Abstract
We explored associations among preterm status (very preterm infant (VPI: <30 weeks), moderate preterm (MPI: 30–336/7 weeks), late preterm (LPI: 34–366/7 weeks), parenting, and 3-year cognitive and behavioral outcomes. We hypothesized that LPIs would demonstrate better health and neurobehavioral outcomes compared with more premature infants, and that preterm status would moderate the association between parenting quality and 3-year outcomes. Sample included 123 preterm infants (gestation < 37 weeks) and their mothers from a larger study of high-risk infants with measures of neonatal and socioeconomic risks at hospital discharge; maternal vocabulary at 9-months, child IQ and behavior at 36-months, and maternal depressive symptoms and parenting at all timepoints. Group differences were explored using MANOVAs while predictors of child outcomes were explored using hierarchical regression analyses. MANOVAs indicated that LPIs had more optimal neonatal health during the hospital stay, yet more externalizing (p= .043), aggressive (p= .006) and oppositional behaviors (p= .008) at 3-years compared with VPIs. There were no IQ differences between VPIs, MPIs and LPIs. However, preterm infants who experienced less negative parenting had higher IQs at 36 months (β = −3.245, p= .017), with the greatest effects seen in VPIs (β = 0.406, p = .01) compared with LPIs (β= 0.148, p= .381). LPIs manifested similar IQ, but more externalizing, oppositional and aggressive behavior symptoms compared to VPIs. VPIs appeared to be differentially susceptible to parenting effects, with VPIs demonstrating the highest cognitive scores in the context of more positive parenting.
Keywords: Behavior, Differential Susceptibility, IQ, Parent-child interaction, Preterm Infant
1. Introduction1
Preterm infants are a vulnerable population, demonstrating increased risk for medical problems and neurobehavioral disabilities (Aylward, 2005; Taylor, Klein, & Hack, 2000) including poorer cognitive outcomes, more learning difficulties, and elevated internalizing and externalizing behavior problems compared to full term infants (Aarnoudse-Moens, Weisglas-Kuperus, van Goudoever, & Oosterlaan, 2009; Bhutta, Cleves, Casey, Cradock, & Anand, 2002; Hack et al., 2004; Johnson, 2007; Saigal, Pinelli, Hoult, Kim, & Boyle, 2003; Schothorst & Van Engeland, 1996). Gestational age and severity of neonatal complications have been documented as determinants of later development, with poorer outcomes described in infants born at earlier gestational ages compared to infants born full-term (Aarnoudse-Moens, et al., 2009; Bhutta, et al., 2002; Johnson, 2007; Taylor, et al., 2000). Although the majority of research in preterm infants has focused on the morbidity and mortality of the highest risk (very preterm) infants, in fact, late preterm infants (LPIs) account for the majority of infants born prematurely, with almost 75% of preterm infants born between 34–366/7 weeks gestation (Davidoff et al., 2006), translating to >400,000 late preterm births per year (Damus, 2008).
Despite the prevalence of LPIs (Raju, Higgins, Stark, & Leveno, 2006), little is known about their outcomes past infancy. It has long been thought that LPIs were a low-risk group, similar to full-term infants, with little to no risk for long-term morbidities. However, only recently has evidence shown that LPIs experience elevated morbidity (Engle, Tomashek, & Wallman, 2007), with poorer neurodevelopmental outcomes (Woythaler, McCormick, & Smith, 2011), and more learning problems compared with their full-term counterparts (Chyi, Lee, Hintz, Gould, & Sutcliffe, 2008; Morse, Zheng, Tang, & Roth, 2009). In addition, although behavior and attention problems are a well-described morbidity among very-preterm infants (VPIs) (Bhutta, et al., 2002; Davis, Burns, Snyder, & Robinson, 2007), emerging evidence suggests that LPIs may also be at increased risk for behavioral difficulties (Gray, Indurkhya, & McCormick, 2004; Huddy, Johnson, & Hope, 2001), although the evidence is mixed (Gurka, LoCasale-Crouch, & Blackman, 2010). There is limited research on the social-emotional outcomes of LPIs. Studies have suggested that low birthweight (LBW) infants exhibit an increased risk for behavior problems compared to normal birthweight infants (Deutscher & Fewell, 2005; Gray, et al., 2004). However, findings were not specific to infants born in the late preterm period. Additional research focusing on behavior problems related to gestational age found that infants born between 32–35 weeks of gestation had elevated rates of school difficulties and hyperactive symptoms compared to estimates previously published with normal population controls (Huddy, et al., 2001), but again the findings were not specifically applicable to late preterm infants (born between 34–366/7 weeks). One study found no differences in cognition or behavior problems (internalizing, externalizing, aggressive or anxiety) between late preterm and full-term infants (Gurka, et al., 2010), but behavioral differences between LPIs and infants born more prematurely were not explored. Taken together, the neurobehavioral (i.e. developmental and behavioral) outcomes of late preterm infants relative to those born more prematurely are not well-elucidated, and little is known about biological and environmental factors that influence those outcomes.
Research on very preterm infants suggests that neurobehavioral outcomes are influenced by the quality of parenting (Landry, Smith, Miller-Loncar, & Swank, 1997; Smith, Landry, & Swank, 2006; Treyvaud et al., 2009). VPIs who experience more responsive early caregiving demonstrate better social competence (Landry, Chapieski, Richardson, Palmer, & Hall, 1990; Landry, Smith, Miller-Loncar, & Swank, 1998; Treyvaud, et al., 2009), language skills (Landry, et al., 1997), cognition (Smith, et al., 2006), self-regulation (C. Clark, Woodward, Horwood, & Moor, 2008; Feldman, Eidelman, & Rotenberg, 2004; Landry, et al., 1997), and fewer behavior problems (Laucht, Esser, & Schmidt, 2001) compared with infants who experience less responsive caregiving. However, the effects of parenting on neurobehavioral outcomes of LPIs compared to infants born more preterm remain unknown. Are LPIs (who typically demonstrate fewer medical complications than infants born more prematurely) more likely to benefit from a responsive caregiving environment, compared with more medically fragile preterm infants? We hypothesized that compared with more preterm infants, LPIs would (1) exhibit fewer neonatal medical complications, (2) demonstrate better cognitive and behavioral outcomes at 36 months post-term, and (3) manifest more optimal developmental outcomes in the context of more positive parenting, and conversely (4) would manifest more adverse outcomes in the context of suboptimal parenting (i.e. preterm status would moderate the association between parenting quality and later neurobehavioral outcomes, with the greatest effects of parenting seen in LPIs compared with VPIs).
2. Methods
2.1 Participants
One-hundred twenty three infant-mother dyads who were part of a larger longitudinal research study (Poehlmann, Schwichtenberg, Bolt, & Dilworth-Bart, 2009) participated in the current research. All 123infants were born preterm (< 37 weeks gestation). For the larger study, 181 mothers and their infants were recruited from three Wisconsin Neonatal Intensive Care Units (NICU)from 2002–2005. Families were invited to participate if infants (a) were preterm or <2500 grams at birth, (b) had no congenital or significant neurological problems or prenatal drug exposuresbased on hospital screening (prenatal maternal interview and post -natal infant meconium assay and urine toxicology screen), and if mothers were (c) at least 17 years of age, (d) could read English, and (e) self-identified as the child’s primary caregiver. For multiple births, one infant was randomly selected to participate.
For the total sample, 94 (76%) of the infants were White, 10 were Black (8%), 1 was Middle Eastern (1%), 1 was Asian (1%), 1 was Latino (1%), and 16 were multiracial (13%). Infant birth-weights ranged from 490 to 3328 g, with a mean of 1732.6 g (SD = 608.2g). Gestational ages ranged from 23.7–35.9 weeks with a mean of 31.3 weeks (SD = 3.2). At NICU discharge, mothers were an average of 30.8 years (SD = 5.9) and had completed an average of 14.8 years of education (SD = 2.5). Most mothers (n = 96, 78%) were married, and average family income was $66,800 (SD = $55,896). Infants were hospitalized an average of 34.2 days (SD = 29.9) and 61 (50%) infants were boys. Infants were stratified by gestational age: very preterm infants (VPI) (n = 39, 32%); moderate preterm infants (MPI) (n = 47, 38%); and late preterm infants (LPI) (n = 37, 30%). Sample characteristics for each gestational age group are presented in Table 1.
Table 1.
