Abstract
This study treated the isolation and passage of muscle-derived stem cells (MDSCs) from rat penile corpora cavernosa, detection of stem cell marker expression, observation of their self-renewal and continuous proliferation, and demonstration of their potential to differentiate into smooth muscle cells in co-culture. Muscle-derived stem cells from the rat penile corpora cavernosa were isolated and purified. The expression of stem cell markers Sca-1 and desmin was detected in PP6 cells, thus confirming that the main components of PP6 cells are MDSCs. The expression of Sca-1 and desmin occurred both in PP6 cells and cells at passages 3, 6, and 8, and there was no significant decrease in the expression level with increasing passage number. The growth curves indicated that the cell doubling time was approximately 48 h. The cells entered the stationary phase after approximately 7 days of culture. The proliferative activity of the cells at passage 8 remained unchanged. After 2 days of co-culture with smooth muscle cells, the DAPI-labeled MDSCs tended to exhibit smooth muscle cell morphology and expression of α-SMA was detected. MDSCs exist in the rat penile corpora cavernosa and possess the potential to differentiate into smooth muscle cells. This discovery serves as the basis in view of the potential use of endogenous stem cells for the treatment of erectile dysfunction (ED).
Keywords: Muscle-derived stem cells, Sca-1, Desmin, Passage, Differentiation induction
Introduction
With constant advances in molecular biology, stem cell technology has become a major focus of biomedical research. Although stem cell research for the treatment of erectile dysfunction (ED) is still at the stage of animal experiments, a new therapy for ED may be developed in the future after further studies have been performed. Some studies confirmed that embryonic stem cells can be used for the treatment of neurogenic ED (Eglitis and Mezey 1997). Studies of the effect of mesenchymal (Bivalacqua et al. 2007; Qiu et al. 2012), human neural crest (Song et al. 2008), and adipose tissue-derived stem cells (Albersen et al. 2010; Garcia et al. 2010; Huang et al. 2010) on ED treatment have also been performed by other researchers using intracavernous injection of these cells in rats. The results indicated that ED could be improved to a certain extent when treated with these cells. However, exogenous stem cell transplantation is associated with transplant rejection, and this treatment involves different degrees of complexity with regard to stem cell harvesting, isolation, and culture. In addition, the application of embryonic stem cells is further limited by the possibility of dysembryoma development and ethical issues.
Vernet et al. (2005) found that cultured penile tunica albuginea cells had the potential to differentiate into bone- and fiber-forming cells in vitro, and also detected the expression of the stem cell antigen CD34 in these cells. Nolazco et al. (2008) also detected the stem cell markers Sca-1 and CD34 in the penile corpora cavernosa of normal rats. Therefore, they concluded that endogenous stem cells existed in the penile corpora cavernosa and believed that the impaired or dysfunctional smooth muscle cells or endothelial cells could be displaced when proliferation and differentiation of these stem cells are stimulated. Based on these findings, we believe that the application of endogenous penile corpora cavernosa stem cells will be a promising treatment option for both the symptoms and causes of ED.
Approximately 40–50 % of the corpora cavernosa tissue is composed of smooth muscle, which serves as a major effector of penile neuromodulation. These smooth muscle cells are widely distributed in the vascular walls and the trabeculae of the corpora cavernosa in a connective tissue framework, and play an important role in penile erection. The majority of the penile corpora cavernosa stem cells are smooth muscle-derived stem cells (MDSCs), which play an essential role in the maintenance and recovery of erectile function. Therefore, in the present study, we focused on the isolation and passage of MDSCs and observed their potential to differentiate into smooth muscle cells. The enzymatic digestion, density gradient centrifugation, and differential adhesion methods were used in this study. Isolation, purification, expansion, and passage of the rat penile corpora cavernosa MDSCs were performed. The expression of stem cell markers was detected, and the process of self-renewal and continuous proliferation of the MDSCs as well as their potential to differentiate into smooth muscle cells in co-culture was observed. The present study was performed to provide empirical evidence to support the theory of the application of endogenous MDSCs for the treatment of ED.
Materials and methods
Ethics statement
All studies adhered to current American Veterinarian Medical Association (AVMA) Guidelines and were approved by the Ethics Committee of the Second Affiliated Hospital of the Suzhou University.
