Abstract
Following infections and environmental exposures, memory T cells are generated that provide long-term protective immunity. Compared to their naïve T cell counterparts, memory T cells possess unique characteristics that endow them with the ability to quickly and robustly respond to foreign antigens. While such memory T cells are beneficial in protecting their hosts from recurrent infection, memory cells reactive to donor antigens pose a major barrier to successful transplantation and tolerance induction. Significant progress has been made over the past several decades contributing to our understanding of memory T cell generation, their distinct biology, and their detrimental impact in clinical and animal models of transplantation. This review focuses on the unique features which make memory T cells relevant to the transplant community and discusses potential therapies targeting memory T cells which may ameliorate allograft rejection.
Keywords: memory T cells, transplant tolerance, homeostatic proliferation, heterologous immunity, animal models, transplantation
INTRODUCTION
Lymphocyte development to memory cells is a keystone feature of the adaptive immune response to protect animals through the unique ability to rapidly recognize and effectively eliminate previously encountered pathogens. An individual’s repertoire of T and B memory cells essentially provides a historical log of infections and environmental exposures that have been encountered over the course of one’s life. While the ability of memory T cells to quickly mobilize and mount a potent recall response is beneficial in protective immunity against recurrent pathogens, memory T cells are a significant barrier to successful transplantation. Pre-existing donor antigen-primed memory T cells or memory T cells generated during responses to infectious or environmental antigens that cross-react with donor allogeneic MHC molecules are quickly activated in response to allografts and express functions mediating graft tissue injury that can directly lead to graft failure or promote sufficient injury to jeopardize patient survival. In this review we will describe fundamental characteristics of memory T cells and their generation, discuss their role in clinical transplantation and animal models of allograft rejection, and relate their unique traits to emerging therapeutic agents.
MEMORY T CELL GENERATION
Memory CD4+ and CD8+ T cells are generated during the course of primary immune responses, such as during infections and vaccinations. Viral and bacterial infection models and models immunizing with model protein antigens have indicated clonal proliferation of antigen-responsive primary effector T cells and the development of some of these proliferating T cells into memory T cells expressing a specific phenotype, CD127hi (IL-7 receptor α chain), CD27hi, Bcl-2hi, KLRG1low (1**). At what point during primary activation, antigen-responsive T cells commit to memory T cell develop remains unclear with several models being tested. Discussion of this commitment is beyond the focus of this review and sources detailing these models are referenced for interested readers (1–4).
Another mechanism of memory T cell generation involves the homeostasis of the peripheral T cell repertoire. In adults, the size and composition of the peripheral lymphocyte pool is tightly regulated and, in the absence of disease, is maintained at relatively constant levels (5). Homeostatic proliferation of lymphocytes, as the name implies, is a physiological process triggered by lymphopenia to maintain a constant level of T cells cell numbers. With normal involution of the thymus, which contributes new naïve T cells through early adulthood, homeostatic proliferation of mature peripheral T cells becomes the predominant mechanism sustaining T cell numbers in adulthood (6). In response to insults that decrease peripheral T cell numbers such as viral infections, cancer chemotherapy, or the peritransplant depletion therapies commonly utilized in higher risk transplant patients, homeostatic proliferation stimulates replenishment of lymphocyte numbers until threshold numbers are re-achieved. Mechanistically, homeostatic proliferation of T cells typically occurs in the absence of any exogenous antigen, but instead is supported by self-peptide-MHC complexes and cytokines, especially IL-7 and IL-15 (7, 8). Interestingly, this spontaneous expansion has a side-effect of converting naïve T cells directly to memory cells, especially those of an effector-memory phenotype (9). As would be expected, this conversion of naïve T cells to memory T cells through homeostatic proliferation is detrimental to transplant recipients. Studies in animal models and human patients have demonstrated that such memory cells generated by homeostatic proliferation vigorously respond to transplanted allografts and mediate accelerated rejection that is highly resistant to tolerization (10–12).
MEMORY T CELL BIOLOGY
Memory T cells possess several inherent characteristics that differentiate them from their naïve counterparts and allow them to robustly respond to foreign antigens such as microbial pathogens and allografts (Table 1). A key characteristic that memory T cells possess is that upon re-encounter with antigen, memory T cells are able to multiply and differentiate into effector T cells much more quickly and effectively than their naïve counterparts. It has been estimated that virus-specific memory CD8+ T cells require only hours to express cytolytic function, compared to the several days needed by naïve CD8+ T cells to differentiate into cytotoxic effector cells (13, 14). Moreover, on a per cell basis, memory T cells give rise to roughly fivefold more effector T cells than naïve T cells do during a primary immune response (15). The ability of memory T cells to mount such a vigorous response stems from both quantitative and qualitative advantages over naïve T cells. Quantitatively, clonal populations of antigen-specific memory cells can exist for many years and far outnumber the precursor frequencies of rare antigen-reactive naive T cells (16, 17). Antigen-reactive memory T cells are not only more abundant within the host, but qualitatively exhibit intrinsic antigen hyperresponsiveness compared to naïve T cells. The mechanisms of this hyperresponsiveness are incompletely understood, but are thought to include enhanced T cell receptor signaling, affinity maturation, and a reduced activation threshold (18). Whereas naïve T cells require CD28/B7-mediated costimulation provided by professional antigen presenting cells (APCs), memory T cells may be fully activated in the absence of costimulation via CD28/B7 and/or CD40/154 pathways and by many nonprofessional APC types such as B cells and macrophages (19, 20). This heightened propensity to activation coupled with a shortened lag time to enter cell cycle and acquiring effector functions allows memory T cells to quickly and robustly mount an effective recall response. Moreover, CD8+effector memory T cells in particular may constitutively express cytolytic functions, further decreasing the response time between antigen re-exposure and immune reaction (21).
Table 1.
Characteristics of memory versus naïve T cells
| Cell Type | Naïve T Cell | Central Memory T Cell | Effector Memory T Cell |
|---|---|---|---|
| Phenotype (human) | CD45RAhiCD45ROlow CD62LhiCCR7hi | CD45RAlowCD45ROhi CD62LhiCCR7hi | CD45RAlowCD45ROhi CD62LlowCCR7low |
| Phenotype (mouse) | CD44low CD62LhiCCR7hi | CD44hiCD62LhiCCR7hi | CD44hiCD62LlowCCR7low |
| Location | Spleen, lymph nodes | Spleen, lymph nodes, bone marrow | Spleen, blood, peripheral tissues |
| Response Time After Antigen | Days | Hours | Hours |
| Costimulation Requirements | +++ | −/+ | −/+ |
| Proliferative Potential | − | +++ | ++ |
| Cytokine Production | − | +++ (IL-2) | +++ (IFN-γ, TNF-α) |
| Effector Functions/ Cytotoxicity | − | + | +++ |
| Sensitivity to Immuno-suppression | +++ | −/+ | −/+ |
An additional advantage that memory T cells possess over naïve T cells is in their distribution and accessibility to antigens. While the circulation of naïve T cells is restricted to secondary lymphoid tissues, memory T cells circulate through both lymphoid and peripheral non-lymphoid tissues (22). Consistent with these properties, memory CD8+ and CD4+ T cells express distinct cell surface homing receptors and comprise heterogeneous populations of CCR7+/CD62Lhigh and CCR7−/CD62Llow subsets characterized as central memory and effector memory, respectively (21). The high expression of CCR7 and CD62L promotes central memory T cell localization within secondary lymph nodes whereas effector memory T cells express integrins and chemokine receptors that specifically promote their localization to distinct peripheral tissue sites such as the lungs, skin or intestinal mucosa. Unlike naïve T cells which require secondary lymphoid organs for full activation by professional APCs, the activation of memory T cells does not require secondary lymphoid organs. Memory CD8+ T cells in peripheral tissues have been demonstrated to spontaneously lyse target cells upon antigen reencounter, without the need for clonal expansion and differentiation, and effectively mediate cardiac allograft rejection in rodent models lacking secondary lymphoid tissue (22, 23). Thus, memory T cells have an anatomic advantage in not only their access to antigen, but are well poised to initiate immediate function at the site of antigen reencounter, bypassing the need for formal antigen presentation in the draining lymph node.
