cell lysates for assay. Nitrotyrosine content was measured by using an ELISA kit (Northwest Life Science, Vancouver, WA, USA).
Measurement of RANKL, TNF-α, and IL-6
The cells were treated, at confluence, with differentiation medium to initiate differentiation. After 6 days, the cells were pre-incubated with AE for 1 h before treatment with antimycin A for 48 h. Receptor activator of NF-κB ligand (RANKL), tumor necrosis factor-alpha (TNF-α), and interleukin-6 (IL-6) contents in the medium were measured using ELISA kits (R&D system Inc., Minneapolis, MN, USA) according to the manufacturer’s recommendation.
Statistical analysis
The results are expressed as mean ± SEM. Statistical significance was determined by analysis of variance and subsequently applying the Dunnett’s t test (P < 0.05).
Results and discussion
Effect of AE on the function of MC3T3-E1 cells
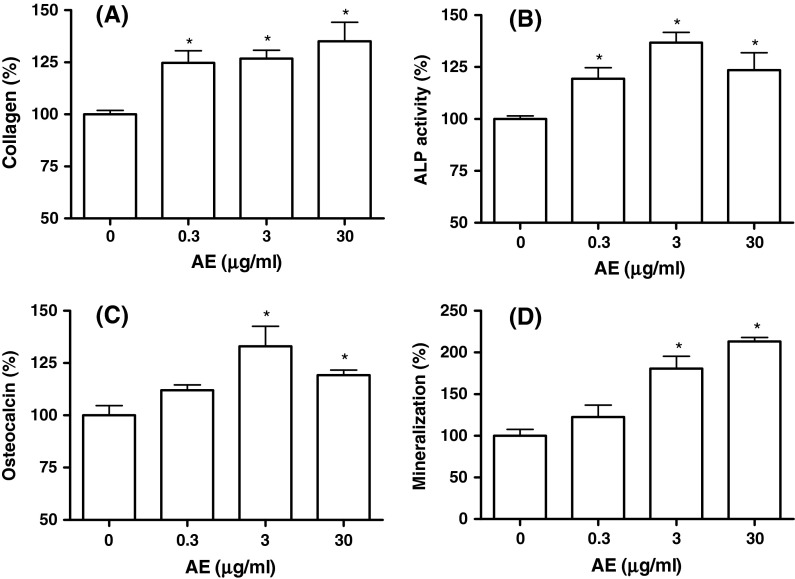
The decrease in osteoblastic differentiation is an important factor in the pathogenesis of osteoporosis. In order to assess the effects of AE on differentiation and mineralization of MC3T3-E1 cells, collagen content, ALP activity, osteocalcin release, and calcium deposition were assayed (Fig. 1). Collagen is an early osteoblastic marker, and as shown in Fig. 1a, incubation of cell with AE (0.3–30 μg/ml) increased collagen synthesis significantly. We then examined the activity of ALP, an early-stage osteogenic differentiation marker, in MC3T3-E1 cells. The cultured cells in the presence of AE (0.3–30 μg/ml) caused a significant increase in the ALP activity of osteoblastic cells (Fig. 1b). When osteocalcin, a late-stage osteogenic differentiation marker, was measured in cytosol, AE (3 and 30 μg/ml) supplementation significantly increased the osteocalcin secretion compared with control (Fig. 1c). Matrix mineralization, the final step in osteoblast differentiation, plays a critical role in maintaining the mechanical integrity of bone tissues. To detect the effect of AE on mineralization, a late-stage osteogenic differentiation marker, Alizarin red S staining was performed in MC3T3-E1 cells. As shown in Fig. 1d, the increase of mineralization was significant at AE concentrations of 3 and 30 μg/ml in MC3T3-E1 cell culture. These results suggest that AE may induce osteogenic differentiation throughout the processes from the early to late phase.
Fig. 1.
