Abstract
Gas exchange is constrained by the whole-plant hydraulic conductance (K plant). Leaves account for an important fraction of K plant and may therefore represent a major determinant of plant productivity. Leaf hydraulic conductance (K leaf) decreases with increasing water stress, which is due to xylem embolism in leaf veins and/or the properties of the extra-xylary pathway. Water flow through living tissues is facilitated and regulated by water channel proteins called aquaporins (AQPs). Here we assessed changes in the hydraulic conductance of Populus trichocarpa leaves during a dehydration-rewatering episode. While leaves were highly sensitive to drought, K leaf recovered only 2 hours after plants were rewatered. Recovery of K leaf was absent when excised leaves were bench-dried and subsequently xylem-perfused with a solution containing AQP inhibitors. We examined the expression patterns of 12 highly expressed AQP genes during a dehydration-rehydration episode to identify isoforms that may be involved in leaf hydraulic adjustments. Among the AQPs tested, several genes encoding tonoplast intrinsic proteins (TIPs) showed large increases in expression in rehydrated leaves, suggesting that TIPs contribute to reversing drought-induced reductions in K leaf. TIPs were localized in xylem parenchyma, consistent with a role in facilitating water exchange between xylem vessels and adjacent living cells. Dye uptake experiments suggested that reversible embolism formation in minor leaf veins contributed to the observed changes in K leaf.
Introduction
High gas exchange rates can only be sustained when leaves are kept well hydrated. This, in turn, depends on the properties of the xylem pipeline and on the way in which water moves through living cells in roots and leaves [1], [2]. Leaf hydraulic conductance is emerging as an important component of whole-plant hydraulic conductance [3]–[7]. Like in roots and stems, the hydraulic conductance of leaves declines as the water potential becomes more negative. This loss of hydraulic conductance is due to embolism formation in leaf veins [8], [9], cell shrinkage [10], collapse of xylem conduits [11], and/or to decline in the permeability of extra-xylary tissues [12]. Compared with stems, leaves [13] and roots [14] are often more vulnerable to hydraulic dysfunction. In some cases, however, the hydraulic conductance of these plant organs may also be able to quickly recover from the effects of drought [3], [15].
This recovery of hydraulic function may be facilitated by the activity of aquaporin (AQP) water channels [16]–[20]. AQPs belong to the major intrinsic protein (MIP) superfamily, a family of protein pores present in the membranes of almost all biological cells to facilitate the diffusion of a wide range of small uncharged solutes. Plant MIPs form a particularly large family of proteins, with 28 members in Vitis vinifera [21], ≥30 members in Arabidopsis thaliana, Picea glauca and Oryza sativa [19], [22], [23], and >50 members in Populus trichocarpa [24]. The plant-specific plasma membrane intrinsic proteins (PIPs), with their highly conserved phylogenetic subgroups PIP1 and PIP2, and tonoplast intrinsic proteins (TIPs) show significant water transport activity in vitro and in planta [25]–[27]. Regulation of AQPs via transcription, translation, post-translational modifications or trafficking allows plant cells and organs to respond to hydraulic changes in their surrounding environment [28].
In this present study, Populus trichocarpa plants were exposed to moderate drought and then rewatered. The objective was to study the recovery of K leaf from water stress at both physiological and molecular levels. We hypothesized that leaves would quickly (i.e., within hours) recover from water stress, and that this would be associated with modulation of AQP activity. To test this hypothesis, we monitored K leaf and Ψleaf during a dehydration-rehydration episode. We also explored the regulation of 12 leaf-expressed AQP isoforms as well as the tissue-specific location of PIP1, PIP2 and TIP2 proteins. Recovery of K leaf was assessed in two ways: (i) intact plants were taken through a drying-rewatering cycle, and (ii) detached leaves were bench-dried and subsequently xylem-perfused with AQPs inhibitors.
Materials and Methods
Plant material and growing conditions
All experiments were carried out with P. trichocarpa clone 664042 cuttings (mother tree planted in Lotbinière, Québec, Canada from a 1973 IUFRO progeny collection in Washougal, Oregon, USA). Rooted cuttings were produced and established in a greenhouse at the University of Alberta for 2 months in 3.8 L containers with sunshine mix 4 (Sun Gro Horticulture Canada Ltd.) under semi-controlled conditions (22/20°C day: night cycle, 18/6 h light: dark, watered daily, and fertilized (2g L-1 NPK15-30-15) once a week).
Leaf hydraulic conductance measurements
Leaf hydraulic conductance was measured using the evaporative flux method (29) on six plants per treatment. A filtered (0.2 µm) 20 mM KCl+1 mM CaCl2 solution (subsequently referred to as ‘artificial xylem sap’, AXS) was used for these measurements. Flow rate through leaves was measured with a balance (model CP 224S, Sartorius, Göttingen, Germany), which logged data every 30 s to a computer. The air was well stirred by a fan as explained by Sack & Scoffoni [29]. Leaves were illuminated with ∼1000 µmol m−2 s−1 photosynthetically active radiation (PAR) at the leaf surface by an LED worklight (Husky, distributed by Home Depot, Atlanta, GA, USA). Leaf temperature was monitored by a thermocouple. Leaf water potential (Ψleaf) was measured using a pressure chamber (PMS Instruments, Albany, OR, USA). For hydrated leaves, the K leaf was calculated as described previously [29] using the final Ψleaf (Ψfinal), which was determined at the end of the measurement of E, immediately after removing the leaf from the tubing system. The K leaf was normalized by leaf area, which was determined with a scanner.
A leaf vulnerability curve was generated with plants experiencing different levels of water stress following methods of Sack & Scoffoni [29]. K leaf was measured on leaves corresponding to leaf plastochron index (LPI) 9 [30]. The initial Ψleaf (ΨO) was measured using a leaf immediately above or below the leaf that was subsequently connected to the tubing system. Once a leaf was connected to the tubing system, a stable flow rate (E) was usually reached in less than 20 minutes; this value of E was subsequently used for calculating K leaf. Leaves that did not provide stable E within 20 min were discarded. When dehydrated leaves are measured with the EFM, their Ψleaf may change because the petiole is connected to water at atmospheric pressure [3]. To test for this, Ψfinal and ΨO were compared. In most cases, ΨO and Ψfinal were similar and the more negative of these two values was used to calculate K leaf. When ΨO and Ψfinal differed by more than 0.2 MPa, the leaf was discarded and K leaf was not calculated. The leaf vulnerability curve (shown in Fig. 1) was fitted with a 3 parameter logistic function: K leaf = a/[1+(Ψleaf/x0)b]. The curve fit was done using SigmaPlot v. 13 (Systat Software, San Jose, CA, USA). The curve fit was used to calculate the maximum leaf hydraulic conductance as well as the leaf water potentials at 50% and 80% loss of hydraulic conductance.