Finally, memory T cells possess unique survival advantages when compared to naïve T cells. By definition, memory cells must provide their hosts with memory of past antigenic experiences and in order to provide protection for future re-exposures, memory T cells must be long-lived cells and persist often for the lifetime of the individual. The long-term maintenance of memory T cells is dependent on constant division in the periphery and the intrinsic ability of memory T cells to survive in a relative resting state for an extended duration. Consequently, memory T cells have been demonstrated to survive lymphoablative therapies with polyclonal antithymocyte globulin (ATG) or monoclonal anti-CD52 antibody (Campath), therapies used in clinical transplantation that very effectively eliminate naïve T cells (24–27).
ALLOREACTIVE MEMORY T CELL GENERATION
Alloreactive memory T cells are generated through three major mechanisms. First, target antigen stimulation of naïve T cells provides the most direct source of memory T cells during sensitization of an individual. In the transplant setting, donor antigen sensitization occurs in individuals through prior blood transfusion, previous transplant, or pregnancy which all generate donor-specific memory T cells (28). In support of this, a study of chronic renal failure patients revealed primed alloreactive T cells in all patients that had received a prior blood transfusion or transplant (29). However, even in the absence of prior alloantigen sensitization, individuals may contain alloreactive precursors within the memory T cell population generated through homeostatic proliferation or via heterologous immunity.
As mentioned earlier, alloreactive memory T cells may also be generated in instances when a lymphopenic environment is imposed on transplant recipients. Current induction therapies include the use of T cell depleting agents such as ATG and Campath. Although these strategies are very efficient in decreasing the number of T cells and increasing the efficacy of standard immunosuppression, the T cells that remain following administration of T cell depletion therapies can be activated to undergo homeostatic proliferation and those with alloreactive TCRs will clonally expand to become donor-reactive memory T cells. Memory T cells surviving the administration of these depletion strategies are associated with acute rejection episodes in treated transplant patients (24, 30). Importantly, elegant studies in mouse models have shown that homeostatically generated memory T cells confer resistance to the costimulatory blockade strategies that promote long term survival of complete MHC mismatched heart allografts in the absence of donor-reactive memory T cells (10–12).
Memory T cells generated from prior immunological exposures have the capacity to influence the course of future immune responses to unrelated pathogens, a phenomenon termed heterologous immunity. Despite being highly specific for their priming antigen, memory T cells maintain a varied TCR repertoire and exhibit degeneracy in their ability to recognize and cross-react with a number of antigens (31). This diversity and degeneracy in memory TCRs is thought to be evolutionarily beneficial to allow the immune system to respond to mutations in previously encountered pathogens. Unfortunately, a major implication of heterologous immunity is that many of the pathogen-specific memory T cells generated during an individual’s history are potentially reactive to previously unseen transplant antigens. Indeed, it has been estimated that while the frequency of pathogen specific naïve T cells is quite rare (about 1:200,000), the proportion that can directly recognize foreign MHC is substantially greater, about 1–10% of the total T cell repertoire (32, 33). The molecular basis of this TCR degeneracy has been explained by observations that while hundreds of peptides in any pathogenic infection have the appropriate sequence to bind MHC class I, data from studies using crystal structures of peptide- MHC complexes suggest that only a few contact residues of the embedded peptide actually interact with the TCR to stimulate an immunogenic response (31, 34). Thus, a TCR may tolerate certain amino acid substitutions in the peptide sequence and still become activated. Aside from this “molecular mimicry”, the degeneracy of the T cell response has also been attributed to the possibility that different regions of the same TCR may interact with different targets and that a T cell may express two different TCRs, due to incomplete allelic exclusion of the TCR alpha chain. Together, these unique features of memory cells make them formidable obstacles in transplantation.
MEMORY T CELLS IN CLINICAL TRANSPLANTATION
In humans, alloreactive memory cells generated either by previous donor antigen sensitization through prior blood transfusion, previous transplant, or pregnancy or through cross-reactive heterologous immunity contribute to both acute rejection episodes and the development of chronic graft injury. Seminal work by Heeger and colleagues have demonstrated that the frequency of donor-reactive memory T cells in the peripheral blood of renal transplant patients prior to transplant correlates with the risk of developing acute rejection episodes and with poor renal allograft function at 1 year (35, 36). Such alloreactive memory cells are detectable by their production of IFN-γ in ELISPOT assays within 24 hours of allostimulation, a technique which has been confirmed to predict post-transplant renal function and even the development of chronic allograft nephropathy (37, 38). Additionally, the detection of memory-phenotype (CD45RO+) cells within biopsies and peripheral blood of heart and kidney allograft patients has been shown to correlate with the incidence and severity of transplant rejection (39–42).
Given the intrinsic advantages that memory T cells possess over their naïve counterparts in terms of survival and activation thresholds, it is perhaps unsurprising that donor-reactive memory T cells are often detectable in organ transplant recipients receiving induction therapy to deplete T cells and/or high levels of immunosuppression (35, 43). Importantly, in a recent phase 3 clinical trial of Belatacept, a second-generation CTLA4-Ig that blocks CD28-mediated costimulation, an increased incidence and severity of acute renal allograft rejection episodes was observed compared to cyclosporine treatment, despite overall improved renal function in patients receiving Belatacept (44). One possible mechanism contributing to this increased frequency of acute rejection in some Belatacept-treated patients may be the presence of memory T cells resistant to costimulatory blockade (45). In recent in vitro studies, we have observed the ability of IL-15, but not IL-2 or IL-7, to provoke the proliferation of human alloreactive CD28− memory CD8+ T cells and their expression of effector functions including production of TNFα and IFN-γ and expression of CD107a indicating cytolytic function (46**). Furthermore, IL-15 conferred CTLA4-Ig resistance to alloreactive CD28+ memory CD8+ T cells. These results are consistent with the ability of a pro-inflammatory cytokine that is likely to be produced during inflammation in grafts to promote the proliferation and effector function of donor-reactive memory CD8+ T cells whether CTLA4-Ig is administered or not.