Isolation and purification of rat penile corpora cavernosa MDSCs
Healthy 2-month-old male Sprague–Dawley rats, weighing 180–200 g, were used for this study. The surgical procedure was performed under general inhalation anesthesia with diethyl ether. An incision was made in the lower abdomen and the tissues were carefully separated to expose the penile corpora cavernosa tissue, which was removed and cut into small pieces measuring 1 mm × 1 mm × 1 mm. The rats were sacrificed via cervical dislocation. The smooth muscle cells were isolated using the enzymatic digestion method as described elsewhere (Nolazco et al. 2008) and the expression of the smooth muscle cell marker alpha smooth muscle actin (α-SMA) was detected using immunohistochemistry methods. Type II collagenase (Shanghai Yuanju Bio-Tech Co., Ltd., Shanghai, China) was used for enzymatic digestion to achieve a single-cell suspension from the penile corpora cavernosa tissue. Then, density gradient centrifugation and the preplate technique based on differential adhesion were used, and adherent cells preplate 1 [PP1] were acquired, followed by repeated preplating procedures, until preplate 6 (PP6) cells were acquired (Lee et al. 2012; Nishimori et al. 2012; Nolazco et al. 2008), then we confirmed that the PP6 cells were primarily composed of MDSCs.
Identification and flow cytometric analysis of PP6 cells to identify whether the main components are MDSCs
Immunofluorescence cytochemistry was used to identify the MDSC markers Sca-1 using anti-Sca-1 (UCL, Houston, TX, USA) and anti-desmin (Dako, Glostrup, Denmark) antibodies in PP6 cells. The adherent PP6 cells were collected and fluorescence staining was performed for Sca-1 and desmin after adjustment of the cell density. Flow cytometric analysis was used to determine the number of positively stained cells. The detail of flow cytometric analysis is as below. The medium was removed, 0.25 % trypsin solution was added, the cell suspension was centrifuged for 5 min, and the pellet was washed twice with PBS. The cell concentration was adjusted to 1 x 107 cells/mL. Cells were incubated in 100 μL of intracellular fixation buffer (PBS containing 4 % paraformaldehyde) for 15 min to fix the cells, washed with PBS, and collected by centrifugation. One hundred microliters of permeabilization buffer (0.1 % saponin and 0.009 % sodium azide) was used to permeabilize the cells. Then, the primary antibodies (anti-Sca-1 and anti-desmin; 1:50) and IgG isotype control antibodies were added and incubated at room temperature for 40 min, cells were washed twice with PBS, the corresponding FITC-labeled secondary antibody (1:50) was added and incubated at room temperature in the dark for 30 min, and cells were washed once with PBS. Then, 500 μL of PBS was added and the number of positive cells was detected using flow cytometry (FACS Calibur, Becton-Dickinson, Franklin Lakes, NJ, USA). The number of Sca-1- and desmin-positive adherent PP6 cells was determined thrice and the mean values were adopted.
MDSC passage and establishment of growth curves to clarify their self-renewal and continuing proliferation capacity of stem cells
The cells were passaged when they reached 70 % confluence 14–16 days after primary culture. The adherent cells were digested and isolated into a single-cell suspension. Then, the cells were seeded into several 25-mL culture flasks at a density of 1 × 106 cells/mL. The cells were randomly sampled each day from the flasks for digestion and counting. Growth curves were established using the mean values of the cell numbers. The cell growth curves at passages 3, 6, and 8 were used for analysis. Digestion, collection, and counting of the cells were performed on days 1–7. The growth curves of the smooth muscle cells and MDSCs were established, and the doubling time was calculated.
DAPI labeling of MDSCs of penile corpora cavernosa
A sterile 4′-6-diamidino-2-phenylindole (DAPI) stock solution was added to the media of selected passage-3 MDSCs at a final concentration of 60 mg/L, then the cells were incubated at 37 °C for at least 30 min. The cells were washed with phosphate-buffered saline (PBS) at least 6 times to remove unbound DAPI. Then, observation of the growth features and biological activity of MDSCs in terms of molecular and cellular structures was performed.