Sample Characteristics
| Maternal variables | VPI (n= 39) Range (mean, SD) |
MPI (n= 47) Range (mean, SD) |
LPI (n= 37) Range (mean, SD) |
|---|---|---|---|
| Maternal age (years) | 17–41 (28.9, 6.02) | 19–42 (31.4, 5.42) | 19–42 (31.9, 6.2) |
| Maternal education (years) | 10–21 (14.49, 2.51) | 12–21 (15.06, 2.16) | 11–21 (14.8, 2.8) |
| Yearly household income | $4320–$500,000 ($64,774, $79247) | $6,000– 200,000 ($72,102, $42,976) | $10,000–170,000 ($62,199, $39190) |
| Number of dependents | 1–5 (1.62, 1.07) | 1–4 (1.83, 0.94) | 1–11 (2.2, 1.8) |
| Family SES risks | 0–5 (1.05, 1.62) | 0–4 (0.53, 1.02) | 0–5 (0.7, 1.4) |
| Marital status | |||
| Married | 11 (28.2%) | 8 (17%) | 29 (78.4%) |
| Not married | 28 (71.8%) | 39(83%) | 8 (21.6%) |
|
| |||
| Child variables | N (%) | N (%) | N (%) |
|
| |||
| Gender | |||
| Male | 14 (35.9%) | 28 (59.6) | 19(51.4%) |
| Female | 25 (64.1%) | 19 (40.4) | 18 (48.6%) |
| Child’s race | |||
| White | 27 (69.2%) | 34 (72.3%) | 33 (89.2%) |
| Black | 5 (12.8%) | 2 (4.3%) | 3 (8.1%) |
| Latino | 1(2.6%) | 0 (0.0%) | 0 (0.0%) |
| Middle Eastern | 0 (0.0%) | 1 (2.1%) | 0 (0.0%) |
| Asian | 0 (0.0%) | 1 (2.1%) | 0 (0.0%) |
| Multiracial | 6 (15.4%) | 9 (19.1%) | 1 (2.7%) |
| Multiple-birth | |||
| No | 30 (76.9%) | 39 (83.0%) | 29 (78.4%) |
| Yes | 9 (23.1%) | 8 (17.0%) | 8 (21.6%) |
2.2 Measures
2.2.1. Cognitive Skills
Child cognitive skills at 36 months were estimated using the Abbreviated Battery IQ scale (ABIQ) from the Stanford-Binet Intelligence Scales, 5th edition (Roid, 2003). This standardized assessment for children age two through adulthood reports cognitive skills as standard scores, with a mean of 100 (SD=15), demonstrating high reliability (α= .90) and strong correlation with full-battery IQ scores (Roid, 2003). The ABIQ is the sum of the Nonverbal Fluid Reasoning (α= .81) and Verbal Knowledge (α= .93) scaled scores. ABIQ scores were available for 123 children in the sample. Scores ranged from 52–130 (Mean=96.4, SD=14.0). Six children (5%) had IQs ≤ 70.
2.2.2. Child Behavior Problems
Maternal report on the Child Behavior Checklist (CBCL 1½–5) (Achenbach & Rescorla, 2000) was used to assess children’s behaviors at 36 months. The CBCL is a standardized parent-report checklist with 99 items rated on a three point scale, not true (0) to very or often true (2). It has been used extensively with preterm infants (Yu, Buka, McCormick, Fitzmaurice, & Indurkhya, 2006). The CBCL describes patterns of behavioral problems that correlate with seven syndrome scales (Ivanova, 2010), and five Diagnostic and Statistical Manual of Mental Disorders (DSM) scales (Achenbach & Rescorla, 2000). Because early externalizing, oppositional, aggressive and hyperactive problems have been associated with behavior problems at school age (Campbell, Shaw, & Gilliom, 2000), in the current study we explored behaviors on one broadband scale (externalizing behavior), two syndrome scales (aggressive behavior and attention problems), and two DSM-oriented scales (attention deficit hyperactivity disorder (ADHD) and oppositional problems). Behaviors were reported as T-scores on the basis of age and gender. Descriptive statistics differentiated referred and non-referred samples using the T-score cutpoint of ≥ 60 for the broadband scale, and ≥ 65 for the syndrome and DSM-oriented scales (Table 2) (Achenbach & Rescorla, 2000). Cronbach’s alpha for the CBCL subscales were as follows: α=.92 for the broadband (externalizing) subscale, α=.68 for attention problems, α=.92 for aggressive behaviors (syndrome subscales), α=.78 for ADHD, and α=.86 for oppositional defiant problems (DSM-oriented subscales) (Achenbach & Rescorla, 2000). One mother did not finish completing the CBCL. Therefore, 122 checklists were ultimately available for inclusion in data analyses.
Table 2.
Descriptive Characteristics of Child Measures
| Child measures | Score ranges | VPI N = 38 |
MPI N = 47 |
LPI N = 37 |
|---|---|---|---|---|
| ABIQ 36 months (chronological) | Range | 61–124 | 52–115 | 70–130 |
| n (%)a | 2 (5.2%) | 3 (6.4%) | 1 (2.7%) | |
| CBCL: externalizing problems | Range | 28–64 | 28–69 | 32–69 |
| n (%)b | 3 (7.9%) | 8 (17.0%) | 12 (32.4%) | |
| CBCL: aggressive behavior | Range | 50–62 | 50–70 | 50–70 |
| n (%)c | 0 (0.0%) | 3 (6.3%) | 3 (8.1%) | |
| CBCL: oppositional defiant | Range | 50–67 | 50–73 | 50–67 |
| n (%)c | 1 (2.6%) | 5 (10.6%) | 2 (5.4%) | |
| CBCL: attention problems | Range | 50–77 | 50–73 | 50–73 |
| n (%)c | 5 (13.1%) | 5 (10.6%) | 6 (16.2%) | |
| CBCL: ADHD problems | Range | 50–71 | 50–71 | 50–71 |
| n (%)c | 3 (7.9%) | 1 (2.1%) | 2 (5.4%) |
Clinical scores on ABIQ include ABIQ ≤ 70.
At risk scores on CBCL-externalizing (broadband) subscale are reflected by T scores ≥ 60.
At risk scores on CBCL-syndrome and DSM-oriented subscales are reflected by T scores ≥ 65.
2.2.3. Preterm Status
Preterm categories were created based on gestational age cut-points that have been previously used to compare preterm groups (Davidoff, et al., 2006; Raju, et al., 2006; Rogers et al., 2012; Spittle et al., 2009): very preterm infants (VPI) born <30 weeks of gestation, moderate preterm infants (MPI) born 30–336/7 weeks of gestation, and late preterm infants (LPI) born 34–366/7 weeks of gestation.
2.2.4. Maternal Parenting Interactions
Infant-mother play interactions at 4, 9, 16, and 24 months were coded with the 29 parenting variables of the Parent Child Early Relational Assessment (PCERA) (R. Clark, 1985). The PCERA is an observational coding system used previously with preterm infants (Pridham, Steward, Thoyre, Brown, & Brown, 2007). Variables are coded on a 1 (negative quality) to 5 (positive quality) scale. Previous studies have reported an acceptable range of internal consistency (.75 to .96) and validity (R. Clark, 1999). Three previously published parent subscales (Durik, Hyde, & Clark, 2000) were calculated as follows: (1) Positive Affect, Involvement and Verbalizations (11 items, α=.91), (2) Negative Affect and Behavior (e.g., anger and criticism, 5 items, α=.89), and (3) Intrusiveness, Insensitivity, and Inconsistency (8 items, α=.78). Within each subscale, we summed parenting scores across the four timepoints, as the scores were highly correlated. Higher scores reflected more positive parenting. Thus, for Parenting Scales 2 and 3, higher scores represented less negativeparenting. At least 10% of interactions at each timepoint were independently coded by two trained researchers. Inter-rater reliability ranged from .83–.97, with a mean of .88, similar to previous studies (R. Clark, Hyde, Essex, & Klein, 1997; Pridham, Brown, Clark, Sondel, & Green, 2002). Kappas for individual codes ranged from 0.60–1.00 with a mean of 0.83.
2.2.5. Neonatal Health Risk
A neonatal health risk index (Poehlmann, et al., 2009) was created from review of NICU medical records. Infant gestational age and birthweight were standardized, reverse-scored, and combined with the standardized sum of the presence of ten neonatal medical risks. Those risks, along with the percentage of infants experiencing them, included: apnea (69.1%), respiratory distress (53.7%), chronic lung disease (10.6%), gastroesophageal reflux (11.4%), multiple birth (20.3%), supplementary oxygen at NICU discharge (8.9%), apnea monitor at NICU discharge (46.3%), 5-minute Apgar score < 6 (4.1%), ventilation during NICU stay (55.3%), and NICU stay > 30 days (40.7%). The index had a Cronbach’s α of .72, similar to previous studies (e.g., Shah, Clements, & Poehlmann, 2011), with higher scores reflecting more prematurity and health risks.