STUDYING MEMORY T CELLS IN ANIMAL MODELS
While a correlation between high numbers of alloreactive memory T cells and poor transplant outcomes has been observed clinically, studies in numerous animal models have been able to directly demonstrate a detrimental impact of memory T cells on allograft function and survival. Several approaches have been used to study the impact of donor-reactive memory T cells on allograft outcome in rodent transplant models. The most commonly used approach has involved priming naïve animals directly to donor antigens with a donor graft such as skin allograft to generate reactive T cells that develop into memory T cells 6–8 weeks later. The sensitized animals are then either challenged with a second allograft or the memory T cells are isolated from the sensitized animal and transferred to a naïve animal that is then challenged with the allograft. A complementary approach has been to utilize memory T cells expressing a transgenic TCR with known reactivity to graft alloantigens. Memory T cells generated by such means have all been demonstrated to be capable of mediating transplant rejection. It is well established that mice that have previously rejected an allograft develop donor-specific memory T cells that reject a second graft from the same donor with accelerated kinetics, a phenomenon known as second set rejection (47). The potency of these memory T cells alone in mediating rejection is further supported by the demonstration that accelerated rejection of secondary allografts in such donor-antigen primed animals can occur in the complete absence of B cells and circulating antibody (48, 49). Moreover, unlike naïve T cells, memory T cells are able to exert their effector functions and cause allograft rejection without first homing to secondary lymphoid tissues (23).
The generation of alloreactive memory T cells by homeostatic proliferation and cross-reactive heterologous immunity has also been used to study the impact of donor-reactive memory T cells in animal models of transplantation. Experimentally, the most commonly used approach to induce homeostatic proliferation utilizes the transfer of purified naïve CD4+ and/or CD8+ T cells into immunodeficient mice, such as RAG-1−/− or RAG-2−/− mice, which induces a robust expansion of memory phenotype cells including those with alloreactive TCRs. When T cell receptor (TCR) transgenic or polyclonal congenically marked T cells are harvested following homeostatic proliferation within such lymphopenic hosts they can be shown to express the functions of antigen-driven memory T cells. An additional approach to study alloreactive memory T cells in rodents has been to sensitize candidate recipients through infection with a virus or other infectious entity that induces antigen-specific memory CD4+ or CD8+ T cells that cross-react with the target allogeneic MHC molecules expressed by the allograft. Following recovery from the infection and the development of memory T cells, the sensitized animals are then challenged with the target allograft or an allograft to which the generated memory T cells do not react as a control. This approach has been important in directly demonstrating the generation and consequence of heterologous immunity on the immune response to an allograft. Memory T cells generated through homeostatic proliferation or parasitic or virally induced heterologous immunity are similarly capable of directly mediating rejection of cardiac and skin allografts (19, 49, 50).
Memory T cells are increasingly being appreciated as significant barriers to the induction of donor-specific immunologic tolerance which remains the ultimate goal in transplantation. While numerous strategies have been developed to significantly prolong allograft survival or induce tolerance in rodent models, few, if any, have been successfully translated to non-human primate (NHP) models or human patients (51, 52). One of the factors postulated to contribute to the success of tolerance induction strategies in pathogen-free lab mice versus their failure in larger animals is the level of environmental exposure and consequent development of alloreactive memory T cells (53). In support of this, costimulation blockade of the CD154/CD40 pathway in the presence of donor-specific transfusion (DST) has been extremely successful in promoting long-term or permanent survival of various allografts in rodent models of transplantation (54). However, this strategy fails to prevent allograft rejection in mice with high levels of donor-reactive memory T cells. Specifically, prior donor antigen sensitization of recipient animals, transfer of donor-specific memory T cells into naïve mice, and induction of cross-reactive memory T cells by viral infection all render recipient animals refractory to tolerance induction strategies that are extremely effective in naïve animals (55–58).
This manipulation of rodent recipients to induce high numbers of donor-specific memory T cells has clearly demonstrated that memory T cells are able to directly mediate aggressive rejection of transplanted organs that is resistant to costimulatory blockade therapies and is reminiscent of the increased acute allograft rejection observed clinically. The antithesis of these observations is that the rejection of allografts in unsensitized rodent recipients takes longer and that such rejection can be obviated using many different costimulatory blockade strategies and other tolerance inducing strategies. The observation that such strategies are not efficacious when applied to NHP or clinical transplant patients has emphasized the importance of memory T cells induced through supposed greater environmental antigen exposure. This implies that the numbers of these memory T cells in rodents housed in Specific Pathogen Free environments in research facilities are not sufficient to mediate rejection of an allograft independently of the primary effector T cells developing de novo in response to an allograft and are not sufficient to confer resistance to the costimulatory blockade strategies that fail in donor alloantigen sensitized recipients.
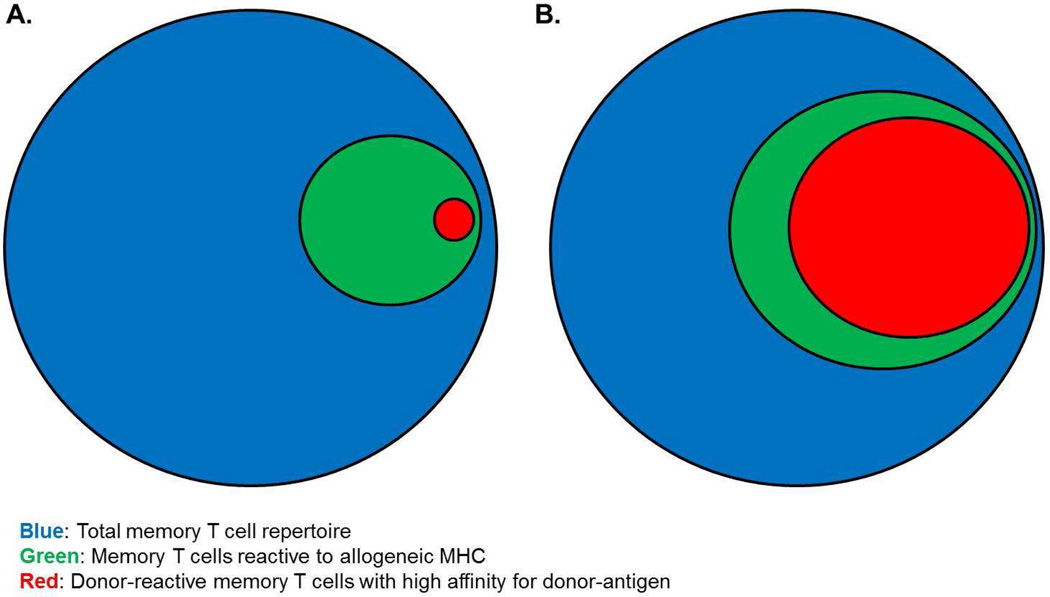
It has been previously demonstrated that naïve unmanipulated adult laboratory mice possess a repertoire of endogenous memory T cells which are naturally generated from environmental exposures. While these endogenous memory CD8+ T cells have not been previously primed to donor antigens, a proportion of this endogenous memory T cell repertoire in naïve mice are reactive to donor class I MHC molecules and are able to rapidly detect and infiltrate cardiac allografts within just hours of graft reperfusion (59). Endogenous memory CD4+ T cells also rapidly infiltrate the allografts but their activation to express function is unclear at this time. While further studies detailed the ability of these endogenous memory CD8+ T cells to undergo proliferation and produce IFN-γ within the allograft upon specifically reacting against donor class I MHC (59), their expression of effector functions were found to be insufficient to directly mediate allograft rejection, findings consistent with prior observations (60). This discrepancy in the ability to effect transplant rejection between naturally generated endogenous memory T cells and those deliberately generated to be donor-antigen reactive suggest that the priming strategies used to generate the latter disproportionately bias the endogenous T cell receptor repertoire to strong reactivity to donor antigens (Figure 1). While these rodent models inducing high numbers donor-reactive memory T cells yield accelerated allograft rejection and resistance to costimulatory blockade induced tolerance, the extent to which the endogenous memory T cell repertoire has been skewed is rarely seen naturally in NHPs and human patients.