Differentiation of MDSCs induced by co-culture and differentiation marker identification to confirm their differentiation potential
Passage-3 MDSCs were digested and centrifuged, and the supernatant was removed. The cells were resuspended in complete medium and the density of the cells was adjusted to 1 × 103 cells/mL. The cells were seeded into 6-well plates, and an equal number of penile corpora cavernosa smooth muscle cells was added to each well for co-culture. Plates with wells containing only the passage-3 MDSCs were prepared as the control group. The co-cultured cells were observed under an inverted microscope. After 2 days of co-culture, the expression of α-SMA was detected in both the experimental group and the control group.
Statistical analysis
The data were expressed as mean and standard deviation (X ± SD). The Statistical Package for the Social Sciences (SPSS) version 17.0 software was used for t tests. Results were considered statistically significant when P < 0.05.
Results
Morphological changes of PP6 cells
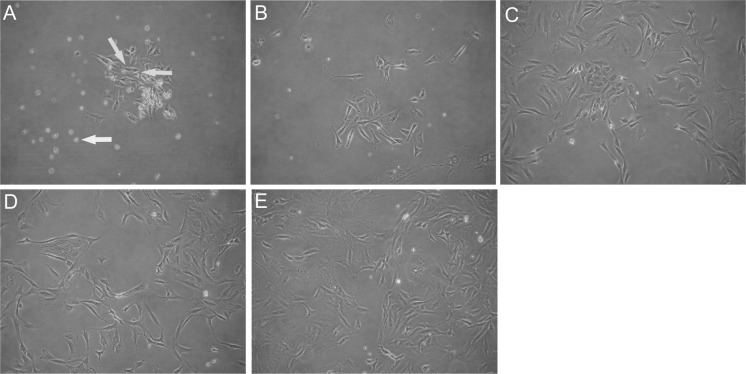
The adherent PP6 cells decreased significantly in number and size compared to the PP1 to PP5 cells. The speed of cell attachment was slow and the refractive index of the cells was high. These cells were primarily round, fusiform, or spindle-shaped. The proliferation rate was low, and there were also a few cells observed in suspension (Fig. 1a). After 72 h of culture, the PP6 cells increased in number and were scattered evenly. The cells were primarily fusiform or spindle-shaped, while some appeared morphologically irregular (Fig. 1b). After 5–7 days of culture, there were essentially no cells in suspension, and the adherent cells showed pronounced proliferation, with numerous cells at different phases of cell division. The density and size of the cells increased significantly after gradual expansion. The cells showed striking morphological changes, with the presence of long spindle-shaped adherent cells (Fig. 1c). The proliferation of these adherent cells was still observed at days 12–14 and the cell density increased significantly (Fig. 1d). At days 14–16 of culture, a sufficiently high confluence percentage was observed, thus enabling further passaging (Fig. 1e).
Fig. 1.
PP6 cell morphology at various culture time points. a Round, fusiform, or spindle-shaped adherent cells (green arrows), and a small number of cells in suspension (yellow arrow). b After 72 h of culture, the number of cells in suspension decreased and the number of adherent cells increased, most of which were fusiform or spindle-shaped. c At days 5–7 of culture, cell proliferation occurred and the size of the cells increased. d At days 12–14, the cell density increased. e At days 14–16, high confluence percentage was observed. (Color figure online)
Passage and establishment of growth curves of PP6 cells
After multiple passages, the cells grew and attached in an even distribution with a high proliferation rate. The numbers of PP6 cells at days 1–7 of culture were calculated using a cell counting method. The growth curves were established based on the numbers of the cells (Fig. 2). The cell doubling time was approximately 48 h. The cells entered stationary phase after about 7 days of culture. The proliferation capacity of the cells remained unchanged up to passage 8. As the passage number increased, the doubling time slightly increased, while the proliferation activity remained high.
Fig. 2.