2.2.6. Family Socioeconomic Risks
A socioeconomic (SES) risk index (Poehlmann et al., 2011) was created by summing the presence of the following risks identified from the NICU demographic questionnaire: family income below federal poverty guidelines (adjusted for family size), unemployment for both parents, single and/or adolescent mother, greater than four dependent children, and less than high school education for mother or father. Scores ranged from 0 to 7, with higher scores reflecting more risks (Cronbach’s α= 0.75).
2.2.7. Maternal Vocabulary
The Peabody Picture Vocabulary Test, 3rd Edition (Dunn & Dunn, 1997) was used to measure maternal receptive vocabulary as an indicator of maternal cognition. The PPVT-III is a widely-used, individually-administered assessment for individuals between the ages of 2 through 90. The PPVT-III has a mean of 100 (SD=15) and correlates highly with IQ.
2.2.8. Maternal Depression
The Center for Epidemiologic Studies-Depression Scale (Radloff, 1977) was used to assess maternal depressive symptoms at 4, 9, 16, 24, and 36-months. The CES-D is a 20-item self-report questionnaire that asks respondents to rate their symptoms on a 4-point scale ranging from rarely/none of the time (0) to most/all of the time (3) during the past week. Scores greater than or equal to 16 suggest need for further evaluation. The CES-D is a well-validated measure used in high and low risk samples (Radloff, 1977). For the present study we averaged depression scores across all timepoints for each mother in the study (Cronbach’s α=.88).
2.3 Procedure
This study was approved by hospital and university Institutional Review Boards. Infants were assessed at six timepoints: NICU discharge and 4, 9, 16, 24, and 36-months corrected age. Corrected ages are calculated on the basis of the infant’s due date and they are a commonly used assessment of preterm infants (DiPietro & Allen, 1991). At Time 6, 83% of families remained in the study. Multivariate analysis of variance (MANOVA) was used to examine potential differences between families who continued in the study for three years and familieswho were lost to attrition. The MANOVA examining differences in participation at the three year assessment for infant health variables revealed no significant differencesin the following areas: infant gestational age, birthweight, 1- and 5-minute Apgar scores, days hospitalized, and neonatal health risk index (multivariate F(6, 165) = 0.96, p = .45). The MANOVA conducted on family SES variables (measured at Time 1) revealed significant differences between families who participated in the study for three years and families lost to attrition (multivariate F (7, 164) = 5.06, p < .05). Follow-up univariate tests indicated that mothers who were lost to attrition were younger (F (1, 170) = 7.24, p < .05) and had completed fewer years of education (F (1, 170) = 13.19, p < .05). In addition, families were more likely to be lost to attrition when the fathers had completed fewer years of education (F (1, 170) = 8.24, p < .05) and when the families had more SES risks, (F (1, 170) = 8.28, p < .05). There were no significant difference between mothers who participated in the study for three years and mothers who were lost to attrition in: number of children in the family, father’s age, or family income. However, chi-square analyses revealed that mothers lost to attrition were more likely to be single (χ2(1) = 8.12, p < .05) (Poehlmann et al., 2010).
The current analyses utilized data from all six timepoints. At NICU discharge, medical records and demographic information were reviewed. The 4-, 9-, 16-, and 24-month visits included video-taped observations of infant-mother play in which mothers were instructed to play “as they normally would” for 15 minutes. These interactions were later coded. At the 9-month visit, maternal vocabularywas assessed. At 36 -months, child cognition was measured, and mothers completed a child behavior checklist. Screenings for maternal depressive symptoms occurred at all timepoints.
2.4 Analyses
Data were analyzed using PASW Statistics (Version 17.0) (SPSS, Chicago, IL). Following data screening to detect any statistical assumption violations, we conducted a one-way multivariate analysis of variance (MANOVA), with follow-up univariate F and Tukey HSD tests when the multivariate F was significant, to compare health and neurobehavioral outcomes for each preterm group (i.e. VPI, MPI, and LPI). Using hierarchical linear regression, we then tested for the contribution of preterm status (i.e., degree of prematurity) and parenting on outcomes of interest, including covariates (SES risk, maternal vocabulary, and maternal depression) identified a priori due to the well-established associations that exist between these covariates and child outcomes.
Covariates were entered into a hierarchical linear regression model in the first step, and preterm status and parenting variables were added in the second step to determine the unique contribution of each variable on child outcomes. In step 3 of our regression models, we explored whether an interaction between parenting and preterm status was a significant predictor of child outcomes. When an interaction term in step 3 was statistically significant, we ran simple regressions looking at the parenting slopes for the VPI, MPI, and LPI groups to determine whether there were differential effects of parenting on outcomes based on preterm status. We also examined bivariate correlations between predictors, outcomes and covariates, as is required to test the mechanism of interaction effects using methods previously described (Belsky, Bakersman-Kranenburg, & van IJzendoorn, 2007) (Table 3). Results of significant interaction analyses are presented graphically (Figure 1). Results reflect 2-tailed analyses and a cut-off p value of .05.
Table 3.
Unadjusted Bivariate Correlations Between Predictor, Moderator, Outcome and Control Variables
| Variables | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. Total SES risk | -- | ||||||||||||
| 2. Maternal PPVT score | −.535** | -- | |||||||||||
| 3. Maternal depression (CES-D) | .144 | −.109 | -- | ||||||||||
| 4. Preterm Status | −0.07 | 0.12 | 0.59 | -- | |||||||||
| 5. PCERA 1 (positive parenting) | −0.482** | .309** | −.094 | .134 | -- | ||||||||
| 6. PCERA 2 (negative parenting) | −.400** | .383** | −.203* | .125 | .528** | -- | |||||||
| 7. PCERA 3 (intrusive parenting) | −.510 | .372 | −.168 | −.138 | .744** | .719** | -- | ||||||
| 8. 36 month IQ | −.398** | .359** | −0.18 | .111 | .309** | .220* | .366* | -- | |||||
| 9. Externalizing Behavior T-Score | .097 | −.113 | .373** | .215* | .006 | −.229* | −.124 | −.071 | -- | ||||
| 10. Aggressive behavior - T Score | −.077 | .007 | .285** | .277** | .143 | −.116 | −.008 | .082 | .840** | -- | |||
| 11. Oppositional problems - T Score | −.167 | .092 | .218* | .233** | .084 | −.102 | −.023 | .087 | .708** | .828** | -- | ||
| 12. Attention problems - T Score | .218* | −.125 | .307** | .022 | −.088 | −.224* | −.183* | −.203* | .694** | .541** | −.434** | -- | |
| 13. ADHD problems - T Score | .203* | −.188* | .356** | .048 | −0.62 | −.215 | −.170 | −.156 | .729** | .639** | .490** | .872** | -- |
Correlation is significant at the 0.05 level (2-tailed).
Correlation is significant at the 0.01 level (2-tailed).
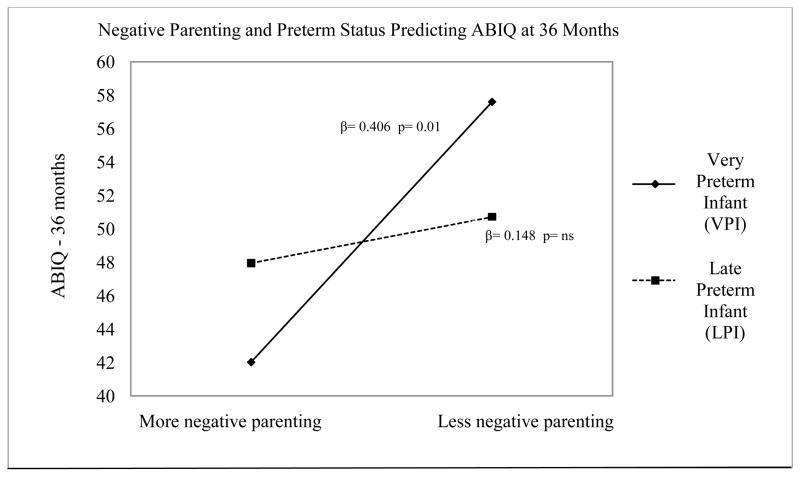
Figure 1.
This figure illustrates how the relationship between parenting quality (more or less negative parenting) and 36 month cognitive outcomes (ABIQ) is moderated by preterm status (VPI vs. LPI).
3. Results
3.1. Neonatal Health and Neurobehavioral Outcomes of Preterm Infants by Degree of Prematurity
One-way multivariate analysis of variance (MANOVA) was used to compare preterm groups (very preterm, moderate preterm, and late preterm infants) on infant health outcomes prior to NICU discharge, and neurobehavioral outcomes at 36 months. Infant health outcomes included APGAR scores, length of hospitalization, and level of neonatal health risk, and neurobehavioral outcomes were characterized by IQ and behavioral symptoms. A significant multivariate statistic indicated that preterm groups differed on outcomes (F (24,216) = 19.63, p < 0.001). Results are presented in Table 4.