Figure 1.
Repertoires of donor-reactive memory T cells in unprimed vs. donor-antigen primed individuals. A hypothetical representation of the endogenous memory T cell repertoire (blue) within (A) unmanipulated animals and (B) following priming by strategies typically used to induce high numbers of donor-reactive memory T cells. While such priming approaches certainly expand the population of memory T cells reactive to donor-MHC (green), it is likely that the frequency of strongly donor-reactive memory T cells (ie, those with high affinity TCRs for donor antigen) (red) is also significantly increased.
We realized, however, that cardiac transplantation performed in most laboratories, including our own, is performed as quickly as possible with cold ischemic storage time minimized. One reason for this approach is to optimize results testing allograft-prolonging strategies such as costimulatory blockade. Ischemia-reperfusion injury is an inherent pathological challenge of transplantation and numerous studies have demonstrated that organs from deceased donors and grafts subjected to longer periods of ischemia correlate with poorer graft function and survival following transplantation (61–68). With these studies and our results in mind, we hypothesized that increasing the duration of cold ischemic storage would generate an inflammatory environment within the allograft to promote endogenous memory CD8+ T cell activation to sufficient levels that increased effector functions and provoked allograft failure in “naïve” unprimed mice. Increasing the duration of cold ischemic storage in a clinically relevant manner resulted in heightened early infiltration of endogenous memory CD8+ T cells into the allograft and their increased expression of effector functions (69). Furthermore, allografts subjected to prolonged ischemia conferred endogenous CD8+ memory T cells resistance to the effects of CTLA4-Ig and the ability to reject the allograft independently of donor-reactive T cells that developed de novo from naïve precursor T cells. While further work is needed to define the impact of the post-ischemic inflammatory environment on the clonal expansion and/or function of endogenous memory CD8+ T cells, these initial findings recapitulate the detrimental impact of endogenous alloreactive memory T cell responses observed in NHP recipients and clinical transplant patients in a mouse model without prior recipient priming to bias the endogenous memory T cell repertoire to strong donor reactivity. In light of these studies, an interesting point to consider is whether the priming strategies currently used to generate high frequencies of donor-antigen reactive or cross-reactive memory T cells in rodent models are physiologic recapitulations of the clinical impact of endogenous memory T cells in transplantation.
TARGETING MEMORY T CELLS IN TRANSPLANTATION
Considering the negative impact of memory T cells in transplantation and their unique characteristics hindering tolerance induction, strategies to selectively target alloreactive memory cells should improve transplant outcomes. The need for memory T cell-directed therapies in transplantation has arisen from observations that immunosuppressive agents that are effective against naive T cells often have minimal effects against memory T cells (70). In particular, treatments directed at traditional T cell activation and depletion therapies have been found to be less effective in preventing allograft rejection in the presence of alloreactive memory T cells. However, studies in several experimental transplant models have suggested that targeting the trafficking and infiltration of memory T cells into the allograft and their subsequent activation and proliferation may be promising therapeutic approaches.
Once within the graft, alloreactive memory T cells proliferate and are capable of mediating significant injury and thus inhibiting their initial trafficking into the transplanted organ itself has been demonstrated to be beneficial to allograft survival. The administration of FTY720, a sphingosine-1 phosphate receptor agonist, prevents lymphocyte migration from the thymus and peripheral lymphoid tissues, effectively sequestering T cells in the lymph nodes and inhibiting them from trafficking to peripheral graft sites. In mouse models, treatment with FTY720 has been shown to sequester memory CD4+ T cells in the lymph nodes and delay heart allograft rejection (71). However, this sequestration does not affect the ability of donor antigen-reactive CD4+ T cells to provide the helper signals needed to drive a donor-specific antibody response and it is this antibody that underlies the rejection of the allograft in such FTY720-treated recipients. Numerous studies have also shown that disruption of adhesion molecules, notably leukocyte integrins such as LFA-1 and VLA-4, are quite effective in inhibiting memory T cell infiltration into grafts. Mouse models of transplantation have shown that anti-LFA-1 or anti-VLA-4 monoclonal antibodies result in attenuation of donor-reactive memory recall responses and decreased T cell trafficking to the allograft, ultimately resulting in prolonged survival of allografts (69, 72, 73). While testing of anti-LFA-1 in NHP models of transplantation were also promising (74), clinical testing of the anti-LFA-1 agent efalizumab in renal transplantation suggested that this agent evoked a higher rate of EBV-associated malignancy (75). Thus, although anti-LFA-1 antibody is not currently clinically available, these findings in animal models suggest that targeting other trafficking molecules on memory T cells, such as VLA-4, may be similarly efficacious and warrant further investigation.
While there is a growing body of evidence to suggest that memory T cells are relatively resistant to traditional costimulation blockade, some studies have suggested that memory T cells may use unique alternative costimulatory pathways for activation and effector functions. For CD8+ T cells, the engagement of 4-1BB (CD137) by its ligand has been shown to provide both CD28-dependent and CD28-independent signals that result in proliferation, cytokine production, augmented cytotoxic effector activity, and enhanced cell survival. Blockade of the 4-1BB costimulatory pathway in mouse models has shown some efficacy in prolonging survival of intestinal, skin, and cardiac allografts (76, 77). Another co-stimulatory pathway that may be important for the recall of memory T cells, particularly the CD4+ population, is the OX40/OX40L pathway. Deficiency or blockade of OX40 has been shown to result in impaired memory CD4+ T cell formation and in transplantation models was found to prolong survival of heart and skin allografts in recipients possessing donor-antigen primed or memory T cells (19, 78, 79).
Targeting the proliferation of memory T cells induced by cytokine and TCR signaling is another potential therapeutic approach. Janus kinases (JAK) are a family of kinases which transduce cytokine mediated signals via the JAK-STAT pathway. JAK3, expressed primarily in hematopoietic cells, is downstream of the common γ chain (CD132) which binds a broad array of cytokines including IL-2, IL-7, IL-15, and IL-21. These cytokines have been demonstrated to have important roles in the generation, maintenance, and proliferation of memory T cells, making JAK inhibitors a potential molecular target for blockade of cytokine signals. A highly selective and potent JAK3 inhibitor, tofacitinib (CP-690550), has been shown to prevent allograft rejection in both murine and NHP models and is currently finishing phase II clinical trials of renal transplantation (80–82). Signals for cell proliferation and survival may also be induced through TCR signaling and attempts to inhibit nuclear factor-κB (NF-κB), a protein complex that regulates DNA transcription, have also been promising. Inhibition of NF-κB has been shown to prevent activation of donor-specific memory CD8+ T cells and synergize with costimulatory blockade to prolong skin allograft survival in rodent models and suppress the proliferation of rapamycin-resistant memory T cell populations in NHPs (83, 84).
