Abstract
Human gingival stem cells (HGSCs) can be easily isolated and manipulated in culture to investigate their multipotency. Osteogenic differentiation of bone-marrow-derived mesenchymal stem/stromal cells has been well documented. HGSCs derive from neural crests, however, and their differentiation capacity has not been fully established. The aim of the present report was to investigate whether HGSCs can be induced to differentiate to osteoblasts and chondrocytes. HGSCs were cultured either in a classical monolayer culture or in three-dimensional floating micromass pellet cultures in specific differentiation media. HGSC differentiation to osteogenic and chondrogenic lineages was determined by protein and gene expression analyses, and also by specific staining of cells and tissue pellets. HGSCs cultured in osteogenic differentiation medium showed induction of Runx2, alkaline phosphatase (ALPL), and osterix expression, and subsequently formed mineralized nodules consistent with osteogenic differentiation. Interestingly, HGSC micromass cultures maintained in chondrogenic differentiation medium showed SOX9-dependent differentiation to both chondrocyte and synoviocyte lineages. Chondrocytes at different stages of differentiation were identified by gene expression profiles and by histochemical and immunohistochemical staining. In 3-week-old cultures, peripheral cells in the micromass cultures organized in layers of cuboidal cells with villous structures facing the medium. These cells were strongly positive for cadherin-11, a marker of synoviocytes. In summary, the findings indicate that HGSCs have the capacity to differentiate to osteogenic, chondrogenic, and synoviocyte lineages. Therefore, HGSCs could serve as an alternative source for stem cell therapies in regenerative medicine for patients with cartilage and joint destructions, such as observed in rheumatoid arthritis.
Introduction
Reconstruction and regeneration of lost skeletal substance and joint structures that are composed of multiple differentiated tissues is a major challenge in osteoarticular surgery and regenerative medicine. In this respect, stem cell therapy could offer a new approach for treatment. One promising approach of stem cell therapy is to induce in stem cells in vitro the developmental processes that generate different tissue components. These preconditioned cells or tissue constructs would then be used in vivo to generate appropriate functional tissues [1].
Skeleton anlagen constitute the primordia of the bone and cartilage through two successive processes, namely, condensation of undifferentiated mesenchymal cells and an early differentiation into chondrocytes allowing the formation of a cartilage template. These primordia can lead to two skeleton derivatives, specifically growth plate and joint structures.
In growth plates in axial and appendicular bones, cells maturate into hypertrophic chondrocytes leading to cartilage mineralization by a process called endochondral ossification [2,3]. Other bone anlagen mature via intramembranous ossification process that takes place in the majority of the craniofacial skeleton. In this process, undifferentiated mesenchymal cells condensate and directly differentiate into osteoblast cells, allowing bone matrix production and mineralization.
Another fate of these primordia is the generation of joint structures. Synovial joints in limbs contain several distinct components, including the synovial cavity, the articular cartilage, and the synovial membrane [4]. Their morphogenesis occurs through segmentation of the cartilage template. During this process, chondrocytes change their phenotype and become elongated and start to express type I collagen [5,6]. Proliferation of these cells leads to an interzone formation within the cartilage primordium [5]. Then, the interzone cavitates and gives rise to the synovial space [5,6]. This cavity is surrounded by a synovial membrane that is composed of two types of synoviocytes. The first type is derived from macrophage-like cells, while the second type is called fibroblast-like synoviocytes [7]. These latter cells provide nutrients and lubrication for cartilage. Especially, they secrete a high amount of hyaluronic acid (HA). Little is known about commitment of these cells [4], but cadherin-11 is necessary for synovial membrane formation, and is a highly specific marker for fibroblast-like synoviocytes during adulthood [7].
Formation of the cartilaginous template critical for both endochondral ossification and articular cartilage development needs well-coordinated multiple cues, including cell–cell and cell–extracellular matrix (ECM) interactions and growth factors, that act in concert to induce the cells into a specific lineage. Replicating the early processes of articular cartilage development during in vitro engineering of cartilage for regenerative therapy would potentially help to optimize the fate of the construct when grafted. Moreover, recreating these developmentally regulated steps in vitro facilitates systematical studies about mechanisms of cell commitment and role of master genes during cartilage differentiation in a controlled setting.
To recreate a functional cartilage, the cell source is critical. In this context, bone-marrow-derived mesenchymal stem/stromal cells (BMMSCs) have been the most studied cells. They are able to differentiate into both chondroblasts and osteoblasts, and to form a mineralized matrix through endochondral differentiation in vitro and in vivo [1,8–10]. However, chondrocytes differentiated from BMMSCs are unstable and tend to follow an endochondral process characterized by type X collagen synthesis and ALPL activity in vitro and in vivo [11,12]. This indicates that the cells acquired a hypertrophic phenotype and that BMMSCs are more predisposed for osteogenic commitment rather than cartilage formation [13], which may limit their utility for joint therapy.
An alternative source of stem cells with bone/cartilage differentiation potential is the neural crest stem cells. Neural crest cells (NCCs) arise from the border of the neural plate and the non-neural ectoderm [14]. Cranial neural crest cells (CNCCs) are multipotent and can generate both neural and mesenchymal derivatives [15], whereas trunk NCCs do not form skeleton derivatives in vivo [16,17]. CNCCs generate facial and oral mesenchymal tissue, such as bone, cartilage, dental pulp, dentin, cementum, and periodontal ligament, as well as certain mesenchymal cells associated with distinct tissue compartments, including myofibroblasts and adipocytes. Facial bone is mainly produced by an intramembranous process although some endochondral ossification may occur in specific areas. These latter include the condylar and coronoid processes. Further, cartilages of the face, such as nasal septum and mandibular condyle, also derive from the neural crests [14]. We hypothesized that the high plasticity during morphogenesis of the CNCCs could be partially recreated in vitro by using postmigratory adult CNCCs. To better understand neural crest cartilage differentiation and to assess utility of CNCCs in cartilage engineering, we used adult stem cells from gingival tissue of oral mucosa. Several studies have shown that neural-crest-derived stem cells persist during adulthood and are multipotent [18]. In particular, recent studies have demonstrated the presence of stem cells in gingival connective tissue and in gingival fibroblast (GF) cultures. These cells express neural crest markers; have self-renewal, multipotency, and immunomodulation properties; and have been used with success in several animal models of cell therapy [19–26].
Materials and Methods
Cell isolation and culture
GF cultures were obtained from human gingival biopsies from three healthy patients with no gingival inflammation. All patients gave their informed consent according to the Helsinki Declaration (1975) and denied having recently taken drugs that could affect connective tissue metabolism. For culture of cells, each biopsy was divided into two identical pieces that were then used to establish cell cultures using the explant or enzymatic digestion with collagenase II (2 mg/mL; Sigma-Aldrich, Oakville, ON, Canada) methods, respectively. Cells were cultured in Dulbecco's modified Eagle's medium (DMEM) containing 10% fetal bovine serum (FBS) with 50 μg/mL of ascorbic acid, nonessential amino acids, and penicillin/streptomycin (Gibco, Life Technologies, Inc. Burlington, ON, Canada). For colony-forming unit fibroblasts (CFU-F)-enriched cultures, single-cell suspensions obtained by incubation of the primary plastic-adherent monolayer cultures with trypsin/EDTA were seeded in 10-cm, tissue-culture-treated plates at low density (<50 cells/cm2), and cultured as earlier until colonies that contained at least 50 cells were formed. The growing colonies in each plate were collected by trypsination and pooled. These pooled CFU-F populations were considered to represent human gingival stem cells (HGSCs). Three HGSC strains from three cell lines isolated by the digestion method from three different donors, and five HGSC strains from explant cultures from five different biopsies were used to assess the osteogenic and adipogenic differentiation capabilities. In addition, three palatal gingival cell lines were used for micromass cultures (two from the enzymatic digestion method and one obtained by the explant method). Two of the cell lines from palatal gingiva were used for SOX9 siRNA experiments.