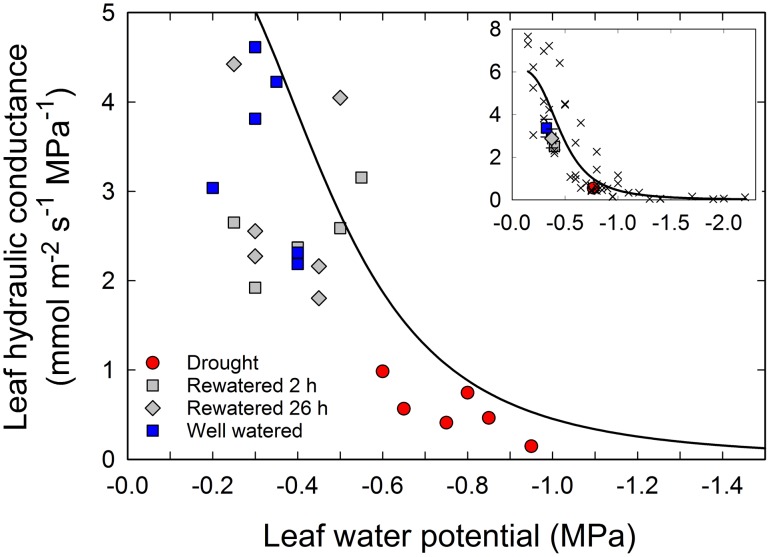
Figure 1. Effect of a change in water availability on leaf hydraulic conductance (K leaf) in Populus trichocarpa saplings.
K leaf and the associated leaf water potential (Ψleaf) were measured in 6 well-watered control plants (blue squares), 6 drought-stressed plants (red circles), and drought-stressed plants 2 and 26 h after rewatering (grey squares and diamonds, respectively). Each data point represents a single measurement of K leaf. The solid line shows the previously established vulnerability curve for K leaf. A 3 parameter logistic function was used for the curve fit: K leaf = 6.154/[1+(Ψleaf/0.469)3.337]. An overview of the complete vulnerability curve is shown in the upper right corner of the figure. Individual measurements are shown as crosses; the mean values for each group (±SE, n = 6) are shown using the same symbols as explained above.
Recovery of leaf hydraulic conductance after dehydration
To study the recovery of K leaf in intact plants, plants were randomly assigned to different watering regimes in the greenhouse. One group of plants was kept well watered (control). Another group of plants was subjected to a drought treatment. Water was withheld for several days until plants reached a Ψleaf of −0.77±0.05 MPa (mean ± SE, n = 6). This Ψleaf was associated with a substantial reduction in K leaf. A subset of drought-stressed plants was then rewatered, and Ψleaf and K leaf were re-measured 2 h and 26 h after rewatering.
To assess the effect of AQP inhibitors and abscisic acid (ABA) on the recovery of K leaf, excised leaves were bench-dried for 1 h (Ψleaf reached −1.00±0.09 MPa (mean ± SE, n = 6)) and then perfused for 2 h with AXS, AXS+0.2 mM HgCl2, AXS+50 mM H2O2 or AXS+50 µM ABA. Solutions were introduced into the transpiring leaf by immersing the petiole inside 50 mL containers. Leaves were placed near a fan; light was provided at a light level of ∼1,000 µmol m−2 s−1 PAR. Mercury chloride and H2O2 have been widely used as AQP inhibitors; ABA may also reduce AQP activity in leaves (reviewed in 28; 12). Control leaves were always kept hydrated and were perfused with pure AXS for 2 h. Immediately after perfusion with these solutions, K leaf was determined as described above. All measurements were conducted at the same time of day (10∶00–11∶30 h).
After perfusion with the different solutions, stomatal pore aperture of leaves was measured as described in Laur & Hacke [31]. Images were recorded in six randomly selected fields of view of each leaf. Fields of view were located near the point of maximum leaf width on the abaxial leaf surface.
Dye uptake experiments
The extent of dye uptake in excised leaves was used as an additional method to assess xylem refilling in leaf veins during the rehydration phase. We also used the dye uptake experiments in an attempt to study how embolism reversal in leaf veins is impacted by mercury. Excised leaves were bench-dried for 1 h and rehydrated for 2 h by immersion of the petioles in filtered safranin solutions. Transpiration during dye uptake was promoted by placing leaves near a fan at a light level of ∼1,000 µmol m−2 s−1 PAR (i.e., conditions similar to the protocol used to measure K leaf). Dye (0.1% (w/v) safranin) was dissolved in pure AXS or AXS+0.2 mM HgCl2. Control leaves were excised from well-watered plants and then perfused for 2 h with 0.1% safranin-containing AXS without prior dehydration treatment. Images were recorded in six randomly selected fields of view of each leaf. Fields of view were located near the point of maximum leaf width on the abaxial leaf surface.
Gene transcript measurements by quantitative real-time PCR
Fully expanded leaves corresponding to LPI 7–10 were collected, immediately frozen in liquid nitrogen and stored at −80°C until analyzed. Samples were always collected between 10∶00 h and 11∶30 h to minimize any diurnal effect on AQP expression. Total RNA was extracted from 3 plants per treatment following the CTAB method of Pavy et al. [32]. RNA quality was assessed on an agarose gel and quantified with a spectrophotometer (Nanodrop ND-1000, Thermo Scientific, Wilmington, DE, USA). RNA was treated as previously described [19]. cDNA quality was checked by PCR with intron-spanning actin primers. Putative leaf-expressed AQP genes were selected [33]–[35], specific primers (Table S1) were designed according to Rutledge & Stewart [36] using the QuantPrime online tool [37]. PCR efficiency was 100±7% for all primer pairs and specificity was checked using melting curves. Real-time qPCR was performed on a 7900 HT Fast Real-Time PCR system (Applied Biosystems, Foster City, CA, USA) as described previously [31]. Relative gene expression was measured according to Livak & Schmittgen [38] using the 2ΔΔC(t) method. The expression values were normalized to the geometric mean of four housekeeping genes (actin (POPTR_0001s31700), cyclophilin (POPTR_0005s26170), TIP4-like (POPTR_0009s09620.1) and ubiquitin (POPTR_0005s09940)). Relative gene expression was determined as the fold change of an AQP isoform at a given condition relative to its expression under control conditions. Real-time PCR was carried out using three biological replicates each with three technical replicates.
Immunolocalization
Samples were fixed in formaldehyde-acetic acid and embedded in paraffin as described previously [39]. Transverse sections, 10 µm thick, were prepared with a microtome. Immunoreactions were performed following the protocol of Gong et al. [40]. Primary antibodies directed against the 42 N-terminal amino acids of AtPIP1;3 [41] and the conserved 10 amino acids of the C-terminal of PIP2s [19] were used. In addition, we applied a commercially available anti-TIP2 antibody (Sakurai et al. [42]); Agrisera AB, Sweden; alignment shown in Figure S1). AlexaFluo 488-conjugated goat anti-chicken, anti-mouse and anti-rabbit secondary antibodies (Life Technologies Inc., Burlington, ON, Canada) were applied respectively for 2 h at 37°C. Slides were mounted with Permount. Images were taken with a Zeiss LSM 700 confocal microscope (Carl Zeiss, Oberkochen, Germany).
Statistical analysis
All statistical analyses were carried out using SigmaPlot software. Differences due to the effect of physiological treatments were analyzed after testing for normality and equal variance by using a one-way ANOVA followed by a Tukey’s test. A one-way ANOVA followed by Bonferroni’s post test was used for the gene expression analysis. Differences were considered significant at P≤0.05.