CONCLUSION
The unique characteristics that make memory T cells so effective and beneficial in protective immunity against recurrent pathogens also endow them to be formidable barriers to successful transplantation. The anatomic advantages to accessing antigen, increased frequency, hyper-responsiveness, and ability to exhibit heterologous immunity are critical features enabling memory T cells to vigorously defend their hosts which unfortunately subvert strategies directed at inducing long-term allograft survival and transplant tolerance. Further advances in our understanding of mechanisms of memory T cell generation, activation, function, and trafficking to allografts are necessary to develop clinically translatable approaches targeting donor-reactive memory T cells to eliminate or attenuate allograft rejection.
ACKNOWLEDGMENTS
This work was supported by NIH RO1-AI40459 and PO1-AI087586 (R.F.). C.S was supported in part by NIH TL1-24991, T32-AI089474, and the Case Western University School of Medicine MSTP.
Footnotes
Compliance with Ethics Guidelines
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Conflict of Interest
Charles A. Su and Robert L. Fairchild declare that they have no conflict of interest.
References
- 1. Kaech SM, Cui W. Transcriptional control of effector and memory CD8+ T cell differentiation. Nat Rev Immunol. 2012;12:749–761. doi: 10.1038/nri3307. This paper provides a great review of the signaling pathways, transcriptional programs, and metabolic factors regulating effector and memory CD8 T cell differentiation.
- 2.Kaech SM, Wherry EJ, Ahmed R. Effector and memory T-cell differentiation: implications for vaccine development. Nat Rev Immunol. 2002;2:251–262. doi: 10.1038/nri778. [DOI] [PubMed] [Google Scholar]
- 3.Moulton VR, Farber DL. Committed to memory: lineage choices for activated T cells. Trends Immunol. 2006;27:261–267. doi: 10.1016/j.it.2006.04.006. [DOI] [PubMed] [Google Scholar]
- 4.Joshi NS, Cui W, Chandele A, Lee HK, Urso DR, Hagman J, Gapin L, Kaech SM. Inflammation directs memory precursor and short-lived effector CD8(+) T cell fates via the graded expression of T-bet transcription factor. Immunity. 2007;27:281–295. doi: 10.1016/j.immuni.2007.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sprent J, Tough DF. Lymphocyte life-span and memory. Science. 1994;265:1395–1400. doi: 10.1126/science.8073282. [DOI] [PubMed] [Google Scholar]
- 6.Jamieson BD, Douek DC, Killian S, Hultin LE, Scripture-Adams DD, Giorgi JV, Marelli D, Koup RA, Zack JA. Generation of functional thymocytes in the human adult. Immunity. 1999;10:569–575. doi: 10.1016/s1074-7613(00)80056-4. [DOI] [PubMed] [Google Scholar]
- 7.Tchao NK, Turka LA. Lymphodepletion and homeostatic proliferation: implications for transplantation. Am J Transplant. 2012;12:1079–1090. doi: 10.1111/j.1600-6143.2012.04008.x. [DOI] [PubMed] [Google Scholar]
- 8.Tan JT, Ernst B, Kieper WC, LeRoy E, Sprent J, Surh CD. Interleukin (IL)-15 and IL-7 jointly regulate homeostatic proliferation of memory phenotype CD8+ cells but are not required for memory phenotype CD4+ cells. J Exp Med. 2002;195:1523–1532. doi: 10.1084/jem.20020066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Goldrath AW, Bogatzki LY, Bevan MJ. Naive T cells transiently acquire a memory-like phenotype during homeostasis-driven proliferation. J Exp Med. 2000;192:557–564. doi: 10.1084/jem.192.4.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Moxham VF, Karegli J, Phillips RE, Brown KL, Tapmeier TT, Hangartner R, Sacks SH, Wong W. Homeostatic proliferation of lymphocytes results in augmented memory-like function and accelerated allograft rejection. J Immunol. 2008;180:3910–3918. doi: 10.4049/jimmunol.180.6.3910. [DOI] [PubMed] [Google Scholar]
- 11.Wu Z, Bensinger SJ, Zhang J, Chen C, Yuan X, Huang X, Markmann JF, Kassaee A, Rosengard BR, Hancock WW, et al. Homeostatic proliferation is a barrier to transplantation tolerance. Nat Med. 2004;10:87–92. doi: 10.1038/nm965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Iida S, Suzuki T, Tanabe K, Valujskikh A, Fairchild RL, Abe R. Transient lymphopenia breaks costimulatory blockade-based peripheral tolerance and initiates cardiac allograft rejection. Am J Transplant. 2013;13:2268–2279. doi: 10.1111/ajt.12342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rogers PR, Dubey C, Swain SL. Qualitative changes accompany memory T cell generation: faster, more effective responses at lower doses of antigen. J Immunol. 2000;164:2338–2346. doi: 10.4049/jimmunol.164.5.2338. [DOI] [PubMed] [Google Scholar]
- 14.Bachmann MF, Barner M, Viola A, Kopf M. Distinct kinetics of cytokine production and cytolysis in effector and memory T cells after viral infection. Eur J Immunol. 1999;29:291–299. doi: 10.1002/(SICI)1521-4141(199901)29:01<291::AID-IMMU291>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 15.Opferman JT, Ober BT, Ashton-Rickardt PG. Linear differentiation of cytotoxic effectors into memory T lymphocytes. Science. 1999;283:1745–1748. doi: 10.1126/science.283.5408.1745. [DOI] [PubMed] [Google Scholar]
- 16.Lee WT, Vitetta ES. Limiting dilution analysis of CD45Rhi and CD45Rlo T cells: further evidence that CD45Rlo cells are memory cells. Cell Immunol. 1990;130:459–471. doi: 10.1016/0008-8749(90)90287-2. [DOI] [PubMed] [Google Scholar]
- 17.Murali-Krishna K, Altman JD, Suresh M, Sourdive DJ, Zajac AJ, Miller JD, Slansky J, Ahmed R. Counting antigen-specific CD8 T cells: a reevaluation of bystander activation during viral infection. Immunity. 1998;8:177–187. doi: 10.1016/s1074-7613(00)80470-7. [DOI] [PubMed] [Google Scholar]
- 18.Lakkis FG, Sayegh MH. Memory T cells: a hurdle to immunologic tolerance. J Am Soc Nephrol. 2003;14:2402–2410. doi: 10.1097/01.asn.0000085020.78117.70. [DOI] [PubMed] [Google Scholar]
- 19.Vu MD, Clarkson MR, Yagita H, Turka LA, Sayegh MH, Li XC. Critical, but conditional, role of OX40 in memory T cell-mediated rejection. J Immunol. 2006;176:1394–1401. doi: 10.4049/jimmunol.176.3.1394. [DOI] [PubMed] [Google Scholar]
- 20.Croft M, Bradley LM, Swain SL. Naive versus memory CD4 T cell response to antigen. Memory cells are less dependent on accessory cell costimulation and can respond to many antigen-presenting cell types including resting B cells. J Immunol. 1994;152:2675–2685. [PubMed] [Google Scholar]
- 21.Sallusto F, Lenig D, Forster R, Lipp M, Lanzavecchia A. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature. 1999;401:708–712. doi: 10.1038/44385. [DOI] [PubMed] [Google Scholar]
- 22.Masopust D, Vezys V, Marzo AL, Lefrancois L. Preferential localization of effector memory cells in nonlymphoid tissue. Science. 2001;291:2413–2417. doi: 10.1126/science.1058867. [DOI] [PubMed] [Google Scholar]