Effect of A. bidentata extract (AE) on the differentiation of MC3T3-E1 cells. The measured values are converted to % of control group. The control values for a collagen content, b ALP activity, c osteocalcin, and d mineralization were 23.45 ± 0.659 μg/106 cells, 0.871 ± 0.016 Unit/mg protein, 42.09 ± 1.958 ng/mg protein, and 0.504 ± 0.006 OD/106 cells, respectively. *P < 0.05 compared with control
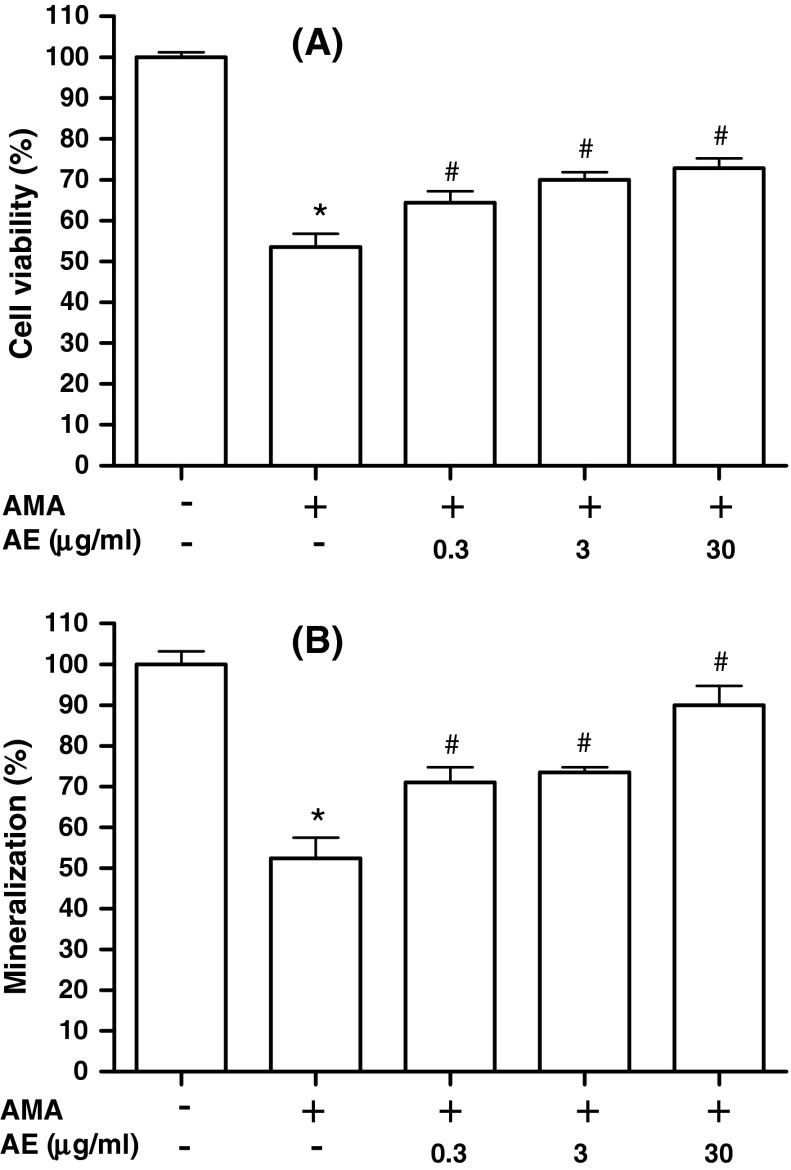
We determined whether AE inhibits the osteoblast dysfunction induced by antimycin A, a mitochondrial inhibitor that increases the generation of ROS. In our system, IC50 for antimycin A in MC3T3-E1 cells was 70 μM, and this concentration was chosen as the optimal dose of antimycin A for the experiments of antimycin A-induced cell damages. When the cells were pretreated with AE in the presence of 70 μM antimycin A, AE (0.3–30 μg/ml) significantly suppressed the antimycin A induced cytotoxicity (Fig. 2a). To investigate the effect of AE on the osteoblast dysfunction induced by antimycin A, calcium deposition was determined by Alizarin Red staining. AE (0.3–30 μg/ml) showed significant recovery effect on mineralization inhibited by antimycin A (Fig. 2b). Our results demonstrate that AE attenuates antimycin A induced dysfunction of osteoblasts.
Fig. 2.
Effect of A. bidentata extract (AE) on the cell viability and mineralization of osteoblasts in the presence of antimycin A (AMA). The cells were treated, at confluence, with culture medium containing 5 mM β-glycerophosphate and 50 μg/ml ascorbic acid to initiate differentiation. After 6 days or 14 days for MTT assay (a) and mineralization (b), respectively, the cells were pre-incubated with AE for 1 h before treatment with 70 μM antimycin A for 48 h. Data were expressed as percentage of control. *P < 0.05; control versus AMA, # P < 0.05; AMA versus AE
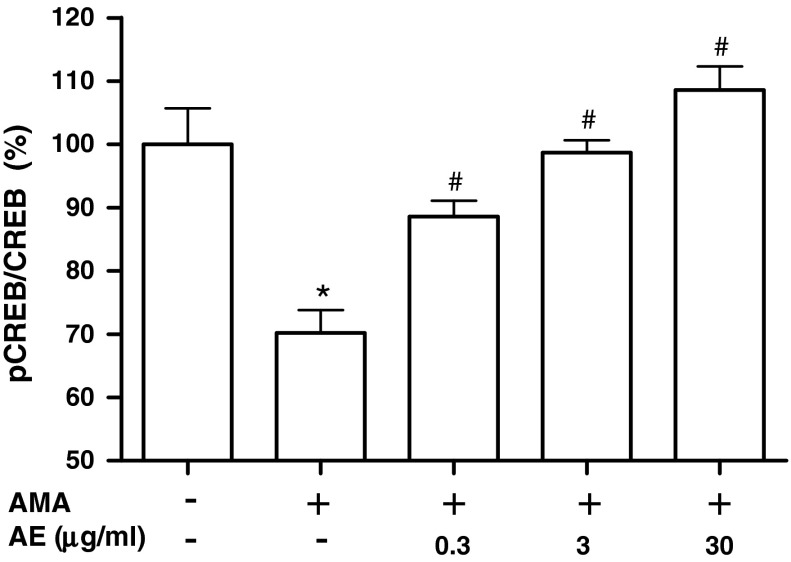
Effect of AE on the CREB phosphorylation of osteoblasts in the presence of antimycin A
As CREB are known to function as pro-survival signal, we determined whether CREB activation is related to the cytoprotective effect of AE in osteoblastic MC3T3-E1 cells. Incubating with antimycin A decreased phosphorylation of CREB and pretreatment with AE (0.3–30 μg/ml) enhanced the antimycin A-inhibited phosphorylation of CREB (Fig. 3). This result suggests that CREB are related to the protective effect of AE. An et al. (2010) have demonstrated that markers of mitochondrial biogenesis are significantly upregulated during osteoblastic differentiation of a murine embryonic mesenchymal cell line, suggesting that mitochondrial biogenesis may play an important role in the osteoblastic differentiation. In their report, mitochondrial biogenesis is mediated by upregulation of peroxisome proliferator-activated receptor gamma coactivator (PGC)-1α. PGC-1α has been known as a key master controller of mitochondrial biogenesis and respiration (Scarpulla 2008). Handschin et al. (2003) reported that CREB signaling regulated PGC-1α expression. Cammarota et al. (1999) presented that CREB is localized in the inner mitochondrial compartment as well as in the nucleus. Mitochondrial CREB appears to regulate pro-survival effects, at least in part, by regulating mitochondrial genes whose expression may be controlled by CRE-like elements within the D-loop of the mitochondrial genome (Ryu et al. 2005). Our results suggest that the protective effect of AE on the cell damage induced by antimycin A is related to the enhancement of CREB phosphorylation.