Osteogenic differentiation assay
Confluent cell cultures were treated with osteogenic induction medium that consisted of DMEM supplemented with 10% FBS, 50 μg/mL of ascorbic acid, 100 nM dexamethasone, 100 nM vitamin D3, 10 mM β-glycerophosphate (Sigma-Aldrich), and antibiotic mixture (Gibco). After 21–35 days of culture, mineralization was analyzed by Alizarin Red S staining.
Chondrogenic differentiation assay
Cells were released from monolayer cultures by trypsin treatment, 500,000 cells were centrifuged (500 g/5 min), and then they were cultured as micromass cultures in 15-mL polypropylene tubes (Falcon, BD Biosciences, Mississauga, ON, Canada). Two media were used successively as previously described [1]. The first serum-free medium contained high-glucose DMEM, 100 nM dexamethasone, 1% ITS+1, l-proline (40 μg/mL), and ascorbic acid (50 μg/mL) (Sigma-Aldrich); sodium pyruvate (1 mM; Gibco); and 10 ng/mL transforming growth factor-β3 (TGF-β3) (BioVision, Edmonton, Alberta, Canada), and was used during the first 3 weeks of culture to induce chondrogenic differentiation. To induce hypoxic conditions, this medium was then replaced for the following 2 weeks with a medium composed of high-glucose DMEM, 1 nM dexamethasone, 1% ITS+1, l-proline (40 μg/mL), ascorbic acid (50 μg/mL), l-thyroxine (50 nM), and β-glycerophosphate (20 mM) (Sigma-Aldrich) and sodium pyruvate (50 nM; Gibco), and cells were cultured in a hypoxic condition that was achieved by closing the cap of the culture tube tightly to prevent gas exchange.
Histology and immunostaining
For histological assessment, micromass cultures were fixed in cold (4°C) ethanol solution (50%), dehydrated in ethanol, and embedded in paraffin. Paraffin sections (7-μm thick) were cut using a microtome. Histological staining was performed using alcian blue (pH 1), alizarin red S, toluidine blue, hematoxylin-eosin, and picrosirius red stains. The following primary antibodies were used for immunostaining: anti-type II collagen (CIIC1; Developmental Studies Hybridoma Bank, University of Iowa, Iowa City, IA), anti-type X collagen (X-AC9; Developmental Studies Hybridoma Bank), anti-cadherin-11 (MAB1790; R&D Systems, Minneapolis, MN), anti-tubulin, anti-α-SMA (ab5694; ABCAM, Paris, France), and anti-Ki67 (M0722; Dako, Les Ulis, France). For HA staining, biotinylated hyaluronic acid binding protein (HABP) was used (Calbiochem, Millipore, Toronto, ON, Canada). Apoptosis was detected using ApopTag kit (Millipore, Molsheim, France). Prior to immunostaining, nonspecific binding of antibodies was blocked by incubating the samples with phosphate-buffered saline containing 3% bovine serum albumin and 0.1% glycine for 30 min. Incubation with primary antibodies was carried out at 4°C overnight, and immunolabeling was detected using Vectastain ABC kit with VIP or DAB substrates (Vector Laboratories, Burlington, ON, Canada). For every immunostaining, a negative control was added without primary antibody. The sections were observed using an Axioplan light microscope (Zeiss, North York, ON, Canada).
For immunofluorescence stainings, goat anti-rabbit Alexafluor 594– and goat anti-mouse Alexafluor 488–conjugated secondary antibodies were used (Life Technologies Corporation). Cell nuclei were stained using DAPI (Life Technologies Corporation). Samples were observed and digital images were recorded on the Axioplan fluorescent light microscope (Zeiss).
Quantitation of Ki67-positive and apoptotic cells in tissue sections (three sections per condition) was performed counting cells in 5–10 acquisition fields per section per culture condition using the ImageJ software (NIH).
Electron microscopy
Paraffin-embedded sections from micromass cultures were rehydrated, rinsed in PIPES, and fixed in 2.5% glutaraldehyde for 1 h. After rinsing, sections were postfixed with 0.5% osmium tetroxide for 20 min. Portions of the glass slides were trimmed to contain only the sections, and then mounted on to aluminum stubs using conductive adhesive and colloidal silver (Canemco-Marivac, St. Laurent, QC, Canada), followed by sputter coating with 5-nm gold/palladium (Leica, EM MED020, Leica Microsystem, Inc., Concord, ON, Canada). The samples were examined using Helios NanoLab 650 Dual Beam Scanning Electron Microscope (FEI, Hillsboro, OR).
Gene expression analysis
Total RNA was extracted from cultured cells using Aurum™ Total & RNA Mini Kit (Bio-Rad Laboratories, Mississauga, ON, Canada), and treated with DNAse (Fermentas, Burlington, ON, Canada) according to the manufacturer's protocol. Total RNA concentration and purity was measured by nanodrop (Agilent, Mississauga, ON, Canada) and only samples with OD260/280 ratio of 1.8 to 2.1 were used for the study. cDNA was synthesized using iScript™ cDNA Synthesis Kit (Bio-Rad Laboratories) according to the manufacturer's instructions: 25°C for 5 min, 42°C for 30 min, and 85°C for 5 min. All primers (Supplementary Table S1; Supplementary Data are available online at www.liebertpub.com/scd) were designed on the boundaries of exons whenever possible, and analyzed by BLASTn for their specificity and by RNAfold software for their secondary structures. Efficiency of target amplification was optimized for each primer set using a 10-fold dilution series of cDNA while standard curves were made. For the reaction, equal amount of retro-transcriptase (RT) product from each sample was diluted to a concentration such that the Ct values were well within the range of their standard curves (in general equivalent to 5 ng/mL RNA), and 3 μL of diluted RT products was mixed with 7.5 μL of 2 X iQ SYBR Green I Supermix (Bio-Rad Laboratories), and 5 pmols of primers, for a final volume of 15 μL. Real-time PCR amplification was performed on the CFX96 System (Bio-Rad Laboratories) using the following program: one cycle at 94°C for 3 min, 35 cycles at 95°C for 5 s, gene-specific annealing for 10–20 s, and reaction completion with reading plate and a melt curve analysis from 65°C to 95°C, 5 s for each 0.5°C. For some genes (collagen type II and aggrecan), a touchdown PCR cycle was implemented. In brief, a cycling program where the annealing temperature was gradually reduced (2°C/every cycle) was followed by the standard quantitative polymerase chain reaction (QPCR) protocol. In preliminary experiments, reaction products were run on agarose gels (1.2%) to confirm the correct amplicon sizes. For a given experiment, a reference gene was chosen based on its variance calculation using CFX manager software (Bio-Rad Laboratories). Nontranscribed RNA samples (nonreverse transcribed sample) and a water control were used as negative controls. The PCRs were performed in triplicate for each sample. The data were analyzed based on 2ΔΔCt method (CFX Manager Software Version 2.1; Bio-Rad Laboratories).
siRNA transfection
Transfections with siRNA were performed using lipofectamine RNAiMax kit (Invitrogen, Life Technologies, Burlington, ON, Canada) in 60-mm culture dishes by the indirect method. The siRNA duplexes were designed using Whitehead Institute's siRNA prediction tool following the N19+dTdT rule. Among these duplexes, three were designed to inhibit SOX9 (iSOX9a, b, c), one was to inhibit a gene unrelated to SOX9 (vimentin: iVIM), and one was composed of a scrambled iSOX9a sequence (iScr) used as a control (Mock) (Supplementary Table S1). In brief, cells were treated with siRNA (2 μL of 10 mM siRNA in 100 μL Opti-MEM, final concentration at 100 nM) in RNAiMax (6 μL in 100 μL Opti-MEM) in serum-free Opti-MEM medium (Gibco) for 12 h, before replacing the medium with the normal growth medium. A set of cultures was also treated with a mixture of all three SOX9 siRNAs at the same time (100 nM each). To assess transfection efficiency by real-time PCR, cells were cultured for various time points and RNA was isolated as earlier. Optimal siRNA and RNAiMax concentrations were determined in preliminary experiments. For a set of experiments to visualize siRNA inside the cells by fluorescence microscopy, iSOX9b was coupled with Alexafluor 488 fluorochrome (Eurogentec, Liège, Belgium). To assess cadherin-11 function in synoviocyte differentiation, an siRNA inhibiting cadherin-11 (iCDH11, see Supplementary Table S1) was used during micromass cultures in the same conditions than the iSOX9.