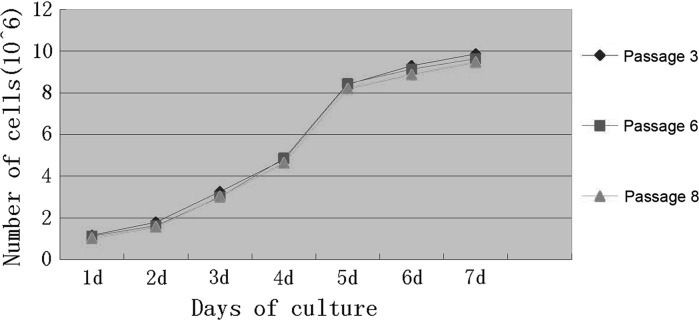
MDSCs growth curves at passages 3, 6, and 8
Identification of penile corpora cavernosa MDSCs
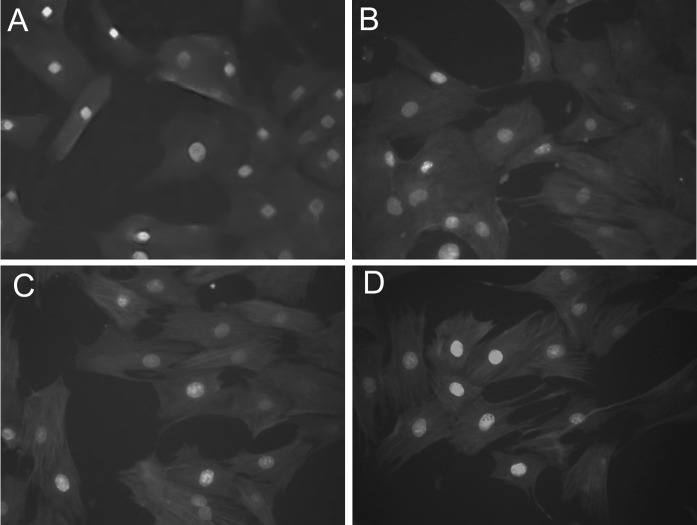
The expression of Sca-1 and desmin was observed both in PP6 cells and in the selected PP6 cells at passages 3, 6, and 8. The expression of Sca-1 and desmin did not decrease with increasing passage number. Figure 3 shows typical MDSCs with positive expression of Sca-1 (red fluorescence) and desmin (green fluorescence), thus confirming that the main components of PP6 cells are MDSCs with continuing proliferation and self-renewal characteristics of stem cells.
Fig. 3.
Immunofluorescence staining of PP6 cells for Sca-1 (red)/desmin (green). a (×400). b Passage 3 PP6 cells (×400). c Passage 6 PP6 cells (×400). d Passage 8 PP6 cells (×400). (Color figure online)
Flow cytometric determination of Sca-1 and desmin expression in PP6 cells
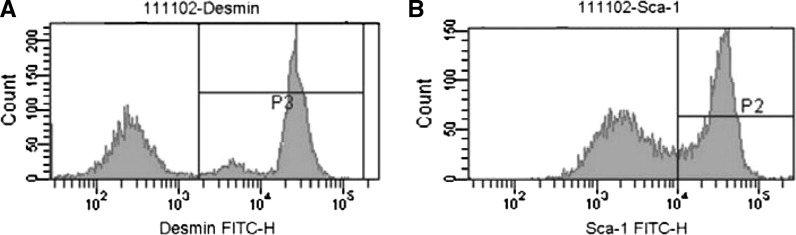
Flow cytometric analysis was employed for the determination of the Sca-1 and desmin expression in PP6 cells. The expression levels of Sca-1 and desmin in PP6 cells were 64.7 ± 0.61 % and 79.6 ± 1.21 %, respectively (Fig. 4), thus reflecting that MDSCs are the main components of PP6 cells.
Fig. 4.
MDSCs-characteristic expression of Desmin (A) and Sca-1 (B) in PP6 cells. The expression levels were 79.6 and 64.7 %, respectively
Induced differentiation of MDSCs into smooth muscle cells
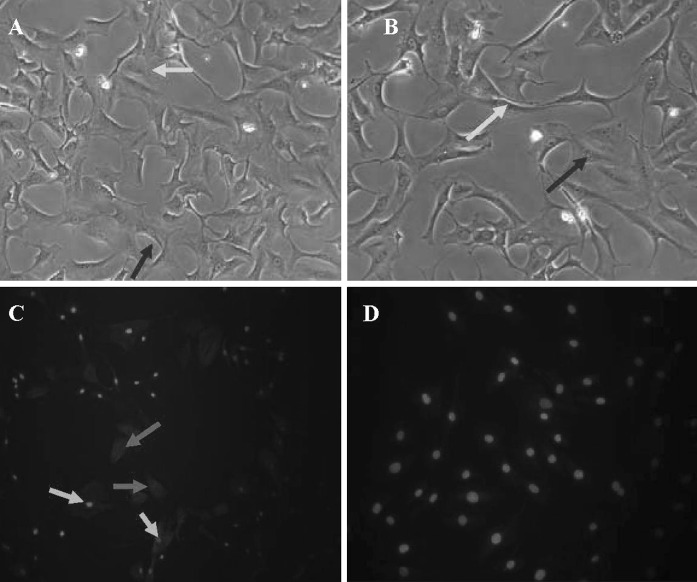
DAPI-labeled penile corpora cavernosa MDSCs (103 cells/ml) were co-cultured with an equal number of penile corpora cavernosa smooth muscle cells (Fig. 5a, b). After 2 days of co-culture, immunofluorescence cytochemistry revealed that α-SMA was expressed in the DAPI-labeled MDSCs in the experimental group, while no expression of α-SMA was detected in the DAPI-labeled MDSCs in the control group (Fig. 5c, d).