Fig. 3.
Effect of A. bidentata extract (AE) on the CREB phosphorylation of osteoblastic MC3T3-E1 cells in the presence of antimycin A. Osteoblasts were treated with AE in the presence of 70 μM antimycin A (AMA) for 48 h. Data are expressed as percentage of the control. The control values for phosphorylated CREB was 0.46 ± 0.026 phospho-CREB/total CREB. *P < 0.05; control versus AMA, # P < 0.05; AMA versus AE
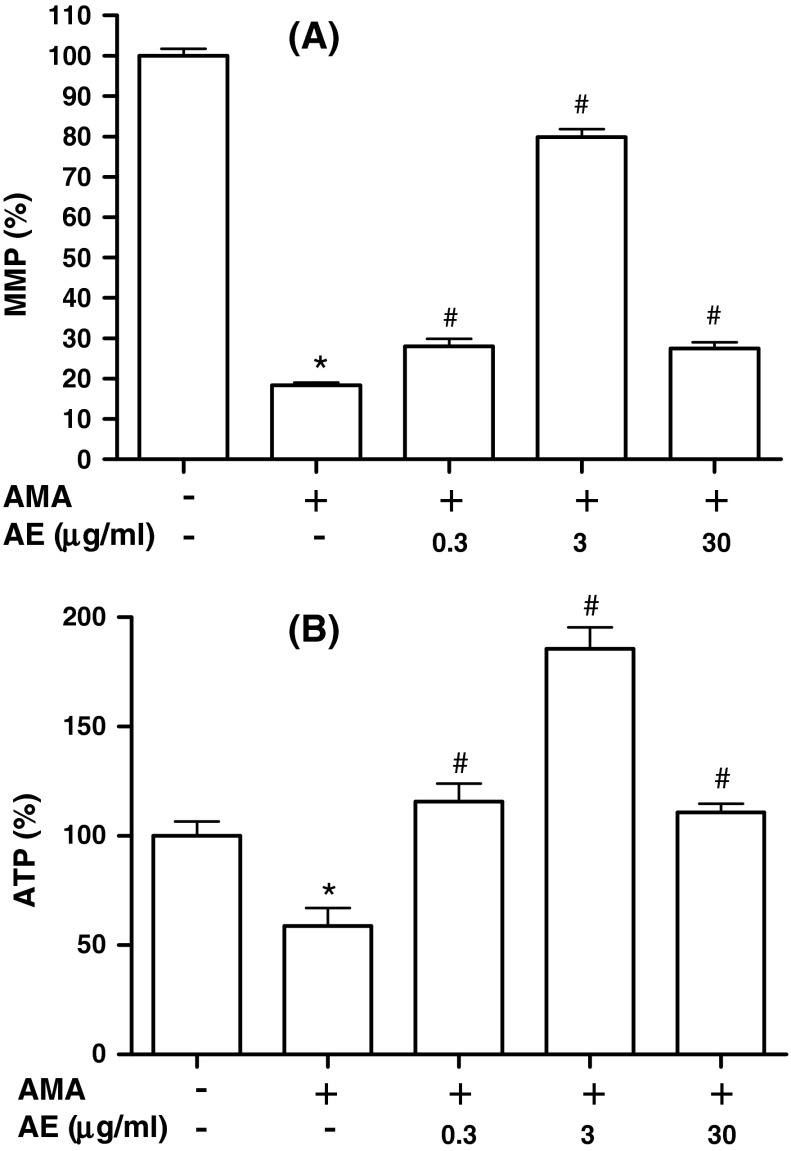
Effect of AE on the antimycin A-induced mitochondrial dysfunction in MC3T3-E1 cells
Mitochondrial membrane integrity is essential for normal cell function. When cells are subjected to oxidative stress, mitochondria lose the membrane integrity, initiating a series of signaling events leading to cell death. To investigate the mitochondrial function of osteoblasts, mitochondrial membrane potential (MMP) and ATP content were assayed after osteoblasts were treated with antimycin A in the presence or absence of AE. MMP is a marker of mitochondrial oxidative phophorylation activity that can be assessed using fluorescent probes accumulating in mitochondria depending on the MMP. JC-1 is a fluorescent dye that is concentrated by respiring mitochondria. It exists as a monomer that fluoresces green and forms J-aggregates with red fluorescence at low and high MMP, respectively. In this study, MMP was measured after osteoblasts were stimulated with antimycin A following pre-treatment with AE (Fig. 4a). The decrease in MMP occurred after exposure to antimycin A and AE (0.3–30 μg/ml) abrogated the reduction of MMP by antimycin A, suggesting that AE pretreatment retained the membrane potential of these cells, thereby preventing cells from undergoing cell death. The levels of ATP were measured using a standard luminescent luciferin/luciferase assay. As illustrated in Fig. 4b, the ATP level of osteoblasts decreased after treatment with antimycin A. However, AE (0.3–30 μg/ml) pre-treatment significantly increased intracellular ATP levels when compared with the antimycin A alone group. The effect of AE seems to be weakened at 30 μg/ml compared to 3 μg/ml, suggesting that a low concentration of AE is more effective on osteoblastic cells.