- 23.Chalasani G, Dai Z, Konieczny BT, Baddoura FK, Lakkis FG. Recall and propagation of allospecific memory T cells independent of secondary lymphoid organs. Proc Natl Acad Sci U S A. 2002;99:6175–6180. doi: 10.1073/pnas.092596999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pearl JP, Parris J, Hale DA, Hoffmann SC, Bernstein WB, McCoy KL, Swanson SJ, Mannon RB, Roederer M, Kirk AD. Immunocompetent T-cells with a memory-like phenotype are the dominant cell type following antibody-mediated T-cell depletion. Am J Transplant. 2005;5:465–474. doi: 10.1111/j.1600-6143.2005.00759.x. [DOI] [PubMed] [Google Scholar]
- 25.Ayasoufi K, Yu H, Fan R, Wang X, Williams J, Valujskikh A. Pretransplant antithymocyte globulin has increased efficacy in controlling donor-reactive memory T cells in mice. Am J Transplant. 2013;13:589–599. doi: 10.1111/ajt.12068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Neujahr DC, Chen C, Huang X, Markmann JF, Cobbold S, Waldmann H, Sayegh MH, Hancock WW, Turka LA. Accelerated memory cell homeostasis during T cell depletion and approaches to overcome it. J Immunol. 2006;176:4632–4639. doi: 10.4049/jimmunol.176.8.4632. [DOI] [PubMed] [Google Scholar]
- 27.Zeevi A, Husain S, Spichty KJ, Raza K, Woodcock JB, Zaldonis D, Carruth LM, Kowalski RJ, Britz JA, McCurry KR. Recovery of functional memory T cells in lung transplant recipients following induction therapy with alemtuzumab. Am J Transplant. 2007;7:471–475. doi: 10.1111/j.1600-6143.2006.01641.x. [DOI] [PubMed] [Google Scholar]
- 28.Li XC, Kloc M, Ghobrial RM. Memory T cells in transplantation - progress and challenges. Curr Opin Organ Transplant. 2013;18:387–392. doi: 10.1097/MOT.0b013e3283626130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Deacock SJ, Lechler RI. Positive correlation of T cell sensitization with frequencies of alloreactive T helper cells in chronic renal failure patients. Transplantation. 1992;54:338–343. doi: 10.1097/00007890-199208000-00026. [DOI] [PubMed] [Google Scholar]
- 30.Haanstra KG, Sick EA, Ringers J, Wubben JA, Kuhn EM, t Hart BA, Boon L, Jonker M. No synergy between ATG induction and costimulation blockade induced kidney allograft survival in rhesus monkeys. Transplantation. 2006;82:1194–1201. doi: 10.1097/01.tp.0000235910.47214.67. [DOI] [PubMed] [Google Scholar]
- 31.Selin LK, Cornberg M, Brehm MA, Kim SK, Calcagno C, Ghersi D, Puzone R, Celada F, Welsh RM. CD8 memory T cells: cross-reactivity and heterologous immunity. Semin Immunol. 2004;16:335–347. doi: 10.1016/j.smim.2004.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Blattman JN, Antia R, Sourdive DJ, Wang X, Kaech SM, Murali-Krishna K, Altman JD, Ahmed R. Estimating the precursor frequency of naive antigenspecific CD8 T cells. J Exp Med. 2002;195:657–664. doi: 10.1084/jem.20001021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Suchin EJ, Langmuir PB, Palmer E, Sayegh MH, Wells AD, Turka LA. Quantifying the frequency of alloreactive T cells in vivo: new answers to an old question. J Immunol. 2001;166:973–981. doi: 10.4049/jimmunol.166.2.973. [DOI] [PubMed] [Google Scholar]
- 34.Selin LK, Brehm MA, Naumov YN, Cornberg M, Kim SK, Clute SC, Welsh RM. Memory of mice and men: CD8+ T-cell cross-reactivity and heterologous immunity. Immunol Rev. 2006;211:164–181. doi: 10.1111/j.0105-2896.2006.00394.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Heeger PS, Greenspan NS, Kuhlenschmidt S, Dejelo C, Hricik DE, Schulak JA, Tary-Lehmann M. Pretransplant frequency of donor-specific, IFN-gamma-producing lymphocytes is a manifestation of immunologic memory and correlates with the risk of posttransplant rejection episodes. J Immunol. 1999;163:2267–2275. [PubMed] [Google Scholar]
- 36.Augustine JJ, Siu DS, Clemente MJ, Schulak JA, Heeger PS, Hricik DE. Pre-transplant IFN-gamma ELISPOTs are associated with post-transplant renal function in African American renal transplant recipients. Am J Transplant. 2005;5:1971–1975. doi: 10.1111/j.1600-6143.2005.00958.x. [DOI] [PubMed] [Google Scholar]
- 37.Hricik DE, Rodriguez V, Riley J, Bryan K, Tary-Lehmann M, Greenspan N, Dejelo C, Schulak JA, Heeger PS. Enzyme linked immunosorbent spot (ELISPOT) assay for interferon-gamma independently predicts renal function in kidney transplant recipients. Am J Transplant. 2003;3:878–884. doi: 10.1034/j.1600-6143.2003.00132.x. [DOI] [PubMed] [Google Scholar]
- 38.Poggio ED, Clemente M, Riley J, Roddy M, Greenspan NS, Dejelo C, Najafian N, Sayegh MH, Hricik DE, Heeger PS. Alloreactivity in renal transplant recipients with and without chronic allograft nephropathy. J Am Soc Nephrol. 2004;15:1952–1960. doi: 10.1097/01.asn.0000129980.83334.79. [DOI] [PubMed] [Google Scholar]
- 39.Azzawi M, Hasleton PS, Geraghty PJ, Yonan N, Krysiak P, El-Gammal A, Deiraniya AK, Hutchinson IV. RANTES chemokine expression is related to acute cardiac cellular rejection and infiltration by CD45RO T-lymphocytes and macrophages. J Heart Lung Transplant. 1998;17:881–887. [PubMed] [Google Scholar]
- 40.Salom RN, Maguire JA, Esmore D, Hancock WW. Analysis of proliferating cell nuclear antigen expression aids histological diagnosis and is predictive of progression of human cardiac allograft rejection. Am J Pathol. 1994;145:876–882. [PMC free article] [PubMed] [Google Scholar]
- 41.Akbar AN, Amlot PL, Timms A, Lombardi G, Lechler R, Janossy G. The development of primed/memory CD8+ lymphocytes in vitro and in rejecting kidneys after transplantation. Clin Exp Immunol. 1990;81:225–231. doi: 10.1111/j.1365-2249.1990.tb03322.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ibrahim S, Dawson DV, Sanfilippo F. Predominant infiltration of rejecting human renal allografts with T cells expressing CD8 and CD45RO. Transplantation. 1995;59:724–728. doi: 10.1097/00007890-199503150-00015. [DOI] [PubMed] [Google Scholar]
- 43.Najafian N, Salama AD, Fedoseyeva EV, Benichou G, Sayegh MH. Enzyme-linked immunosorbent spot assay analysis of peripheral blood lymphocyte reactivity to donor HLA-DR peptides: potential novel assay for prediction of outcomes for renal transplant recipients. J Am Soc Nephrol. 2002;13:252–259. doi: 10.1681/ASN.V131252. [DOI] [PubMed] [Google Scholar]
- 44.Vincenti F, Charpentier B, Vanrenterghem Y, Rostaing L, Bresnahan B, Darji P, Massari P, Mondragon-Ramirez GA, Agarwal M, Di Russo G, et al. A phase III study of belatacept-based immunosuppression regimens versus cyclosporine in renal transplant recipients (BENEFIT study) Am J Transplant. 2010;10:535–546. doi: 10.1111/j.1600-6143.2009.03005.x. [DOI] [PubMed] [Google Scholar]
- 45.Vincenti F. Costimulation blockade--what will the future bring? Nephrol Dial Transplant. 2007;22:1293–1296. doi: 10.1093/ndt/gfl830. [DOI] [PubMed] [Google Scholar]
- 46. Traitanon O, Gorbachev A, Bechtel JJ, Keslar KS, III, W MB, Poggio ED, Fairchild RL. IL-15 induces alloreactive CD28- memory CD8 T cell proliferation and CTLA4-Ig resistant memory CD8 T cell activation. American Journal of Transplantation. 2014 doi: 10.1111/ajt.12719. This paper documents the ability of IL-15, a pro-inflammatory cytokine present in allografts, to induce the activation of CD28− memory CD8 T cells to proliferate and express effector functions in response to allogeneic cells. Furthermore, IL-15 conferred resistance of donor-reactive CD28+ memory CD8 T cells to CTLA4-Ig.