Fig. 5.
Co-cultured MDSCs and smooth muscle cells. (a) ×100; (b) ×200: DAPI-labeled MDSCs with blue-stained nuclei (green arrows) and smooth muscle cells with unstained nuclei (red arrows). c Immunofluorescence staining showing expression of α-SMA in the DAPI-labeled MDSCs (green arrows) and co-cultured smooth muscle cells (red arrows). d No α-SMA expression was detected in the DAPI-labeled MDSCs in the control group. (Color figure online)
Discussion
Muscle-derived stem cells have generated great interest in recent adult stem cell studies. MDSCs tend to differentiate into muscle cells and muscle tissue in the absence of specific differentiation-induction, and differentiate into bone cells, cartilage cells, smooth muscle cells, endothelial cells, and neurons in vitro when specific interventions are performed (Gates et al. 2008; Smaldone et al. 2009). Therefore, MDSCs are valuable in treating muscular dystrophy (Ambrosio et al. 2009), heart disease (Shibuya et al. 2010), stress urinary incontinence (Stangel-Wojcikiewicz et al. 2010), neurogenic bladder (Nitta et al. 2010), bone disease, and central nervous system disease (Matsumoto et al. 2009; Xu et al. 2010). Nolazco et al. (2008) infused MDSCs into penile corpora cavernosa of old rats and observed that they differentiated into smooth muscle cells, resulting in significant improvement of erectile function. In addition to muscle disorders, diseases of the nervous system or endothelium are also the main causes of ED. Since MDSCs can differentiate into muscle, endothelial, and nerve cells, they might serve as a promising tool for the treatment of ED.
According to Huard et al. (2003), MDSCs possess the biological characteristics of low adhesion, round shape, small size, high refractive index, and low proliferation rate. After 48 h of culture, cell attachment was accomplished and the cells gradually expanded into a polygonal shape. Regardless of the passage number, the cell proliferative activity remained high. In the present study, the cellular morphology, attachment time, and growth features were similar to the aforementioned MDSC characteristics reported by Huard et al. (2003).
In our study, penile corpora cavernosa MDSCs were co-cultured with smooth muscle cells that were isolated and identified simultaneously. DAPI labeling was performed on the isolated MDSCs. We found that the growth activity of the MDSCs before and after DAPI staining remained unchanged, and that the in vitro tracer test demonstrated a 100 % DAPI staining rate. After 2 days of co-culture of the isolated MDSCs and smooth muscle cells, immunofluorescence cytochemistry demonstrated that the specific marker of smooth muscle cells, α-SMA, was expressed in the labeled MDSCs, while no α-SMA expression was detected in the control group. Therefore, we believe that the induction of MDSC differentiation into smooth muscle cells was achieved successfully in co-culture. The theoretical basis might be that, in a microenvironment containing abundant smooth muscle cells, differentiation of MDSCs will be induced by the interactions between the cells and the growth factors produced by the target cells.
However, further studies are needed regarding the differentiation of MDSCs into other cell types and MDSC transdifferentiation, which will be the focus of our future work. Like other adult stem cells, MDSCs have the ability to proliferate endlessly and differentiate into cells from different germ layers (Choi et al. 2012; Kwon et al. 2011; Ota et al. 2011). Therefore, MDSCs can differentiate into specific cell types under specific circumstances and play a role in the treatment of diseases such as ED (Usas et al. 2011). However, the exact mechanisms of MDSC self-renewal, selective differentiation, and activation remain unknown. Therefore, these issues will be the focus for our future studies. We believe that the proliferation and differentiation of endogenous penile corpora cavernosa MDSCs can be stimulated by regulation of their activity, and the impaired or dysfunctional cells of corpora cavernosa tissue can be displaced and, consequently, penile erectile function will be improved. The results of this study provide the basis for future research regarding a promising new ED treatment.