Table 4.
Overall Group Differences: Health and Neurobehavioral Outcomes by Preterm Status
| NEONATAL HEALTH OUTCOMES Mean (SD) |
VPIa N = 38 |
MPI N = 47 |
LPI N = 37 |
F(df) | p | Partial η2 |
|---|---|---|---|---|---|---|
| Gestational age (weeks) | 27.3 (1.6) | 32.1 (1.1) | 34.5 (0.6) | 395.65 (2,119) | <0.001 | 0.869 |
| Birthweight (grams) | 1031.3 (302.2) | 1857.9 (339.3) | 2314.7 (328.6) | 151.38 (2,119) | <0.001 | 0.718 |
| APGAR scores: 1 Minute | 4.8 (1.9) | 6.2 (1.7) | 6.5 (2.0) | 9.03 (2,119) | <0.001 | 0.132 |
| APGAR scores: 5 Minute | 7.1 (1.5) | 8.1 (1.2) | 8.54 (0.9) | 14.32 (2,119) | <0.001 | 0.194 |
| Number of days hospitalized | 71.8 (22.7) | 22.4 (11.1) | 9.7 (4.8) | 191.89 (2,119) | <0.001 | 0.763 |
| Neonatal health risks | 5.4 (1.8) | 2.6 (1.7) | 1.6 (1.3) | 57.87 (2,119) | <0.001 | 0.493 |
| NEUROBEHAVIORAL OUTCOMES | ||||||
| ABIQ 36 months (chronological) | 95.8 (14.7) | 95.4 (13.9) | 99.1 (12.8) | 0.84 (2,119) | 0.433 | 0.014 |
| CBCL: externalizing problems | 48.1 (9.1) | 52.1 (9.0) | 53.3 (9.9) | 3.24 (2,119) | 0.043 | 0.052 |
| CBCL: aggressive behavior | 52.2 (3.4) | 54.8 (5.3) | 55.8 (6.1) | 5.31 (2,119) | 0.006 | 0.082 |
| CBCL: oppositional defiant | 51.9 (3.5) | 55.3 (6.4) | 55.1 (5.0) | 5.05 (2,119) | 0.008 | 0.078 |
| CBCL: attention problems | 56.1 (7.4) | 55.9 (6.3) | 56.5 (7.2) | 0.08 (2,119) | 0.926 | 0.001 |
| CBCL: ADHD problems | 54.3 (6.0) | 54.7 (5.1) | 55.0 (5.7) | 0.14 (2,119) | 0.869 | 0.002 |
1 mother did not complete the CBCL, so 38 VPI were included in this analysis.
Post hoc tests with univariate F and Tukey HSD tests indicated that, compared with VPIs and MPIs, LPIs demonstrated shorter NICU stays and fewer neonatal health risks (p < .001). LPIs also evidenced higher 1 and 5–minute APGARs than VPIs (p < .001). In addition, LPIs differed from more preterm infants on select Child Behavior Checklist (CBCL) outcomes, but not on cognitive outcomes. At 36-months, LPIs demonstrated more externalizing behaviors (p = .043), oppositional behaviors (p = .008), and aggressive behaviors (p = .006) compared to VPIs, but demonstrated similar behaviors to MPIs. Although the MANOVA showed that the means were significantly different among preterm groups defined by gestational age, the effect sizes were small to modest. The partial Eta squared statistics for behavior problems ranged from .05 to .08, indicating that preterm status by itself accounted for only 5% to 8% of the overall variance in behavior problems. LPIs demonstrated similar cognitive skills to both VPIs (p = .559) and MPIs (p = .445), with no differences between groups identified.
3.2. Predictors of 36-month Cognitive and Behavioral Outcomes
Hierarchical linear regression analyses were conducted to examine predictors of behavior problems and cognitive outcomes at 36 months, including testing the potential moderating effect of preterm group on the relation between parenting and child outcomes. Step 1 predictors included SES risk, maternal vocabulary (PPVT), and maternal depression as controls. Step 2 predictors were preterm status and parenting behaviors (PCERA factors 1,2, and 3) to test for main effects. The preterm status x PCERA interaction terms (for each PCERA factor) were added in step 3 to test for moderation. Separate analyses were conducted for each behavior problem scale and for IQ scores. Results indicated that late preterm infants were more likely to demonstrate externalizing (β = .223, p = 014), aggressive (β = 0.249, p = 0.004) and . oppositional behaviors (β = 0.214, p = 0.016) compared with infants born more prematurely (Table 5), although prematurity was not a significant predictor of IQ. Negative parenting (PCERA-2) was a significant predictor of externalizing behavior problems (β = −.228, p = 0.028), although the quality of parent interactive styles was not a significant predictor of other behavior problem scales (Table 5) or IQ (Table 6, step 2).
Table 5.
Preterm Status and Parenting Quality Predicting Behavior Outcomes of Preterm Infants
| Step 2 Linear regression results | Predictor | Standardized coefficients Beta
|
t | sig. | 95.0% Confidence interval for B
|
R2 (Step 2 results) | |
|---|---|---|---|---|---|---|---|
| Lower bound | Upper bound | ||||||
| Externalizing behavior | SES risk | 0.059 | 0.512 | 0.610 | −1.208 | 2.050 | .231 |
| Maternal PPVT | −0.002 | −0.019 | 0.985 | −0.156 | 0.153 | ||
| Maternal depression 36m | 0.079 | 0.882 | 0.380 | −0.102 | 0.267 | ||
| Preterm status | 0.223 | 2.486 | 0.014 | 0.544 | 4.821 | ||
| PCERA 1: positive parenting | 0.212 | 1.557 | 0.122 | −0.288 | 2.400 | ||
| PCERA 2: negative parenting | −0.288 | −2.225 | 0.028 | −3.061 | −0.177 | ||
| PCERA 3: intrusive parenting | −0.075 | −0.457 | 0.649 | −2.660 | 1.664 | ||
| Aggressive behavior | SES risk | −0.082 | −0.743 | 0.459 | −1.180 | 0.536 | .218 |
| Maternal PPVT | 0.027 | 0.266 | 0.790 | −0.070 | 0.092 | ||
| Maternal depression 36m | 0.257 | 2.999 | 0.003 | 0.075 | 0.370 | ||
| Preterm status | 0.249 | 2.920 | 0.004 | 0.532 | 2.775 | ||
| PCERA 1: positive parenting | 0.282 | 2.177 | 0.032 | 0.070 | 1.483 | ||
| PCERA 2: negative parenting | −0.224 | −1.809 | 0.073 | −1.455 | 0.066 | ||
| PCERA 3: intrusive parenting | −0.105 | −0.674 | 0.502 | −1.524 | 0.750 | ||
| Oppositional behavior | SES risk | −0.223 | −1.961 | 0.052 | −1.812 | 0.009 | .169 |
| Maternal PPVT | 0.070 | 0.670 | 0.504 | −0.057 | 0.115 | ||
| Maternal depression | 0.197 | 2.227 | 0.028 | 0.019 | 0.331 | ||
| Preterm status | 0.214 | 2.438 | 0.016 | 0.274 | 2.655 | ||
| PCERA 1: positive parenting | 0.122 | 0.913 | 0.363 | −0.404 | 1.096 | ||
| PCERA 2: negative parenting | −0.197 | −1.545 | 0.125 | −1.437 | 0.178 | ||
| PCERA 3: intrusive parenting | −0.107 | −0.664 | 0.508 | −1.611 | 0.803 | ||
| Attention problems | SES risk | 0.174 | 1.511 | 0.134 | −0.277 | 2.060 | .152 |
| Maternal PPVT | 0.039 | 0.372 | 0.710 | −0.090 | 0.131 | ||
| Maternal depression | 0.264 | 2.958 | 0.004 | 0.099 | 0.499 | ||
| Preterm status | 0.026 | 0.298 | 0.766 | −1.297 | 1.757 | ||
| PCERA 1: positive parenting | 0.132 | 0.982 | 0.328 | −0.485 | 1.439 | ||
| PCERA 2: negative parenting | −0.159 | −1.236 | 0.219 | −1.681 | 0.390 | ||
| PCERA 3: intrusive parenting | −0.059 | −0.361 | 0.719 | −1.830 | 1.266 | ||
| ADHD problems | SES risk | 0.120 | 1.062 | 0.290 | −0.433 | 1.432 | .129 |
| Maternal PPVT | −0.065 | −0.627 | 0.532 | −0.116 | 0.060 | ||
| Maternal depression | 0.312 | 3.559 | 0.001 | 0.127 | 0.447 | ||
| Preterm status | 0.039 | 0.447 | 0.656 | −0.943 | 1.493 | ||
| PCERA 1: positive parenting | 0.168 | 1.263 | 0.209 | −0.278 | 1.257 | ||
| PCERA 2: negative parenting | −0.126 | −0.997 | 0.321 | −1.242 | 0.410 | ||
| PCERA 3: intrusive parenting | −0.075 | −0.467 | 0.641 | −1.526 | 0.944 | ||
Table 6.