Results and Discussion
Leaf hydraulic conductance is highly sensitive to drought
To assess how K leaf declines as a function of Ψleaf, we first constructed a vulnerability curve. Water was withheld from plants in the greenhouse until plants reached different levels of water stress. Leaves were highly vulnerable with 50% and 80% loss of hydraulic conductance occurring at Ψleaf = −0.47 MPa and −0.71 MPa, respectively (Figure 1, insert). According to the curve fit in Figure 1 (insert), the maximum K leaf measured during this experiment was 6.15 mmol m−2 s−1 MPa−1. The drought-induced loss in K leaf shown in Figure 1 may have been due to xylem cavitation, reduced water permeability of cell membranes and/or other factors [6], [7]. The water potentials at 50% and 80% loss of hydraulic conductance (P 50 and P 80, respectively) are well within the range of water potentials that trees experience under natural conditions [43], [44]. It therefore appears that K leaf is subject to substantial diurnal changes under natural conditions, similar to what has been observed in rice and other species [3], [15], [45], [46]. Our data also indicates that leaf hydraulic conductance is more sensitive to decreasing water potentials than the hydraulic conductance of stems [43]. However, since we only worked with young greenhouse-grown plants, it remains to be seen whether leaves of field-grown trees are similar in their response to water stress.
Leaves of intact plants quickly recover from drought
We next tested whether K leaf would recover after a drought treatment when plants were left intact during the dehydration-rehydration episode. In this experiment, leaves of well-watered control plants had a Ψleaf of −0.33±0.03 MPa (±SE, n = 6), which was associated with a K leaf of 3.37±0.41 mmol m−2 s−1 MPa−1 (±SE, n = 6) (Figure 1, blue squares). The drought treatment resulted in a drop of Ψleaf to −0.77±0.05 MPa (± SE, n = 6) and a six-fold drop of K leaf to 0.55±0.12 mmol m−2 s−1 MPa−1 (±SE, n = 6) (Figure 1, red circles). These values were in good agreement with the previously established vulnerability curve (Figure 1, insert). Only 2 h after rewatering (Figure 1, grey squares), both Ψleaf and K leaf reached values that were not statistically different from well-watered control plants (t test, P = 0.083 for K leaf), indicating that leaves completely recovered their hydraulic function.
AQP expression in leaves collected from intact plants
To study the role of water channels in the recovery of K leaf, AQP expression was measured in leaves at different stages during the dehydration-rehydration experiment. Three PIP1, three PIP2, and six TIP candidate genes were selected for analysis. Among them, PtPIP1;1, PtPIP1;2, PtPIP1;3; PtPIP2;4 and PtTIP2;1 exhibited the highest total number of mRNA molecules in leaves of control plants (Table 1).
Table 1. Transcript abundance of 12 aquaporin genes expressed in leaves of well-watered control plants.
| Aquaporin name | Expression (copies µg−1 of total RNA) |
| PtPIP1;1 | 112,960±9,067 |
| PtPIP1;2 | 272,111±32,575 |
| PtPIP1;3 | 229,960±44,252 |
| PtPIP2;3 | 85,667±15,402 |
| PtPIP2;4 | 273,655±33,728 |
| PtPIP2;5 | 11,536±1,738 |
| PtTIP1;3 | 23,105±2,540 |
| PtTIP1;5 | 11,840±1,675 |
| PtTIP1;6 | 2,330±121 |
| PtTIP2;1 | 153,689±19,669 |
| PtTIP2;2 | 24,863±3,451 |
| PtTIP4;1 | 517±9 |
Values are the means ± SE from three biological samples which were tested in triplicate.
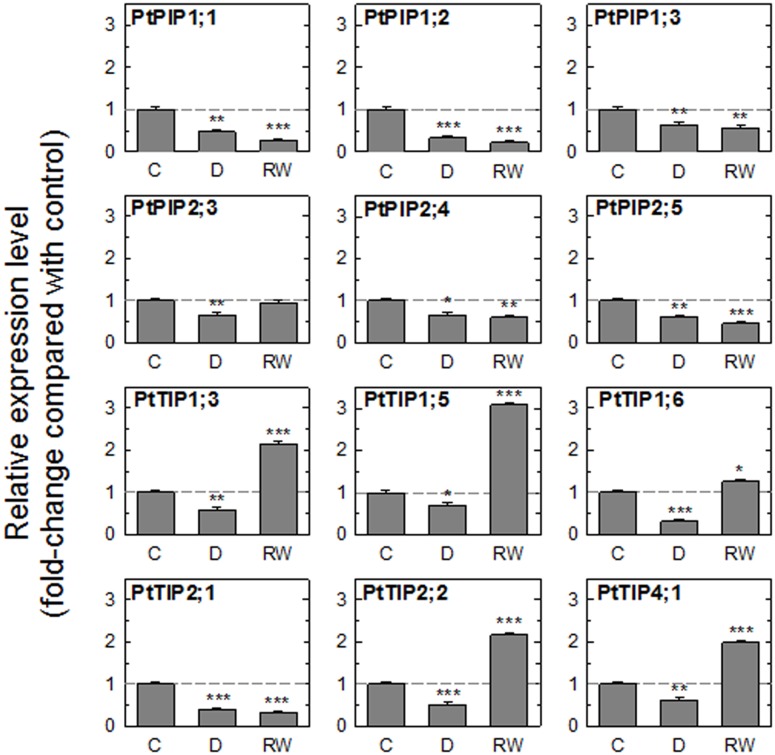
The drought treatment resulted in a significant reduction in the expression of all tested genes (Fig. 2). In leaves collected 2 h after rewatering, there were two patterns of expression between the 12 isoforms. One group of genes (among them all PIP1s) remained down-regulated while the expression of a second group of genes matched or exceeded the transcript levels measured in control leaves. With the exception of PtTIP2;1, all tested TIPs were significantly up-regulated after 2 h. Among the PIPs, only the expression level of PtPIP2;3 increased to match the control level.
Figure 2. Relative expression of aquaporin genes in leaves of plants exposed to a drying-rewatering cycle.
Gene expression was measured in leaves of well-watered control plants (C), drought-stressed plants (D), and 3 h after drought-stressed plants were rewatered (RW). The geometric mean of the expression levels of four reference genes (ACT2, CYC063, TIP41-like, UBQ7) was used to normalize the results. Asterisks denote significant differences in expression level compared to control levels (one-way ANOVA, followed by Bonferroni’s post test, *P≤0.05; **P≤0.01***P≤0.001). Data are means ± SE of three biological replicates.
Recovery of K leaf in detached leaves is impaired by inhibitors
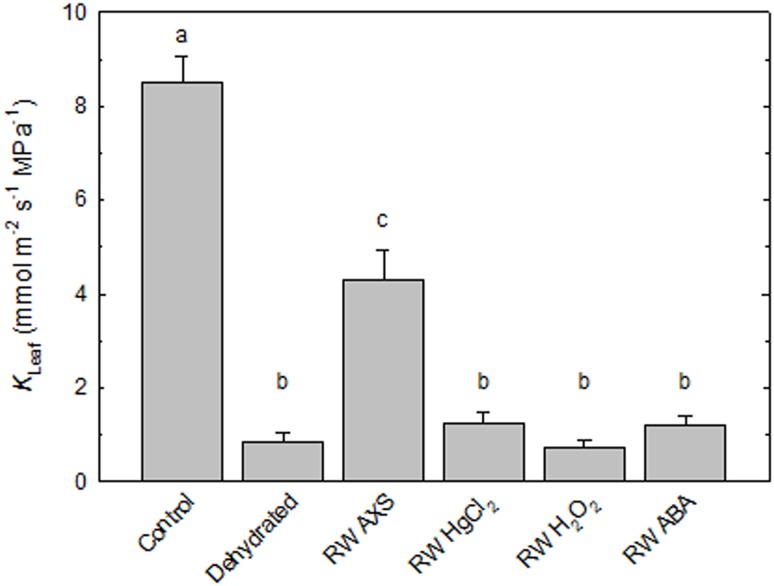
Another set of experiments was conducted on leaves that were excised from the plant prior to the dehydration-rehydration treatment. Working with detached leaves allowed us to study the effect of AQP inhibitors and ABA on the recovery of K leaf. Control leaves exhibited a K leaf of 8.49±0.57 mmol m−2 s−1 MPa−1 (±SE, n = 6), which is higher than the values shown in Figure 1. One difference between the data shown in Figures 1 and 3 is that all data in Figure 1 was derived from leaves that were excised (petioles were cut under water) from transpiring plants immediately before K leaf was measured while the control leaves in Figure 3 were perfused with AXS for 2 h prior to measuring K leaf. Hence, the absolute K leaf values shown in Figures 1 and 3 are not readily comparable.