Fig. 4.
Protective effect of A. bidentata extract (AE) against the antimycin A-induced mitochondrial dysfunction in osteoblastic MC3T3-E1 cells. Osteoblasts were pre-incubated with AE before treatment with 50 μM antimycin A (AMA) for 48 h. Data were expressed as percentage of control. The control values for a mitochondrial membrane potential (MMP) b ATP level were 1.58 ± 0.06 red:green ratio, 18.96 ± 1.55 mOD/min/mg, and 0.582 ± 0.036 nmole/105 cells, respectively. *P < 0.05; control versus AMA, # P < 0.05; AMA versus AE
The mitochondrion is a key organelle in the control of cell death, and a collapse in MMP has been associated with this process (Kroemer and Reed 2000). During cell respiration, the flow of electrons through the respiratory chain is used ultimately to reduce oxygen. This process is coupled to the extrusion of H+ from the mitochondrial matrix to the intermembrane space, generating an electrochemical gradient also known as the proton motive force. The energy stored in this gradient can be used to drive the synthesis of ATP by ATP synthase as well as other energy-requiring mitochondrial activities (Boyer 1975). Thus MMP is a good indicator of the energy status of the mitochondrion in particular and of cellular homeostasis in general. Opening of the mitochondrial transition pore (MTP) allows the passage of any molecule of >1,500 Da across the inner mitochondrial membrane. Because the MTP also allows rapid passage of protons, its opening is accompanied by depolarization of the mitochondria and uncoupling of oxidative phosphorylation. In addition, the equilibration of small solutes across the inner mitochondrial membrane leaves behind high concentrations of proteins in the matrix and these exert a colloidal osmotic pressure that is responsible for the extensive swelling of mitochondria associated with MTP opening (Bernardi 1999).
Cellular antioxidant effect of AE in osteoblastic MC3T3-E1 cells
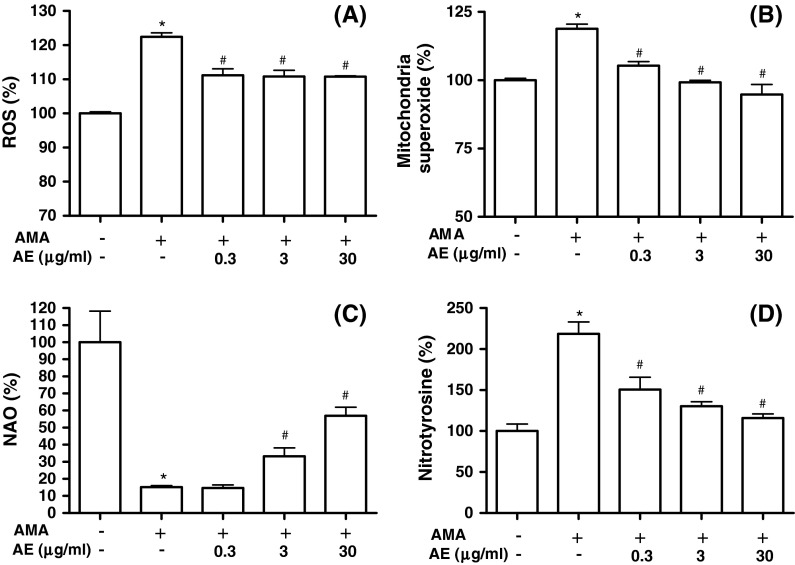
Mitochondria-mediated ROS generation leads to primary reaction and damage in the immediate area surrounding where these ROS are produced due to their highly reactive and short-lived life span. Therefore, as major sources of ROS production, mitochondria could also be major targets of ROS attack. To investigate whether cytoprotective action of AE is related to its antioxidant activity, we measured ROS production in MC3T3-E1 cells. As shown in Fig. 5a, ROS generation was increased in antimycin A-treated cells, and was suppressed by treatment with 0.3–30 μg/ml AE. Increased mitochondrial ROS production has traditionally been attributed to deficits in electron transport chain function. To examine whether AE suppresses antimycin A-induced mitochondrial 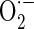 production, we assessed the changes of mitochondrial
production, we assessed the changes of mitochondrial 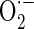 production using MitoSOX, a live-cell-permeable and mitochondrial localizing
production using MitoSOX, a live-cell-permeable and mitochondrial localizing 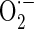 indicator. Mitochondrial MitoSOX fluorescence was increased by antimycin A treatment; however AE (0.3–30 μg/ml) pretreatment inhibited mitochondrial
indicator. Mitochondrial MitoSOX fluorescence was increased by antimycin A treatment; however AE (0.3–30 μg/ml) pretreatment inhibited mitochondrial 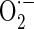 production increased by antimycin A (Fig. 5b). To study if peroxidation of cardiolipin took place in antimycin A-treated cells, binding of NAO, a fluorescent probe that binds specifically to cardiolipin, was measured in cells. Results showed a decrease of NAO binding to antimycin A-treated cells, which reflect the peroxidation of mitochondrial cardiolipin (Fig. 5c). However, AE (3 and 30 μg/ml) reduced cardiolipin peroxidation induced by antimycin A. Cardiolipin, a phospholipid located almost uniquely within the inner mitochondrial membrane, is sensitive to oxidative stress because rich in unsaturated fatty acids. Thus, mitochondrial cardiolipin molecules are a possible early target of ROS attack, either because of their high content of unsaturated fatty acids or because of their location in inner mitochondrial membrane near to the site of ROS production, mainly at the level of complexes of the respiratory chain (Paradies et al. 2002). With the correlation of ROS production, the content of cardiolipin, and the activities of respiratory chain complexes (Paradies et al. 2004), our study also demonstrated that AE can limit the source of ROS and reduce its generation by restoring the activity of complexes, whose optimal activity requires a cardiolipin rich environment in the inner mitochondrial membrane. AE treatment leads to decreased ROS generation and less cardiolipin peroxidation, supporting the fact that AE provided cytoprotection by manipulating mitochondrial function during mitochondrial dysfunction.