Differential Effects of Parenting and Preterm Status on Cognitive Outcomes (IQ) of Preterms
| Regression results | Predictor | Standardized coefficients beta | t | sig | 95.0% Confidence interval for B | R2 | |
|---|---|---|---|---|---|---|---|
| Lower bound | Upper bound | ||||||
| Step 1 | SES risk | −0.291 | 2.938 | 0.004 | −5.087 | −0.990 | .189 |
| Maternal PPVT | 0.208 | 2.108 | 0.037 | 0.014 | 0.433 | ||
| Maternal depression | 0.046 | 0.551 | 0.583 | −0.276 | 0.488 | ||
| Step 2 | SES risk | −0.188 | −1.730 | 0.086 | −4.216 | 0.285 | .233 |
| Maternal PPVT | 0.205 | 2.040 | 0.044 | 0.006 | 0.433 | ||
| Maternal depression | 0.048 | 0.569 | 0.571 | −0.274 | 0.495 | ||
| Preterm status | 0.063 | 0.749 | 0.456 | −1.824 | 4.040 | ||
| PCERA 1: positive parenting | 0.034 | 0.267 | 0.790 | −1.608 | 2.107 | ||
| PCERA 2: negative parenting | −0.138 | −1.135 | 0.259 | −3.076 | 0.835 | ||
| PCERA 3: intrusive parenting | 0.269 | 1.743 | 0.084 | −0.360 | 5.621 | ||
| Step 3 | SES risk | −0.175 | −1.636 | 0.105 | −4.036 | 0.386 | .281 |
| Maternal PPVT | 0.217 | 2.159 | 0.033 | 0.019 | 0.447 | ||
| Maternal depression | 0.088 | 1.043 | 0.299 | −0.182 | 0.586 | ||
| Preterm status | −0.014 | −0.013 | 0.990 | −36.755 | 36.271 | ||
| PCERA 1: positive parenting | −0.201 | −0.535 | 0.594 | −6.962 | 4.003 | ||
| PCERA 2: negative parenting | 0.563 | 1.828 | 0.070 | −0.384 | 9.549 | ||
| PCERA 3: intrusive parenting | −0.221 | −0.474 | 0.637 | −11.225 | 6.896 | ||
| Preterm status × positive parenting | 0.880 | 0.754 | 0.452 | −1.528 | 3.405 | ||
| Preterm status × negative parenting | −3.245 | −2.430 | 0.017 | −5.820 | −0.591 | ||
| Preterm status × intrusive parenting | 2.469 | 1.277 | 0.204 | −1.438 | 6.647 | ||
In step 3, we explored whether the effects of prematurity in combination with certain parenting interactive styles could predict 36-month behavior and IQ, after controlling for the effects of sociodemographic and maternal characteristics. No interaction effects were seen between preterm status and parenting quality on 36-month behavior outcomes. However, the combined effects of parenting, sociodemographic risks, and degree of prematurity accounted for 13%–23% of the variance in behavior outcomes (overall model R2= 0.13–0.23, Table 5). In contrast to the behavior problem findings, regression analyses indicated that the effects of parenting on children’s 36-month cognitive outcomes were moderated by the degree of prematurity (Table 6, step 3). A significant interaction effect was found to exist between very preterm status and negative parenting (PCERA-2) on 36-month ABIQ scores (β = −3.245, p = .017). Post-hoc tests indicated that variations in maternal negative behavior significantly related to less optimal child IQ for VPIs, (β = 0.406, p = .01), but not for MPIs, (β = 0.031, p =. 834) or LPIs (β = 0.148, p = .381). VPIs had higher cognitive skills when mothers exhibited less negative interactions during infancy, and lower cognitive skills with more negative interactions (Figure 1). No interaction effects were seen between preterm status and positive (PCERA-1) or angry parenting (PCERA-3) on 36-month cognitive outcomes.
4. Discussion
The present study contributes to our understanding of neurobehavioral outcomes of preterm infants. Further, it addresses how the quality of early parenting and degree of prematurity relate to such outcomes. We discovered that late preterm infants, previously thought to be a relatively low-risk population among preterm infants, may be at risk for behavioral vulnerabilities. In addition, our results indicated that the cognitive deficits well-described in very preterm infants (Johnson, 2007) may be related to the quality of early caregiving. Our results also suggest that the degree of prematurity (characterized by “preterm status”) is an independent predictor of externalizing behavior outcomes at 36 months. Although the late preterm infants in our sample demonstrated better neonatal health, fewer medical complications, and similar IQs compared to infants born more prematurely, LPIs demonstrated an increased risk of externalizing, aggressive, and oppositional behaviors at 36 months compared to infants born more preterm. This finding challenges previous literature suggesting that behavior problems in preterm infants are largely a function of degree of prematurity, with behavior problems most notable in infants who were born at lower gestational ages and with more medical complications (Kelly, Nazroo, McMunn, Boreham, & Marmot, 2001; Salt & Redshaw, 2006; Spittle, et al., 2009). Whereas it has been presumed that infants born in the late preterm period (i.e. 34–366/7 weeks gestation) are at lower risk for negative outcomes (Gurka, et al., 2010), our findings suggest that late preterms (LPIs) may be at higher risk for externalizing behavior, aggressive, and oppositional symptoms (at least at 3-years postterm), although effect sizes were modest. Although the late preterm infants in our sample demonstrated more externalizing, aggressive, and oppositional symptoms than infants born preterm, the degree of prematurity (characterized by”preterm status”) accounted for only 5–8% of the variance. This suggests that additional factors contribute to the development of behavior problems in toddlerhood. Previous research in a non-preterm population highlights how behavior problems in early childhood emerge within the context of multiple biopsychosocial risk factors including history of psychiatric illness, family stress, and harsh parenting (Campbell, et al., 2000). Our findings also support the association between negative parenting and the development of externalizing behavior problems at 36 months. Because preterm infants are at risk for behavior problems, additional research is needed to explore how family processes, in combination with the degree of prematurity, contribute to the development of externalizing behavioral symptoms in preterm infants. Moreover, the lack of group differences among preterm groups regarding attentional symptoms is consistent with previous research suggesting that preterm infants, regardless of degree of prematurity, appear to have an increased risk for the development of attention problems (Msall & Park, 2008; Rowland, Lesesne, & Abramowitz, 2002).
Because LPIs generally experience fewer neonatal health complications compared to more preterm infants, we also hypothesized that they would demonstrate better cognitive outcomes compared to VPIs, and would be most influenced by positive caregiving. However, contrary to expectations, differences between cognitive skills among preterm groups were not found in this sample of preterm infants. Moreover, the 36-month cognitive skills of verypreterm infants (not late preterms) were the most affected by quality of parenting. Very preterm infants demonstrated the lowest cognitive scores in the context of more negative caregiving, but exhibited higher cognitive scores in the context of less negative parenting. In contrast, late preterm infants demonstrated similar cognitive outcomes whether they experienced more or less negative parenting (Figure 1). It is possible that parental expressions of negative affect, notably frustration or criticism during interactions (described by PCERA-2), may be particularly dysregulating for some preterm infants, and may thereby affect subsequent learning and cognitive development. These findings also extend our knowledge of cognitive susceptibility to parenting in preterm infants while supporting previous research demonstrating that preterm infants exhibit more optimal cognitive skills in the context of more positive parenting, and conversely, less optimal cognitive skills when parenting is less positive (Poehlmann et al., in press).