Figure 3. Response of leaf hydraulic conductance (A) and stomatal aperture (B) to different perfusion solutions.
Control conditions refer to the K leaf that was measured after leaves were xylem perfused with filtered (0.2 µm) 20 mM KCl+1 mM CaCl2 solution (subsequently referred to as ‘artificial xylem sap’, AXS) for 2 h. K leaf was also measured on leaves that were bench-dried for 1 h (Dehydrated) and on leaves that were bench-dried for 1 h and subsequently perfused for 2 h with AXS (RW AXS), AXS+0.2 mM HgCl2 (RW HgCl2), AXS+50 mM H2O2 (RW H2O2) or AXS+50 µM ABA (RW ABA). Values are means ± SE (n = 6). Different letters denote statistically significant differences by one-way ANOVA with Tukey’s test.
Bench-drying of leaves caused a ∼10-fold decline in K leaf relative to fully hydrated control leaves (Figure 3A). Dehydrated leaves that were subsequently xylem-perfused for 2 h with AXS exhibited a significant recovery to 50% of the hydraulic conductance measured in control leaves. The fact that recovery remained incomplete in detached leaves is consistent with an involvement of phloem transport in embolism repair [47], [48]. Application of commonly used inhibitors allowed us to assess the impact of AQPs on K leaf during leaf rehydration. Leaves fed with HgCl2 and H2O2 did not exhibit any recovery of hydraulic conductance, indicating that AQPs were involved in the recovery of K leaf after dehydration. A role of AQPs in embolism repair has also been proposed for other species and plant organs [18], [19], [49]–[51].
We next asked whether differences in K leaf were associated with different degrees of stomatal closure. Stomatal apertures in fully hydrated control leaves were 9.5±0.1 µm (±SE, n = 6), similar to the value of ∼10 µm previously reported for P. trichocarpa leaves [52]. Schulte and Hinckley [52] found that stomatal aperture in this species was not affected by a wide range of epidermal water potentials. Our data supports these findings as we also did not observe complete stomatal closure in any of our experimental treatments (Figure 3B). Even in dehydrated leaves and in leaves that were perfused with AQP inhibitors and ABA, stomatal aperture remained at ∼6 µm. This value is similar to the maximum apertures found in P. deltoides and in P. trichocarpa x deltoides hybrids [31], [52]. We conclude that the magnitude of the decline in K leaf in dehydrated leaves and in leaves that were perfused with AQP inhibitors is greater than that of changes in stomatal aperture. Guyot et al. [53] also found a discrepancy between patterns of stomatal conductance and K leaf, and they discuss possible reasons for a mechanistic independence of stomatal and leaf hydraulic conductance.
We used the dye uptake experiments in an attempt to study how embolism reversal in leaf veins is impacted by mercury. Nearly all veins of well-watered control leaves were stained and functional (Figure 4A). In leaves that were bench-dried and subsequently supplied with ASX + safranin for 2 h, many minor veins exhibited incomplete staining (Figure 4B). Staining was even less complete in leaves that were bench-dried and subsequently perfused with ASX + safranin + HgCl2 (Figure 4C).
Figure 4. Typical images of transpiring P. trichocarpa leaves that were allowed to take up safranin solution.
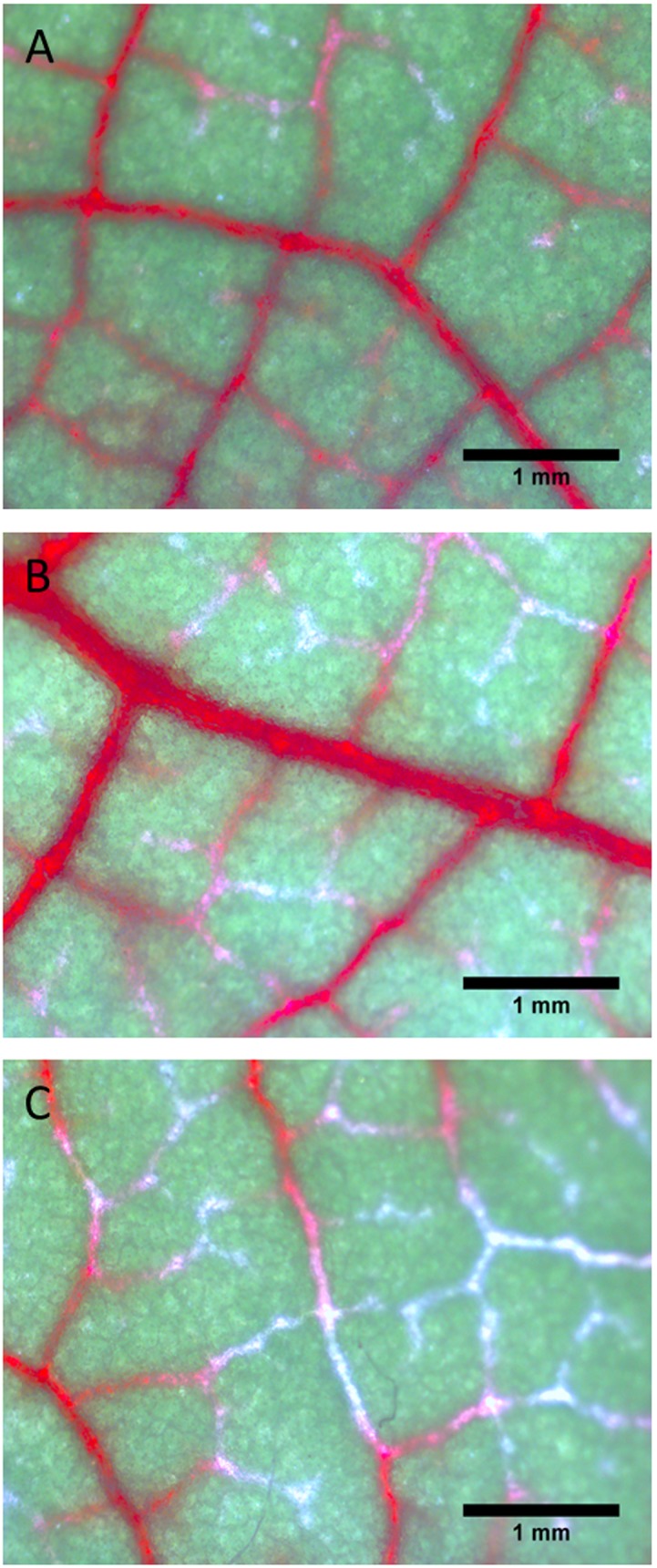
(A) A control leaf was excised from a well-watered plant, and the petiole was immersed for 2 h in safranin solution. Transpiration during dye uptake was promoted by placing the leaf near a fan at ∼1,000 µmol m−2 s−1 photosynthetic active radiation. Most leaf veins were stained indicating minimal xylem embolism. (B) Dye uptake in a bench-dried leaf that was subsequently perfused with safranin solution for 2 h. Minor veins exhibited incomplete staining indicating the presence of embolized xylem conduits in minor veins. (C) Dye uptake of a bench-dried leaf subsequently perfused with safranin + HgCl2 solution for 2 h. Mercury is an aquaporin inhibitor. Staining remained even more incomplete than in (B).