production increased by antimycin A (Fig. 5b). To study if peroxidation of cardiolipin took place in antimycin A-treated cells, binding of NAO, a fluorescent probe that binds specifically to cardiolipin, was measured in cells. Results showed a decrease of NAO binding to antimycin A-treated cells, which reflect the peroxidation of mitochondrial cardiolipin (Fig. 5c). However, AE (3 and 30 μg/ml) reduced cardiolipin peroxidation induced by antimycin A. Cardiolipin, a phospholipid located almost uniquely within the inner mitochondrial membrane, is sensitive to oxidative stress because rich in unsaturated fatty acids. Thus, mitochondrial cardiolipin molecules are a possible early target of ROS attack, either because of their high content of unsaturated fatty acids or because of their location in inner mitochondrial membrane near to the site of ROS production, mainly at the level of complexes of the respiratory chain (Paradies et al. 2002). With the correlation of ROS production, the content of cardiolipin, and the activities of respiratory chain complexes (Paradies et al. 2004), our study also demonstrated that AE can limit the source of ROS and reduce its generation by restoring the activity of complexes, whose optimal activity requires a cardiolipin rich environment in the inner mitochondrial membrane. AE treatment leads to decreased ROS generation and less cardiolipin peroxidation, supporting the fact that AE provided cytoprotection by manipulating mitochondrial function during mitochondrial dysfunction.
Fig. 5.
Cellular antioxidant effect of A. bidentata extract (AE) in osteoblastic MC3T3-E1 cells. Osteoblasts were pre-incubated with AE before treatment with 70 μM antimycin A (AMA) for 48 h. a The data show changes in levels of ROS, which was measured by DCF fluorescence method. b Mitochondrial 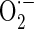 levels were detected using MitoSOX™ Red mitochondrial
levels were detected using MitoSOX™ Red mitochondrial 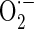 indicator. c Cardiolipin oxidation was measured using 5 μM NAO. Data are expressed as relative percentages of the fluorescence emitted by bound NAO with respect to non-treated control cells. Note that AMA treatment gives rise to a decrease in NAO binding, which is related with cardiolipin peroxidation. d Nitrotyrosine content was measured by using an ELISA assay. Data were expressed as percentage of control. The control value for intracellular nitrotyrosine was 1.05 ± 0.05 pmol/mg. *P < 0.05; control versus AMA, #
P < 0.05; AMA versus AE
indicator. c Cardiolipin oxidation was measured using 5 μM NAO. Data are expressed as relative percentages of the fluorescence emitted by bound NAO with respect to non-treated control cells. Note that AMA treatment gives rise to a decrease in NAO binding, which is related with cardiolipin peroxidation. d Nitrotyrosine content was measured by using an ELISA assay. Data were expressed as percentage of control. The control value for intracellular nitrotyrosine was 1.05 ± 0.05 pmol/mg. *P < 0.05; control versus AMA, #
P < 0.05; AMA versus AE
The presence of nitrotyrosine is thought to represent oxidative damage of cellular proteins (Aulak et al. 2001). Therefore, we investigated whether AE could reduce antimycin A-induced production of nitrotyrosine (Fig. 5d). Antimycin A markedly increased the production of nitrotyrosine, whereas pretreatment with AE (0.3–30 μg/ml) prevented nitrotyrosine production increased by antimycin A. 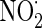 is a powerful oxidant that can nitrate tyrosine protein residues to form 3-nitrotyrosine. At higher concentrations, NO promotes ubiquinol autooxidation with the concomitant production of O2−, which then reacts with NO, in a diffusion-limited reaction, to form the reactive nitrogen species peroxynitrite (ONOO−) (Poderoso et al. 1999). ONOO−, as a strong oxidant, has the potential to cause persistent inhibition of complex I (due to nitration of the complex), followed by inhibition of complex II (due to iron removal from ironsulfur clusters), complex IV, the ATP synthase, MnSOD, and other proteins, and to promote mitochondrial permeability transition pore opening, cytochrome c release, and cell death (Cadenas 2004). Our data support the hypothesis that the cytoprotective effect of AE may be associated with its antioxidative capacity.
is a powerful oxidant that can nitrate tyrosine protein residues to form 3-nitrotyrosine. At higher concentrations, NO promotes ubiquinol autooxidation with the concomitant production of O2−, which then reacts with NO, in a diffusion-limited reaction, to form the reactive nitrogen species peroxynitrite (ONOO−) (Poderoso et al. 1999). ONOO−, as a strong oxidant, has the potential to cause persistent inhibition of complex I (due to nitration of the complex), followed by inhibition of complex II (due to iron removal from ironsulfur clusters), complex IV, the ATP synthase, MnSOD, and other proteins, and to promote mitochondrial permeability transition pore opening, cytochrome c release, and cell death (Cadenas 2004). Our data support the hypothesis that the cytoprotective effect of AE may be associated with its antioxidative capacity.