Statistical analysis
Real-time PCR results were analyzed using the nonparametric Friedman test with Graph Pad software (Graphpad Software, Inc., La Jolla, CA).
Results
Gingival cell characterization
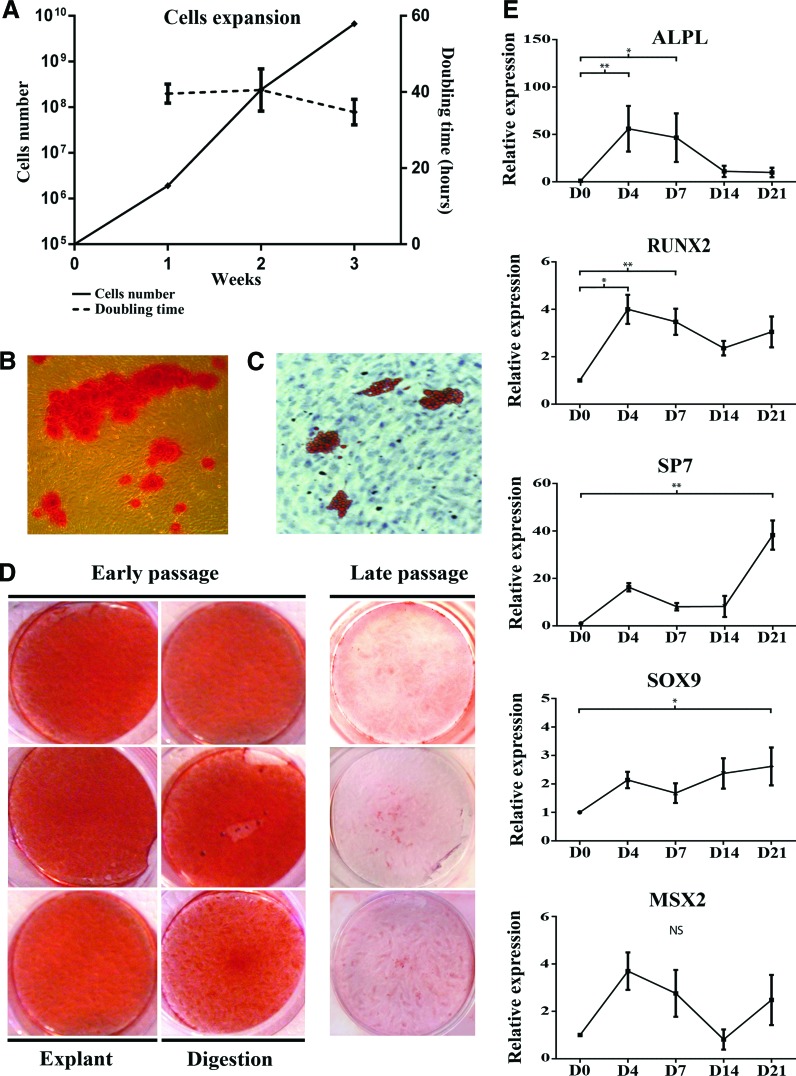
Human gingival cell lines were derived from independent donors, and each biopsy was divided into two samples in order to assess two cell-harvesting strategies (enzyme digestion and explant culture). Because long-term in vitro culture impairs progenitor cell properties [27,28], all experiments were performed using cells up to the third passage. As previously described [20], GFs were able to form CFU-F when cultured at low density (<50/cm2) as cell-culture-plastic-adherent cells. For all subsequent experiments, CFU-F-pooled cells, named HGSCs, were used. Analysis of mRNA expression of neural crest markers by PCR showed that the cells expressed TWIST1 and SNAIL1 (n=5 cell lines, three from palatal and two from buccal gingiva) (Supplementary Fig. S1). Cells were also positive for osteochondro-lineage markers Runt-related transcription factor 2 (RUNX2) and SOX9 (n=5) (Supplementary Fig. S1). Further, they displayed stable cell proliferation rate with a constant about 40-h population doubling time over 3 weeks (Fig. 1A), and with osteogenic and adipogenic differentiation potential in vitro (n=3) (Fig. 1B, C). Both enzymatically and explant-derived cell cultures from these three different patients showed similar osteogenic differentiation capacity (Fig. 1D). For the subsequent experiments, we used the three HGSC lines, which were all derived from palatal gingiva.
FIG. 1.
Characterization of gingival-connective-tissue-derived cells. Analysis of growth kinetics showed an increase in cell numbers with a constant doubling time over 3 weeks (A). When incubated in the appropriate differentiation medium, cells showed osteogenic-like differentiation with deposition of mineralized matrix (B, alizarin red S staining) and differentiation into adipocyte-like cells (C, oil red O staining). Osteogenic differentiation at low (P≤3) and high passages (P≥6) was further tested with three HGSC lines from three different donors and isolated by the explant or enzymatic digestion method. All low-passage cell lines showed similar osteogenic response (D), while cells at high passage showed a decreased differentiation potential. Gene expression analysis of key genes involved in osteodifferentiation was also followed over time in osteogenic medium. Results from real-time PCR showed that the master genes were upregulated during this process and followed classical kinetics over time (E). The results show mRNA expression relative to GAPDH as housekeeping gene according to the 2ΔΔCt method. Each QPCR was performed in triplicate. Results show mean±standard deviation from four parallel cell lines (*P<0.05; **P<0.01). NS, no statistically significant differences. HGSCs, human gingival stem cells; QPCR, quantitative polymerase chain reaction. Color images available online at www.liebertpub.com/scd
Osteogenic differentiation of HGSC monolayer cultures
To further characterize the HGSCs, we tested their osteogenic differentiation capacity in more detail, and assessed expression of osteogenic-differentiation-related genes over time in monolayer cultures. The cells were able to form mineralized nodules when stimulated by an osteoinductive medium for 3–5 weeks (Fig. 1D). This involved an early upregulation of ALPL (days 4 and 7), an early and extended upregulation of RUNX2 (days 4–21), and an about 20-fold late upregulation of SP7 (day 21). Expression of SOX9 remained moderately upregulated from day 4 to 21, while MSX2 showed an early (day 4) and late (day 21) peak, but these changes in gene expression were not statistically significant (Fig. 1E). Gene expression studies showed comparable mRNA expression kinetics over time among the different cell strains. The capability to form mineralized nodules decreased at late passage (>P6) (Fig. 1D), which is consistent with data from dental-pulp-derived stem cells [29].