These findings suggest that embolism formation in minor veins had a substantial impact on the dynamics of K leaf. Studying water transport in rice leaves, Stiller et al. [8] reported that the leaf xylem experienced high embolism levels, even in watered controls. Nardini et al. [54] found that minor veins of Cercis siliquastrum leaves underwent extensive embolism at leaf water potentials <−1.5 MPa, indicating that leaf vein embolism was closely related to K leaf changes. Recently, Johnson et al. [55] suggested that reductions in K leaf are directly related to vein embolism. On the other hand, a recent study found that hydraulic decline during mild dehydration was associated with leaf shrinkage [10]. The changes in K leaf we observed in this present study were likely caused by xylem and extra-xylem components; it is difficult to determine the relative importance of either component. In addition, both xylem refilling in leaf veins and the permeability of extra-xylem tissues may be impacted by AQP function.
AQP expression in detached leaves
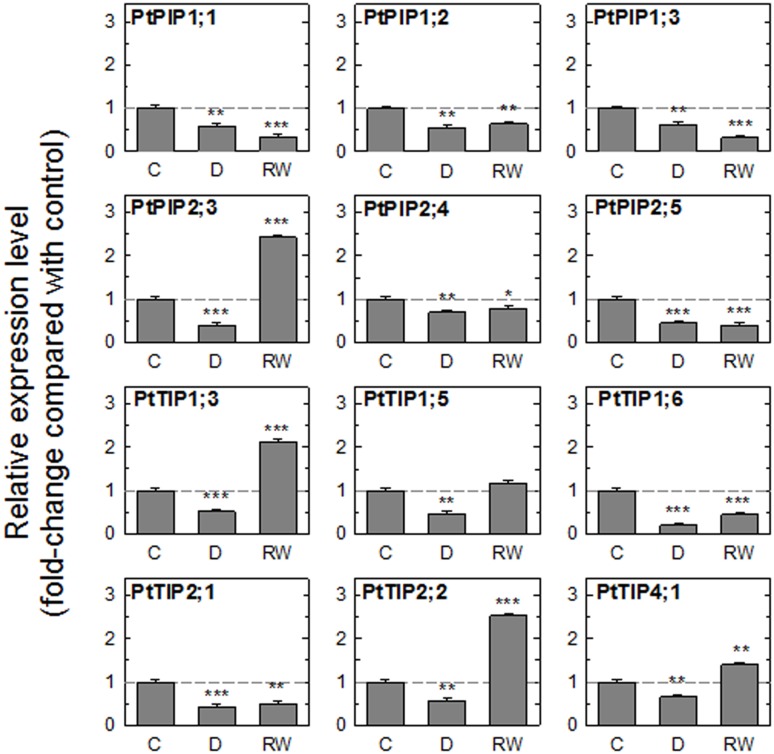
Aquaporin expression was measured in detached leaves undergoing a dehydration-rehydration cycle (Figure 5). Control leaves were perfused with AXS for 2 h before leaf tissue was sampled for the gene expression analysis. As previously seen in intact plants (Figure 2), water stress caused down-regulation of all tested AQPs (Figure 5). This agrees with several previous studies [19], [56], [57].
Figure 5. Relative expression of aquaporin genes in detached leaves during a dehydration-rehydration experiment.
Data are from control leaves (C) after they were perfused with artificial xylem sap (AXS) for 2 h, leaves that were dehydrated on the bench top for 1 h (D), and leaves that were dehydrated on the bench top for 1 h and then perfused for 2 h with AXS (RW). The geometric mean of the expression levels of four reference genes (ACT2, CYC063, TIP41-like, UBQ7) was used to normalize the results. Asterisks denote significant differences in expression level compared to control levels (one-way ANOVA, followed by Bonferroni’s post test, *P≤0.05; **P≤0.01***P≤0.001). Data are means ± SE of three biological replicates.
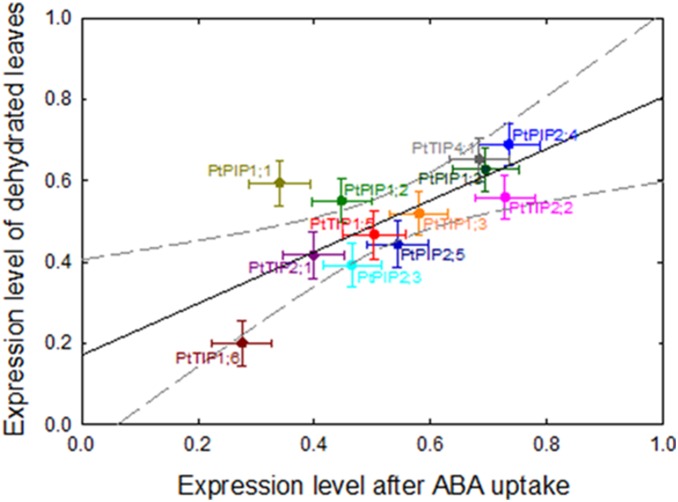
Notably, very similar degrees of down-regulation were found in bench-dried leaves and in dried leaves that were subsequently xylem-perfused with AXS + ABA (Figure 6, r = 0.725, P<0.01). Genes that were strongly down-regulated by dehydration, such as PtTIP1;6 also exhibited strong down-regulation after perfusion with ABA solution while the expression of other genes, such as PtPIP2;4, changed less in response to either of these factors (Figure 6). Excluding PtPIP1;1 from the analysis shown in Figure 6 further increased the strength of the linear relationship (r = 0.89, P<0.001).
Figure 6. Relative expression of 12 aquaporin genes in response to dehydration (y-axis) and dehydration + perfusion with abscisic acid (x-axis).
Detached leaves were either dehydrated on the bench top for 1 h or dehydrated for 1 h and subsequently perfused for 1 h with 50 µM abscisic solution (ABA). Data from fully hydrated detached leaves (perfused for 3 h with 20 mM KCl+1 mM CaCl2 solution) were used as the control group, and their expression refers to a value of 1. Pearson’s r = 0.725; P≤0.01. Data are means ± SE of three biological replicates.