Effect of AE on antimycin A induced cytokines production in MC3T3-E1 cells
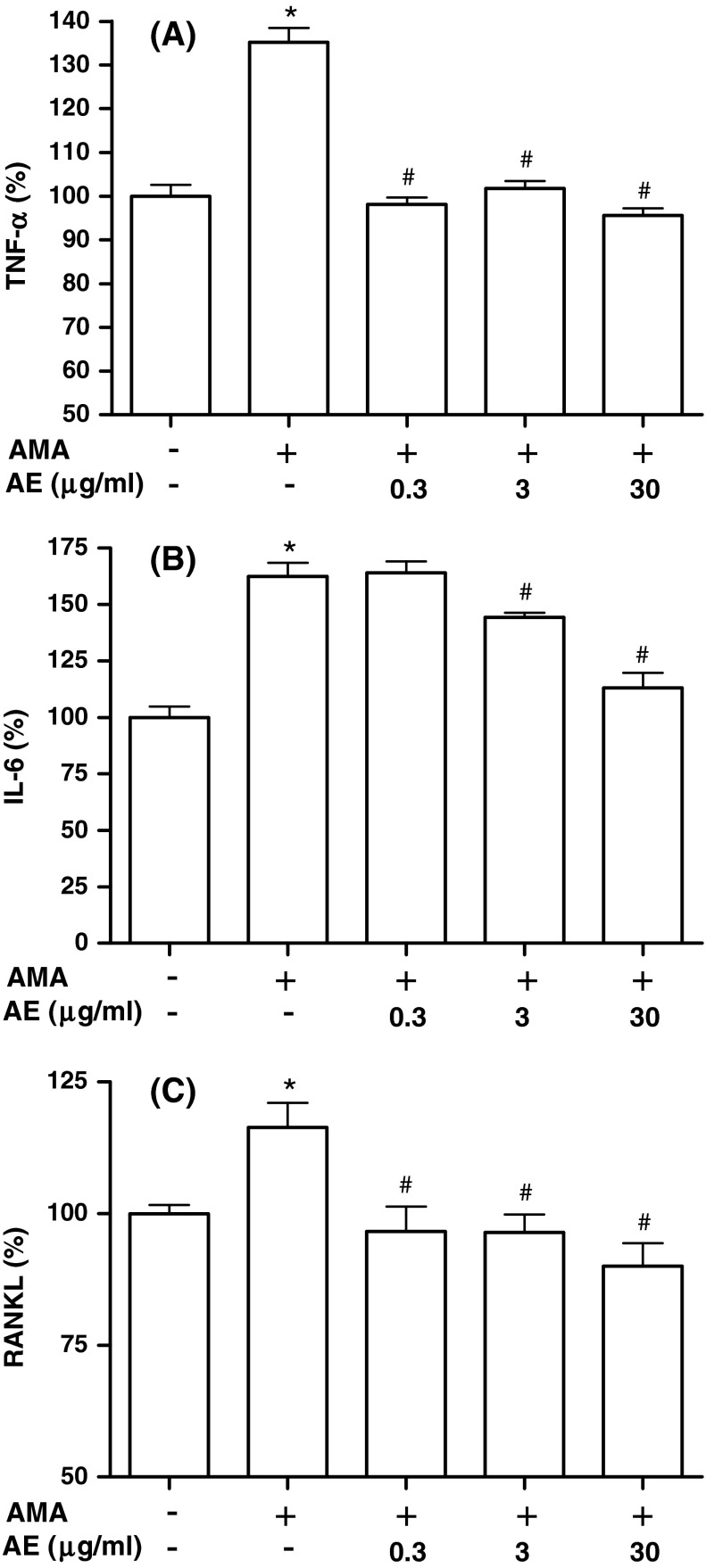
TNF-α and IL-6 have been demonstrated to increase osteoclastic activity. Thus, we investigated whether AE modulates antimycin A-induced production of TNF-α and IL-6 in MC3T3-E1 cells (Fig. 6a, b). When 70 μM antimycin A was added to the cells, production of TNF-α and IL-6 increased significantly. However, antimycin A-induced TNF-α and IL-6 productions were significantly inhibited by treatment of AE at 0.3–30 and 3–30 μg/ml, respectively. In order to further determine the regulator of osteoclast differentiation in osteoblasts, we examined the production of RANKL in the osteoblastic MC3T3-E1 cells. When 70 μM antimycin A was added to cells, the production of RANKL increased significantly (Fig. 6c). However, antimycin A-induced RANKL production was significantly inhibited by treatment of AE (0.3–30 μg/ml). RANKL produced by osteoblastic lineage cells and activated T lymphocytes are key signals of osteoclast formation, fusion, activation and survival, thus resulting in bone resorption and bone loss (Hofbauer and Heufelder 2001). Several family members are produced by bone cells and have been implicated to be involved in regulation of bone metabolism. In the present report, we showed that inflammatory cytokines releases induced by antimycin A were decreased by AE. The inhibitory effect on these cytokines production by AE may contribute to the bone anti-resorbing effect of AE, and possibly also play a role in the reduction of bone loss seen in the vicinity of inflammatory processes such as rheumatoid arthritis and periodontal disease.