- 47.Gordon RD, Mathieson BJ, Samelson LE, Boyse EA, Simpson E. The effect of allogeneic presensitization on H-Y graft survival and in vitro cell-mediated responses to H-y antigen. J Exp Med. 1976;144:810–820. doi: 10.1084/jem.144.3.810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cerny A, Ramseier H, Bazin H, Zinkernagel RM. Unimpaired first-set and second-set skin graft rejection in agammaglobulinemic mice. Transplantation. 1988;45:1111–1113. doi: 10.1097/00007890-198806000-00022. [DOI] [PubMed] [Google Scholar]
- 49.Hancock WW, Gao W, Shemmeri N, Shen XD, Gao F, Busuttil RW, Zhai Y, Kupiec-Weglinski JW. Immunopathogenesis of accelerated allograft rejection in sensitized recipients: humoral and nonhumoral mechanisms. Transplantation. 2002;73:1392–1397. doi: 10.1097/00007890-200205150-00006. [DOI] [PubMed] [Google Scholar]
- 50.Pantenburg B, Heinzel F, Das L, Heeger PS, Valujskikh A. T cells primed by Leishmania major infection cross-react with alloantigens and alter the course of allograft rejection. J Immunol. 2002;169:3686–3693. doi: 10.4049/jimmunol.169.7.3686. [DOI] [PubMed] [Google Scholar]
- 51.Hancock WW, Wang L, Ye Q, Han R, Lee I. Chemokines and their receptors as markers of allograft rejection and targets for immunosuppression. Curr Opin Immunol. 2003;15:479–486. doi: 10.1016/s0952-7915(03)00103-1. [DOI] [PubMed] [Google Scholar]
- 52.Sayegh MH, Turka LA. The role of T-cell costimulatory activation pathways in transplant rejection. N Engl J Med. 1998;338:1813–1821. doi: 10.1056/NEJM199806183382506. [DOI] [PubMed] [Google Scholar]
- 53.Sachs DH. Tolerance: of mice and men. J Clin Invest. 2003;111:1819–1821. doi: 10.1172/JCI18926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Larsen CP, Elwood ET, Alexander DZ, Ritchie SC, Hendrix R, Tucker-Burden C, Cho HR, Aruffo A, Hollenbaugh D, Linsley PS, et al. Long-term acceptance of skin and cardiac allografts after blocking CD40 and CD28 pathways. Nature. 1996;381:434–438. doi: 10.1038/381434a0. [DOI] [PubMed] [Google Scholar]
- 55.Valujskikh A, Pantenburg B, Heeger PS. Primed allospecific T cells prevent the effects of costimulatory blockade on prolonged cardiac allograft survival in mice. Am J Transplant. 2002;2:501–509. doi: 10.1034/j.1600-6143.2002.20603.x. [DOI] [PubMed] [Google Scholar]
- 56.Zhai Y, Meng L, Gao F, Busuttil RW, Kupiec-Weglinski JW. Allograft rejection by primed/memory CD8+ T cells is CD154 blockade resistant: therapeutic implications for sensitized transplant recipients. J Immunol. 2002;169:4667–4673. doi: 10.4049/jimmunol.169.8.4667. [DOI] [PubMed] [Google Scholar]
- 57.Adams AB, Williams MA, Jones TR, Shirasugi N, Durham MM, Kaech SM, Wherry EJ, Onami T, Lanier JG, Kokko KE, et al. Heterologous immunity provides a potent barrier to transplantation tolerance. J Clin Invest. 2003;111:1887–1895. doi: 10.1172/JCI17477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Welsh RM, Markees TG, Woda BA, Daniels KA, Brehm MA, Mordes JP, Greiner DL, Rossini AA. Virus-induced abrogation of transplantation tolerance induced by donor-specific transfusion and anti-CD154 antibody. J Virol. 2000;74:2210–2218. doi: 10.1128/jvi.74.5.2210-2218.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Schenk AD, Nozaki T, Rabant M, Valujskikh A, Fairchild RL. Donor-reactive CD8 memory T cells infiltrate cardiac allografts within 24-h posttransplant in naive recipients. Am J Transplant. 2008;8:1652–1661. doi: 10.1111/j.1600-6143.2008.02302.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Setoguchi K, Hattori Y, Iida S, Baldwin WM, 3rd, Fairchild RL. Endogenous memory CD8 T cells are activated within cardiac allografts without mediating rejection. Am J Transplant. 2013;13:2293–2307. doi: 10.1111/ajt.12372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Terasaki PI, Cecka JM, Gjertson DW, Takemoto S. High survival rates of kidney transplants from spousal and living unrelated donors. N Engl J Med. 1995;333:333–336. doi: 10.1056/NEJM199508103330601. [DOI] [PubMed] [Google Scholar]
- 62.Jassem W, Koo DD, Cerundolo L, Rela M, Heaton ND, Fuggle SV. Cadaveric versus living-donor livers: differences in inflammatory markers after transplantation. Transplantation. 2003;76:1599–1603. doi: 10.1097/01.TP.0000100400.82135.DC. [DOI] [PubMed] [Google Scholar]
- 63.Hauptman PJ, Aranki S, Mudge GH, Jr, Couper GS, Loh E. Early cardiac allograft failure after orthotopic heart transplantation. Am Heart J. 1994;127:179–186. doi: 10.1016/0002-8703(94)90523-1. [DOI] [PubMed] [Google Scholar]
- 64.Ojo AO, Wolfe RA, Held PJ, Port FK, Schmouder RL. Delayed graft function: risk factors and implications for renal allograft survival. Transplantation. 1997;63:968–974. doi: 10.1097/00007890-199704150-00011. [DOI] [PubMed] [Google Scholar]
- 65.Shoskes DA, Halloran PF. Delayed graft function in renal transplantation: etiology, management and long-term significance. J Urol. 1996;155:1831–1840. doi: 10.1016/s0022-5347(01)66023-3. [DOI] [PubMed] [Google Scholar]
- 66.Strasberg SM, Howard TK, Molmenti EP, Hertl M. Selecting the donor liver: risk factors for poor function after orthotopic liver transplantation. Hepatology. 1994;20:829–838. doi: 10.1002/hep.1840200410. [DOI] [PubMed] [Google Scholar]