Chondrogenic differentiation and maturation of HGSC micromass cultures
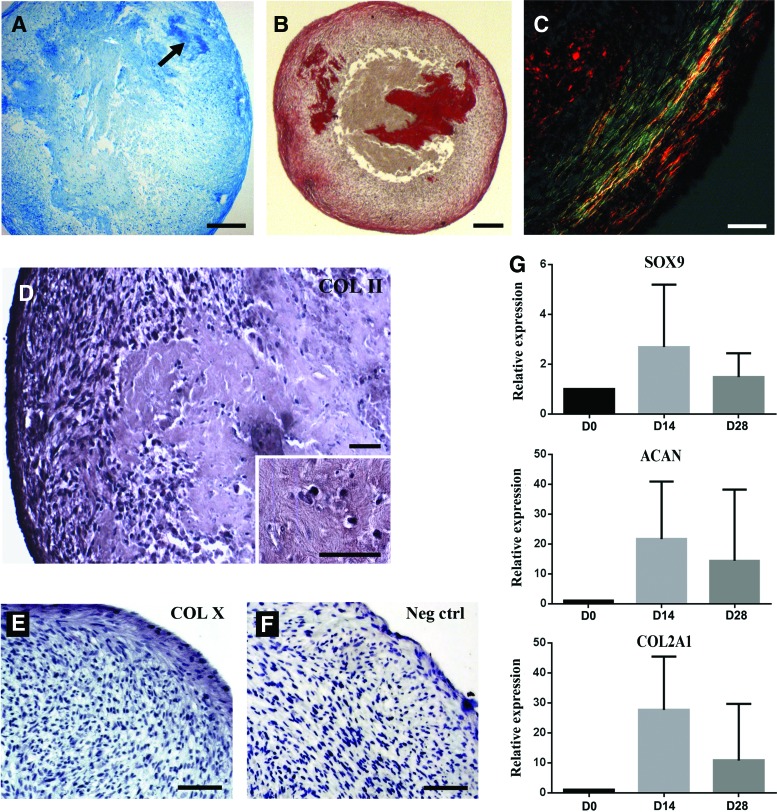
To assess the capability of the cells for chondrogenic differentiation along the endochondral pathway, HGSCs were cultured in chondrogenic induction medium as micromass cultures for 3 weeks, followed by 2 weeks of hypoxic condition to induce hypertrophy (n=3). When expression of key genes involved in chondrogenesis was followed over time, an upregulation of SOX9, ACAN, and COL2A1 mRNA expression appeared after 2 weeks of chondrogenic induction (Fig. 2G). At day 21, histological sections from the cultures showed strong inhomogeneous glycosaminoglycan staining (Fig. 2A). Organization of the collagen-rich ECM was then assessed by picrosirius red staining (Fig. 2B, C). In the most peripheral surface layers of the cultures, collagen was parallel to the surface, while in the more-central areas it was randomly oriented (Supplementary Fig. S2). The cultures were positive for type II collagen (Fig. 2D) especially in the more-central areas of the micromass that were relatively acellular reminiscent of natural cartilage. Immunostaining for type X collagen was mostly negative especially in the central areas of the cultures, but areas closest to the outer surface showed some weak staining (Fig. 2E, F). Examination with a higher magnification showed a heterogeneous composition of the micromass culture with a central cell-free area located next to typical chondrocyte-like cells embedded in a cartilaginous-like matrix (Fig. 2E). This differentiation is comparable to what is classically obtained with BMMSCs (Supplementary Fig. S3).
FIG. 2.
Analysis of chondrogenic differentiation. After 21 days of micromass culture in chondrogenic induction medium, cultures generated cartilage-like tissue. (A) Alcian-blue-stained (pH=1) histological sections from the cultures that displayed a cartilage-like ECM particularly at the center of the micromass. Positive area is outlined by the arrow. (B) Picrosirius red staining showed collagen-rich (red) areas in both periphery and center of the culture. (C) Observation under polarized light of the periphery of the culture revealed collagen fibers that were aligned parallel to the micromass surface, and showed a more intense green color, indicating a difference in the diameter of the collagens fibers in the peripheral and central areas. Cultures showed also positive immunostaining for type II collagen (D), while the staining for type X collagen was mostly negative, with some areas closest to the outer surface showing a weak staining (E). (F) Negative control staining without primary antibody showed no positive immunoreaction. Representative images from three experiments performed with three parallel HGSC lines are shown. Magnification bar: (B, C) 200 μm; (D, E) 100 μm; and (F, G) 30 μm. (G) In the micromass cultures, expression of type II collagen, aggrecan, and SOX9 mRNA was strongly upregulated after 14 days of culture in chondrogenic medium, and was then downregulated after 1 week under hypoxia (corresponding to day 28 after initiation of the experiment). Results show QPCR analysis of mRNA expression relative to GAPDH (2ΔΔCt method). ECM, extracellular matrix. Color images available online at www.liebertpub.com/scd
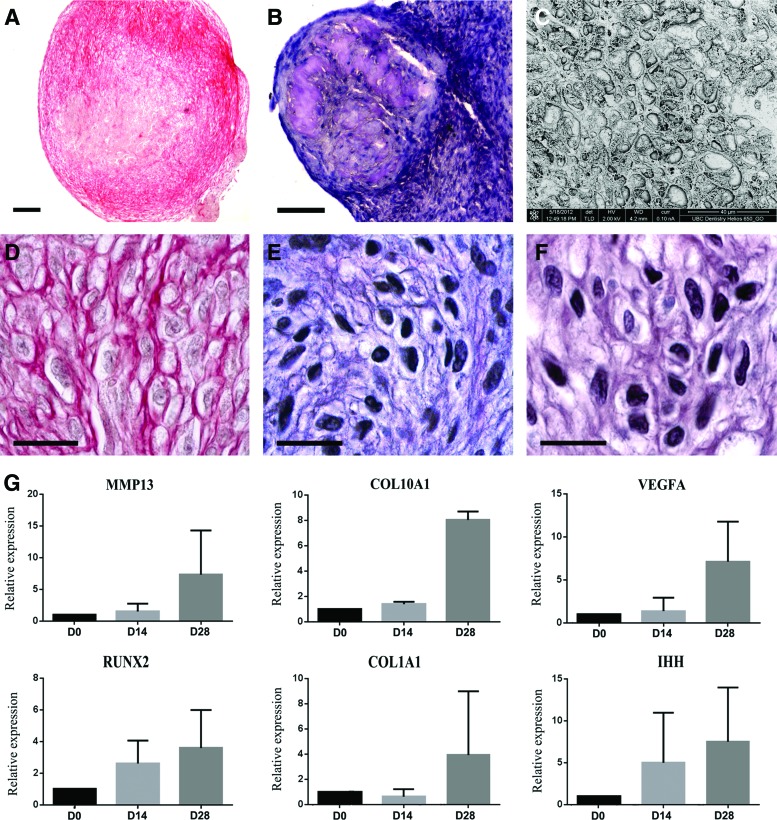
During cartilage development, hypoxic condition due to a lack of blood supply within the cartilage induces hypertrophy [30]. Hypertrophic cells are characterized by an increase of the cell volume and a rounded cell shape [31]. To assess the effect of hypoxia in our micromass culture model, cells grown for 21 days in the chondrogenic induction medium in normoxic conditions were switched to hypoxic condition for 14 days by adding l-thyroxine and closing the caps of the culture tubes. After 1 week, expression of genes implicated in the maturation of hypertrophic chondrocytes, that is, Indian Hedgehog (IHH), collagen type X alpha 1 (COL10A1), vascular endothelial growth factor α (VEGFA), matrix metalloproteinase 13 (MMP13), RUNX2, and collagen type I alpha 1 (COL1A1), was upregulated (Fig. 3G), while chondroblast-related genes SOX9, ACAN, and COL2A1 were downregulated (Fig. 2G). These differences did not reach statistical significance due to variation in the magnitude of gene expression changes in the examined three different HGSC cultures. At day 35, a fibrillar collagen network was reorganized compared with day 21 (Fig. 3A), and was characterized by a lower fiber density and absence of peripheral aligned fibers. A glycosaminoglycan-rich ECM (Fig. 3B) was observed in two out of three micromass cultures. In all the cultures, cells acquired a hypertrophic phenotype characterized by a round shape and an increased cell volume (Fig. 3C, D). In addition, cells were surrounded by a collagen network composed of cartilage-specific type II collagen (Fig. 3E) and hypertrophy-associated type X collagen (Fig. 3F). However, cells were unable to complete endochondral ossification, and to form mineralized matrix, even when treated with β-glycerophosphate. Moreover, alkaline phosphatase activity was negative (data not shown). Thus, HGSCs were able to give rise to hypertrophic chondrocytes, but did not induce ossification in vitro.