The lack of recovery in ABA-perfused leaves and down-regulation of AQPs in leaves supplied with AXS + ABA is consistent with the model of Shatil-Cohen et al. [12]. Working with Arabidopsis, these authors also used a ‘detached leaf’ approach to feed ABA to the xylem via the petiole. Feeding the leaf with ABA decreased K leaf by nearly 50%. In contrast, smearing ABA on the leaf surface, while reducing transpiration, had no effect on K leaf. Shatil-Cohen et al. [12] proposed that the membrane water permeability of bundle sheath cells is controlled by AQPs, and that the bundle sheath would act like a control center regulating K leaf in response to signals from the xylem. As the concentration of ABA increases in the xylem, AQP activity in the bundle sheath would be down-regulated, reducing water flow into the leaf mesophyll. Bundle sheath cells, and perhaps xylem parenchyma cells, seem to have a specific responsiveness to ABA, which likely explains the negative effects of this hormone on K leaf (for a recent review see 7). While our data is consistent with these observations, it is not clear yet which cells may perform the role of a ‘control center’ in P. trichocarpa leaves (Figure S2). While we previously observed prominent PIP1 and PIP2 labeling of the endodermis-like bundle sheath in Picea glauca needles [19], no such pattern was found in this present study.
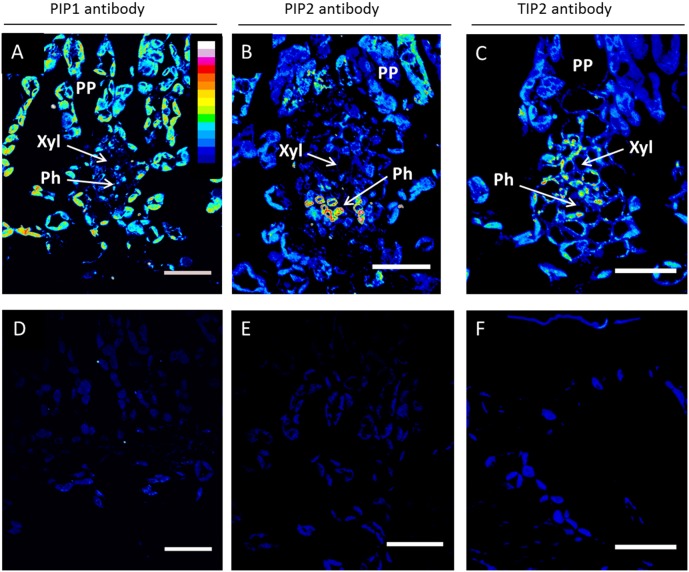
In rehydrated leaves, four genes showed increased expression levels relative to control leaves (Figure 5). Three of these AQPs (PtTIP1;3, PtTIP2;2, and PtTIP4;1) were TIPs and were also found to be up-regulated when intact plants were rewatered after a drought (compare Figures 2 and 5). While TIPs have rarely been studied in the context of water flow through tissues and embolism repair, a recent study on grapevine plants found a striking positive correlation between K leaf and the transcript abundance of VvTIP2;1 [58]. Our immunolocalization experiments indicate that TIP2 protein was present in xylem parenchyma cells (Figure 7). This agrees with the expression pattern of ZmTIP1 in leaves and stems of maize. In situ localization revealed that this tonoplast AQP was highly expressed in parenchyma cells surrounding xylem vessels, in phloem companion cells, and between the phloem and the xylem strands [59]. Barrieu et al. [59] hypothesized that the high expression of the ZmTIP1 tonoplast AQP in xylem parenchyma cells would allow these cells to control water movement in and out of the xylem vessels. Daniels et al. [60] found that AtTIP2 expression in mature leaves was generally restricted to vascular tissues. In stem xylem of hybrid poplar, a TIP2 AQP was highly expressed in contact cells, suggesting a role in increasing water exchange between vessels and xylem rays [61].
Figure 7. Immunolocalization of AQP proteins in leaves of P. trichocarpa saplings.
Confocal laser scanning micrographs showing the localization of PIP1, PIP2, TIP2 proteins in minor veins of leaf transverse sections (A, B, C respectively). Controls with no primary antibody indicate minimal background fluorescence (D, E, F respectively). Images were taken at an identical setting and were color-coded with an intensity look-up-table (LUT; displayed in A), in which black was used to encode background, and blue, green, yellow, red and white to encode increasing signal intensities. Ph, phloem; PP, palisade parenchyma; Xyl, xylem. Scale bars = 20 µm.
In this present study, we also determined the cell- and tissue-level localization of PIP1 and PIP2 proteins (Figure 7). All sections were taken from leaves of well-watered plants. Strong PIP1 signals were present in the palisade parenchyma (Figure 7A). PIP1 antibody was also detected in vein cells, including phloem and xylem parenchyma. This labeling pattern is consistent with a dual role of PIP1s in influencing permeability to water and CO2 [62]. PIP2 was mostly localized in the phloem, which agrees with previous studies [19], [27], [61], [63], [64]. Weaker PIP2 labelling was evident in palisade parenchyma cells (Figure 7B).
Conclusions
We studied how AQPs may be involved in the recovery of water stress-induced declines in K leaf. We examined how K leaf responds to known AQP inhibitors and xylem-fed ABA. We also examined the expression of 12 highly expressed AQP genes during dehydration-rehydration experiments. Hydraulic measurements and gene expression assays were complemented by dye uptake and immunolocalization experiments. This has revealed that, while P. trichocarpa leaves are highly sensitive to dehydration, leaf hydraulic conductance can quickly recover when water becomes available again. Recovery of K leaf was absent when excised leaves were xylem-perfused with AQP inhibitors, suggesting that the recovery of leaf hydraulic function is associated with AQP activity. Among the AQPs tested, several TIPs showed large increases in expression in rehydrated leaves, suggesting that TIPs play an important role in reversing drought-induced reductions in K leaf.
Supporting Information
(a) Amino acid multiple sequence alignment of the N-terminal region of the Arabidopsis thaliana AtPIP1;3 and the Populus trichocarpa PtPIP1s; (b) of the conserved the C-terminal region of PIP2s, and (c) TIP2s. Consensus amino acids are underlined in black.
(DOCX)
Transverse section of a Populus trichocarpa leaf showing minor veins with (left) and without (right) bundle sheath cell extensions. Scale bar = 20 µm.
(DOCX)
Primer sequences used for the gene expression study.
(DOCX)
Acknowledgments
We are grateful to Dr. Barb R. Thomas (University of Alberta - Alberta-Pacific Forest Industries, Canada) and Pierre Périnet (Québec Ministry of Forests) for providing the plant material.
Data Availability
The authors confirm that all data underlying the findings are fully available without restriction. All relevant data are within the paper and its Supporting Information files.