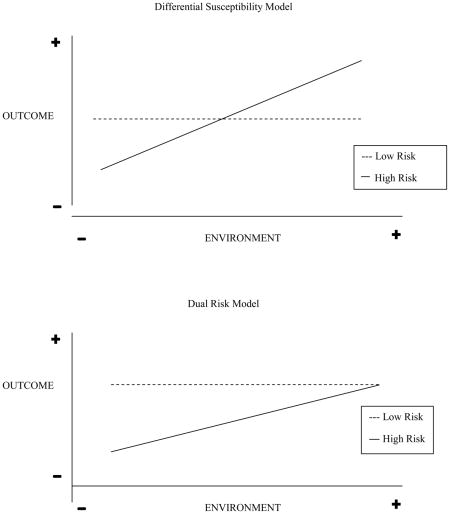
Findings from this study support a growing body of evidence indicating that children vary in their responsiveness to environmental influences, and that biological differences can moderate the effects of family environments on later outcomes (Ellis & Boyce, 2011; Ellis, Boyce, Belsky, Bakermans-Kranenburg, & van Ijzendoorn, 2011). This notion of “biologic sensitivity to context” (Ellis & Boyce, 2008) or “differential susceptibility” to environmental influences (Belsky, 2005; Belsky, et al., 2007) is grounded in an evolutionary framework. It suggests that some children, because of genetic or other biological characteristics, are more adversely affected by negative caregiving environments, and conversely, are more positively affected by supportive caregiving environments (Belsky, et al., 2007). This framework challenges the logic of traditional dual risk or cumulative risk models which highlight the detrimental effects of adversity on developmental outcomes, but unlike the differential susceptibility hypothesis, do not address the potential benefits of supportive caregiving environments. The “dual risk” model arises when the most “vulnerable” individuals are disproportionately affected in an adverse manner by a negative environment, but conversely, do not benefit to the same extent in a positive environment (Appendix B) (Belsky, et al., 2007). Similarly, the cumulative risk model asserts that the accumulation of risk factors impacts developmental outcomes, such that the greater the number of risk factors, the greater the prevalence of clinical problems (Appleyard, Egeland, van Dulmen, & Alan Sroufe, 2005). There is support for the perspective that children with a history of biological vulnerability (e.g. children born prematurely) manifest compromised development when exposed to contextual adversity (e.g. suboptimal parenting). However, this “dual-risk model” of development may underestimate the potential of human plasticity by failing to consider that the very children who are vulnerable to problems in development may also be disproportionately susceptible to the effects of supportive rearing environments (Belsky & Pluess, 2009).
Although degree of prematurity has not been explored as a potential “susceptibility factor,” previous research has demonstrated that more reactive infants demonstrate the best behavioral outcomes in the context of more positive parenting and worse outcomes in the context of less positive parenting, but that these effects are not seen in less reactive infants (Belsky, Hsieh, & Crnic, 1998; Mesman et al., 2009; Stright, Gallagher, & Kelley, 2008). Broadening outcomes to include cognitive development, our results suggest that the caregiving environment may exert differential effects on cognition, depending upon the degree of prematurity. Although being born very preterm is a risk factor for adverse neurodevelopment, particularly in the context of psychosocial adversity (Ross, Lipper, & Auld, 1991), a “resilience” framework suggests that, “… regarding cognitive development, in some cases, it may be possible to “reprogram” these systems to operate more normally when a positive caregiving environment is provided.”(Masten, 2007). Our understanding of the phenomenon of resilience has been shaped by the recognition that individuals can demonstrate trajectories of unexpectedly positive adaptation and recovery after adverse experiences, and that the processes which foster optimal adaptation in at-risk individuals involve environmental processes external to the individual (Masten, 2007; Masten & Obradovic, 2006). Articulated another way, resilience describes the phenomena of successful adaptation in the context of significant threats to development (Masten et al., 1999). Although being born very preterm is a well-known “threat to development” (Johnson, 2007), it appears that variations in the quality of the caregiving environment can help obviate the effects of biological risk and contribute to optimal cognitive development in infants born very prematurely. However, additional research is needed to further examine the processes that contribute to resilience in preterm infants.
4.1. Limitations
One notable limitation of the current research was that the sample size, small size of each gestational age group, and population characteristics limit the generalizability of the findings. Future research should include a larger sample of LPIs and inclusion of an LPI group not managed in the NICU. A full-term group was not included so that we could focus on factors facilitating resilience processes within preterm infants. However, inclusion of a full-term group could help determine if similar or different processes occur in infants born full-term and preterm. In the current study, behavior problems were assessed using maternal report. Future research should also use teacher report to supplement parental reports. Although the behavior outcomes reported reflect statistically significant behavioral concerns, further clinical research is warranted to determine whether these behaviors correspond to clinical indicators of emerging psychopathology. Moreover, in addressing environmental influences, we focused on maternal parenting behaviors. Future research should also examine father-infant interactions as well as co-parenting and full family interactions.
5. Conclusions
Findings from our study provide further evidence that the cognitive and behavioral outcomes of preterm infants are varied, and may be influenced by both degree of biological risk (i.e. degree of prematurity) and quality of the early caregiving environment. Our results suggest that late preterm infants, previously thought to be a low-risk population, may be at risk for behavioral difficulties. Furthermore, our findings suggest that the cognitive deficits well described in very preterm infants (Johnson, 2007) may be diminished by the quality of early caregiving, with very preterm infants demonstrating differentially susceptibility to the effects of caregiving, manifest by higher cognitive skills in the context of more optimal parenting. These findings may inform the type of care and anticipatory guidance provided in the care of preterm infants. Because late preterm infants demonstrated elevated externalizing, oppositional, and aggressive symptoms at 36 months, consistent with some of the previous literature (Gray, et al., 2004; Huddy, et al., 2001), they may benefit from closer behavioral surveillance during pediatric visits. Specific areas of potential intervention during routine health surveillance visits could include monitoring for signs of behavioral difficulties in the toddler and preschool years using parent-report checklists and screening for potential psychosocial issues that can influence behavior (Garg & Dworkin, 2011).
Furthermore, because the very preterm infants in our study demonstrated higher IQ in the context of more optimal parenting, VPIs may uniquely benefit from interventions supporting the quality of early-parent child interactions. However, further research is needed. Taken together, these findings highlight the importance of pediatric providers recognizing the potential neurobehavioral risks associated with infants at various degrees of prematurity, such that they may provide individualized developmental and behavioral surveillance for these infants.
Highlights.
36-month outcomes in preterm infants vary by degree of prematurity and parenting
Late preterms exhibit more behavior problems compared to very preterm infants (VPI)
Very preterm infants are differentially susceptible to the quality of parenting
VPIs exhibit lower IQ with more negative parenting and higher IQ with more positive
Late preterms demonstrate similar cognition regardless of early parenting quality
Acknowledgments
Special thanks to the children and families who generously gave of their time to participate in this study.
Role of the funding source: This research was supported by grants from the National Institutes of Health (HD44163) and the University of Wisconsin to Julie Poehlmann, and by grants from the National Institutes of Health (K12 RR17665) and University of Michigan to Prachi Shah.
Appendix B. Graphical Representation of Differential Susceptibility Hypothesis versus Dual Risk Model (Adapted from Belsky, et al., 2007, p. 303)
Footnotes
Abbreviations: VPI: very preterm infant; MPI: moderate preterm infant; LPI: late preterm infant; PPVT: Peabody Picture Vocabulary Test; PCERA: Parent-Child Early Relational Assessment; CBCL: Child Behavior Checklist; ABIQ: Abbreviated Battery IQ Scale; NICU: neonatal intensive care unit; CES-D: Center for Epidemiologic Studies-Depression Scale; SES: Socio-Economic Status; APGAR: American Pediatric Gross Assessment Record; ADHD: Attention Deficit Hyperactivity Disorder
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Prachi E. Shah, Division of Child Behavioral Health, Department of Pediatrics, & Center for Human Growth and Development, University of Michigan, Ann Arbor, MI
Natashia Robbins, Department of Pediatrics, University of Michigan, Ann Arbor, MI.
Renuka B. Coelho, Department of Pediatrics, University of Michigan, Ann Arbor, MI
Julie Poehlmann, Human Development and Family Studies and the Waisman Center, University of Wisconsin, Madison, WI.