Fig. 6.
Effect of A. bidentata extract (AE) on antimycin A-induced cytokines production of MC3T3-E1 cells. Osteoblasts were pre-incubated with AE before treatment with 70 μM antimycin A (AMA) for 48 h. Data were expressed as percentage of control. The control values for TNF-α (a), IL-6 (b), and RANKL (c) were 0.228 ± 0.006, 0.655 ± 0.033, and 3.378 ± 0.074 ng/mg, respectively. *P < 0.05; control versus AMA, # P < 0.05; antimycin A versus AE
Conclusion
In summary, AE stimulated ALP activity and bone matrix proteins, such as collagen and osteocalcin, and these increases trigger mineralized nodule formation. Moreover, our data indicate that AE suppresses the production of the bone resorbing factors in the presence of antimycin A, a mitochondrial inhibitor that increases the generation of ROS. AE protected osteoblasts from antimycin A-induced cell damage via improved mitochondrial function and activation of CREB. The results indicate that A. bidentata may protect oxidative stress-induced damage and dysfunction in osteoblasts.
Acknowledgments
This research was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (2013004361).
Conflict of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- An JH, Yang JY, Ahn BY, Cho SW, Jung JY, Cho HY, Cho YM, Kim SW, Park KS, Kim SY, Lee HK, Shin CS. Enhanced mitochondrial biogenesis contributes to Wnt induced osteoblastic differentiation of C3H10T1/2 cells. Bone. 2010;47:140–150. doi: 10.1016/j.bone.2010.04.593. [DOI] [PubMed] [Google Scholar]
- Aon MA, Cortassa S, Marban E, O’Rourke B. Synchronized whole cell oscillations in mitochondrial metabolism triggered by a local release of reactive oxygen species in cardiac myocytes. J Biol Chem. 2003;278:44735–44744. doi: 10.1074/jbc.M302673200. [DOI] [PubMed] [Google Scholar]
- Aulak KS, Miyagi M, Yan L, West KA, Massillon D, Crabb JW, Stuehr DJ. Proteomic method identifies proteins nitrated in vivo during inflammatory challenge. Proc Natl Acad Sci USA. 2001;98:12056–12061. doi: 10.1073/pnas.221269198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barja G, Herrero A. Oxidative damage to mitochondrial DNA is inversely related to maximum life span in the heart and brain of mammals. FASEB J. 2000;14:312–318. doi: 10.1096/fasebj.14.2.312. [DOI] [PubMed] [Google Scholar]
- Bernardi P. Mitochondrial transport of cations: channels, exchangers and permeability transition. Physiol Rev. 1999;79:1127–1155. doi: 10.1152/physrev.1999.79.4.1127. [DOI] [PubMed] [Google Scholar]
- Boyer PD. A model for conformational coupling of membrane potential and proton translocation to ATP synthesis and to active transport. FEBS Lett. 1975;58:1–6. doi: 10.1016/0014-5793(75)80212-2. [DOI] [PubMed] [Google Scholar]
- Cadenas E. Mitochondrial free radical production and cell signaling. Mol Aspects Med. 2004;25:17–26. doi: 10.1016/j.mam.2004.02.005. [DOI] [PubMed] [Google Scholar]
- Cammarota M, Paratcha G, Bevilaqua LR, Levi de Stein M, Lopez M, Pellegrino de Iraldi A, Izquierdo I, Medina JH, Pellegrinode Iraldi A, Izquierdo I, Medina JH. Cyclic AMP-responsive element binding protein in brain mitochondria. J Neurochem. 1999;72:2272–2277. doi: 10.1046/j.1471-4159.1999.0722272.x. [DOI] [PubMed] [Google Scholar]
- Deng HB, Cui DP, Jiang JM, Feng YC, Cai NS, Li DD. Inhibiting effects of Achyranthes bidentata polysaccharide and Lycium barbarum polysaccharide on nonenzyme glycation in D-galactose induced mouse aging model. Biomed Environ Sci. 2003;6:267–275. [PubMed] [Google Scholar]
- Droge W. Free radicals in the physiological control of cell function. Physiol Rev. 2002;82:47–95. doi: 10.1152/physrev.00018.2001. [DOI] [PubMed] [Google Scholar]
- Fiskum G, Rosenthal RE, Vereczki V, Martin E, Hoffman GE, Chinopoulos C, Kowaltowski A. Protection against ischemic brain injury by inhibition of mitochondrial oxidative stress. J Bioenerg Biomembr. 2004;36:347–352. doi: 10.1023/B:JOBB.0000041766.71376.81. [DOI] [PubMed] [Google Scholar]
- Gao XY, Wang DW, Li FM. Determination of ecdysterone in Achyranthes bidentata BL. and its activity promoting proliferation of osteoblast-like cells. Yao Xue Xue Bao. 2000;35:868–870. [PubMed] [Google Scholar]