FIG. 3.
Analysis of chondrocyte maturation. After 3 weeks of chondrogenic differentiation, followed by 2 weeks of hypoxic conditions, chondrogenic cells acquired a hypertrophic phenotype. Histological sections stained with picrosirius red showed that cells inside the micromass cultures were surrounded by a mature cartilage-like fibrillar collagen network (A) and (D) associated with a toluidine-blue-positive ECM (B). (C–F) Examination with higher magnification revealed a typical organization of mature cartilage. Many cells displayed morphology reminiscent of hypertrophic chondrocytes when examined by SEM analysis (C), and were surrounded by a collagen network composed of type II (E) and type X collagens (F). However, no mineralization of the matrix was noted (data not shown). Representative images from three experiments performed with three parallel HGSC lines are shown. Magnification bar: (B) 200 μm; (C) 100 μm; and (E–G) 20 μm. (G) QPCR analysis showed an upregulation of key genes associated or involved in chondrocyte hypertrophy after 1 week of hypoxia (corresponding to day 28 after initiation of the experiment). Results show mRNA expression relative to GAPDH (2ΔΔCt method). IHH, Indian Hedgehog; COL10A1, collagen type X alpha 1; VEGFA, vascular endothelial growth factor α; MMP13, matrix metalloproteinase 13; RUNX2, Runt-related transcription factor 2; COL1A1, collagen type I alpha 1. Results show mean of relative mRNA expression from two parallel HGSC lines. SEM, scanning electron microscopy. Color images available online at www.liebertpub.com/scd
Peripheral cell lining in the HGSC micromass cultures differentiates into fibroblast-like synoviocytes
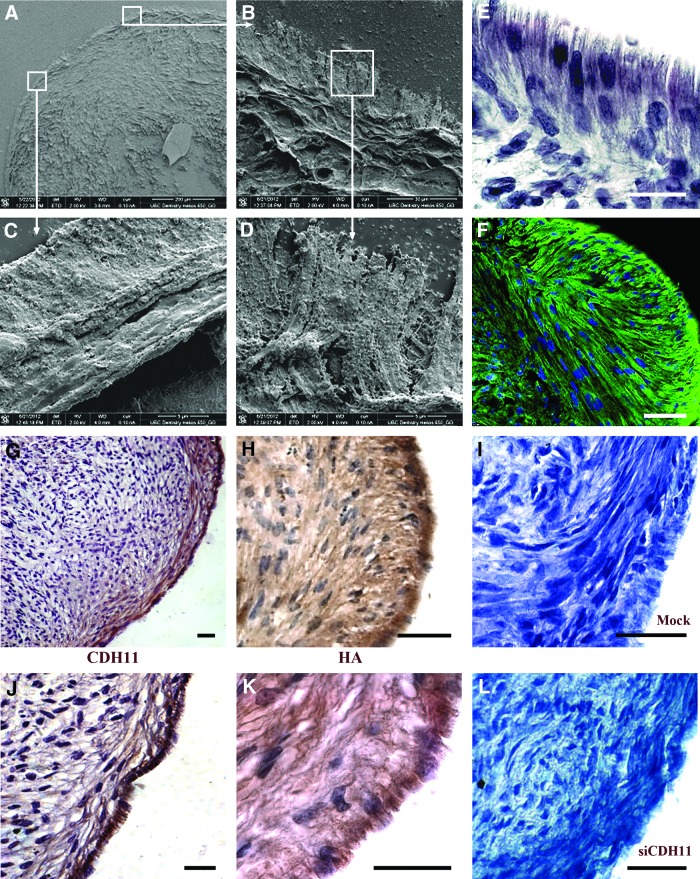
At day 21, a peripheral surface cell lining had formed and was oriented perpendicularly to the underlying layers of the micromass cultures (Fig. 4A–F). The cell lining was mainly composed of only one layer of polarized cells (Fig. 4E), but in some locations it reached a thickness of up to five layers (not shown). Scanning electron microscopy showed that cells of the external layers had a cuboidal shape and presented an irregular villous outer surface (Fig. 4A–D). To find out whether these cells shared key properties of fibroblast-like synoviocytes found in vivo, we performed immunostaining for cadherin-11 [7]. Results showed that the peripheral cell lining was strongly positive for cadherin-11, and that the subperipheral layer also contained positive cells (Fig. 4G, J). The first cadherin-11-positive cells appeared in the peripheral surface layers at day 14 after induction of the micromass cultures (Supplementary Fig. S4). Moreover, similar to normal fibroblast-like synoviocytes [4] these cells produced HA as indicated by strong HABP reactivity (Fig. 4H, K).
FIG. 4.
Fibroblast-like synoviocyte differentiation. (A–D) SEM analysis of the histological sections from the micromass cultures at day 21 showed a distinct peripheral lining cell layer that was formed on the outer surface of the micromass culture. Hematoxylin staining (E) and tubulin immunostaining (F) showed that the cells in the peripheral layer were polarized and oriented perpendicularly to the underlying layer that was aligned more parallel to the outer surface of the micromass culture. Immunostaining showed that the peripheral cell lining was positive for cadherin-11 (CDH-11) (G, J) and hyaluronic acid (HA) (H, K). Inhibition of cadherin-11 by siRNA transfection showed an inhibition of the formation of the peripheral cell lining (I, L). Representative images from experiments with three parallel HGSC lines are shown. Magnification bar: (E) 20 μm; (F) 50 μm; (G) 100 μm; and (H–J) 50 μm. Color images available online at www.liebertpub.com/scd
SOX9 plays a critical role in gingival cell chondrogenic micromass culture self-organization
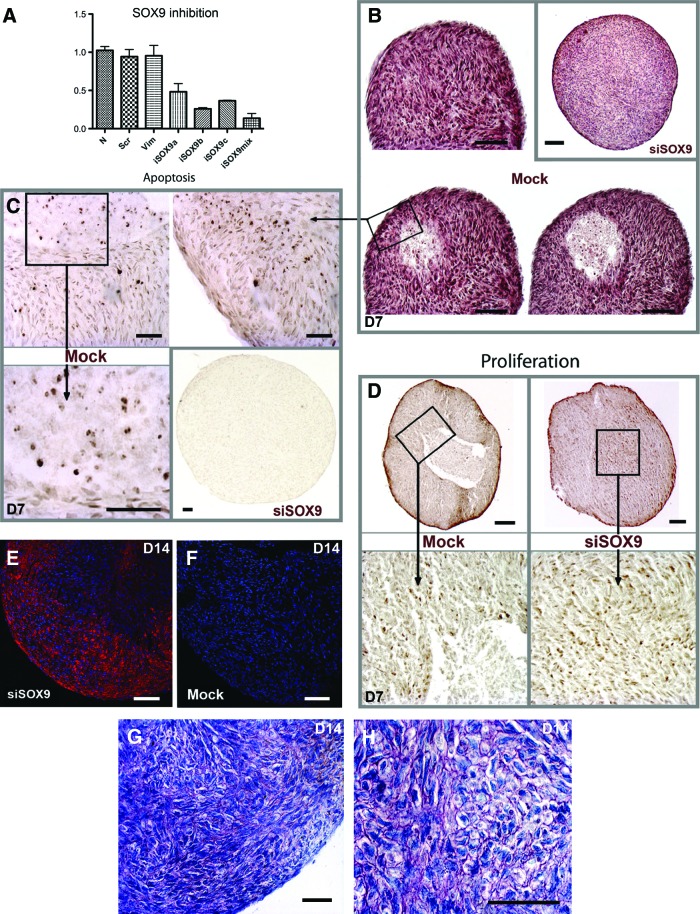
Role of SOX9, a key transcription factor expressed by chondrocyte precursors that regulates COL2A1 and ACAN expression in chondrogenesis [32], was studied by using SOX9 siRNA transfection of the micromass cultures. Control cultures were transfected with siRNA against an unrelated vimentin gene (iVIM), and using scrambled (mock) SOX9 siRNA. Real-time PCR showed that different SOX9 siRNAs caused variable SOX9 downregulation (Fig. 5A). iSOX9a had a marked effect on SOX9 expression (52% downregulation of SOX9), while iSOX9b and iSOX9c caused a 74% and 64% downregulation, respectively (Fig. 5A). Combining all three SOX9 siRNAs caused the most potent SOX9 downregulation (87% downregulation) (Fig. 5A), but it also resulted to poor generation of micromass cultures likely due to toxicity (data not shown). Downregulation of SOX9 by the different duplexes of siRNA lasted at least for 8 days (Supplementary Fig. S5). Based on the aforementioned data, iSOX9b was chosen for the further experiments. To assess the role of SOX9 in the development of the micromass cultures, we compared them histologically with and without SOX9 siRNA treatment. Histological analysis showed that compartmentalization of the micromass culture was already underway in control samples at day 7. In particular, control cultures had formed a well-defined area containing fewer cells and increased apoptotic activity (Fig. 5B, C). Accordingly, in the peripheral area, around 3.5% of the cells were apoptotic, while 30.9% of the cells near the central acellular area were apoptotic in the control cultures. In contrast, this apoptotic compartment was almost missing from SOX9-siRNA-treated samples (<1% of apoptotic cells) (Fig. 5B, C). Proliferating cells were present in the peripheral area for both mock (Fig. 5D, left panel) and SOX9-siRNA-treated micromass cultures (Fig. 5D, right panel) with similar proportions (11.44%±5.25% for mock vs. 10.5%±5.69% for siSOX9-treated cultures). However, while proliferating cells were present in the center of SOX9-siRNA-treated cultures, they were missing from the control cultures (Fig. 5D). Immunostaining of α-smooth muscle actin, a marker for myofibroblasts, showed that inhibition of SOX9 resulted to areas with high numbers of α-SMA-positive myofibroblasts (Fig. 5E) while these cells were missing from the control cultures (Fig. 5F). To further assess cell morphology and abundance of glycosaminoglycans, the cultures were stained with toluidine blue. In 14-day-old cultures, toluidine blue staining showed cells with well-defined polarized surface layer, a distinct elongated layer underneath the surface layers, and chondrocyte-like cells in the deeper areas of the cultures (Fig. 4I). In contrast, some areas of the SOX9-siRNA-treated cultures harbored predominantly cells with a prehypertrophic-like phenotype (round shape) and the surface layers were less well-defined (Fig. 5G, H).