References
- Albersen M, Fandel TM, Lin G, Wang G, Banie L, Lin CS, Lue TF. Injections of adipose tissue-derived stem cells and stem cell lysate improve recovery of erectile function in a rat model of cavernous nerve injury. J Sex Med. 2010;7:3331–3340. doi: 10.1111/j.1743-6109.2010.01875.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ambrosio F, Ferrari RJ, Fitzgerald GK, Carvell G, Boninger ML, Huard J. Functional overloading of dystrophic mice enhances muscle-derived stem cell contribution to muscle contractile capacity. Arch Phys Med Rehabil. 2009;90:66–73. doi: 10.1016/j.apmr.2008.06.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bivalacqua TJ, Deng W, Kendirci M, Usta MF, Robinson C, Taylor BK, Murthy SN, Champion HC, Hellstrom WJ, Kadowitz PJ. Mesenchymal stem cells alone or ex vivo gene modified with endothelial nitric oxide synthase reverse age-associated erectile dysfunction. Am J Physiol Heart Circ Physiol. 2007;292:H1278–H1290. doi: 10.1152/ajpheart.00685.2006. [DOI] [PubMed] [Google Scholar]
- Choi YJ, Lee JY, Lee SJ, Chung CP, Park YJ. Determination of osteogenic or adipogenic lineages in muscle-derived stem cells (MDSCs) by a collagen-binding peptide (CBP) derived from bone sialoprotein (BSP) Biochem Biophys Res Commun. 2012;419:326–332. doi: 10.1016/j.bbrc.2012.02.022. [DOI] [PubMed] [Google Scholar]
- Eglitis MA, Mezey E. Hematopoietic cells differentiate into both microglia and macroglia in the brains of adult mice. Proc Natl Acad Sci USA. 1997;94:4080–4085. doi: 10.1073/pnas.94.8.4080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia MM, Fandel TM, Lin G, Shindel AW, Banie L, Lin CS, Lue TF. Treatment of erectile dysfunction in the obese type 2 diabetic ZDF rat with adipose tissue-derived stem cells. J Sex Med. 2010;7:89–98. doi: 10.1111/j.1743-6109.2009.01541.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gates CB, Karthikeyan T, Fu F, Huard J. Regenerative medicine for the musculoskeletal system based on muscle-derived stem cells. J Am Acad Orthop Surg. 2008;16:68–76. doi: 10.5435/00124635-200802000-00004. [DOI] [PubMed] [Google Scholar]
- Huang YC, Ning H, Shindel AW, Fandel TM, Lin G, Harraz AM, Lue TF, Lin CS. The effect of intracavernous injection of adipose tissue-derived stem cells on hyperlipidemia-associated erectile dysfunction in a rat model. J Sex Med. 2010;7:1391–1400. doi: 10.1111/j.1743-6109.2009.01697.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huard J, Cao B, Qu-Petersen Z. Muscle-derived stem cells: potential for muscle regeneration. Birth Defects Res C Embryo Today. 2003;69:230–237. doi: 10.1002/bdrc.10020. [DOI] [PubMed] [Google Scholar]
- Kwon EB, Lee JY, Piao S, Kim IG, Ra JC. Comparison of human muscle-derived stem cells and human adipose-derived stem cells in neurogenic trans-differentiation. Korean J Urol. 2011;52:852–857. doi: 10.4111/kju.2011.52.12.852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JY, Piao S, Kim IG, Byun SS, Hwang JH, Hong SH, Kim SW, Hwang TK. Effect of human muscle-derived stem cells on cryoinjured mouse bladder contractility. Urology. 2012;80:e224–e227. doi: 10.1016/j.urology.2012.03.037. [DOI] [PubMed] [Google Scholar]