Funding Statement
U. G. H. acknowledges funding from a Natural Sciences and Engineering Research Council (NSERC) Discovery grant, the Canada Foundation for Innovation and the Canada Research Chair program. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Sperry JS, Hacke UG, Oren R, Comstock JP (2002) Water deficits and hydraulic limits to leaf water supply. Plant, Cell Environ 25(2): 251–63. [DOI] [PubMed] [Google Scholar]
- 2. Tyree MT, Sperry JS (1988) Do woody plants operate near the point of catastrophic xylem dysfunction caused by dynamic water stress? answers from a model. Plant Physiol (88): 574–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Scoffoni C, McKown AD, Rawls M, Sack L (2012) Dynamics of leaf hydraulic conductance with water status: quantification and analysis of species differences under steady state. J Exp Bot 63: 643–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Nardini A, Luglio J (2014) Leaf hydraulic capacity and drought vulnerability: possible trade-offs and correlations with climate across three major biomes. Funct Ecol 28(4): 810–818. [Google Scholar]
- 5. Brodribb TJ, Holbrook NM (2004) Stomatal protection against hydraulic failure: a comparison of coexisting ferns and angiosperms. New Phytol 162(3): 663–70. [DOI] [PubMed] [Google Scholar]
- 6. Heinen RB, Ye Q, Chaumont F (2009) Role of aquaporins in leaf physiology. J Exp Bot 60(11): 2971–85. [DOI] [PubMed] [Google Scholar]
- 7. Prado K, Maurel C (2013) Regulation of leaf hydraulics: from molecular to whole plant levels. Front Plant Sci 4: 255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Stiller V, Lafitte HR, Sperry JS (2003) Hydraulic properties of rice and the response of gas exchange to water stress. Plant Physiol 132: 1698–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Johnson DM, McCulloh KA, Meinzer FC, Woodruff DR, Eissenstat DM (2011) Hydraulic patterns and safety margins, from stem to stomata, in three eastern U.S. tree species. Tree Physiol 31(6): 659–68. [DOI] [PubMed] [Google Scholar]
- 10. Scoffoni C, Vuong C, Diep S, Cochard H, Sack L (2014) Leaf shrinkage with dehydration: coordination with hydraulic vulnerability and drought tolerance. Plant Physiol 164(4): 1772–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Brodribb TJ, Holbrook NM (2005) Water stress deforms tracheids peripheral to the leaf vein of a tropical conifer. Plant Physiol 137: 1139–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Shatil-Cohen A, Attia Z, Moshelion M (2011) Bundle-sheath cell regulation of xylem-mesophyll water transport via aquaporins under drought stress: a target of xylem-borne ABA? Plant J 67(1): 72–80. [DOI] [PubMed] [Google Scholar]
- 13. Brodribb TJ, Holbrook NM, Edwards EJ, Gutiérrez MV (2003) Relations between stomatal closure, leaf turgor and xylem vulnerability in eight tropical dry forest trees. Plant, Cell Environ 26: 443–50. [Google Scholar]
- 14. Hacke UG, Sperry JS, Ewers BE, Ellsworth DS, Schäfer KVR, et al. (2000) Influence of soil porosity on water use in Pinus taeda . Oecologia 124(4): 495–505. [DOI] [PubMed] [Google Scholar]
- 15. Stiller V, Sperry JS, Lafitte R (2005) Embolized conduits of rice (Oryza sativa, Poaceae) refill despite negative xylem pressure. Am J Bot 92(12): 1970–4. [DOI] [PubMed] [Google Scholar]
- 16. Galmés J, Pou A, Alsina MM, Tomàs M, Medrano H, et al. (2007) Aquaporin expression in response to different water stress intensities and recovery in Richter-110 (Vitis sp.): relationship with ecophysiological status. Planta 226(3): 671–81. [DOI] [PubMed] [Google Scholar]
- 17. Jang HY, Yang SW, Carlson JE, Ku YG, Ahn SJ (2013) Two aquaporins of Jatropha are regulated differentially during drought stress and subsequent recovery. J Plant Physiol 170(11): 1028–38. [DOI] [PubMed] [Google Scholar]
- 18. Martre P, Morillon R, Barrieu F, North GB, Nobel PS, et al. (2002) Plasma membrane aquaporins play a significant role during recovery from water deficit. Plant Physiol 130(4): 2101–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Laur J, Hacke UG (2014) Exploring Picea glauca aquaporins in the context of needle water uptake and xylem refilling. New Phytol 203 (2): 388–400. [DOI] [PubMed] [Google Scholar]
- 20. North GB, Martre P, Nobel PS (2004) Aquaporins account for variations in hydraulic conductance for metabolically active root regions of Agave deserti in wet, dry, and rewetted soil. Plant, Cell Environ 27(2): 219–28. [Google Scholar]
- 21. Fouquet R, Léon C, Ollat N, Barrieu F (2008) Identification of grapevine aquaporins and expression analysis in developing berries. Plant Cell Rep 27(9): 1541–50. [DOI] [PubMed] [Google Scholar]
- 22. Quigley F, Rosenberg JM, Shachar-Hill Y, Bohnert HJ (2002) From genome to function: the Arabidopsis aquaporins. Genome Biol 3(1): research0001.1–research0001.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Sakurai J, Ishikawa F, Yamaguchi T, Uemura M, Maeshima M (2005) Identification of 33 rice aquaporin genes and analysis of their expression and function. Plant Cell Physiol 46(9): 1568–77. [DOI] [PubMed] [Google Scholar]
- 24. Gupta AB, Sankararamakrishnan R (2009) Genome-wide analysis of major intrinsic proteins in the tree plant Populus trichocarpa: characterization of XIP subfamily of aquaporins from evolutionary perspective. BMC Plant Biol 9: 134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Daniels MJ, Mirkov TE, Chrispeels MJ (1994) The plasma membrane of Arabidopsis thaliana contains a mercury-insensitive aquaporin that is a homolog of the tonoplast water channel protein TIP. Plant Physiol 106(4): 1325–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Postaire O, Tournaire-Roux C, Grondin A, Boursiac Y, Morillon R, et al. (2010) A PIP1 aquaporin contributes to hydrostatic pressure-induced water transport in both the root and rosette of Arabidopsis . Plant Physiol 152(3): 1418–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Vandeleur RK, Mayo G, Shelden MC, Gilliham M, Kaiser BN, et al. (2009) The role of plasma membrane intrinsic protein aquaporins in water transport through roots: diurnal and drought stress responses reveal different strategies between isohydric and anisohydric cultivars of grapevine. Plant Physiol 149(1): 445–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Chaumont F, Tyerman SD (2014) Aquaporins: Highly regulated channels controlling plant water relations. Plant Physiol 164: 1600–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Sack L, Scoffoni C (2012) Measurement of leaf hydraulic conductance and stomatal conductance and their responses to irradiance and dehydration using the Evaporative Flux Method (EFM). J Vis Exp (70): 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Larson PR, Isebrands JG (1971) The plastochron index as applied to developmental studies of cottonwood. Can J For Res 1(1): 1–11. [Google Scholar]
- 31. Laur J, Hacke UG (2013) Transpirational demand affects aquaporin expression in poplar roots. J Exp Bot 64(8): 2283–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Pavy N, Boyle B, Nelson C, Paule C, Giguère I, et al. (2000) Identification of conserved core xylem gene sets: conifer cDNA microarray development, transcript profiling and computational analyses. New Phytol 180(4): 766–86. [DOI] [PubMed] [Google Scholar]
- 33. Almeida-Rodriguez AM, Cooke JEK, Yeh F, Zwiazek JJ (2010) Functional characterization of drought-responsive aquaporins in Populus balsamifera and Populus simonii×balsamifera clones with different drought resistance strategies. Physiol Plant 140(4): 321–33. [DOI] [PubMed] [Google Scholar]