FIG. 5.
SOX9 regulates morphogenesis of cartilage-like micromass cultures. (A) Real-time PCR of SOX9 mRNA expression in SOX9 siRNA (siSOX9)– and control siRNA (mock)–transfected cells 1 day after siRNA transfection showed a downregulation of SOX9 expression. Results show mean and standard deviation from two experiments performed with two parallel HGSC lines. iSOX9a, first duplex against SOX9; iSOX9b, second duplex; iSOX9c, third duplex; N, cells treated without siRNA; Scr, transfection with a scrambled iSOX9a siRNA; iSOX9mix, cells were transfected with all three SOX9 siRNAs (iSOX9a, iSOX9b, and iSOX9c) at the same time. (B) Hematoxylin-eosin staining shows the presence of an early compartmentalization of micromass cultures at day 7 with the presence of a cell-free area at the center. (C) Staining of apoptotic cells by TUNEL assay at day 7. Control cultures showed areas that contained abundantly apoptotic cells especially at the center. However, apoptosis was not evident in SOX9-siRNA samples (B, C). (D) Analysis of cell proliferation by Ki67 immunostaining at day 7 showed that proliferating cells were present in the peripheral area for both mock and siSOX9 micromass cultures. They were also present in the center of SOX9-siRNA cultures, but missing from the control cultures. (E, F) Immunostaining of α-SMA (red fluorescence) at day 14 showed immunoreactivity only in SOX9-siRNA-treated samples. Blue color indicates nuclear staining with DAPI. (G, H) Toluidine blue staining shows the presence of round cells in SOX9-siRNA micromass cultures, but not in mock conditions. Toluidine blue metachromasia (purple staining) was mainly observed in SOX9-siRNA conditions. Magnification bar: (B, D, E, F) 100 μm; (C, G, H) 50 μm. The siRNA experiment was performed twice on two different cell strains. Color images available online at www.liebertpub.com/scd
Discussion
This study provides novel insight into the properties of postmigratory NCCs. First, while these cells were derived from adult human gingival soft tissue, they were able to differentiate into hypertrophic chondrocytes. Second, when cultured as micromasses, they self-organized to generate distinct areas that resembled native cartilage templates. These included a peripheral area composed of two distinct layers that mimic synovial cell lining and the perpendicularly oriented subintimal layer. This area also contained aligned fibrils of collagen. These characteristics are similar to chondrodifferentiation achieved using induced pluripotent stem cells [33]. In the micromass cultures in the present study, the central area also contained a type II-collagen-rich cartilaginous matrix where the cells further differentiated in hypertrophic chondrocytes. Thus, this in vitro chondrogenesis by HGSCs reproduced a set of physiological developmental processes resulting in different cell phenotypes, highlighting the plasticity of gingival cells.
To our knowledge, this is the first report that describes fibroblast-like synoviocyte differentiation during micromass chondrodifferentiation in vitro with cells other than those derived from the synovium. Distinct mechanical and other cell-adhesion-related stimuli due to lack of adhesion to the culture plate and abundant cell–cell contacts in the floating micromass cultures may act synergistically with cytokine signaling to polarize the most external cell lining to form this fibroblast-like synoviocyte layer. This is supported by the finding that no synoviocyte differentiation was noted in cultures where the micromass was attached to the culture dish (data not shown). Whether the fibroblast-like synoviocyte differentiation is a distinct property of neural-crest-derived gingival cells or a more general characteristic of connective tissue cells from other tissues in this culture environment remains to be shown.
SOX9 is considered a mastergene for differentiation of chondrocytes [34]. Moreover, SOX9 drives chondrodifferentiation of stem cells derived from diverse sources [34]. Our results confirm the upregulation of this gene during chondrogenesis of HGSCs. In addition, SOX9 was also expressed by these cells at basal level without chondrogenic stimulation. This basal expression may explain the ability of HGSCs for efficient chondrogenesis.
In SOX9-knockout mice, cells are unable to generate normal cartilage after mesenchymal condensation but acquired a hypertrophic phenotype [34]. This is similar to the SOX9-siRNA-treated micromass cultures in the present study. However, in the knockout mice, inhibition of SOX9 was associated with apoptosis [34], which was not noted in our study. These opposite effects might be linked either to differences between the embryonic origins of the cell populations in the mice and in our study or to incomplete SOX9 inhibition in our siRNA-treated samples. Regardless, chondrogenesis was disturbed in the SOX9-siRNA-treated micromass cultures, and associated with a change of cell phenotype involving cell rounding and differentiation toward myofibroblast-like cells. TGF-β3-supplemented medium in combination with SOX9 inhibition may explain this phenomenon. TGF-β3 is a potent regulator of neural-crest-derived cell fate [35], and known to be an inducer of myofibroblast phenotype [32]. Moreover, inhibition of SOX9 is required for maturation of hypertrophic cells [34]. Nevertheless, our results confirmed that SOX9 is crucial for chondrogenesis in vitro, mimicking chondrogenesis in vivo.