References
- Aarnoudse-Moens CSH, Weisglas-Kuperus N, van Goudoever JB, Oosterlaan J. Meta-analysis of neurobehavioral outcomes in very preterm and/or very low birth weight children. Pediatrics. 2009;124(2):717–728. doi: 10.1542/peds.2008-2816. [DOI] [PubMed] [Google Scholar]
- Achenbach TM, Rescorla LA. Manual for ASEBA Child Behavior Checklist forms & profiles. Burlington, VT: University of Vermont, Research Center for Children, Youth, & Families; 2000. [Google Scholar]
- Appleyard K, Egeland B, van Dulmen MHM, Alan Sroufe L. When more is not better: The role of cumulative risk in child behavior outcomes. Journal of Child Psychology and Psychiatry. 2005;46(3):235–245. doi: 10.1111/j.1469-7610.2004.00351.x. [DOI] [PubMed] [Google Scholar]
- Aylward GP. Neurodevelopmental outcomes of infants born prematurely. Journal of Developmental and Behavioral Pediatrics. 2005;26(6):427–440. doi: 10.1097/00004703-200512000-00008. [DOI] [PubMed] [Google Scholar]
- Belsky J. Differential susceptibility to rearing influence: An evolutionary hypothesis and some evidence. In: Ellis BJ, Bjorklund DF, editors. Origins of the social mind: Evolutionary psychology and child development. New York: Guilford Press; 2005. pp. 139–163. [Google Scholar]
- Belsky J, Bakersman-Kranenburg MJ, van IJzendoorn MH. For better and for worse: Differential susceptibility to environmental influences. Current Directions in Psychological Science. 2007;16(6):300–304. [Google Scholar]
- Belsky J, Hsieh K, Crnic K. Mothering, fathering and infant negativity as antecedents of boys’ externalizing problems and inhibition at 3 years: Differential susceptibility to rearing experience? Development and Psychopathology. 1998;10:301–319. doi: 10.1017/s095457949800162x. [DOI] [PubMed] [Google Scholar]
- Belsky J, Pluess M. The nature (and nurture?) of plasticity in early human development. Perspectives on Psychological Science. 2009;4(4):345–351. doi: 10.1111/j.1745-6924.2009.01136.x. [DOI] [PubMed] [Google Scholar]
- Bhutta AT, Cleves MA, Casey PH, Cradock MM, Anand KJS. Cognitive and behavioral outcomes of school aged children who were born preterm. Journal of the American Medical Association. 2002;288(6):728–737. doi: 10.1001/jama.288.6.728. [DOI] [PubMed] [Google Scholar]
- Campbell SB, Shaw DS, Gilliom M. Early externalizing behavior problems: Toddlers and preschoolers at risk for later maladjustment. Development and Psychopathology. 2000;12(03):467–488. doi: 10.1017/s0954579400003114. [DOI] [PubMed] [Google Scholar]
- Chyi LJ, Lee HC, Hintz SR, Gould JB, Sutcliffe TL. School outcomes of late preterm infants: Special needs and challenges for infants born at 32 to 36 weeks gestation. The Journal of Pediatrics. 2008;153(1):25–31. doi: 10.1016/j.jpeds.2008.01.027. [DOI] [PubMed] [Google Scholar]
- Clark C, Woodward L, Horwood L, Moor S. Development of emotional and behavioral regulation in children born extremely preterm and very preterm: Biological and social influences. Child Development. 2008;79(5):1444–1462. doi: 10.1111/j.1467-8624.2008.01198.x. [DOI] [PubMed] [Google Scholar]
- Clark R. The Parent-Child Early Relational Assessment, instrument and manual. Department of Psychiatry, University of Wisconsin Medical School; Madison, WI: 1985. [Google Scholar]
- Clark R. The Parent Child Early Relational Assessment: A factorial validity study. Educational and Psychological Measurement. 1999;59(5):821–846. [Google Scholar]
- Clark R, Hyde JS, Essex MJ, Klein MH. Length of maternity leave and quality of mother-infant interactions. Child Development. 1997;68(2):364–383. doi: 10.1111/j.1467-8624.1997.tb01945.x. [DOI] [PubMed] [Google Scholar]
- Damus K. Prevention of preterm birth: A renewed national priority. Current Opinions in Obstetrics and Gynecology. 2008;20:590–596. doi: 10.1097/GCO.0b013e3283186964. [DOI] [PubMed] [Google Scholar]
- Davidoff M, Dias T, Damus K, Russell R, Bettegowda V, Dolan S, Petrini J. Changes in the gestational age distribution among US singleton births: Impact on rates of late preterm birth, 1992 to 2002. Seminars in Perinatology. 2006;30(1):8–15. doi: 10.1053/j.semperi.2006.01.009. [DOI] [PubMed] [Google Scholar]
- Davis DW, Burns B, Snyder E, Robinson J. Attention problems in VLBW preschoolers: Are new screening measures needed for this special population. Journal of Child and Adolescent Psychiatric Nursing. 2007;20(2):75–85. doi: 10.1111/j.1744-6171.2007.00089.x. [DOI] [PubMed] [Google Scholar]
- Deutscher B, Fewell RR. Early predictors of attention-deficit/hyperactivity disorder and school difficulties in low-birthweight, premature children. Topics in Early Childhood Special Education. 2005;25(2):71–79. doi: 10.1177/02711214050250020401. [DOI] [Google Scholar]
- DiPietro JA, Allen MC. Estimation of gestational age: Implications for developmental research. Child Development. 1991;62:1184–1199. [PubMed] [Google Scholar]
- Dunn L, Dunn L. Peabody Picture Vocabulary Test. 3. Circle Pines, MN: American Guidance Service; 1997. [Google Scholar]
- Durik AM, Hyde JS, Clark R. Sequelae of cesarean and vaginal deliveries: Psychosocial outcomes for mothers and infants. Developmental Psychology. 2000;36(2):251–260. doi: 10.1037//0012-1649.36.2.251. [DOI] [PubMed] [Google Scholar]
- Ellis BJ, Boyce WT. Biological sensitivity to context. Current Directions in Psychological Science. 2008;17(3):183–187. [Google Scholar]
- Ellis BJ, Boyce WT. Differential susceptibility to the environment: Toward an understanding of sensitivity to developmental experiences and context. Development and Psychopathology. 2011;23(1):1–5. doi: 10.1017/S095457941000060X. [DOI] [PubMed] [Google Scholar]
- Ellis BJ, Boyce WT, Belsky J, Bakermans-Kranenburg MJ, van Ijzendoorn MH. Differential susceptibility to the environment: An evolutionary–neurodevelopmental theory. Development and Psychopathology. 2011;23(1):7–28. doi: 10.1017/S0954579410000611. [DOI] [PubMed] [Google Scholar]
- Engle WA, Tomashek KM, Wallman C. “Late-preterm” infants: A population at risk. Pediatrics. 2007;120(6):1390–1401. doi: 10.1542/peds.2007-2952. [DOI] [PubMed] [Google Scholar]
- Feldman R, Eidelman AI, Rotenberg N. Parenting stress, infant emotion regulation, maternal sensitivity, and the cognitive development of triplets: A model for parent and child influences in a unique ecology. Child Development. 2004;75(6):1774–1791. doi: 10.1111/j.1467-8624.2004.00816.x. [DOI] [PubMed] [Google Scholar]
- Garg A, Dworkin PH. Applying surveillance and screening to family psychosocial issues: Implications for the medical home. Journal of Developmental & Behavioral Pediatrics. 2011;32(5):418–426. doi: 10.1097/DBP.0b013e3182196726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray RF, Indurkhya A, McCormick MC. Prevalence, stability, and predictors of clinically significant behavior problems in low birth weight children at 3, 5, and 8 years of age. Pediatrics. 2004;114(3):736–743. doi: 10.1542/peds.2003-1150-L. [DOI] [PubMed] [Google Scholar]
- Gurka MJ, LoCasale-Crouch J, Blackman JA. Long-term cognition, achievement, socioemotional, and behavioral development of healthy late-preterm infants. Archives of Pediatric & Adolescent Medicine. 2010;164(6):525–532. doi: 10.1001/archpediatrics.2010.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hack M, Youngstrom EA, Cartar L, Schlucter M, Taylor HG, Flannery D. Behavioral outcomes and evidence of psychopathology among very low birthweight infants at age 20 years. Pediatrics. 2004;114(4):932–940. doi: 10.1542/peds.2003-1017-L. [DOI] [PubMed] [Google Scholar]
- Huddy CLJ, Johnson A, Hope PL. Educational and behavioural problems in babies of 32–35 weeks gestation. Archives of Disease in Childhood - Fetal and Neonatal Edition. 2001;85(1):F23–F28. doi: 10.1136/fn.85.1.F23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanova MY. Preschool psychopathology reported by parents in 23 societies: Testing the Seven-Syndrome Model of the child behavior checklist for ages 1.5–5. Journal of the American Academy of Child and Adolescent Psychiatry. 2010;49(12):1215–1224. doi: 10.1016/j.jaac.2010.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson S. Cognitive and behavioral outcomes following very preterm birth. Seminars in Fetal and Neontal Medicine. 2007;12(5):363–373. doi: 10.1016/j.siny.2007.05.004. [DOI] [PubMed] [Google Scholar]
- Kelly YJ, Nazroo JY, McMunn A, Boreham R, Marmot M. Birthweight and behavior problems in children: A modifiable effect? International Journal of Epidemiology. 2001;2001(30):88–94. doi: 10.1093/ije/30.1.88. [DOI] [PubMed] [Google Scholar]