- Matsumoto T, Cooper GM, Gharaibeh B, Meszaros LB, Li G, Usas A, Fu FH, Huard J. Cartilage repair in a rat model of osteoarthritis through intraarticular transplantation of muscle-derived stem cells expressing bone morphogenetic protein 4 and soluble Flt-1. Arthritis Rheum. 2009;60:1390–1405. doi: 10.1002/art.24443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimori M, Matsumoto T, Ota S, Kopf S, Mifune Y, Harner C, Ochi M, Fu FH, Huard J. Role of angiogenesis after muscle derived stem cell transplantation in injured medial collateral ligament. J Orthop Res. 2012;30:627–633. doi: 10.1002/jor.21551. [DOI] [PubMed] [Google Scholar]
- Nitta M, Tamaki T, Tono K, Okada Y, Masuda M, Akatsuka A, Hoshi A, Usui Y, Terachi T. Reconstitution of experimental neurogenic bladder dysfunction using skeletal muscle-derived multipotent stem cells. Transplantation. 2010;89:1043–1049. doi: 10.1097/TP.0b013e3181d45a7f. [DOI] [PubMed] [Google Scholar]
- Nolazco G, Kovanecz I, Vernet D, Gelfand RA, Tsao J, Ferrini MG, Magee T, Rajfer J, Gonzalez-Cadavid NF. Effect of muscle-derived stem cells on the restoration of corpora cavernosa smooth muscle and erectile function in the aged rat. BJU Int. 2008;101:1156–1164. doi: 10.1111/j.1464-410X.2008.07507.x. [DOI] [PubMed] [Google Scholar]
- Ota S, Uehara K, Nozaki M, Kobayashi T, Terada S, Tobita K, Fu FH, Huard J. Intramuscular transplantation of muscle-derived stem cells accelerates skeletal muscle healing after contusion injury via enhancement of angiogenesis. Am J Sports Med. 2011;39:1912–1922. doi: 10.1177/0363546511415239. [DOI] [PubMed] [Google Scholar]
- Qiu X, Sun C, Yu W, Lin H, Sun Z, Chen Y, Wang R, Dai Y. Combined strategy of mesenchymal stem cell injection with vascular endothelial growth factor gene therapy for the treatment of diabetes-associated erectile dysfunction. J Androl. 2012;33:37–44. doi: 10.2164/jandrol.110.012666. [DOI] [PubMed] [Google Scholar]
- Shibuya M, Miura T, Fukagawa Y, Akashi S, Oda T, Kawamura S, Ikeda Y, Matsuzaki M. Tongue muscle-derived stem cells express connexin 43 and improve cardiac remodeling and survival after myocardial infarction in mice. Circ J. 2010;74:1219–1226. doi: 10.1253/circj.CJ-10-0033. [DOI] [PubMed] [Google Scholar]
- Smaldone MC, Chen ML, Chancellor MB. Stem cell therapy for urethral sphincter regeneration. Minerva Urol Nefrol. 2009;61:27–40. [PubMed] [Google Scholar]
- Song YS, Lee HJ, Park IH, Lim IS, Ku JH, Kim SU. Human neural crest stem cells transplanted in rat penile corpus cavernosum to repair erectile dysfunction. BJU Int. 2008;102:220–224. doi: 10.1111/j.1464-410X.2008.07469.x. [DOI] [PubMed] [Google Scholar]
- Stangel-Wojcikiewicz K, Majka M, Basta A, Stec M, Pabian W, Piwowar M, Chancellor MB. Adult stem cells therapy for urine incontinence in women. Ginekol Pol. 2010;81:378–381. [PubMed] [Google Scholar]
- Usas A, Maciulaitis J, Maciulaitis R, Jakuboniene N, Milasius A, Huard J. Skeletal muscle-derived stem cells: implications for cell-mediated therapies. Medicina (Kaunas) 2011;47:469–479. [PubMed] [Google Scholar]
- Vernet D, Nolazco G, Cantini L, Magee TR, Qian A, Rajfer J, Gonzalez-Cadavid NF. Evidence that osteogenic progenitor cells in the human tunica albuginea may originate from stem cells: implications for peyronie disease. Biol Reprod. 2005;73:1199–1210. doi: 10.1095/biolreprod.105.041038. [DOI] [PubMed] [Google Scholar]
- Xu Y, Song YF, Lin ZX. Transplantation of muscle-derived stem cells plus biodegradable fibrin glue restores the urethral sphincter in a pudendal nerve-transected rat model. Braz J Med Biol Res. 2010;43:1076–1083. doi: 10.1590/S0100-879X2010007500112. [DOI] [PubMed] [Google Scholar]