Human and animal studies have suggested that the synovial-derived cells, rather than chondrocytes or cartilage-derived cells, possess stem cell characteristics. These stem cells proliferate and participate in cartilage formation and remodeling after injuries [36–38]. However, cartilage repair and remodeling in adults is usually inefficient likely due to low number of these stem cells [13,36]. Further, joint injury caused by trauma or disease, including osteoarthritis, involves several tissue compartments in the joints and may thus need multiple cell lineages to regenerate these tissues. Using autologous chondrocytes in therapy is challenging because they are difficult to obtain, and can lead to damage of the donor site [39]. Moreover, isolated chondrocytes dedifferentiate during in vitro expansion losing their chondrogenic potential [40]. More recently, the use of embryonic stem cells [41], or induced pluripotent stem cells [33,42], to generate cells that possess chondrodifferentiation capability has been reported. The pluripotency of these cells is an advantage and offers a new model to recapitulate tissue development. However, use of these genetically manipulated cells poses still many challenges, including potential for teratome formation [33,42]. Purification protocols to remove cells prone to teratoma formation have been developed, but they employ antibodies that cannot be used in human cell therapy. Therefore, HGSCs would provide an attractive source of cells to study chondrogenesis and generate cells for therapeutic applications as they can be easily obtained with practically no morbidity of the donor site and quickly expanded in culture without a need for animal serum supplementation, and they are chromosomally stable [43].
To our knowledge, this is the first study to provide evidence that, in addition to chondrogenic cells, HGSCs are also able to differentiate into type X-collagen-secreting hypertrophic chondrocytes and fibroblast-like synoviocytes. However, unlike BMMSCs cultured in identical conditions [1,8], HGSCs showed no capability to form mineral deposits. It is possible that contrary to the mesodermal bone marrow MSCs, neural-crest-originating HGSCs need other cells and/or a distinct ECM niche to promote appropriate terminal differentiation and produce mineralized matrix. Interestingly, gingival cells expressed high levels of VEGFA, a potent proangiogenic factor, under the hypoxic condition. This characteristic may be of advantage to promote revascularization critical for regenerative therapies in the joint. The gingival cell population that had cartilage and synoviocyte-like differentiation potential was derived from pooled CFU population without further selection or fractionation. Although such cell population has been shown to contain stem cells, it can be heterogeneous [44]. Therefore, future fractionation studies of this cell population may provide further details about the identity of the cells that specifically generate cartilage and synoviocyte-like cells.
Conclusions
In conclusion, use of HGSCs for cartilage morphogenesis and differentiation offers interesting perspectives concerning joint and bone repair. Based on their ability to recapitulate processes involved in cartilage and joint development, HGSCs may also be used as a novel model to study NCC commitment and differentiation to cartilage and bone cell fate. Easy access and low morbidity, and fast and efficient wound healing after gingival biopsy [42] provides also an advantage over often difficult and painful sampling of bone or cartilage cells from patients. Finally, use of HGSCs may provide a novel alternative for drug screening or to explore pathological mechanisms involved in cartilage formation.
Supplementary Material
Acknowledgments
This study was supported by the INSERM, ANR program (no. R12118DD), the Canadian Institutes of Health Research, and the “Gueules cassées” Foundation. The authors thank Mr. Cristian Sperantia and Dr. Guoqiao Jiang for their technical help and Dr. Hélène Rouard and Dr. Nathalie Chevallier for providing BMMSCs. The authors thank the Developmental Studies Hybridoma Bank for providing antibodies.
Author Disclosure Statement
The authors indicate no potential conflict of interest.
References
- 1.Scotti C, Tonnarelli B, Papadimitropoulos A, Scherberich A, Schaeren S, Schauerte A, Lopez-Rios J, Zeller R, Barbero A. and Martin I. (2010). Recapitulation of endochondral bone formation using human adult mesenchymal stem cells as a paradigm for developmental engineering. Proc Natl Acad Sci U S A 107:7251–7256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ornitz DM. and Marie PJ. (2002). FGF signaling pathways in endochondral and intramembranous bone development and human genetic disease. Genes Dev 16:1446–1465 [DOI] [PubMed] [Google Scholar]
- 3.Kronenberg HM. (2003). Developmental regulation of the growth plate. Nature 423:332–336 [DOI] [PubMed] [Google Scholar]
- 4.Lefebvre V. and Bhattaram P. (2010). Vertebrate skeletogenesis. Curr Top Dev Biol 90:291–317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Archer CW, Dowthwaite GP. and Francis-West P. (2003). Development of synovial joints. Birth Defects Res C Embryo Today 69:144–155 [DOI] [PubMed] [Google Scholar]
- 6.Spagnoli A, O'Rear L, Chandler RL, Granero-Molto F, Mortlock DP, Gorska AE, Weis JA, Longobardi L, Chytil A, Shimer K. and Moses HL. (2007). TGF-beta signaling is essential for joint morphogenesis. J Cell Biol 177:1105–1117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lee DM, Kiener HP, Agarwal SK, Noss EH, Watts GF, Chisaka O, Takeichi M. and Brenner MB. (2007). Cadherin-11 in synovial lining formation and pathology in arthritis. Science 315:1006–1010 [DOI] [PubMed] [Google Scholar]
- 8.Mueller MB. and Tuan RS. (2008). Functional characterization of hypertrophy in chondrogenesis of human mesenchymal stem cells. Arthritis Rheum 58:1377–1388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Oliveira SM, Mijares DQ, Turner G, Amaral IF, Barbosa MA. and Teixeira CC. (2009). Engineering endochondral bone: in vivo studies. Tissue Eng Part A 15:635–643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Oliveira SM, Amaral IF, Barbosa MA. and Teixeira CC. (2009). Engineering endochondral bone: in vitro studies. Tissue Eng Part A 15:625–634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Johnstone B, Hering TM, Caplan AI, Goldberg VM. and Yoo JU. (1998). In vitro chondrogenesis of bone marrow-derived mesenchymal progenitor cells. Exp Cell Res 238:265–272 [DOI] [PubMed] [Google Scholar]
- 12.Kafienah W, Mistry S, Dickinson SC, Sims TJ, Learmonth I. and Hollander AP. (2007). Three-dimensional cartilage tissue engineering using adult stem cells from osteoarthritis patients. Arthritis Rheum 56:177–187 [DOI] [PubMed] [Google Scholar]
- 13.De Bari C, Kurth TB. and Augello A. (2010). Mesenchymal stem cells from development to postnatal joint homeostasis, aging, and disease. Birth Defects Res C Embryo Today 90:257–271 [DOI] [PubMed] [Google Scholar]
- 14.Le Douarin NM, Creuzet S, Couly G. and Dupin E. (2004). Neural crest cell plasticity and its limits. Development 131:4637–4650 [DOI] [PubMed] [Google Scholar]