- Landry SH, Chapieski ML, Richardson MA, Palmer J, Hall S. The social competence of children born prematurely: Effects of medical complications and parent behaviors. Child Development. 1990;61(5):1605–1616. doi: 10.1111/j.1467-8624.1990.tb02887.x. [DOI] [PubMed] [Google Scholar]
- Landry SH, Smith KE, Miller-Loncar C, Swank P. Predicting cognitive language and social growth curves from early maternal behaviors in children at varying degrees of biological risk. Developmental Psychology. 1997;6:1040–1053. doi: 10.1037//0012-1649.33.6.1040. [DOI] [PubMed] [Google Scholar]
- Landry SH, Smith KE, Miller-Loncar C, Swank P. The relation of change in maternal interactive styles to the developing social competence of full term and preterm children. Child Development. 1998;69(1):105–123. [PubMed] [Google Scholar]
- Laucht M, Esser G, Schmidt MH. Differential development of infants at risk for psychopathology: The moderating role of early maternal responsivity. Developmental Medicine and Child Neurology. 2001;43(5):292–300. doi: 10.1017/s0012162201000561. [DOI] [PubMed] [Google Scholar]
- Masten AS. Resilience in developing systems: Progress and promise as the fourth wave rises. Development and Psychopathology. 2007;19(3):921–930. doi: 10.1017/S0954579407000442. [DOI] [PubMed] [Google Scholar]
- Masten AS, Hubbard JJ, Gest SD, Tellegen A, Garmezy N, Ramirez M. Competence in the context of adversity: Pathways to resilience and maladaptation from childhood to late adolescence. Development and Psychopathology. 1999;11(1):143–169. doi: 10.1017/s0954579499001996. [DOI] [PubMed] [Google Scholar]
- Masten AS, Obradovic J. Competence and resilience in development. Annals of the New York Academy of Sciences. 2006;1094(1):13–27. doi: 10.1196/annals.1376.003. [DOI] [PubMed] [Google Scholar]
- Mesman J, Stoel R, Bakermans-Kranenburg MJ, Ijzendoorn MH, Juffer F, Koot HM, Alink LRA. Predicting growth curves of early childhood externalizing problems: Differential susceptibility of children with difficult temperament. Journal of Abnormal Child Psychology. 2009;37(5):625–636. doi: 10.1007/s10802-009-9298-0. [DOI] [PubMed] [Google Scholar]
- Morse SB, Zheng H, Tang Y, Roth J. Early school-age outcomes of late preterm infants. Pediatrics. 2009;123(4):e622–629. doi: 10.1542/peds.2008-1405. [DOI] [PubMed] [Google Scholar]
- Msall ME, Park JJ. The spectrum of behavioral outcomes after extreme prematurity: Regulatory, attention, social, and adaptive dimensions. Seminars in Perinatology. 2008;32(1):42–50. doi: 10.1053/j.semperi.2007.12.006. [DOI] [PubMed] [Google Scholar]
- Poehlmann J, Hane A, Burnson C, Maleck S, Hamburger E, Shah PE. Preterm infants who are prone to distress: Differential effects of parenting on 36-month behavioral and cognitive outcomes. Journal of Child Psychology and Psychiatry. doi: 10.1111/j.1469-7610.2012.02564.x. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poehlmann J, Schwichtenberg AJ, Bolt D, Dilworth-Bart J. Predictors of depressive symptom trajectories in mothers of preterm or low birth weight infants. Journal of Family Psychology. 2009;23(5):690–704. doi: 10.1037/a0016117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poehlmann J, Schwichtenberg AJM, Shah P, Schlafer RJ, Hahn E, Janus S. The development of effortful control in high risk infants born preterm. Journal of Clinical Child and Adolescent Psychology. 2010;39(4):522–536. doi: 10.1080/15374416.2010.486319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poehlmann J, Schwichtenberg AJM, Shlafer RJ, Hahn E, Bianchi J-P, Warner R. Emerging self-regulation in toddlers born preterm or low birth weight: Differential susceptibility to parenting? Development and Psychopathology. 2011;23(01):177–193. doi: 10.1017/S0954579410000726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pridham K, Brown R, Clark R, Sondel S, Green C. Infant and caregiving factors affecting weight-for-age and motor development of full-term and premature infants at 1 year post-term. Research in nursing & health. 2002;25(5):394–410. doi: 10.1002/nur.10047. [DOI] [PubMed] [Google Scholar]
- Pridham K, Steward D, Thoyre S, Brown R, Brown L. Feeding skill performance in premature infants during the first year. Early Human Development. 2007;83(5):293–305. doi: 10.1016/j.earlhumdev.2006.06.004. [DOI] [PubMed] [Google Scholar]
- Radloff LS. The CES-D Scale: A self-report depression scale for research in the general population. Applied Psychological Measurement. 1977;1(3):385–401. doi: 10.1177/014662167700100306. [DOI] [Google Scholar]
- Raju TNK, Higgins RD, Stark AR, Leveno KJ. Optimizing care and outcome for late-preterm (near-term) infants: A summary of the workshop sponsored by the National Institute of Child Health and Human Development. Pediatrics. 2006;118(3):1207–1214. doi: 10.1542/peds.2006-0018. [DOI] [PubMed] [Google Scholar]
- Rogers CE, Anderson PJ, Thompson DK, Kidokoro H, Wallendorf M, Treyvaud K, Inder TE. Regional cerebral development at term relates to school-age social–emotional development in very preterm children. Journal of the American Academy of Child and Adolescent Psychiatry. 2012;51(2):181–191. doi: 10.1016/j.jaac.2011.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roid G. Stanford-Binet Intelligence Scales technical manual (SB5) 5. Itasca, IL: Riverside Publishers; 2003. [Google Scholar]
- Ross G, Lipper EG, Auld PAM. Educational status and school-related abilities of very low birth weight premature children. Pediatrics. 1991;88(6):1125–1134. [PubMed] [Google Scholar]
- Rowland AS, Lesesne CA, Abramowitz AJ. The epidemiology of attention-deficit/hyperactivity disorder (ADHD): A public health view. Mental Retardation and Developmental Disabilities Research Reviews. 2002;8(3):162–170. doi: 10.1002/mrdd.10036. [DOI] [PubMed] [Google Scholar]
- Saigal S, Pinelli J, Hoult L, Kim MM, Boyle M. Psychopathology and social competencies of adolescents who were extremely low birthweight. Pediatrics. 2003;111(5):969–975. doi: 10.1542/peds.111.5.969. [DOI] [PubMed] [Google Scholar]
- Salt A, Redshaw M. Neurodevelopmental followup after preterm bith: Followup after two years. Early Human Development. 2006;82:185–197. doi: 10.1016/j.earlhumdev.2005.12.015. [DOI] [PubMed] [Google Scholar]
- Schothorst PF, Van Engeland H. Long-term behavioral sequelae of prematurity. Journal of the American Academy of Child and Adolescent Psychiatry. 1996;35(2):175–183. doi: 10.1097/00004583-199602000-00011. [DOI] [PubMed] [Google Scholar]
- Smith K, Landry SH, Swank PR. The role of early maternal responsiveness in supporting school aged cognitive development for children who vary in birth status. Pediatrics. 2006;117:1608–1617. doi: 10.1542/peds.2005-1284. [DOI] [PubMed] [Google Scholar]
- Spittle AJ, Treyvaud K, Doyle LW, Roberts G, Lee KJ, Inder TE, Anderson PJ. Early emergence of behavior and social-emotional problems in very preterm infants. Journal of the American Academy of Child & Adolescent Psychiatry. 2009;48(9):909–918. doi: 10.1097/CHI.0b013e3181af8235. [DOI] [PubMed] [Google Scholar]
- Stright AD, Gallagher KC, Kelley K. Infant temperament moderates relations between maternal parenting in early childhood and childrens’s adjustment in first grade. Child Development. 2008;79(1):186–200. doi: 10.1111/j.1467-8624.2007.01119.x. [DOI] [PubMed] [Google Scholar]
- Taylor HG, Klein N, Hack M. School age consequences of birthweight less than 750g: A review and update. Developmental Psychology. 2000;17(3):289–321. doi: 10.1207/S15326942DN1703_2. [DOI] [PubMed] [Google Scholar]
- Treyvaud K, Anderson VA, Howard K, Bear M, Hunt RW, Doyle LW, Anderson PJ. Parenting behavior is associated with the early neurobehavioral development of very preterm children. Pediatrics. 2009;123(2):555–561. doi: 10.1542/peds.2008-0477. [DOI] [PubMed] [Google Scholar]
- Woythaler MA, McCormick MC, Smith VC. Late preterm infants have worse 24-month neurodevelopmental outcomes than term infants. Pediatrics. 2011;127(3):e622–e629. doi: 10.1542/peds.2009-3598. [DOI] [PubMed] [Google Scholar]
- Yu J, Buka S, McCormick M, Fitzmaurice G, Indurkhya A. Behavioral problems and the effects of early intervention on eight-year-old children with learning disabilities. Maternal and Child Health Journal. 2006;10(4):329–338. doi: 10.1007/s10995-005-0066-7. [DOI] [PubMed] [Google Scholar]