- 67.Banner NR, Thomas HL, Curnow E, Hussey JC, Rogers CA, Bonser RS. The importance of cold and warm cardiac ischemia for survival after heart transplantation. Transplantation. 2008;86:542–547. doi: 10.1097/TP.0b013e31818149b9. [DOI] [PubMed] [Google Scholar]
- 68.Russo MJ, Chen JM, Sorabella RA, Martens TP, Garrido M, Davies RR, George I, Cheema FH, Mosca RS, Mital S, et al. The effect of ischemic time on survival after heart transplantation varies by donor age: an analysis of the United Network for Organ Sharing database. J Thorac Cardiovasc Surg. 2007;133:554–559. doi: 10.1016/j.jtcvs.2006.09.019. [DOI] [PubMed] [Google Scholar]
- 69.Su CA, Iida S, Abe T, Fairchild RL. Endogenous memory CD8 T cells directly mediate cardiac allograft rejection. Am J Transplant. 2014;14:568–579. doi: 10.1111/ajt.12605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Page AJ, Ford ML, Kirk AD. Memory T-cell-specific therapeutics in organ transplantation. Curr Opin Organ Transplant. 2009;14:643–649. doi: 10.1097/MOT.0b013e328332bd4a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zhang Q, Chen Y, Fairchild RL, Heeger PS, Valujskikh A. Lymphoid sequestration of alloreactive memory CD4 T cells promotes cardiac allograft survival. J Immunol. 2006;176:770–777. doi: 10.4049/jimmunol.176.2.770. [DOI] [PubMed] [Google Scholar]
- 72.Setoguchi K, Schenk AD, Ishii D, Hattori Y, Baldwin WM, 3rd, Tanabe K, Fairchild RL. LFA-1 antagonism inhibits early infiltration of endogenous memory CD8 T cells into cardiac allografts and donor-reactive T cell priming. Am J Transplant. 2011;11:923–935. doi: 10.1111/j.1600-6143.2011.03492.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kitchens WH, Haridas D, Wagener ME, Song M, Kirk AD, Larsen CP, Ford ML. Integrin antagonists prevent costimulatory blockade-resistant transplant rejection by CD8(+) memory T cells. Am J Transplant. 2012;12:69–80. doi: 10.1111/j.1600-6143.2011.03762.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Badell IR, Russell MC, Thompson PW, Turner AP, Weaver TA, Robertson JM, Avila JG, Cano JA, Johnson BE, Song M, et al. LFA-1-specific therapy prolongs allograft survival in rhesus macaques. J Clin Invest. 2010;120:4520–4531. doi: 10.1172/JCI43895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Vincenti F, Mendez R, Pescovitz M, Rajagopalan PR, Wilkinson AH, Butt K, Laskow D, Slakey DP, Lorber MI, Garg JP, et al. A phase I/II randomized open-label multicenter trial of efalizumab, a humanized anti-CD11a, anti-LFA-1 in renal transplantation. Am J Transplant. 2007;7:1770–1777. doi: 10.1111/j.1600-6143.2007.01845.x. [DOI] [PubMed] [Google Scholar]
- 76.Wang J, Guo Z, Dong Y, Kim O, Hart J, Adams A, Larsen CP, Mittler RS, Newell KA. Role of 4-1BB in allograft rejection mediated by CD8+ T cells. Am J Transplant. 2003;3:543–551. doi: 10.1034/j.1600-6143.2003.00088.x. [DOI] [PubMed] [Google Scholar]
- 77.Cho HR, Kwon B, Yagita H, La S, Lee EA, Kim JE, Akiba H, Kim J, Suh JH, Vinay DS, et al. Blockade of 4-1BB (CD137)/4-1BB ligand interactions increases allograft survival. Transpl Int. 2004;17:351–361. doi: 10.1007/s00147-004-0726-3. [DOI] [PubMed] [Google Scholar]
- 78.Salek-Ardakani S, Song J, Halteman BS, Jember AG, Akiba H, Yagita H, Croft M. OX40 (CD134) controls memory T helper 2 cells that drive lung inflammation. J Exp Med. 2003;198:315–324. doi: 10.1084/jem.20021937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Yuan X, Salama AD, Dong V, Schmitt I, Najafian N, Chandraker A, Akiba H, Yagita H, Sayegh MH. The role of the CD134-CD134 ligand costimulatory pathway in alloimmune responses in vivo. J Immunol. 2003;170:2949–2955. doi: 10.4049/jimmunol.170.6.2949. [DOI] [PubMed] [Google Scholar]
- 80.Borie DC, Larson MJ, Flores MG, Campbell A, Rousvoal G, Zhang S, Higgins JP, Ball DJ, Kudlacz EM, Brissette WH, et al. Combined use of the JAK3 inhibitor CP-690,550 with mycophenolate mofetil to prevent kidney allograft rejection in nonhuman primates. Transplantation. 2005;80:1756–1764. doi: 10.1097/01.tp.0000184634.25042.ea. [DOI] [PubMed] [Google Scholar]
- 81.Changelian PS, Flanagan ME, Ball DJ, Kent CR, Magnuson KS, Martin WH, Rizzuti BJ, Sawyer PS, Perry BD, Brissette WH, et al. Prevention of organ allograft rejection by a specific Janus kinase 3 inhibitor. Science. 2003;302:875–878. doi: 10.1126/science.1087061. [DOI] [PubMed] [Google Scholar]
- 82.Vincenti F, Tedesco Silva H, Busque S, O'Connell P, Friedewald J, Cibrik D, Budde K, Yoshida A, Cohney S, Weimar W, et al. Randomized phase 2b trial of tofacitinib (CP-690,550) in de novo kidney transplant patients: efficacy, renal function and safety at 1 year. Am J Transplant. 2012;12:2446–2456. doi: 10.1111/j.1600-6143.2012.04127.x. [DOI] [PubMed] [Google Scholar]
- 83.Kim JS, Lee JI, Shin JY, Kim SY, Shin JS, Lim JH, Cho HS, Yoon IH, Kim KH, Kim SJ, et al. Bortezomib can suppress activation of rapamycin-resistant memory T cells without affecting regulatory T-cell viability in non-human primates. Transplantation. 2009;88:1349–1359. doi: 10.1097/TP.0b013e3181bd7b3a. [DOI] [PubMed] [Google Scholar]
- 84.Chiffoleau E, Beriou G, Dutartre P, Usal C, Soulillou JP, Cuturi MC. Role for thymic and splenic regulatory CD4+ T cells induced by donor dendritic cells in allograft tolerance by LF15-0195 treatment. J Immunol. 2002;168:5058–5069. doi: 10.4049/jimmunol.168.10.5058. [DOI] [PubMed] [Google Scholar]