- 15.Calloni GW, Le Douarin NM. and Dupin E. (2009). High frequency of cephalic neural crest cells shows coexistence of neurogenic, melanogenic, and osteogenic differentiation capacities. Proc Natl Acad Sci U S A 106:8947–8952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McGonnell IM. and Graham A. (2002). Trunk neural crest has skeletogenic potential. Curr Biol 12:767–771 [DOI] [PubMed] [Google Scholar]
- 17.Helms JA. and Schneider RA. (2003). Cranial skeletal biology. Nature 423:326–331 [DOI] [PubMed] [Google Scholar]
- 18.Kaltschmidt B, Kaltschmidt C. and Widera D. (2012). Adult craniofacial stem cells: sources and relation to the neural crest. Stem Cell Rev 8:658–671 [DOI] [PubMed] [Google Scholar]
- 19.Zhang Q, Shi S, Liu Y, Uyanne J, Shi Y. and Le AD. (2009). Mesenchymal stem cells derived from human gingiva are capable of immunomodulatory functions and ameliorate inflammation-related tissue destruction in experimental colitis. J Immunol 183:7787–7798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fournier BP, Ferre FC, Couty L, Lataillade JJ, Gourven M, Naveau A, Coulomb B, Lafont A. and Gogly B. (2010). Multipotent progenitor cells in gingival connective tissue. Tissue Eng Part A 16:2891–2899 [DOI] [PubMed] [Google Scholar]
- 21.Widera D, Zander C, Heidbreder M, Kasperek Y, Noll T, Seitz O, Saldamli B, Sudhoff H, Sader R, Kaltschmidt C. and Kaltschmidt B. (2009). Adult palatum as a novel source of neural crest-related stem cells. Stem Cells 27:1899–1910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Marynka-Kalmani K, Treves S, Yafee M, Rachima H, Gafni Y, Cohen MA. and Pitaru S. (2010). The lamina propria of adult human oral mucosa harbors a novel stem cell population. Stem Cells 28:984–995 [DOI] [PubMed] [Google Scholar]
- 23.Durand E, Fournier B, Couty L, Lemitre M, Achouh P, Julia P, Trinquart L, Fabiani JN, Seguier S, et al. (2012). Endoluminal gingival fibroblast transfer reduces the size of rabbit carotid aneurisms via elastin repair. Arterioscler Thromb Vasc Biol 32:1892–1901 [DOI] [PubMed] [Google Scholar]
- 24.Kobayashi K, Suzuki T, Nomoto Y, Tada Y, Miyake M, Hazama A, Wada I, Nakamura T. and Omori K. (2010). A tissue-engineered trachea derived from a framed collagen scaffold, gingival fibroblasts and adipose-derived stem cells. Biomaterials 31:4855–4863 [DOI] [PubMed] [Google Scholar]
- 25.Zhang QZ, Su WR, Shi SH, Wilder-Smith P, Xiang AP, Wong A, Nguyen AL, Kwon CW. and Le AD. (2010). Human gingiva-derived mesenchymal stem cells elicit polarization of m2 macrophages and enhance cutaneous wound healing. Stem Cells 28:1856–1868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fournier BP, Larjava H. and Hakkinen L. (2013). Gingiva as a source of stem cells with therapeutic potential. Stem Cells Dev 22:3157–3177 [DOI] [PubMed] [Google Scholar]
- 27.Bianco P, Riminucci M, Gronthos S. and Robey PG. (2001). Bone marrow stromal stem cells: nature, biology, and potential applications. Stem Cells 19:180–192 [DOI] [PubMed] [Google Scholar]
- 28.Shi S, Gronthos S, Chen S, Reddi A, Counter CM, Robey PG. and Wang CY. (2002). Bone formation by human postnatal bone marrow stromal stem cells is enhanced by telomerase expression. Nat Biotechnol 20:587–591 [DOI] [PubMed] [Google Scholar]
- 29.Mehrazarin S, Oh JE, Chung CL, Chen W, Kim RH, Shi S, Park NH. and Kang MK. (2011). Impaired odontogenic differentiation of senescent dental mesenchymal stem cells is associated with loss of Bmi-1 expression. J Endod 37:662–666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schipani E, Ryan HE, Didrickson S, Kobayashi T, Knight M. and Johnson RS. (2001). Hypoxia in cartilage: HIF-1alpha is essential for chondrocyte growth arrest and survival. Genes Dev 15:2865–2876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nurminsky D, Magee C, Faverman L. and Nurminskaya M. (2007). Regulation of chondrocyte differentiation by actin-severing protein adseverin. Dev Biol 302:427–437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sobral LM, Montan PF, Martelli-Junior H, Graner E. and Coletta RD. (2007). Opposite effects of TGF-beta1 and IFN-gamma on transdifferentiation of myofibroblast in human gingival cell cultures. J Clin Periodontol 34:397–406 [DOI] [PubMed] [Google Scholar]
- 33.Diekman BO, Christoforou N, Willard VP, Sun H, Sanchez-Adams J, Leong KW. and Guilak F. (2012). Cartilage tissue engineering using differentiated and purified induced pluripotent stem cells. Proc Natl Acad Sci U S A 109:19172–19177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Akiyama H, Chaboissier MC, Martin JF, Schedl A. and de Crombrugghe B. (2002). The transcription factor Sox9 has essential roles in successive steps of the chondrocyte differentiation pathway and is required for expression of Sox5 and Sox6. Genes Dev 16:2813–2828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shah NM, Groves AK. and Anderson DJ. (1996). Alternative neural crest cell fates are instructively promoted by TGFbeta superfamily members. Cell 85:331–343 [DOI] [PubMed] [Google Scholar]
- 36.De Bari C, Dell'Accio F, Tylzanowski P. and Luyten FP. (2001). Multipotent mesenchymal stem cells from adult human synovial membrane. Arthritis Rheum 44:1928–1942 [DOI] [PubMed] [Google Scholar]
- 37.Alsalameh S, Amin R, Gemba T. and Lotz M. (2004). Identification of mesenchymal progenitor cells in normal and osteoarthritic human articular cartilage. Arthritis Rheum 50:1522–1532 [DOI] [PubMed] [Google Scholar]
- 38.Kurth TB, Dell'accio F, Crouch V, Augello A, Sharpe PT. and De Bari C. (2011). Functional mesenchymal stem cell niches in the adult knee joint synovium in vivo. Arthritis Rheum 63:1289–1300 [DOI] [PubMed] [Google Scholar]
- 39.Fischer J, Dickhut A, Rickert M. and Richter W. (2010). Human articular chondrocytes secrete parathyroid hormone-related protein and inhibit hypertrophy of mesenchymal stem cells in coculture during chondrogenesis. Arthritis Rheum 62:2696–2706 [DOI] [PubMed] [Google Scholar]
- 40.von der Mark K, Gauss V, von der Mark H. and Muller P. (1977). Relationship between cell shape and type of collagen synthesised as chondrocytes lose their cartilage phenotype in culture. Nature 267:531–532 [DOI] [PubMed] [Google Scholar]
- 41.Oldershaw RA, Baxter MA, Lowe ET, Bates N, Grady LM, Soncin F, Brison DR, Hardingham TE. and Kimber SJ. (2010). Directed differentiation of human embryonic stem cells toward chondrocytes. Nat Biotechnol 28:1187–1194 [DOI] [PubMed] [Google Scholar]
- 42.Hiramatsu K, Sasagawa S, Outani H, Nakagawa K, Yoshikawa H. and Tsumaki N. (2011). Generation of hyaline cartilaginous tissue from mouse adult dermal fibroblast culture by defined factors. J Clin Invest 121:640–657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Naveau A, Lataillade JJ, Fournier BP, Couty L, Prat M, Ferre FC, Gourven M, Durand E, Coulomb B, Lafont A. and Gogly B. (2011). Phenotypic study of human gingival fibroblasts in a medium enriched with platelet lysate. J Periodontol 82:632–641 [DOI] [PubMed] [Google Scholar]
- 44.Häkkinen LLH. and Fournier BPJ. (2014). Distinct phenotype and therapeutic potential of gingival fibroblasts. Cytotherapy [Epub ahead of print]; DOI: 10.1016/j.jcyt.2014.04.004 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.