- 34. Wilkins O, Nahal H, Foong J, Provart NJ, Campbell MM (2009) Expansion and diversification of the Populus R2R3-MYB family of transcription factors. Plant Physiol 149(2): 981–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Cohen D, Bogeat-Triboulot M-B, Vialet-Chabrand S, Merret R, Courty P-E, et al. (2013) Developmental and environmental regulation of aquaporin gene expression across Populus species: Divergence or Redundancy? PLoS One 8(2): e55506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Rutledge RG, Stewart D (2010) Assessing the performance capabilities of LRE-based assays for absolute quantitative real-time PCR. PLoS One 5(3): e9731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Arvidsson S, Kwasniewski M, Riaño-Pachón DM, Mueller-Roeber B (2008) QuantPrime–a flexible tool for reliable high-throughput primer design for quantitative PCR. BMC Bioinformatics 9: 465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 25(4): 402–8. [DOI] [PubMed] [Google Scholar]
- 39. Almeida-Rodriguez AM, Hacke UG, Laur J (2011) Influence of evaporative demand on aquaporin expression and root hydraulics of hybrid poplar. Plant Cell Environ 34(8): 1318–31. [DOI] [PubMed] [Google Scholar]
- 40. Gong H, Peng Y, Zou C, Wang D (2006) A simple treatment to significantly increase signal specificity in immunohistochemistry. Plant Mol Biol Report 24: 93–101. [Google Scholar]
- 41. Kammerloher W, Fischer U, Piechottka GP, Schäffner AR (1994) Water channels in the plant plasma membrane cloned by immunoselection from a mammalian expression system. Plant J 6: 187–99. [DOI] [PubMed] [Google Scholar]
- 42. Sakurai J, Ahamed A, Murai M, Maeshima M, Uemura M (2008) Tissue and cell-specific localization of rice aquaporins and their water transport activities. Plant Cell Physiol 49(1): 30–9. [DOI] [PubMed] [Google Scholar]
- 43. Sparks JP, Black RA (1999) Regulation of water loss in populations of Populus trichocarpa: The role of stomatal control in preventing xylem cavitation. Tree Physiol 19: 453–9. [DOI] [PubMed] [Google Scholar]
- 44. Pezeshki SR, Hinckley TM (1982) The stomatal response of red alder and black cottonwood to changing water status. Can J For Res 12(4): 761–71. [Google Scholar]
- 45. Trifilò P, Gascó A, Raimondo F, Nardini A, Salleo S (2003) Kinetics of recovery of leaf hydraulic conductance and vein functionality from cavitation-induced embolism in sunflower. J Exp Bot 54(391): 2323–30. [DOI] [PubMed] [Google Scholar]
- 46. Martorell S, Diaz-Espejo A, Medrano H, Ball MC, Choat B (2014) Rapid hydraulic recovery in Eucalyptus pauciflora after drought: linkages between stem hydraulics and leaf gas exchange. Plant Cell Environ 37(3): 617–26. [DOI] [PubMed] [Google Scholar]
- 47. Nardini A, Lo Gullo MA, Salleo S (2011) Refilling embolized xylem conduits: is it a matter of phloem unloading? Plant Sci 180(4): 604–11. [DOI] [PubMed] [Google Scholar]
- 48. Christman MA, Sperry JS, Smith DD (2012) Rare pits, large vessels and extreme vulnerability to cavitation in a ring-porous tree species. New Phytol 193: 713–20. [DOI] [PubMed] [Google Scholar]
- 49. Mayr S, Schmid P, Laur J, Rosner S, Charra-Vaskou K, et al. (2014) Uptake of water via branches helps timberline conifers refill embolized xylem in late winter. Plant Physiol 164(4): 1731–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Secchi F, Zwieniecki MA (2010) Patterns of PIP gene expression in Populus trichocarpa during recovery from xylem embolism suggest a major role for the PIP1 aquaporin subfamily as moderators of refilling process. Plant Cell Environ 33(8): 1285–97. [DOI] [PubMed] [Google Scholar]
- 51. Chitarra W, Balestrini R, Vitali M, Pagliarani C, Perrone I, et al. (2014) Gene expression in vessel-associated cells upon xylem embolism repair in Vitis vinifera L. petioles. Planta 239: 887–99. [DOI] [PubMed] [Google Scholar]
- 52. Schulte PJ, Hinckley TM (1987) The relationship between guard cell water potential and the aperture of stomata in Populus . Plant Cell Environ 10: 313–8. [Google Scholar]
- 53. Guyot G, Scoffoni C, Sack L (2012) Combined impacts of irradiance and dehydration on leaf hydraulic conductance: insights into vulnerability and stomatal control. Plant Cell Environ 35(5): 857–71. [DOI] [PubMed] [Google Scholar]
- 54. Nardini A, Salleo S, Raimondo F (2003) Changes in leaf hydraulic conductance correlate with leaf vein embolism in Cercis siliquastrum L. Trees. 17(6): 529–34. [Google Scholar]
- 55. Johnson DM, Mcculloh KA, Woodruff DR, Meinzer FC (2012) Evidence for xylem embolism as a primary factor in dehydration-induced declines in leaf hydraulic conductance. Plant, Cell Environ 35: 760–9. [DOI] [PubMed] [Google Scholar]
- 56. Alexandersson E, Fraysse L, Sjövall-Larsen S, Gustavsson S, Fellert M, et al. (2005) Whole gene family expression and drought stress regulation of aquaporins. Plant Mol Biol 59(3): 469–84. [DOI] [PubMed] [Google Scholar]
- 57. Secchi F, Lovisolo C, Schubert A (2007) Expression of OePIP2.1 aquaporin gene and water relations of Olea europaea twigs during drought stress and recovery. Ann Appl Biol 150(2): 163–7. [Google Scholar]
- 58. Pou A, Medrano H, Flexas J, Tyerman SD (2012) A putative role for TIP and PIP aquaporins in dynamics of leaf hydraulic and stomatal conductances in grapevine under water stress and re-watering. Plant Cell Environ 36(4): 828–43. [DOI] [PubMed] [Google Scholar]
- 59. Barrieu F, Chaumont F, Chrispeels MJ (1998) High expression of the tonoplast aquaporin ZmTIP1 in epidermal and conducting tissues of maize. Plant Physiol 117(4): 1153–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Daniels MJ, Chaumont F, Mirkov TE, Chrispeels MJ (1996) Characterization of a new vacuolar membrane aquaporin sensitive to mercury at a unique Site. Plant Cell 8: 587–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Almeida-Rodriguez AM, Hacke UG (2012) Cellular localization of aquaporin mRNA in hybrid poplar stems. Am J Bot 99(7): 1249–54. [DOI] [PubMed] [Google Scholar]
- 62. Secchi F, Zwieniecki MA (2013) The physiological response of Populus tremula x alba leaves to the down-regulation of PIP1 aquaporin gene expression under no water stress. Front Plant Sci 4: 507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Kirch H, Vera-estrella R, Golldack D, Quigley F, Michalowski CB, et al. (2000) Expression of water channel proteins in Mesembryanthemum crystallinum . Plant Physiol 123: 111–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Yamada S, Bohnert HJ (2000) Expression of the PIP aquaporin promoter-MipA from the common ice plant in tobacco. Plant Cell Physiol 41(6): 719–25. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(a) Amino acid multiple sequence alignment of the N-terminal region of the Arabidopsis thaliana AtPIP1;3 and the Populus trichocarpa PtPIP1s; (b) of the conserved the C-terminal region of PIP2s, and (c) TIP2s. Consensus amino acids are underlined in black.
(DOCX)
Transverse section of a Populus trichocarpa leaf showing minor veins with (left) and without (right) bundle sheath cell extensions. Scale bar = 20 µm.
(DOCX)
Primer sequences used for the gene expression study.
(DOCX)
Data Availability Statement
The authors confirm that all data underlying the findings are fully available without restriction. All relevant data are within the paper and its Supporting Information files.