- Handschin C, Rhee J, Lin J, Tarr PT, Spiegelman BM. An autoregulatory loop controls peroxisome proliferator-activated receptor gamma coactivator 1 alpha expression in muscle. Proc Natl Acad Sci USA. 2003;100:7111–7116. doi: 10.1073/pnas.1232352100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofbauer LC, Heufelder AE. Role of receptor activator of nuclear factor-kB ligand and osteoprotegerin in bone cell biology. J Mol Med. 2001;79:243–253. doi: 10.1007/s001090100226. [DOI] [PubMed] [Google Scholar]
- Kroemer G, Reed JC. Mitochondrial control of cell death. Nat Med. 2000;6:513–519. doi: 10.1038/74994. [DOI] [PubMed] [Google Scholar]
- Li JX, Hareyama T, Tezuka Y, Zhang Y, Miyahara T, Kadota S. Five new oleanolic acid glycosides from Achyranthes bidentata with inhibitory activity on osteoclast formation. Planta Med. 2005;71:673–679. doi: 10.1055/s-2005-871275. [DOI] [PubMed] [Google Scholar]
- Meng DL, Li X. The research development in the chemical constituents and pharmacological activities of Achyranthes bidentata Bl. Chin J Med Chem. 2001;11:120–124. [Google Scholar]
- Paradies G, Petrosillo G, Pistolese M, Ruggiero FM. Reactive oxygen species affect mitochondrial electron transport complex I activity through oxidative cardiolipin damage. Gene. 2002;286:135–141. doi: 10.1016/S0378-1119(01)00814-9. [DOI] [PubMed] [Google Scholar]
- Paradies G, Petrosillo G, Pistolese M, Di Venosa N, Federici A, Ruggiero FM. Decrease in mitochondrial complex I activity in ischemic/reperfused rat heart: involvement of reactive oxygen species and cardiolipin. Circ Res. 2004;94:53–59. doi: 10.1161/01.RES.0000109416.56608.64. [DOI] [PubMed] [Google Scholar]
- Peng ZG, Chen HS, Guo ZM, Dong B, Tian GY, Wang GQ. Anti-HIV activities of Achyranthes bidentata polysaccharide sulfate in vitro and in vivo. Yaoxue Xuebao. 2008;43:702–706. [PubMed] [Google Scholar]
- Poderoso JJ, Lisdero C, Schopfer F, Riobó N, Carreras MC, Cadenas E, Boveris A. The regulation of mitochondrial oxygen uptake by redox reactions involving nitric oxide and ubiquinol. J Biol Chem. 1999;274:37709–37716. doi: 10.1074/jbc.274.53.37709. [DOI] [PubMed] [Google Scholar]
- Ryu H, Lee J, Impey S, Ratan RR, Ferrante RJ. Antioxidants modulate mitochondrial PKA and increase CREB binding to D-loop DNA of the mitochondrial genome in neurons. Proc Natl Acad Sci USA. 2005;102:13915–13920. doi: 10.1073/pnas.0502878102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scarpulla RC. Transcriptional paradigms in mammalian mitochondrial biogenesis and function. Physiol Rev. 2008;88:611–638. doi: 10.1152/physrev.00025.2007. [DOI] [PubMed] [Google Scholar]
- Stefan N, Ngugen T, Valcho Z (1996) Flavonoids from Achyranthes bidentata BI. International Symposium on Medicinal and Aromatic Plants, ISHS Acta Horticulturae 426
- Teitelbaum SL. Osteoclasts: what do they do and how do they do it? Am J Pathol. 2007;170:427–435. doi: 10.2353/ajpath.2007.060834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wattel A, Kamel S, Prouillet C, Petit JP, Lorget F, Offord E, Brazier M. Flavonoid quercetin decreases osteoclastic differentiation induced by RANKL via a mechanism involving NF kappa B and AP-1. J Cell Biochem. 2004;15:285–295. doi: 10.1002/jcb.20071. [DOI] [PubMed] [Google Scholar]
- Xiang DB, Li XY. Antitumor activity and immuno-potentiating actions of Achyranthes bidentata polysaccharides. Acta Pharmacol Sin. 1993;14:556–561. [PubMed] [Google Scholar]
- Xue SX, Chen XM, Lu JX. Protective effect of sulfated Achyranthes bidentata polysaccharides on streptozotocin-induced oxidative stress in rats. Carbohydr Polym. 2009;75:415–419. doi: 10.1016/j.carbpol.2008.08.003. [DOI] [Google Scholar]
- Yang Q, Yang G, Zhang J, Masuoka N, Riffle YZ, Wang Z, Ebinuma H, Kodama H. Effect of Agkistroden blomhoffi (mamushi) on the proliferation of human fibroblasts. Clin Biochem. 2004;37:138–145. doi: 10.1016/j.clinbiochem.2003.10.012. [DOI] [PubMed] [Google Scholar]
- Zhang R, Hu SJ, Li C, Zhang F, Gan HQ, Mei QB. Achyranthes bidentata root extract prevent OVX-induced osteoporosis in rats. J Ethnopharmacol. 2012;139:12–18. doi: 10.1016/j.jep.2011.05.034. [DOI] [PubMed] [Google Scholar]
- Zheng Y, Liu B, Chen M, Chen T. Supercritical fluid extraction of ecdysterone from the roots of Achyranthes bidentata BL. J Sep Sci. 2008;31:1393–1398. doi: 10.1002/jssc.200700468. [DOI] [PubMed] [Google Scholar]